Abstract
The recommended treatment for latent tuberculosis (TB) infection in adults is a daily dose of isoniazid (INH) 300 mg for six months. In Brazil, INH was formulated as 100 mg tablets. The treatment duration and the high pill burden compromised patient adherence to the treatment. The Brazilian National Programme for Tuberculosis requested a new 300 mg INH formulation. The aim of our study was to compare the bioavailability of the new INH 300 mg formulation and three 100 mg tablets of the reference formulation. We conducted a randomised, single dose, open label, two-phase crossover bioequivalence study in 28 healthy human volunteers. The 90% confidence interval for the INH maximum concentration of drug observed in plasma and area under the plasma concentration vs. time curve from time zero to the last measurable concentration “time t” was 89.61-115.92 and 94.82-119.44, respectively. The main limitation of our study was that neither adherence nor the safety profile of multiple doses was evaluated. To determine the level of INH in human plasma, we developed and validated a sensitive, simple and rapid high-performance liquid chromatography-tandem mass spectrometry method. Our results showed that the new formulation was bioequivalent to the 100 mg reference product. This finding supports the use of a single 300 mg tablet daily strategy to treat latent TB. This new formulation may increase patients’ adherence to the treatment and quality of life.
latent tuberculosis; isoniazid; bioavailability; bioequivalence; HPLC-MS/MS method
The greatest public health goal in tuberculosis (TB) control is stopping the transmission of Koch bacilli. The most efficient approach to stop transmission is treating infectious cases using an appropriate drug regimen. However, this approach does not benefit patients who have already been infected and present with latent TB infection. An important secondary objective towards eliminating TB is treating patients with latent infection who are at high risk of developing TB disease and becoming infectious. (IUATCP 1982IUATCP - International Union Against Tuberculosis Committee on Prophylaxis 1982. Efficacy of various durations of isoniazid preventive therapy for tuberculosis: five years of follow-up in the IUAT trial. Bull World Health Organ 60: 555-564., WHO 2012a) TB is a co-morbidity associated with human immunodeficiency virus (HIV) infection and the treatment of latent TB infection in HIV epidemic areas is essential (WHO 2012b).
In the 1950s, Horwitz et al. (1966)Horwitz O, Payne PG, Wilbek E 1966. Epidemiological basis of tuberculosis eradication. 4. The isoniazid trial in Greenland. Bull World Health Organ 35: 509-526. evaluated isoniazid (INH) as TB prophylaxis in a double blind, randomised, placebo controlled clinical trial in Greenland. The results demonstrated a reduced incidence of active infection in the treated arm after six years of follow-up. However, they concluded that chemoprophylaxis recommendations should be restricted to selected population groups (Horwitz et al. 1966Horwitz O, Payne PG, Wilbek E 1966. Epidemiological basis of tuberculosis eradication. 4. The isoniazid trial in Greenland. Bull World Health Organ 35: 509-526.).
The results of subsequent clinical studies support that 300 mg INH treatment for one year is effective at preventing the progression of latent TB to active infection (Comstock 1962Comstock GW 1962. Isoniazid prophylaxis in an undeveloped area.Am Rev Respir Dis 86: 810-822., Bush Jr et al. 1965, Del Castillo et al. 1965Del Castillo H, Bautista LD, Jacinto CP, Lorenzo CE, Lapuz S, Legaspi B 1965. Chemoprophylaxis in the Philippines: a controlled pilot study among household contacts of tuberculosis cases. Bull Quezon Institute 7: 277-290., Egsmose et al. 1965Egsmose T, Ang’awa JO, Poti SJ 1965. The use of isoniazid among household contacts of open cases of pulmonary tuberculosis. Bull World Health Organ 33: 419-433., Akolo et al. 2010Akolo C, Adetifa I, Shepperd S, Volmink J 2010. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev 1: CD000171.). In 1982, the International Union Against Tuberculosis and Lung Diseases published a study comparing six, nine and 12 months of treatments with a five-year follow up of 28,000 patients (IUATCP 1982IUATCP - International Union Against Tuberculosis Committee on Prophylaxis 1982. Efficacy of various durations of isoniazid preventive therapy for tuberculosis: five years of follow-up in the IUAT trial. Bull World Health Organ 60: 555-564.). This study supports the reduction of chemoprophylaxis to six months, especially given the improved adherence to the proposed six months of treatment. The Brazilian guidelines for the treatment of latent TB recommend 5-10 mg per kg weight with a 300 mg maximum dose for at least six months (PNCT 2011PNCT - Programa Nacional de Controle da Tuberculose 2011.Manual de recomendações para o controle da tuberculose no Brasil, Ministério da Saúde/Secretaria de Vigilância em Saúde/Departamento de Vigilância Epidemiológica, Brasília, 284 pp.). Selected populations should be exposed to INH if there are risk factors for the progression to active TB disease. Thus, chemoprophylaxis is recommended in the following situations: immunodeficiency or under immunosuppressive treatment, age under two years and above 60 years and malnutrition. Chemoprophylaxis for patients with HIV infection is especially highlighted (PNCT 2011PNCT - Programa Nacional de Controle da Tuberculose 2011.Manual de recomendações para o controle da tuberculose no Brasil, Ministério da Saúde/Secretaria de Vigilância em Saúde/Departamento de Vigilância Epidemiológica, Brasília, 284 pp.).
Reduced immunocompetence in acquired immune deficiency syndrome (AIDS) patients increases the risk of reactivation of a remote TB infection. A study conducted with Haitian subjects who were likely infected with TB during childhood showed that AIDS was related to the progression to active TB in 60% of the cases (Pitchenik et al. 1984Pitchenik AE, Cole C, Russell BW, Fischl MA, Spira TJ, Snider Jr DE 1984. Tuberculosis, atypical mycobacteriosis and the acquired immunodeficiency syndrome among Haitian and non-Haitian patients in south Florida. Ann Intern Med 101: 641-645.).
If left untreated, most HIV patients with a positive purified protein derivative test will progress to active disease. The therapeutic recommendation for this population is wider than the other groups because they have a higher risk of mortality and a higher risk of becoming infectious (MS/SVS/PN DST/aids 2008MS/SVS/PN DST/aids - Ministério da Saúde/Secretaria de Vigilância em Saúde/Programa Nacional de DST e Aids 2008. Recomendações para terapia anti-retroviral em adultos infectados pelo HIV: 2008, MS, Brasília, 244 pp.).
In Brazil, the governmental national control programmes supply highly active anti-retroviral therapy (HAART) and anti-TB drugs free of charge to the population. Until now, INH has only been available as 100 mg tablets in the country. The number of necessary tablets to complete the recommended six months of treatment may compromise patient adherence to the treatment (WHO 2003WHO - World Health Organization 2003. Adherence to long-term therapies: evidence for action. Available from: whqlibdoc.who.int/publications/2003/9241545992.pdf.
whqlibdoc.who.int/publications/2003/9241...
). This issue is even more serious in HIV patients on HAART because they already have a high pill burden. For these reasons, the Brazilian National Programme for Tuberculosis (PNCT) requested the development of a new 300 mg INH formulation. This new formulation provides an important pill burden reduction to Brazilian patients (from 540-180 tablets for the whole treatment). This reduction may have a considerable impact on patient adherence to INH.
We conducted this study to support the registration of the new product by the Brazilian regulatory agency. The aim of the study was to compare the bioavailability of the new INH 300 mg formulation manufactured by Institute of Drug Technology (Farmanguinhos)/Oswaldo Cruz Foundation (Fiocruz) and three 100 mg tablets of the reference formulation. As a secondary objective, we assessed the tolerability of a single dose of the formulations.
SUBJECTS, MATERIALS AND METHODS
Clinical protocol - The protocol for this trial is available asSupplementary data. The clinical study protocol and informed consent were reviewed and approved by the Ethical Committee at the University of Campinas (CAAE 3652.0.000.146-08). This committee is accredited by the Brazilian National Council on Ethics in Research of the Ministry of Health. The clinical study was conducted in accordance with the Helsinky Declaration revised in 2000, Good Clinical Practice (ICH 1996), Guidance on the Investigation of Bioavailability and Bioequivalence (CPMP/EWP/QWP/1401 1998) and Brazilian legislation (RES 196/96/MS/CNS 1996, RES 251/97/MS/CNS 1997, RES 1170/06/MS/CNS 2006). Written informed consent was obtained for every subject prior to enrolment. All of the subjects were informed about the nature of the trial, the possible risks and that they were free to withdraw their consent of participation at any time. The investigators and study staff observed the confidentiality of the records. The clinical part of the study was conducted at the Synchrophar clinical unit at Irmãos Penteado Hospital, Campinas, state of São Paulo, Brazil, which is accredited by the Brazilian Health Surveillance Agency (ANVISA).
The trial was designed as a single centre, randomised, single dose, open label, fasting, two-phase crossover bioequivalence study with an washout period of seven days (> 7 half-life). The trial was registered at the Brazilian National Information System of Pharmaceutical Equivalence and Bioequivalence studies (SINEB) (registry CEF 03/08) and in the clinicaltrials.gov database (registry NCT02043314). The authors confirm that all of the ongoing and related trials for this intervention are registered.
The inclusion criteria were predefined as follows: healthy men and women, aged between 18-50 years and body-mass-index (BMI) between 18.5-29.9 kg/m2. The exclusion criteria included known hypersensitivity to INH, pregnancy, use of other drugs other than contraceptives, health problems, alcohol or drug abuse and any other condition that could alter absorption, distribution, metabolism and excretion of INH.
Study procedures - The study participants were recruited and enrolled from the study volunteers’ pool at Synchrophar. All of the volunteers met the inclusion and the exclusion criteria defined in the study protocol. Healthy volunteers were considered to be eligible for the study based on medical history, demographic data, medication history, physical examination and clinical laboratory tests [haematology, biochemistry, urinalysis, HIV and hepatitis B and C, parasitological faeces test, β-HCG (female subjects before each administration of study drugs and post study) and a 12-lead electrocardiogram].
The volunteers were recruited and included in the study on the 21 October 2008, the second phase was conducted after the washout period of seven days and the follow-up adverse event (AE) evaluation was conducted 10 days after the last blood collection. The Labsefar statistician performed the randomisation. Each subject was assigned to one of two study treatment sequences (RT or TR). The trial was open label and thus no blinding was needed. After 10 h of fasting overnight, each subject received either the test formulation (INH 300 mg manufactured by Farmanguinhos - batch 06102382) or three tablets of the reference formulation (INH 100 mg manufactured by Aeronautics Chemical and Pharmaceutical Laboratory - batch 07090034). The study drugs were administered with 200 mL of mineral water. Mouth checks were performed following drug administration to ensure ingestion of each dose. Subjects continued to fast until after 4 h after the administration and controlled meals were served afterwards. Fluid intake was allowed ad libitum until 6 h before dosing and 2 h after dosing.
Serial blood samples for the pharmacokinetic assessments were collected over a period that was greater than three half-lives at the following time points: 0 (pre-dose 30 min before dosing), 0:20, 0:40, 1, 1:20, 1:40, 2, 2:30, 3, 3:30, 4, 5, 6, 8, 12 and 24 h after oral administration of each treatment. Blood samples (7.5 mL each) were drawn into heparinised tubes for further processing. Immediately after blood collection, the tube was inverted by hand to ensure thorough mixing with the anticoagulant and centrifuged for 10 min at 3,000 rpm (+4ºC). After centrifugation, the plasma samples were immediately transferred to separate appropriately labelled plastic cryotubes and stored at -25ºC until analysis.
AEs were assessed either by periodic physical examination or by clinical laboratory tests at baseline and at the end of the study. Registration of AEs included duration, severity, intensity and causality relationship with the drug as well as the outcome. Neither serious deviation nor violation of the study protocol occurred.
Fig. 1 shows the flow diagram of participants throughout the trial.
Drug analysis - The analytical part of the study was performed from 11 December 2008-14 January 2009. The statistical analysis was performed from 11 December 2008-16 March 2009. The study was finalised in March 2009.
Chemicals and reagents - The INH reference standard and fluconazole (FLC) [internal standard (IS)] were identified and supplied by the United States Pharmacopoeia (USA). Methanol, acetonitrile and isopropanol were of chromatographically pure grade and the formic acid was of analytical grade. All of the chemicals were purchased from Tédia Company (USA). Ultra purified water (type I) was separated and used for preparing the solutions and the mobile phase study.
Instrumentation and operating conditions - Liquid chromatography was performed using a Varian System (USA) equipped with two Varian 212-LC pumps. A CTC Analytics HTS auto-injector was used. Chromatographic separation was conducted using an Inertsil Sil 100A (5 μm, 50 x 2.1 mm I.D.) column at an ambient temperature. The mobile phase consisted of a mixture of the following two solutions: (i) water containing formic acid 0,046% and (ii) isopropanol-acetonitrile. The composition of the mobile phase was 20A/80B v/v and was set at a flow rate of 0.3 mL/min.
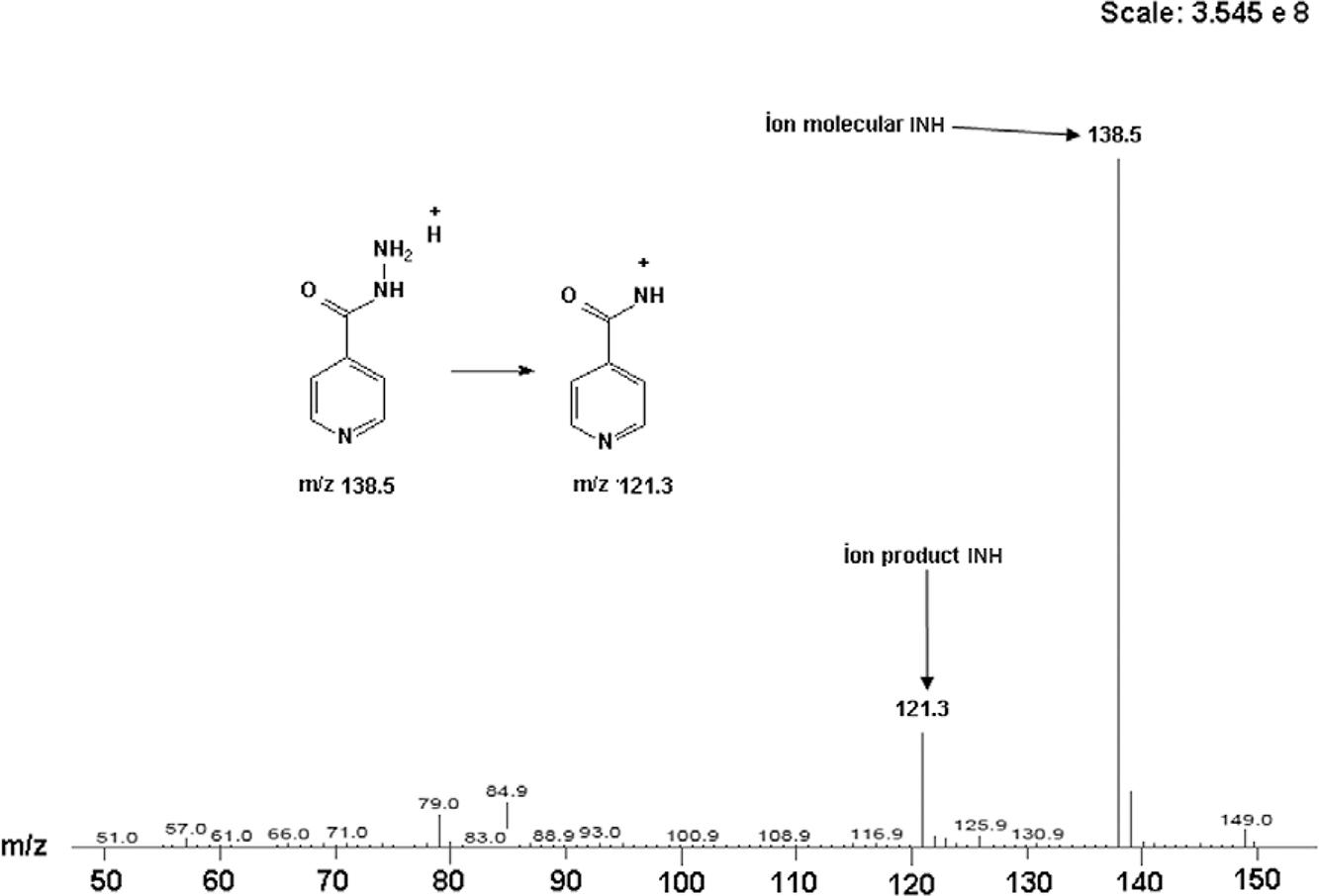
The mass spectrometric detection was performed using a Varian 310-MS quadrupole LC-MS/MS system (USA) equipped with an electrospray ion source and operated in the positive ionisation mode. The ion spray voltage and source temperature were 3,000 V and 400ºC, respectively. The parameters for the other gas source were set, as follows: drying gas 20 psi, nebulizer gas 55 psi and collision induced dissociation 2.00 mTorr. Quantification was performed using multiple reaction monitoring of the fragmentation transitions m/z 138.50→121.30 for INH and m/z 307.30→238.00 for FLC (IS) (Figs 2, 3). Data acquisition and analysis were achieved using the Varian MS Workstation software v.6.6 (USA).
Preparation of stock and work solutions - The stock solution of INH was prepared at a concentration of 1.0 mg/mL in ultrapure water. Stock solutions of FLC (IS) were prepared only in methanol/water - 50/50 - v/v at a concentration of 1.0 mg/mL. All of the solutions were stored at -25ºC.
The standard working solutions of INH analytes were prepared in 200, 400, 1,000, 2,000, 4,000, 10,000 and 15,000 ng/mL concentrations. They were obtained by serial dilution of the stock solution with ultrapure water. Quality control (QC) working solutions of INH in 200, 400, 4,000 and 10,000 ng/mL concentrations were similarly prepared as the standard working solution. The working IS solution containing 1,000 ng/mL of FLC was prepared in methanol/water - 50/50 - v/v by diluting the stock solutions. When not in use, all of the solutions were stored at -25ºC in an amber glass bottle.
Sample preparation - QCs, calibration curve and clinical plasma samples were extracted using a precipitation extraction technique. A 0.10 mL aliquot of the collected plasma sample from healthy volunteers, QCs or calibration curve was pipetted into a 2 mL polypropylene tube. The working solution of the IS (1,000 ng/mL) and 0.4 mL of acetonitrile were added and then vortexed for 1 min. Then, the samples were centrifuged at 14,400 rpm for 5 min at +4ºC. The organic layer was transferred to another 2 mL polypropylene tube, 0.1 mL ultrapure water and formic acid 0.046% were added and the mixture was vortexed for 30 s. An aliquot of 0.3 mL was transferred to vial glasses and 10 µL was injected into the LC-MS/MS system.
Method validation - The validation process was conducted according to guidance (Bioanalytical Method Validation) provided by the US Food Drug Administration (FDA 2001FDA - Food and Drug Administration 2001. Guidance for industry: bioanalytical method validation. Available from: fda.gov/downloads/Drugs/Guidances/ucm070107.pdf.
fda.gov/downloads/Drugs/Guidances/ucm070...
). This guidance is in accordance with resolution 899 of 23 May 2003 by the ANVISA (ANVISA 2003b).
Selectivity was assessed by comparing chromatograms of six different batches of blank human plasma with the corresponding spiked human plasma. In this test, no significant interference was detected in the retention times of the analyte and the IS. Intra and inter-day precision [the relative standard deviation (RSD)] and accuracy (the relative error) were determined by analysing low, medium and high QC samples (n = 8) on three different days. The extraction recovery was determined by comparing the mean peak areas of eight extracted samples at low, medium and high QC concentrations with the mean peak areas of Spike after the extraction samples.
Stability was assessed by analysing the replicates (n = 8) of the low and high QC samples during sample storage and processing procedures. The freeze-thaw stability was determined after four freeze-thaw cycles. Post-preparation stability was estimated by analysing QC samples at 24 h at +4ºC. Six aliquots of QC samples were stored at -20ºC for 63 days and at ambient temperature for 6 h to determine long-term and short-term stability, respectively.
Pharmacokinetics analysis - Pharmacokinetic analysis was performed with the data from subjects who completed both periods of the study using the WinNonlin® software v.5.01 (Pharsight Co, USA). Concentration values below the quantification limit (100 ng/mL) at the initial absorption or at the terminal elimination phase were considered to be zero. Missing values were not included in the pharmacokinetic evaluation calculation.
The following pharmacokinetic parameters were estimated: maximum concentration of drug observed in plasma (Cmax), area under the plasma concentration vs. time curve from time zero to the last measurable concentration “time t” (AUC0-t) calculated using a linear trapezoidal method, area under the plasma concentration vs. time curve from time zero to time infinity (AUC0-inf) calculated as where cycle threshold is the last concentration of the drug, the time required to reach maximum concentration of drug in plasma (Tmax), elimination rate constant, Lambda_z and the time it took the plasma concentration to reduce to 50% during the elimination phase. A noncompartmental method was used to estimate the pharmacokinetic parameters.
The pharmacokinetic parameters were calculated from the plasma concentrations using a noncompartmental method for INH. The area under the curve was obtained by the linear trapezoidal method. The Cmax and the Tmax were obtained by directly reading the concentration results.
Statistical analysis - Summary statistics were calculated for the concentration-time data of the test and reference products for all of the pharmacokinetic parameters, including the arithmetic mean [mean, SD, coefficient of variation (CV), minimum (min), maximum (max) and median].
The bioequivalence acceptance interval was set at 80-125% as required by the regulatory agency (ANVISA 2003b). The 90% confidence interval (CI) of the test/reference mean ratio of INH was computed for the log-transformed pharmacokinetic parameters of Cmax, AUC0-t and AUC0-inf. The ANOVA model included the following factors: sequence, subject with sequence, period and treatment.
Tables I, II show the pharmacokinetic parameters of the test product and the reference, respectively.
RESULTS
Drug analysis - INH is a hydrophilic compound that is easily eluted from a normal reversed phase C18 column. Therefore, to chromatically resolve these compounds, we used a highly mobile phase and an Inertsil Sil 100A (5 μm, 50 x 2.1 mm I.D.) column that provided appropriate retention and selectivity for polar compounds.
The best analysis conditions were achieved using a mixture of solutions, including (i) water containing formic acid 0.046% and (ii) isopropanol-acetonitrile. This mixture provided a good chromatographic peak shape of the analyte and the IS as well as their elution in a short period of analysis when compared with literature (Panchagnula et al. 2003Panchagnula R, Sancheti P, Rungta S, Agrawal S, Kaul CL 2003. Evaluation of bioequivalence of isoniazid and pyrazinamide in three and four drugs fixed dose combinations using WHO simplified protocol. Pharmacol Res 48: 383-387., Wang et al. 2007Wang A, Zhang W, Sun J, Liu J, Sang Y, Gao S, He Z 2007. HPLC-MS analysis of isoniazid in dog plasma. Chromatographia 66: 741-745.). The extraction method by precipitation with acetonitrile was adequate for the study. However, the analyst chose to perform a procedure of division in the acquisition of masses at the beginning of the analysis up to 0.5 min to eliminate potential contaminants that could suppress the signal when in contact with the ion source (Fig. 4). The linearity of the method set from the LLQ of 100 ng/mL to the upper limit of 7,500 ng/mL (Table III) was sufficient to define the pharmacokinetic parameters of the study. The linearity limits of the bioanalytical method were defined based on published data of the bioavailability of INH.
Table III describes the calibration curve of INH with precision and accuracy according to regulatory agency requirements [20% accuracy for the lower limit of quantification (LLQ) (100 ng/mL) and 15% for the other concentration values] (ANVISA 2003b). The LLQ of 100 ng/mL was established as a function of signal to noise ratio greater than 10 and quantifiable analytical signal with acceptable precision and accuracy.
Intra-day, inter-day precisions and accuracies were within the pre-defined acceptance limits (Table IV). The precision and accuracy were according to the acceptable criteria recommended by the ANVISA. The precision at each concentration level did not exceed a CV of 15% and its accuracy was within the range established (i.e., 85-115%) for each concentration level tested. The mean extraction recoveries of INH (n = 8) were 80.35%, 88.59%, 94.58% at concentrations of 200, 2,000 and 5,000 ng/mL, respectively (Table IV).
The plasma stability tests were reproduced under the conditions of analysis in which the subject samples were studied. The results presented in Table V were obtained comparing freshly prepared samples with those undergoing short-term stability tests (6 h, room temperature), post-preparative stability (48 h, +7ºC), freeze-thaw stability (4 cycles - 96 h, -20ºC) and long-term stability (63 days, -20ºC).
Bioequivalence analysis - Twenty-eight healthy subjects of both genders between 21-50 years of age (µ 33 and SD 8.42), weight 54.3-84 kg (µ 65.88 and SD 8.29) and BMI ≥ 19.79 kg/m2 and ≤ 28.04 kg/m2 (µ 24.09 and SD 2.08) were enrolled and dosed in the study. All of the subjects completed both periods of the study.
The mean plasma concentration-time profile for the test and reference product of INH is shown in Fig. 5. The mean pharmacokinetic parameters of INH are provided in Tables I,II for the test and reference product, respectively.
The average bioequivalence was evaluated based on the 90% CI for the intra-individual mean ratio of log-transformed Cmax and AUC0-t of the test and reference formulation. The 90% CI interval was planned in the protocol and was recommended by the regulatory agencies (ANVISA 2003a, FDA 2006FDA - Food and Drug Administration 2006. Guidance for industry: bioequivalence guidance. Available from: fda.gov/downloads/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/ucm052363.pdf.
fda.gov/downloads/AnimalVeterinary/Guida...
). The 90% CI for the INH log transformed Cmax and AUC0-t was 89.61, 115.92 and 94.82-119.44, respectively. Hence, all of the pharmacokinetic parameters were within the accepted bioequivalence range 80-125% for Cmax and AUC0-t of INH.
Tolerability analysis - Fourteen volunteers (50%) presented with 25 AEs. The most frequent AEs were anaemia or low haemoglobin levels (9 cases) and headache (8 cases). These events might be related to study procedures as the sample collection. The distribution of AEs was similar between the reference and test groups, as follows: 12 AEs were reported after the administration of the reference formulation and 13 were reported after the test dosing formulation. Symptomatic treatment was prescribed in nine cases. Causality was defined as probable in 14 cases, possible in six cases and unrelated in five cases. No serious AEs were reported during the study.
DISCUSSION
This manuscript addresses a new treatment approach for latent TB that is highly relevant for clinical practice in Brazil. Because there have been no radical innovations for TB treatment for a long time, incremental innovations are important to increase patients’ quality of life. Public health policies that reduce pill burden have been recommended to simplify treatments (Blomberg et al. 2001Blomberg B, Spinaci S, Fourie B, Laing R 2001. The rationale for recommending fixed-dose combination tablets for treatment of tuberculosis.Bull World Health Organ 79: 61-68.) increase compliance and even prevent the emergence of resistance (Mitchison 1998Mitchison DA 1998. How drug resistance emerges as a result of poor compliance during short course chemotherapy for tuberculosis. Int J Tuberc Lung Dis 2: 10-15.). A well-known approach is the use of fixed dose combinations (Blomberg et al. 2001Blomberg B, Spinaci S, Fourie B, Laing R 2001. The rationale for recommending fixed-dose combination tablets for treatment of tuberculosis.Bull World Health Organ 79: 61-68.). Because the new formulation will reduce pill burden compared to the old formulation, it is expected to improve care by simplifying treatment.
The main limitation of our study is that neither adherence nor multiple doses safety profile was evaluated. However, an increased risk of AEs is not expected because the daily and total dose of treatment remains the same. The bioavailability results confirm that exposure to the drug using the old formulation and the new formulation is equivalent.
For this bioequivalence study, a sensitive, simple and rapid high-performance liquid chromatography-tandem mass spectrometry method to determine INH levels in human plasma was developed and validated. The described method showed good specificity, precision, accuracy and linearity over the range of 100-7,500 mg/mL for INH. This method provided a simple sample preparation using protein precipitation with acetonitrile. The established LLQ of 100 ng/mL for INH was adequate to determine concentrations in human plasma during the pharmacokinetic study. No significant interferences caused by endogenous compounds were observed.
The stability tests demonstrated that the INH analyte showed no signs of degradation when present in the biological matrix under plasma conditions of storage and analysis in short and long term. The developed method using tandem mass spectrometry demonstrated the advantage of a short run time and high selectivity for application to high throughput analysis. Contrary to usual practice, this analytical method is thoroughly reported in this paper. Researchers will benefit from this simple method, which can be used to explore the pharmacokinetic aspects of INH in future research studies, clinical practice and therapeutic drug monitoring.
By applying this method, the new formulation of INH 300 mg has been shown to be bioequivalent to three tablets of the 100 mg reference product. This new product in Brazil may increase adherence to treatment for latent TB. In 2013, the PNCT made the standard dose formulation recommended worldwide available to Brazilians. While the new formulation is made available in the country, the medical community needs to be aware of the new treatment strategy adopted by the PNCT, namely a single tablet of 300 mg INH daily for six months. Increasing medical awareness is important to avoid medication errors when prescribing INH because the wrong use of the new formulation could lead to a higher frequency of AEs. Further pharmacovigilance studies may explore the misuse of INH formulations.
This bioequivalence study reports important data on the pharmacokinetics of INH to consider including INH as a Class I drug in the Biopharmaceutical Classification System (BCS). The BCS may avoid unnecessary exposure of healthy human volunteers to clinical trials. The BCS may also lower the costs of developing generic products and improve the population’s access to these products. INH has been regarded as a product that can be considered as a biowaiver, i.e., a product can be approved in a regulatory agency based on in vitro dissolution tests rather than requiring bioequivalence studies in human subjects (Lindenberg et al. 2004Lindenberg M, Kopp S, Dressman JB 2004. Classification of orally administered drugs on the World Health Organization Model List of Essential Medicines according to the biopharmaceutics classification system. Eur J Pharm Biopharm 58: 265-278.). However, regulatory agencies have been refusing this approach because standard permeability tests have not been defined. The historic series of bioequivalence studies have been the actual gold standard to define the permeability of a product. In 2011, the Brazilian regulatory agency published a list of biowaivers that included INH (ANVISA 2011ANVISA - Agência Nacional de Vigilância Sanitária 2011. Instrução Normativa 4. Available from: bvsms.saude.gov.br/bvs/saudelegis/anvisa/2011/int0004_03_08_2011.html.
bvsms.saude.gov.br/bvs/saudelegis/anvisa...
). This study may provide additional support for this decision that can lower the costs of future INH formulation developments. These results could also support the acceptance of INH as a biowaiver by regulatory agencies worldwide.
This bioavailability study demonstrates that the new formulation of INH 300 mg and three 100 mg tablets of the reference formulation are interchangeable. The study led to the registration of the new product by the Brazilian regulatory agency. By increasing patient adherence to the treatment, it is expected that the new formulation will improve patient care for latent TB.
The analytical method reported provides a sensitive, simple and rapid way of measuring INH in human plasma that will help future research on INH worldwide.
ACKNOWLEDGEMENTS
To all the volunteers that participated in this study.
REFERENCES
- Akolo C, Adetifa I, Shepperd S, Volmink J 2010. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev 1: CD000171.
- ANVISA - Agência Nacional de Vigilância Sanitária 2003a. Resolução 898. Guia para planejamento e realização da etapa estatística de estudos de biodisponiblidade relativa/bioequivalência. Available from: anvisa.gov.br/medicamentos/bioequivalencia/legis.htm.
» anvisa.gov.br/medicamentos/bioequivalencia/legis.htm - ANVISA - Agência Nacional de Vigilância Sanitária 2003b. Resolução 899. Guia para validação de métodos analíticos e bioanalíticos. Available from: anvisa.gov.br/medicamentos/bioequivalencia/legis.htm.
» anvisa.gov.br/medicamentos/bioequivalencia/legis.htm - ANVISA - Agência Nacional de Vigilância Sanitária 2011. Instrução Normativa 4. Available from: bvsms.saude.gov.br/bvs/saudelegis/anvisa/2011/int0004_03_08_2011.html.
» bvsms.saude.gov.br/bvs/saudelegis/anvisa/2011/int0004_03_08_2011.html - Blomberg B, Spinaci S, Fourie B, Laing R 2001. The rationale for recommending fixed-dose combination tablets for treatment of tuberculosis.Bull World Health Organ 79: 61-68.
- Bush Jr OB, Sugimoto M, Fujii Y, Brown Jr FA 1965. Isoniazid pro- phylaxis in contacts of persons with known tuberculosis. Second report.Am Rev Respir Dis 92: 732-740.
- Comstock GW 1962. Isoniazid prophylaxis in an undeveloped area.Am Rev Respir Dis 86: 810-822.
- Del Castillo H, Bautista LD, Jacinto CP, Lorenzo CE, Lapuz S, Legaspi B 1965. Chemoprophylaxis in the Philippines: a controlled pilot study among household contacts of tuberculosis cases. Bull Quezon Institute 7: 277-290.
- Egsmose T, Ang’awa JO, Poti SJ 1965. The use of isoniazid among household contacts of open cases of pulmonary tuberculosis. Bull World Health Organ 33: 419-433.
- FDA - Food and Drug Administration 2001. Guidance for industry: bioanalytical method validation. Available from: fda.gov/downloads/Drugs/Guidances/ucm070107.pdf.
» fda.gov/downloads/Drugs/Guidances/ucm070107.pdf - FDA - Food and Drug Administration 2006. Guidance for industry: bioequivalence guidance. Available from: fda.gov/downloads/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/ucm052363.pdf.
» fda.gov/downloads/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/ucm052363.pdf - Horwitz O, Payne PG, Wilbek E 1966. Epidemiological basis of tuberculosis eradication. 4. The isoniazid trial in Greenland. Bull World Health Organ 35: 509-526.
- IUATCP - International Union Against Tuberculosis Committee on Prophylaxis 1982. Efficacy of various durations of isoniazid preventive therapy for tuberculosis: five years of follow-up in the IUAT trial. Bull World Health Organ 60: 555-564.
- Lindenberg M, Kopp S, Dressman JB 2004. Classification of orally administered drugs on the World Health Organization Model List of Essential Medicines according to the biopharmaceutics classification system. Eur J Pharm Biopharm 58: 265-278.
- Mitchison DA 1998. How drug resistance emerges as a result of poor compliance during short course chemotherapy for tuberculosis. Int J Tuberc Lung Dis 2: 10-15.
- MS/SVS/PN DST/aids - Ministério da Saúde/Secretaria de Vigilância em Saúde/Programa Nacional de DST e Aids 2008. Recomendações para terapia anti-retroviral em adultos infectados pelo HIV: 2008, MS, Brasília, 244 pp.
- Panchagnula R, Sancheti P, Rungta S, Agrawal S, Kaul CL 2003. Evaluation of bioequivalence of isoniazid and pyrazinamide in three and four drugs fixed dose combinations using WHO simplified protocol. Pharmacol Res 48: 383-387.
- Pitchenik AE, Cole C, Russell BW, Fischl MA, Spira TJ, Snider Jr DE 1984. Tuberculosis, atypical mycobacteriosis and the acquired immunodeficiency syndrome among Haitian and non-Haitian patients in south Florida. Ann Intern Med 101: 641-645.
- PNCT - Programa Nacional de Controle da Tuberculose 2011.Manual de recomendações para o controle da tuberculose no Brasil, Ministério da Saúde/Secretaria de Vigilância em Saúde/Departamento de Vigilância Epidemiológica, Brasília, 284 pp.
- Wang A, Zhang W, Sun J, Liu J, Sang Y, Gao S, He Z 2007. HPLC-MS analysis of isoniazid in dog plasma. Chromatographia 66: 741-745.
- WHO - World Health Organization 2003. Adherence to long-term therapies: evidence for action. Available from: whqlibdoc.who.int/publications/2003/9241545992.pdf.
» whqlibdoc.who.int/publications/2003/9241545992.pdf - WHO - World Health Organization 2012a. Global tuberculosis report. Available from: apps.who.int/iris/bitstream/10665/75938/1/9789241564502_eng.pdf.
» apps.who.int/iris/bitstream/10665/75938/1/9789241564502_eng.pdf - WHO - World Health Organization 2012b. WHO policy on collaborative TB/HIV activities: guidelines for national programmes and other stakeholders. Available from: apps.who.int/iris/bitstream/10665/44789/1/9789241503006_eng.pdf?ua=1&ua=1.
» apps.who.int/iris/bitstream/10665/44789/1/9789241503006_eng.pdf?ua=1&ua=1
-
Erratum
Vol. 110 (4): 543-550, 2015.p. 543André Daher/+, Luciana Pitta, Tereza Santos, Draurio Barreira, Douglas PintoFarmanguinhos, Fundação Oswaldo Cruz, Rio de Janeiro, RJ, Brasilshould read:André Daher1/+, Luciana Pitta1, Tereza Santos1, Draurio Barreira2, Douglas Pinto31Farmanguinhos 3Laboratório da Farmacocinética, Fundação Oswaldo Cruz, Rio de Janeiro, RJ, Brasil 2Programa Nacional de Controle de Tuberculose, Ministério da Saúde, Brasília, DF, Brasil
Publication Dates
-
Publication in this collection
02 June 2015 -
Date of issue
June 2015
History
-
Received
2 Dec 2014 -
Accepted
10 Feb 2015