Abstract
The purpose of this study is to evaluate four rapid colourimetric methods, including the resazurin microtitre assay (REMA), malachite green decolourisation assay (MGDA), microplate nitrate reductase assay (MNRA) and crystal violet decolourisation assay (CVDA), for the rapid detection of multidrug-resistant (MDR) tuberculosis. Fifty Mycobacterium tuberculosis isolates were used in this study. Eighteen isolates were MDR, two isolates were only resistant to isoniazid (INH) and the remaining isolates were susceptible to both INH and rifampicin (RIF). INH and RIF were tested in 0.25 µg/mL and 0.5 µg/mL, respectively. The agar proportion method was used as a reference method. MNRA and REMA were performed with some modifications. MGDA and CVDA were performed as defined in the literature. The agreements of the MNRA for INH and RIF were 96% and 94%, respectively, while the agreement of the other assays for INH and RIF were 98%. In this study, while the specificities of the REMA, MGDA and CVDA were 100%, the specificity of the MNRA was lower than the others (93.3% for INH and 90.9% for RIF). In addition, while the sensitivity of the MNRA was 100%, the sensitivities of the others were lower than that of the MNRA (from 94.1-95%). The results were reported on the seventh-10th day of the incubation. All methods are reliable, easy to perform, inexpensive and easy to evaluate and do not require special equipment.
resazurin microtitre assay; malachite green decolourisation assay; microplate nitrate reductase assay; crystal violet decolourisation assay; multidrug-resistant tuberculosis
Mycobacterium tuberculosis is the oldest known infectious agent and remains a major public health problem (WHO 2014WHO - World Health Organization 2014. Global tuberculosis report 2014. Available from: http://apps.who.int/iris/bitstream/10665/137094/1/9789241564809_eng.pdf?ua=1.
http://apps.who.int/iris/bitstream/10665...
). An early detection of multidrug-resistant tuberculosis (MDR-TB) isolates allows for an appropriate and timely treatment (Martin et al. 2008Martin A, Panaiotov S, Portaels F, Hoffner S, Palomino JC, Angeby K 2008. The nitrate reductase assay for the rapid detection of isoniazid and rifampicin resistance in Mycobacterium tuberculosis: a systematic review and meta-analysis. J Antimicrob Chemother 62: 56-64.). The proportion method, which is performed on Löwenstein-Jensen (LJ) and Middlebrook 7H10 or 7H11 agar media, is recommended as a reference method. However, this method requires at least three-six weeks to obtain results (Kent & Kubica 1985Kent PT, Kubica GP 1985. Public health mycobacteriology: a guide for the level III laboratory, US Department of Health and Human Services, Atlanta, 207 pp.). There are also rapid automated systems for drug susceptibility testing, but these systems are expensive and require equipment (Bwanga et al. 2009Bwanga F, Hoffner S, Haile M, Joloba ML 2009. Direct susceptibility testing for multidrug resistant tuberculosis: a meta-analysis. BMC Infect Dis 9: 67., Friedrich et al. 2011Friedrich SO, Venter A, Kayigire XA, Dawson R, Donald PR, Diacon AH 2011. Suitability of Xpert MTB/RIF and Genotype MTBDRplus for patient selection for a tuberculosis clinical trial. J Clin Microbiol 49: 2827-2831., Chang et al. 2012)Chang K, Lu W, Wang J, Zhang K, Jia S, Li F, Deng S, Chen M 2012. Rapid and effective diagnosis of tuberculosis and rifampicin resistance with Xpert MTB/RIF assay: a meta-analysis. J Infect 64: 580-588.. Recently, many colourimetric methods, including the resazurin microtitre assay (REMA), or resazurin tube assay, malachite green decolourisation assay (MGDA), nitrate reductase assay (NRA) and crystal violet decolourisation assay (CVDA), have been developed. These methods are rapid, inexpensive, reliable and reproducible (Angeby et al. 2002Angeby KA, Klintz L, Hoffner SE 2002. Rapid and inexpensive drug susceptibility testing of Mycobacterium tuberculosis with a nitrate reductase assay. J Clin Microbiol 40: 553-555., Coban et al. 2004Coban AY, Birinci A, Ekinci B, Durupinar B 2004. Drug susceptibility testing of Mycobacterium tuberculosis by the broth microdilution method with 7H9 broth. Mem Inst Oswaldo Cruz 99: 111-113., Martin et al. 2007Martin A, Portaels F, Palomino JC 2007. Colorimetric redox-indicator methods for the rapid detection of multidrug resistance in Mycobacterium tuberculosis: a systematic review and meta-analysis. J Antimicrob Chemother 59: 175-183., 2011Martin A, Paasch F, Docx S, Fissette K, Imperiale B, Ribón W, González LA, Werngren J, Engström A, Skenders G, Juréen P, Hoffner S, Del Portillo P, Morcillo N, Palomino JC 2011. Multicentre laboratory validation of the colorimetric redox indicator (CRI) assay for the rapid detection of extensively drug-resistant (XDR) Mycobacterium tuberculosis. J Antimicrob Chemother 66: 827-833., Palomino et al. 2007)Palomino JC, Martin A, Portaels F 2007. Rapid drug resistance detection in Mycobacterium tuberculosis: a review of colourimetric methods. Clin Microbiol Infect 13: 754-762..
This paper aims to evaluate four rapid colourimetric methods, including the REMA, microplate NRA (MNRA), CVDA and MGDA methods, for the rapid detection of MDR-TB. In this study, these methods were compared with each other for the first time in terms of their sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for isoniazid (INH) and rifampicin (RIF).
MATERIALS AND METHODS
Bacterial isolates - Fifty M. tuberculosis isolates were used in this study. Isolates were obtained from the Istanbul Faculty of Medicine and Samsun Chest Diseases and Thoracic Surgery Hospital. Eighteen isolates were MDR, two isolates were only resistant to INH and the remaining isolates were susceptible to both INH and RIF. All isolates from stocks were subcultured on LJ medium and freshly subcultured isolates were used in the study.
Chemical substances - Resazurin salt powder, sulfanilamide, n-1-naphthylethylenediamine dihydrochloride, crystal violet (CV) dye, INH and RIF were purchased from Sigma-Aldrich (Germany). Malachite green (MG) dye was purchased from Merck (Germany).
Preparation of chemical substances and reagents - Resazurin powder was dissolved in sterile distilled water to a final concentration of 0.02%, sterilised by filtration and stored at 4ºC until use. Griess reagent consisted of one part 50% (vol/vol) concentrated hydrochloric acid, two parts 0.2% (wt/vol) sulfanilamide and two parts 0.1% (wt/vol) n-1-naphthylethylenediamine dihydrochloride. MG dye was dissolved in distilled water to a final concentration of 50 µg/mL. CV dye was prepared in distilled water to a final concentration of 25 µg/mL. All of the chemical substances and reagents were stored at 4ºC until use.
Preparation of media - The REMA, MNRA, CVDA and MGDA susceptibility tests were performed in Middlebrook 7H9S broth containing 0.1% casitone, 0.5% glycerol and 10% OADC. Middlebrook 7H9S broth with 1,000 µg/mL potassium nitrate (KNO3) was used for the MNRA.
Preparation of bacterial inoculum - Bacterial colonies that had been freshly grown on LJ media were transferred into tubes containing 5 mL saline solution and 15-20 glass beads and were vortexed for 1-2 min. The tube was kept in a vertical position for 30 min at room temperature to allow aerosols and large particles to precipitate. The turbidity of the supernatant was adjusted to that of the McFarland 1 standard. The prepared bacterial suspension was then diluted at an 1:10 ratio in Middlebrook 7H9S broth.
Preparation of antibiotics - All tests were performed in 96-well microtitre plates. One hundred microlitres of Middlebrook 7H9S broth (containing 0.1% casitone, 0.5% glycerol and 10% OADC) were added into each well of the plates for the CVDA, MGDA and REMA methods. One hundred microlitres of Middlebrook 7H9S broth with 1,000 µg/mL KNO3 were added into each well of the plate for the MNRA method. For each isolate, three wells containing INH, RIF and media without antibiotics were prepared. All plates were kept at -20ºC for two weeks.
Performing susceptibility tests - The MNRA and REMA were performed with some modifications. Briefly, the MNRA and REMA were performed using a critical concentration of drugs and in liquid media. The MGDA and CVDA were performed as defined in the literature. Each bacterial isolate was tested in each of the three wells containing INH (0.25 µg/mL), RIF (0.5 µg/mL) and an antibiotic-free growth control for each of the susceptibility test methods. One hundred microlitres of bacterial suspension was inoculated into each well of the plates. After bacterial inoculation, all plates were incubated at 37ºC under normal atmospheric conditions.
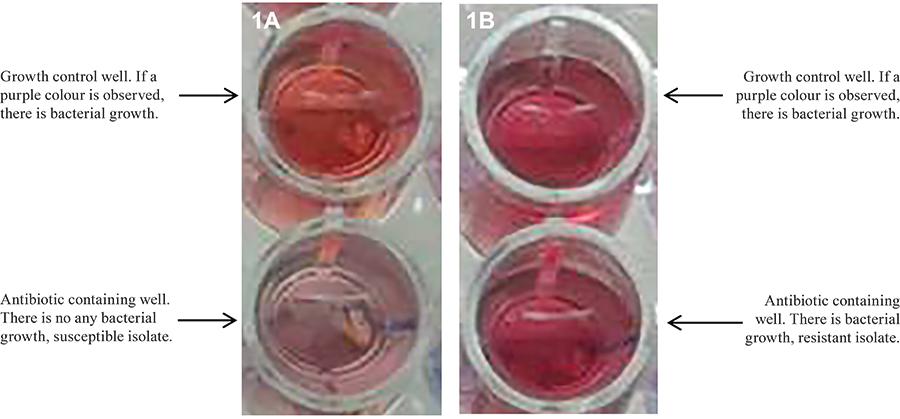
Evaluation of the MNRA method - After seven days of incubation, 50 µL of Griess reagent was added into each of the growth control wells for the MNRA. If a purple colour was observed, Griess reagent was also added into the test wells. If a purple colour was observed in the test wells containing antibiotics, the bacterial isolate was considered to be resistant to the antibiotic that was being tested. The MNRA wells were evaluated for colour change within 3-5 s after the addition of the Griess reagent (Fig. 1A, B).
Evaluation of the REMA method - After seven days of incubation, 30 µL of resazurin solution was added into each well with and without antibiotics. After the reagents were added into the wells, the plates were re-incubated for one-three days under the same conditions. If the blue colour of resazurin changed to pink in the growth control well as in the wells containing antibiotics, the bacterial isolate was reported to be resistant to the antibiotics that were being tested (Fig. 2A, B).
Evaluation of the MGDA method - After seven days of incubation, 30 μL of MG was added into each of the test wells and the plates were incubated for an additional one-three days. After the MG decolourised in the growth control well, the test wells containing antibiotics were evaluated for a colour change. If the MG decolourised as a result of bacterial growth in the wells containing antibiotics, these bacterial isolates were reported to be resistant to the antibiotics (Fig. 3A, B).
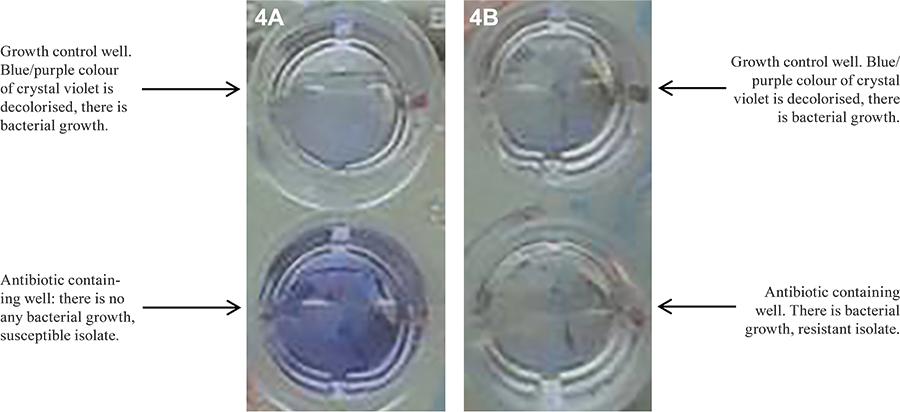
Evaluation of the CVDA method - On the seventh day of incubation, 25 μL of CV was added into the wells and incubated for an additional one-three days. As the blue/purple colour of CV was decolourised by the growth of bacteria, these bacterial isolates were considered to be resistant to that drug (Fig. 4A, B).
RESULTS
All data are summarised in Table. The specificity, sensitivity, PPV, NPV and agreement of the MNRA for INH were 93.3%, 100%, 90.9%, 100% and 96%, respectively. Two isolates were susceptible to INH by the reference method, but resistant by the MNRA. The values for RIF were 90.9%, 100%, 85%, 100% and 94%, respectively. Three isolates were shown to be susceptible to RIF by the reference method, whereas they were resistant according to the MNRA.
The specificity, sensitivity, PPV, NPV and agreement of the REMA for INH were 100%, 95%, 100%, 96.7% and 98%, respectively. One isolate was susceptible to INH by the REMA but resistant by the reference method. These values for RIF were 100%, 94.1%, 100%, 97% and 98%, respectively. One isolate was resistant to RIF by the reference method but susceptible by the REMA.
The specificity, sensitivity, PPV, NPV and agreement of the MGDA for INH were 100%, 95%, 100%, 96.7% and 98%, respectively. One isolate was susceptible to INH by the MGDA, but resistant by the reference method. The values for RIF were 100%, 94.1%, 100%, 97% and 98%, respectively. One isolate was resistant to RIF by the reference method, but susceptible by the MGDA test.
The specificity, sensitivity, PPV, NPV and agreement of the CVDA for INH were 100%, 95%, 100%, 96.7% and 98%, respectively. One isolate was susceptible to INH by the CVDA, but resistant by the reference method. For RIF, these values were 100%, 94.1%, 100%, 97% and 98%, respectively. One isolate was shown to be resistant to RIF by the reference method, whereas it was susceptible according to the CVDA.
The MNRA results were reported immediately after Griess reagent was added on the seventh day of the incubation, while the results of the REMA, MGDA and CVDA methods were reported on the eighth-10th day after inoculation due to the one-three days of incubation that were required.
DISCUSSION
For susceptibility testing of M. tuberculosis isolates, the use of colourimetric methods has increased in recent years because these methods are easy to perform, reproducible, reliable, readily available and very low in cost. In this study, colourimetric methods, including the MGDA, MNRA, REMA and CVDA, were compared with each other in terms of sensitivity, specificity, PPV and NPV.
In this study, the agreement of the MNRA was 96% for INH and 94% for RIF. In the study performed by Coban et al. (2012)Coban AY, Uzun M, Akgunes A, Durupinar B 2012. Comparative evaluation of the microplate nitrate reductase assay and the rezasurin microtitre assay for the rapid detection of multidrug resistant Mycobacterium tuberculosis clinical isolates. Mem Inst Oswaldo Cruz 107: 578-581., the rates for both anti-TB drugs were found to be 100% for the NRA. In another study, Montoro et al. (2005)Montoro E, Lemus D, Echemendia M, Martin A, Portaels F, Palomino JC 2005. Comparative evaluation of the nitrate reductase assay, the MTT test and the resazurin microtiter assay for drug susceptibility testing of clinical isolates of Mycobacterium tuberculosis. J Antimicrob Chemother 55: 500-505. reported that the sensitivity and specificity of the NRA for INH and RIF were 95.6% and 100%, respectively.
Agreements for the REMA were detected to be 98% for both drugs. One study showed that the sensitivities, specificities, PPVs, NPVs and agreements of INH and RIF for the REMA were 100-91.3%, 90.4-100%, 88.5-100%, 100-96.1% and 94.5-97.2%, respectively (Coban et al. 2012Coban AY, Uzun M, Akgunes A, Durupinar B 2012. Comparative evaluation of the microplate nitrate reductase assay and the rezasurin microtitre assay for the rapid detection of multidrug resistant Mycobacterium tuberculosis clinical isolates. Mem Inst Oswaldo Cruz 107: 578-581.). Nour et al. (2013)Nour MS, El-Shokry MH, Shehata IH, Aziz AMA-E 2013. Evaluation of rezasurin microtiter assay and high resolution melting curve analysis for detection of rifampicin and isoniazid resistance of Mycobacterium tuberculosis clinical isolates. Clin Lab 59: 763-771. also reported that sensitivities, specificities, PPVs, NPVs and agreements of INH and RIF were 100% by this method.
In the current study, the agreements for both drugs were 98%. Farnia et al. (2008)Farnia P, Masjedi MR, Mohammadi F, Tabarsei P, Farnia P, Mohammadzadeh AR, Baghei P, Varahram M, Hoffner S, Velayati AA 2008. Colorimetric detection of multidrug-resistant or extensively drug-resistant tuberculosis by use of malachite green indicator dye. J Clin Microbiol 46: 796-799. reported that both INH and RIF sensitivities, specificities and agreements were 100%. In another study, Coban and Uzun (2013)Coban AY, Uzun M 2013. Rapid detection of multidrug-resistant Mycobacterium tuberculosis using the malachite green decolourisation assay. Mem Inst Oswaldo Cruz 108: 1021-1023. reported that the sensitivity, specificity, PPV, NPV and agreement of the MGDA for INH and RIF were 100-95.6%, 97.6-100%, 96.8-100%, 100-98% and 98.6-98.6%, respectively.
In this study, agreements were detected as 98% for both drugs by the CVDA. Earlier studies performed by Coban (2014)Coban AY 2014. A new rapid colourimetric method for testing Mycobacterium tuberculosis susceptibility to isoniazid and rifampicin: a crystal violet decolourisation assay. Mem Inst Oswaldo Cruz 109: 246-249. found that the rates for INH and RIF were 92.5-88.8%, 96.4-100%, 96.1-100%, 93.1-94.8% and 94.5-96.3%, respectively.
The specificity of the MNRA test was found to be lower than that of the other tests because the reference method yielded sensitive results for antibiotics whereas the MNRA test yielded resistant results (2 isolates for INH and 3 isolates for RIF). NRA is usually performed on LJ medium, but it was performed in liquid medium in this study. A false colour change may occur because following the addition of KNO3 and drugs to the liquid medium, viable bacteria reduce KNO3 to nitrite until they are killed by the antibiotic. This change may cause a method to define susceptible isolates as resistant.
In the MNRA, the time required for detection is less than for the other colourimetric methods and this test does not require additional incubation time, but the Griess reagent, which consists of three chemical substances, should be freshly prepared. The REMA requires resazurin powder that should be freshly prepared and this method requires additional incubation time. The MGDA and CVDA have similar characteristics to the REMA, but MG and CV dyes are obtained easily. Moreover, CV (used for Gram staining) is available in clinical microbiology laboratories. Evaluating all of the colourimetric methods, each has some advantages and disadvantages. Nonetheless, all of the above methods are reliable, easy to perform, cheap and easy to evaluate and do not require special equipment. In conclusion, these methods may be used in mycobacteriology laboratories in both developed and developing countries.
REFERENCES
- Angeby KA, Klintz L, Hoffner SE 2002. Rapid and inexpensive drug susceptibility testing of Mycobacterium tuberculosis with a nitrate reductase assay. J Clin Microbiol 40: 553-555.
- Bwanga F, Hoffner S, Haile M, Joloba ML 2009. Direct susceptibility testing for multidrug resistant tuberculosis: a meta-analysis. BMC Infect Dis 9: 67.
- Chang K, Lu W, Wang J, Zhang K, Jia S, Li F, Deng S, Chen M 2012. Rapid and effective diagnosis of tuberculosis and rifampicin resistance with Xpert MTB/RIF assay: a meta-analysis. J Infect 64: 580-588.
- Coban AY 2014. A new rapid colourimetric method for testing Mycobacterium tuberculosis susceptibility to isoniazid and rifampicin: a crystal violet decolourisation assay. Mem Inst Oswaldo Cruz 109: 246-249.
- Coban AY, Birinci A, Ekinci B, Durupinar B 2004. Drug susceptibility testing of Mycobacterium tuberculosis by the broth microdilution method with 7H9 broth. Mem Inst Oswaldo Cruz 99: 111-113.
- Coban AY, Uzun M 2013. Rapid detection of multidrug-resistant Mycobacterium tuberculosis using the malachite green decolourisation assay. Mem Inst Oswaldo Cruz 108: 1021-1023.
- Coban AY, Uzun M, Akgunes A, Durupinar B 2012. Comparative evaluation of the microplate nitrate reductase assay and the rezasurin microtitre assay for the rapid detection of multidrug resistant Mycobacterium tuberculosis clinical isolates. Mem Inst Oswaldo Cruz 107: 578-581.
- Farnia P, Masjedi MR, Mohammadi F, Tabarsei P, Farnia P, Mohammadzadeh AR, Baghei P, Varahram M, Hoffner S, Velayati AA 2008. Colorimetric detection of multidrug-resistant or extensively drug-resistant tuberculosis by use of malachite green indicator dye. J Clin Microbiol 46: 796-799.
- Friedrich SO, Venter A, Kayigire XA, Dawson R, Donald PR, Diacon AH 2011. Suitability of Xpert MTB/RIF and Genotype MTBDRplus for patient selection for a tuberculosis clinical trial. J Clin Microbiol 49: 2827-2831.
- Kent PT, Kubica GP 1985. Public health mycobacteriology: a guide for the level III laboratory, US Department of Health and Human Services, Atlanta, 207 pp.
- Martin A, Paasch F, Docx S, Fissette K, Imperiale B, Ribón W, González LA, Werngren J, Engström A, Skenders G, Juréen P, Hoffner S, Del Portillo P, Morcillo N, Palomino JC 2011. Multicentre laboratory validation of the colorimetric redox indicator (CRI) assay for the rapid detection of extensively drug-resistant (XDR) Mycobacterium tuberculosis J Antimicrob Chemother 66: 827-833.
- Martin A, Panaiotov S, Portaels F, Hoffner S, Palomino JC, Angeby K 2008. The nitrate reductase assay for the rapid detection of isoniazid and rifampicin resistance in Mycobacterium tuberculosis: a systematic review and meta-analysis. J Antimicrob Chemother 62: 56-64.
- Martin A, Portaels F, Palomino JC 2007. Colorimetric redox-indicator methods for the rapid detection of multidrug resistance in Mycobacterium tuberculosis: a systematic review and meta-analysis. J Antimicrob Chemother 59: 175-183.
- Montoro E, Lemus D, Echemendia M, Martin A, Portaels F, Palomino JC 2005. Comparative evaluation of the nitrate reductase assay, the MTT test and the resazurin microtiter assay for drug susceptibility testing of clinical isolates of Mycobacterium tuberculosis J Antimicrob Chemother 55: 500-505.
- Nour MS, El-Shokry MH, Shehata IH, Aziz AMA-E 2013. Evaluation of rezasurin microtiter assay and high resolution melting curve analysis for detection of rifampicin and isoniazid resistance of Mycobacterium tuberculosis clinical isolates. Clin Lab 59: 763-771.
- Palomino JC, Martin A, Portaels F 2007. Rapid drug resistance detection in Mycobacterium tuberculosis: a review of colourimetric methods. Clin Microbiol Infect 13: 754-762.
- WHO - World Health Organization 2014. Global tuberculosis report 2014. Available from: http://apps.who.int/iris/bitstream/10665/137094/1/9789241564809_eng.pdf?ua=1
» http://apps.who.int/iris/bitstream/10665/137094/1/9789241564809_eng.pdf?ua=1
Publication Dates
-
Publication in this collection
24 July 2015 -
Date of issue
Aug 2015
History
-
Received
2 Apr 2015 -
Accepted
26 June 2015