Abstract
BACKGROUND
The northern limits of Trypanosoma cruzi across the territory of the United States remain unknown. The known vectors Triatoma sanguisuga and T. lecticularia find their northernmost limits in Illinois; yet, earlier screenings of those insects did not reveal the presence of the pathogen, which has not been reported in vectors or reservoir hosts in this state.
OBJECTIVES
Five species of medium-sized mammals were screened for the presence of T. cruzi.
METHODS
Genomic DNA was isolated from heart, spleen and skeletal muscle of bobcats (Lynx rufus, n = 60), raccoons (Procyon lotor, n = 37), nine-banded armadillos (Dasypus novemcinctus, n = 5), Virginia opossums (Didelphis virginiana, n = 3), and a red fox (Vulpes vulpes). Infections were detected targeting DNA from the kinetoplast DNA minicircle (kDNA) and satellite DNA (satDNA). The discrete typing unit (DTU) was determined by amplifying two gene regions: the Spliced Leader Intergenic Region (SL), via a multiplex polymerase chain reaction, and the 24Sα ribosomal DNA via a heminested reaction. Resulting sequences were used to calculate their genetic distance against reference DTUs.
FINDINGS
18.9% of raccoons were positive for strain TcIV; the rest of mammals tested negative.
MAIN CONCLUSIONS
These results confirm for the first time the presence of T. cruzi in wildlife from Illinois, suggesting that a sylvatic life cycle is likely to occur in the region. The analyses of sequences of SL suggest that amplicons resulting from a commonly used multiplex reaction may yield non-homologous fragments.
Key words:
Trypanosoma cruzi; zoonotic disease; Midwest; raccoon; bobcat; Illinois
The etiological agent of American trypanosomiasis, Trypanosoma cruzi (Euglenozoa) commonly infects mammals as well as triatomine bugs throughout the tropical and subtropical regions of the New World. In the United States of America, the presence of T. cruzi has been documented in both vectors and in wildlife screened for the presence of the parasite in 16 states (Bi et al. 2010Bi LP, Groce C, Davis C. Molecular analysis of Trypanosoma cruzi isolates obtained from raccoons in Warren and Barren counties of Kentucky. BMC Bioinformatics. 2010; 11(Suppl. 4): P3., Brown et al. 2010Brown EL, Roellig DM, Gompper ME, Monello RJ, Wenning KM, Gabriel MW, et al. Seroprevalence of Trypanosoma cruzi among eleven potential reservoir species from six states across the southern United States. Vector Borne Zoonotic Dis. 2010; 10(8): 757-63., Bern et al. 2011Bern C, Kjos S, Yabsley MJ, Montgomery SP. Trypanosoma cruzi and Chagas' disease in the United States. Clin Microbiol Rev. 2011; 24(4): 655-81.). Additionally, positive vectors have been identified in nine states, including Alabama, Arizona, California, Georgia, Louisiana, New Mexico, Tennessee and Texas (Bern et al. 2011Bern C, Kjos S, Yabsley MJ, Montgomery SP. Trypanosoma cruzi and Chagas' disease in the United States. Clin Microbiol Rev. 2011; 24(4): 655-81.). Contrastingly, the distribution of T. cruzi in wildlife has been reconstructed through the screening of mammals, chiefly raccoons (Procyon lotor) and Virginia opossums (Didelphis virginiana), from Arizona, Florida, Georgia, Kentucky, Louisiana, Maryland, Missouri, North Carolina, Oklahoma, South Carolina, Tennessee, Texas and Virginia (Bi et al. 2010Bi LP, Groce C, Davis C. Molecular analysis of Trypanosoma cruzi isolates obtained from raccoons in Warren and Barren counties of Kentucky. BMC Bioinformatics. 2010; 11(Suppl. 4): P3., Bern et al. 2011Bern C, Kjos S, Yabsley MJ, Montgomery SP. Trypanosoma cruzi and Chagas' disease in the United States. Clin Microbiol Rev. 2011; 24(4): 655-81., Rosypal et al. 2014Rosypal AC, Smith T, Alexander A, Weaver M, Stewart R, Houston A. Serologic survey of antibodies to Trypanosoma cruzi in coyotes and red foxes from Pennsylvania and Tennessee. J Zoo Wildl Med. 2014; 45(4): 991-3., Hodo et al. 2016Hodo CL, Goodwin CC, Mayes BC, Mariscal JA, Waldrup KA, Hamer SA. Trypanosome species, including Trypanosoma cruzi, in sylvatic and peridomestic bats of Texas, USA. Acta Trop. 2016; 164: 259-66.). However, the sylvatic life cycle of T. cruzi has been documented only in southern and coastal states (Garcia et al. 2015Garcia MN, Aguilar D, Gorchakov R, Rossmann SN, Montgomery SP, Rivera H, et al. Evidence of autochthonous Chagas disease in southeastern Texas. Am J Trop Med Hyg. 2015; 92(2): 325-30., Herrera et al. 2015Herrera CP, Licon MH, Nation CS, Jameson SB, Wesson DM. Genotype diversity of Trypanosoma cruzi in small rodents and Triatoma sanguisuga from a rural area in New Orleans, Louisiana. Parasit Vectors. 2015; 8: 123., Hodo et al. 2016Hodo CL, Goodwin CC, Mayes BC, Mariscal JA, Waldrup KA, Hamer SA. Trypanosome species, including Trypanosoma cruzi, in sylvatic and peridomestic bats of Texas, USA. Acta Trop. 2016; 164: 259-66.).
T. cruzi grows chiefly via cellular fission, with occasional recombination. Yet, its genetic diversity across the Americas is relatively high, especially in areas where several competent vectors and mammalian hosts are involved (Brenière et al. 2016Brenière SF, Waleckx E, Barnabê C. Over six thousand Trypanosoma cruzi strains classified into dDiscrete Typing Units (DTUs): attempt at an inventory. PLoS Negl Trop Dis. 2016; 10(8): e19.). To add to this complexity, some strains show clear host preferences (Brenière et al. 2016Brenière SF, Waleckx E, Barnabê C. Over six thousand Trypanosoma cruzi strains classified into dDiscrete Typing Units (DTUs): attempt at an inventory. PLoS Negl Trop Dis. 2016; 10(8): e19.). As a consequence, the species has been subdivided into six universally recognized discrete typing units (DTUs), including TcI through TcVI, and a seventh, TcBat, that cycles through bats (Zingales et al. 2009Zingales B, Andrade SG, Briones MRS, Campbell DA, Chiari E, Fernandes O, et al. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem Inst Oswaldo Cruz. 2009; 104(7): 1051-4., 2012Zingales B, Miles MA, Campbell DA, Tibayrenc M, Macedo AM, Teixeira MM. The revised Trypanosoma cruzi subspecific nomenclature: rationale, epidemiological relevance and research applications. Infect Genet Evol. 2012; 12(2): 240-53., Lima et al. 2015Lima L, Espinosa-Alvarez O, Ortiz PA, Trejo-Varon JA, Carranza JC, Pinto CM, et al. Genetic diversity of Trypanosoma cruzi in bats, and multilocus phylogenetic and phylogeographical analyses supporting Tcbat as an independent DTU (discrete typing unit). Acta Trop. 2015; 151: 166-77., Hodo et al. 2016Hodo CL, Goodwin CC, Mayes BC, Mariscal JA, Waldrup KA, Hamer SA. Trypanosome species, including Trypanosoma cruzi, in sylvatic and peridomestic bats of Texas, USA. Acta Trop. 2016; 164: 259-66.). In the United States, the strains TcI, TcIV, and, less commonly, TcII have been documented in both mammals and triatomines (Herrera et al. 2015Herrera CP, Licon MH, Nation CS, Jameson SB, Wesson DM. Genotype diversity of Trypanosoma cruzi in small rodents and Triatoma sanguisuga from a rural area in New Orleans, Louisiana. Parasit Vectors. 2015; 8: 123.). From these, TcI has been identified as the strain inducing most of the autochthonous infections in humans in Louisiana and Texas (Dorn et al. 2007Dorn PL, Perniciaro L, Yabsley MJ, Roellig DM, Balsamo G, Diaz J, et al. Autochthonous transmission of Trypanosoma cruzi, Louisiana. Emerg Infect Dis. 2007; 13(4): 605-7., Garcia et al. 2015Garcia MN, Aguilar D, Gorchakov R, Rossmann SN, Montgomery SP, Rivera H, et al. Evidence of autochthonous Chagas disease in southeastern Texas. Am J Trop Med Hyg. 2015; 92(2): 325-30., 2017Garcia MN, Burroughs H, Gorchakov R, Gunter SM, Dumonteil E, Murray KO, et al. Molecular identification and genotyping of Trypanosoma cruzi DNA in autochthonous Chagas disease patients from Texas, USA. Infect Genet Evol. 2017; 49: 151-6.), whereas TcIV has been detected in raccoons in Kentucky (Bi et al. 2010Bi LP, Groce C, Davis C. Molecular analysis of Trypanosoma cruzi isolates obtained from raccoons in Warren and Barren counties of Kentucky. BMC Bioinformatics. 2010; 11(Suppl. 4): P3.) and south eastern states (Roellig et al. 2013Roellig DM, Savage MY, Fujita AW, Barnabe C, Tibayrenc M, Steurer FJ, et al. Genetic variation and exchange in Trypanosoma cruzi isolates from the United States. PLoS ONE. 2013; 8(2): 1-12.).
In Illinois, a Midwestern state located in the north central region of the United States, the parasite has been detected in humans who contracted the infection while residing or visiting endemic areas in Latin America (Bern et al. 2011Bern C, Kjos S, Yabsley MJ, Montgomery SP. Trypanosoma cruzi and Chagas' disease in the United States. Clin Microbiol Rev. 2011; 24(4): 655-81.). Relative to potential vectors, museum records account for the presence of both Triatoma lecticularia and T. sanguisuga in the state's territory (Fracker 1913Fracker SB. A systematic outline of the Reduviidae of North America. Des Moines: 1913. p. 217-52., Hagerty & McPherson 1999Hagerty AM, McPherson JE. Survey of the Reduviidae (Heteroptera) of southern Illinois, excluding the Phymatinae, with notes on biology. Great Lakes Entomol. 1999; 32(3): 133-60.). Although these insects are known vectors of T. cruzi elsewhere in the United States (Bern et al. 2011Bern C, Kjos S, Yabsley MJ, Montgomery SP. Trypanosoma cruzi and Chagas' disease in the United States. Clin Microbiol Rev. 2011; 24(4): 655-81.), early screenings failed at revealing any infected individuals (Porter 1965Porter JA. Triatoma sanguisuga (Leconte 1855) in Illinois. J Parasitol. 1965; 51(4): 500.). To that effect, the parasite has yet to be detected in reservoirs or vectors native to Illinois.
Given the negative results obtained during previous parasitological surveys in the vector (Porter 1965Porter JA. Triatoma sanguisuga (Leconte 1855) in Illinois. J Parasitol. 1965; 51(4): 500.), and the relatively greater success in detecting infection in mammals, we concentrated our efforts at screening archived tissues collected from raccoons (Procyon lotor), bobcats (Lynx rufus) and other mammals for the presence of T. cruzi. The purpose of this screening is to document its presence and prevalence for the first time in this state. If successful, these results will facilitate the determination of the northernmost distribution limit of the etiological agent of Chagas disease. To date, the pathogen is expected to occur in Illinois and other states in the American Midwest, yet this expectation is based solely on the distribution of the vectors.
MATERIALS AND METHODS
Animal collection - Sixty bobcats, 37 raccoons, one red fox (Vulpes vulpes), five nine-banded armadillos (Dasypus novemcinctus) and three Virginia opossums were trapped or collected as road-kill in southern and south-central Illinois within 250 miles of Jackson County, Illinois (Fig. 1). Bobcats were collected between the summer of 2003 and 2012 from localities referred elsewhere (Hiestand et al. 2014Hiestand SJ, Nielsen C, Jiménez FA. Epizootic and zoonotic helminths of the bobcat (Lynx rufus) in Illinois and a comparison of helminth component communities across the American Midwest. Parasite. 2014; 21: 4.), with four additional individuals collected as road-kills between 2013-2015 in Jackson County. Thirty-three raccoons were trapped using baited wire-cages (38 × 38 × 76 cm) in Jackson, Madison and Williamson counties, in Illinois, and four in Boone County, Missouri between 2013 and 2015. The organisms were humanely euthanized as described elsewhere (Boyles & Nielsen 2017Boyles E, Nielsen CK. PBDEs and dechloranes in raccoons in the Midwestern United States. Bull Environ Contam Toxicol. 2017; 98(6): 758-62.). Upon necropsy, tissue samples from spleen, liver, muscle, and heart were stored at -80°C.
collection localities for bobcats (circles) and raccoons (crosses) screened for the presence of Trypanosoma cruzi in Illinois and Missouri. Raccoons infected with T. cruzi TcIV were detected in Boone (Missouri) and in Jackson and Williamson counties (Illinois). Positive controls were isolated from raccoons trapped in Barren and Warren counties, Kentucky.
Determination of infection with T. cruzi - DNA was extracted from the heart, skeletal muscle, and spleen of 60 bobcats, skeletal muscle of 37 raccoons and smooth muscle of five armadillos, three opossums and a fox using a DNeasy Blood & Tissue Kit (QIAGEN, Valencia, CA). The presence of T. cruzi was determined via polymerase chain reaction (PCR) using specific primers designed for the amplification of the hypervariable region of kinetoplast DNA minicircle (kDNA) and the highly repetitive genomic satellite DNA (satDNA). The set of primers S35/S36 (Sturm et al. 1989Sturm NR, Degrave W, Morel C, Simpson L. Sensitive detection and schizodeme classification of Trypanosoma cruzi cells by amplification of kinetoplast minicircle DNA sequences: use in diagnosis of Chagas' disease. Mol Biochem Parasitol. 1989; 33(3): 205-14.) was used to amplify a fragment of about 330bp of the kDNA enforcing the following thermal profile 95C/5:00 (95C/1:00, 60C/1:00, 72C/1:00) × 30 with a final extension at 72C/10:00. The primers Tcz1/Tcz2 (Moser et al. 1989Moser DR, Kirchhoff LV, Donelson JE. Detection of Trypanosoma cruzi by DNA amplification using the Polymerase Chain-Reaction. J Clin Microbiol. 1989; 27(7): 1477-82.) targeted a 188bp fragment of the satDNA and was amplified with a thermal profile consisting of 94C/5:00 (94C/0:40, 68C/1:00, 72C/1:00) × 40 with a final extension at 72C/10:00. All reactions were performed on a total volume of 20 µL, including the primers, DNA template and the mix of reagents included in the Taq DNA Polymerase kit (QIAGEN, Valencia, California). All reagents were mixed under a negative pressure laminar flow hood away from area of DNA extraction following strict protocols to prevent and detect contamination. Every set of reactions included a negative control of double distilled water. DNA extracted from T. cruzi strain TcIV collected from raccoons in Kentucky was used as positive control to test all primers (Bi et al. 2010Bi LP, Groce C, Davis C. Molecular analysis of Trypanosoma cruzi isolates obtained from raccoons in Warren and Barren counties of Kentucky. BMC Bioinformatics. 2010; 11(Suppl. 4): P3.). Amplicons were visualized on 2% agarose gels stained with ethidium bromide.
Assignation to a DTU - The DTU or genotype of T. cruzi within the positive samples was determined by amplifying and sequencing two different genetic markers: the intergenic region of the spliced leader intergenic region -SL- (also known as mini-exon intergenic region), and the D7 domain of the 24Sα ribosomal DNA -24Sα rDNA-. The SL was amplified by means of a multiplex PCR with five different primers: Tc1, Tc2, Tc3, Tr, and Me (Fernandes et al. 2001Fernandes O, Santos SS, Cupolillo E, Mendonca B, Derre R, Junqueira ACV, et al. A mini-exon multiplex polymerase chain reaction to distinguish the major groups of Trypanosoma cruzi and T. rangeli in the Brazilian Amazon. Trans R Soc Trop Med Hyg. 2001; 95(1): 97-9.). From this set, primer ME binds to the most conserved region of SL whereas the rest of the primers bind at upstream regions, resulting in bands of different size that are used to identify the DTUs. If positive, primer Tc1 would yield a fragment of about 200bp and allow the determination of DTU Tc1; Tc2 would yield a fragment of about 250bp and it would amplify DNA of DTUs TcII, TcV, and TcVI; Tc3 would yield a fragment 150bps and allow the determination of DTUs TcIII and TcIV. Finally, Tr would yield a fragment of 100bp of Trypanosoma rangeli. These reactions were completed in a total volume of 20 µL per reaction using the five primers, 40 ng template DNA mixed with the reagents included in the Taq DNA Polymerase kit (QIAGEN, Valencia, California). Every set of reactions was carried out using stringent controls and they were mixed under a hood dedicated to this process. Thermal profile for this multiplex reaction consisted of 94C/5:00 (94C/0:30, 55C/0:30, 72C/0:30) × 35 with a final extension step of 72C/7:00.
The 24Sα rDNA was amplified via two reactions consisting of a first reaction targeting a 300bp fragment using primers D75-D76 (Briones et al. 1999Briones MRS, Souto RP, Stolf BS, Zingales B. The evolution of two Trypanosoma cruzi subgroups inferred from rRNA genes can be correlated with the interchange of American mammalian faunas in the Cenozoic and has implications to pathogenicity and host specificity. Mol Biochem Parasitol. 1999; 104(2): 219-32.), using a thermal profile described elsewhere (Marcet et al. 2006Marcet PL, Duffy T, Cardinal MV, Burgos JM, Lauricella MA, Levin MJ, et al. PCR-based screening and lineage identification of Trypanosoma cruzi directly from faecal samples of triatomine bugs from northwestern Argentina. Parasitology. 2006; 132(1): 57-65.). An aliquote of 1 µL of this solution was used in a subsequent heminested reaction, targeting a 145bp fragment using primers D71 and D76 (Souto et al. 1996Souto RP, Fernandes O, Macedo AM, Campbell DA, Zingales B. DNA markers define two major phylogenetic lineages of Trypanosoma cruzi. Mol Biochem Parasitol. 1996; 83(2): 141-52.) and the thermal profile described above. Amplicons were visualized on 2% agarose gels stained with ethidium bromide.
Positive PCR products were cleaned using exonuclease I-shrimp phosphatase, Exo SAP-IT (GE Healthcare, Cleveland, Ohio) to remove excess of nucleotides following manufacturer recommendations. Sequencing was conducted in both directions using 0.75 µL of Terminator BigDye 3.2 (BigDye™ Chemistry Perkin-Elmer Applied Biosystems, Norwalk, Connecticut), 3.0 µL 5X BigDye Buffer, 9.25 µL molecular grade water, 1.0 µL DNA template, and 1.0 µL of primer (either Me or Tc3 at 3.2 pM) for a final volume of 15 µL. The thermal profile consisted of 96C/1:00 (96C/0:15, 50C/0:10, 60C/4:00) × 40. Products were purified with the aid of Sephadex columns (GE Healthcare, Buckinghampshire, UK), treated with 10 µL highly deionized formamide (Hi-Di, The Gel Company, San Francisco, CA), and direct sequenced in a 3130XL Genetic Analyzer (Applied Biosystems, Grand Island, NY). Products were directly sequenced in an ABI 3130xl gene sequencer in the Conservation Genetics Laboratory of Southern Illinois University (Carbondale, Illinois). Resulting sequences were uploaded to universal repositories (Table I).
Accession numbers for sequences of Trypanosoma cruzi strain TcIV detected in raccoons from Illinois, Kentucky and Missouri. Sequences from Kentucky (RB and RW) were hemoculture isolates from blood. DNA for all other samples was obtained from muscular tissues. The accession numbers correspond to the database of the National Center for Biotechnology Information (NCBI) or GenBank
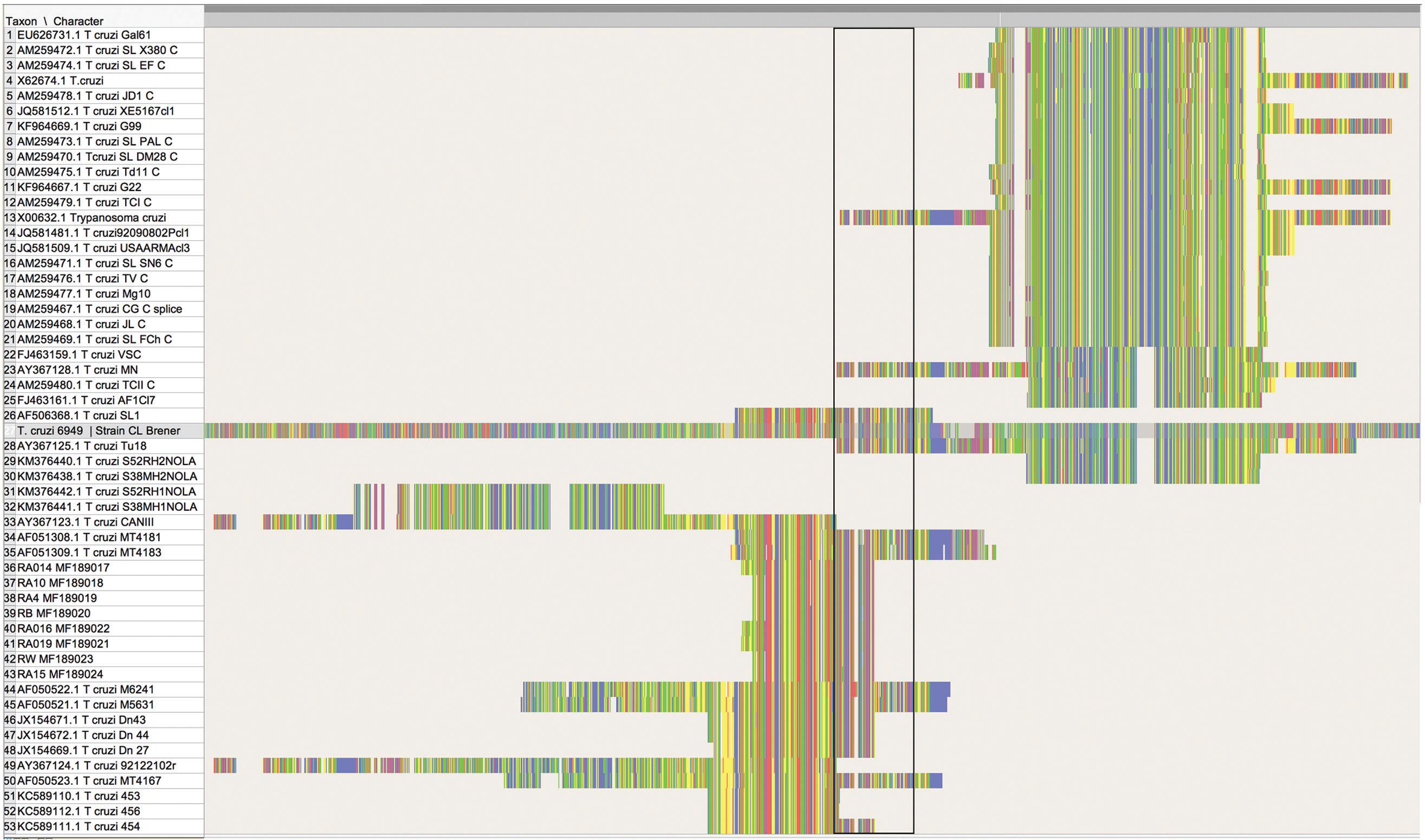
A matrix was assembled with known strains available in GenBank (Fernandes et al. 1998Fernandes O, Sturm NR, Derre R, Campbell DA. The mini-exon gene: a genetic marker for zymodeme III of Trypanosoma cruzi. Mol Biochem Parasitol. 1998; 95(1): 129-33., Sturm et al. 2003Sturm NR, Vargas NS, Westenberger SJ, Zingales B, Campbell DA. Evidence for multiple hybrid groups in Trypanosoma cruzi. Int J Parasitol. 2003; 33(3): 269-79., Cribb et al. 2004Cribb P, Tapia E, Diosque P, Serra E. Spliced leader RNA gene promoter sequence heterogeneity in CL-Brener Trypanosoma cruzi reference strain. Infect Genet Evol. 2004; 4(2): 153-7., Herrera et al. 2015Herrera CP, Licon MH, Nation CS, Jameson SB, Wesson DM. Genotype diversity of Trypanosoma cruzi in small rodents and Triatoma sanguisuga from a rural area in New Orleans, Louisiana. Parasit Vectors. 2015; 8: 123.). Sequences were aligned using Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/). This matrix was used to calculate genetic distance between sequences generated in this study against reference sequences of TcIV, including AY367124 (isolated from a raccoon in Georgia) and AY367123 (isolated from a patient from Brazil) (Sturm et al. 2003Sturm NR, Vargas NS, Westenberger SJ, Zingales B, Campbell DA. Evidence for multiple hybrid groups in Trypanosoma cruzi. Int J Parasitol. 2003; 33(3): 269-79.). This calculation was made using homologous sequences and eliminating all ambiguous base pairs in PAUP* Vers. 4.0a152 (Swofford 2003Swofford DL. PAUP*. Phylogenetic Analysis Using Parsimony (*and other methods) 4.0b10. Sinauer Associates: Sunderland; 2003.). Following alignment, the matrix was trimmed to include only homologous sequences (Fig. 2). This matrix includes 23 taxa and 153 nucleotides and it is available in the permanent repository OpenSIUC (http://opensiuc.lib.siu.edu/zool_data/13/). The best-fitting substitution model calculated for homologous regions was determined using JModelTest 2 (Darriba et al. 2012Darriba D, Taboada GL, Doallo R, Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods. 2012; 9(8): 772.), which selected the Jukes-Cantor as the best fitting model via Akaike information criterion. Posterior probabilities of branches were reconstructed using MrBayes v. 3.2 (Ronquist et al. 2012Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Hohna S, et al. MrBayes 3.2: efficient bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012; 61(3): 539-42.). The Bayesian analyses were run under the following conditions; 4 chains, 3 runs, and 10,000,000 generations. Each chain was sampled every 1,000 generations.
alignment showing the homologous sites for the sequences of strain TcIV detected in Illinois, and those available in GenBank identified as TcI, TcII, and TcIV from the United States. The fragment shown corresponds with region 11,500 to 13,030 nt in reference Trypanosoma cruzi CL-Brener (6949) available from: http://tritrypdb.org/tritrypdb/. The rectangle marks the region between 12,300 to 12,400nt, and it is used as reference. This matrix can be found in OPEN SIUC (http://opensiuc.lib.siu.edu/zool_data/12/).
Bootstrap support for the branches was estimated enforcing the Jukes-Cantor model, with 1,000 replicates in in PAUP* Vers. 4.0a152 (Swofford 2003Swofford DL. PAUP*. Phylogenetic Analysis Using Parsimony (*and other methods) 4.0b10. Sinauer Associates: Sunderland; 2003.).
Ethics - All methods were approved by the Institutional Animal Care and Use Committee of Southern Illinois University Carbondale (Assurance number A-3078-01, protocol numbers 11-042, 13-054 and 14-060).
RESULTS
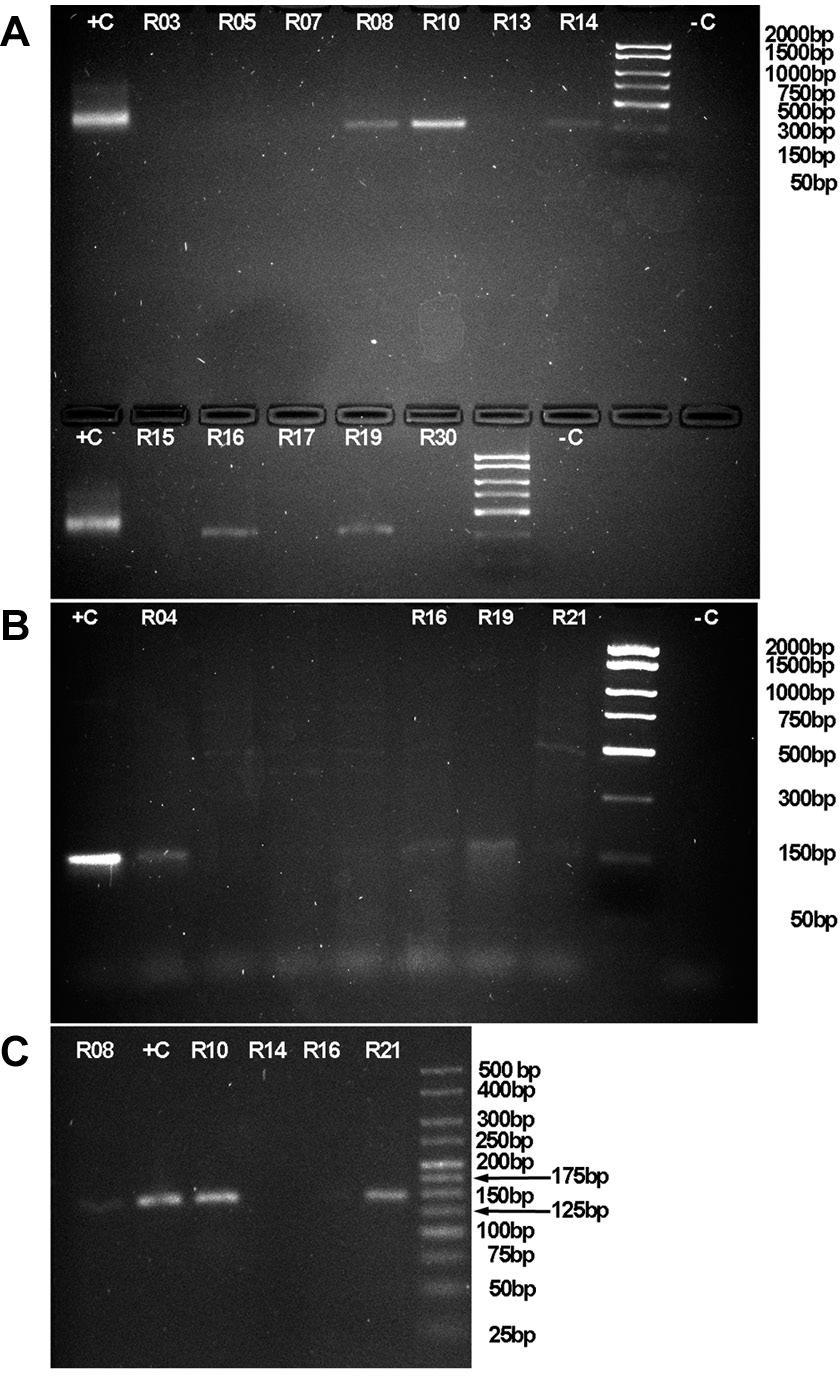
Prevalence of T. cruzi in tissue samples - Seven out of 37 (18.9%) raccoons were positive for infection with T. cruzi, whereas no bobcat, armadillo, fox or opossum tested positive for infection. Five out of the seven infected raccoons were collected in Jackson County, Illinois, while the remaining individuals were collected from Williamson County, Illinois and Boone County, Missouri. All positive were detected by amplification of a 330bp fragment of kDNA, using primers S35/S36 (Fig. 3A). No amplicons resulted from the use of the primer set targeting satDNA.
representative images of amplicons targeted at sites in the genome of Trypanosoma cruzi, imaged after electrophoresis in 2% agarose gels stained with ethidium bromide. (A) Fragments of about 330bp of kinetoplast DNA minicircle kDNA. (B) Amplicons of about 150bp of the intergenic region of the spliced leader intergenic region -SL- (also known as mini-exon intergenic region), and (C) amplicons of about 145bp targeting the D7 domain of the 24Sα ribosomal DNA -24Sα rDNA-.
Genotyping of T. cruzi in tissue samples - The size of the amplified SL was approximately 150bp (Fig. 3B), which is expected for DTU TcIII and TcIV. Further, the size of the amplified 24Sα rDNA fragment following the heminested reaction is about 145bp (Fig. 3C). Upon alignment and analysis of the SL sequences for phylogenetic signal we obtained the tree shown on Fig. 4. In this tree, the sequences resulting from the screening of wildlife in Illinois and Missouri, as well as the positive controls from Kentucky, formed a clade with reference sequences for TcIV, chiefly AY367124 and AY367123.
phylogenetic reconstruction of the homologous sequences for TcIV. The tree shows the topology resulting from the Bayesian inference of nodes and it includes the bootstrap support (above) and posterior probability values (below) for each node.
The sequenced amplicons isolated from raccoons in Illinois and Missouri include two polymorphisms not seen in reference AY367124. Yet, when the sequences from Illinois, Kentucky and Missouri are pooled together, the average intraspecific genetic distance is 0%; these pooled samples show an average distance of 0% when compared to reference AY367124 (isolated from a raccoon in Georgia, USA). The pooled sequences had an average genetic distance of 5% when compared against the reference sequence AY367123, also considered TcIV and isolated from a human being in Brazil. Furthermore, the pooled sequences average 10% genetic distance when compared against the CL-Brener isolate identified as the TcVI reference. Finally, the pooled sequences average a genetic distance of 19% when compared against the reference for strain TcIII (AF050521), isolated from an armadillo in the Brazilian Amazon (Table II).
Table of genetic distances for a set of sequences o Trypanosoma cruzi identified as TcIV in Illinois, Missouri, Kentucky compared to references for this DTU (AY367123 and AY367124) plus a reference for CL-Brener (Entry 6949 in Tritypdb.org). Distances based on the Jukes-Cantor model are shown to the left of the diagonal; uncorrected distances are shown to the right of said diagonal. The Nexus file including the commands used to complete these calculations is available at OPEN SIUC (http://opensiuc.lib.siu.edu/zool_data/12/)
DISCUSSION
We document for the first time the presence of T. cruzi in wild raccoons from Illinois. From the seven positive samples, it was possible to sequence and determine six as DTU TcIV. Thus, our results demonstrate that T. cruzi is present in Illinois as a fairly homogeneous strain, and it cycles in raccoons. This finding is consistent with the available genotypic characterizations of T. cruzi in the United States, which indicates that all DTUs with the exception of TcIII, are present in the country; with TcIV predominantly causing infections in raccoons (Bern et al. 2011Bern C, Kjos S, Yabsley MJ, Montgomery SP. Trypanosoma cruzi and Chagas' disease in the United States. Clin Microbiol Rev. 2011; 24(4): 655-81., Roellig et al. 2013Roellig DM, Savage MY, Fujita AW, Barnabe C, Tibayrenc M, Steurer FJ, et al. Genetic variation and exchange in Trypanosoma cruzi isolates from the United States. PLoS ONE. 2013; 8(2): 1-12., Garcia et al. 2017Garcia MN, Burroughs H, Gorchakov R, Gunter SM, Dumonteil E, Murray KO, et al. Molecular identification and genotyping of Trypanosoma cruzi DNA in autochthonous Chagas disease patients from Texas, USA. Infect Genet Evol. 2017; 49: 151-6.).
T. cruzi was detected in two of 62 bobcats sampled in Georgia (Brown et al. 2010Brown EL, Roellig DM, Gompper ME, Monello RJ, Wenning KM, Gabriel MW, et al. Seroprevalence of Trypanosoma cruzi among eleven potential reservoir species from six states across the southern United States. Vector Borne Zoonotic Dis. 2010; 10(8): 757-63.), and was not detected in any bobcats in our study. Contrastingly, infections in raccoons appear to be common across the southern half of the United States. The difference in the prevalence of T. cruzi in bobcats compared to raccoons, may be a result of the diet preferences of the former. Bobcats only opportunistically consume insects, whereas a raccoon's diet may consist of up to 40% insects, depending on the season (Llewellyn & Uhler 1952Llewellyn LM, Uhler FM. The foods of fur animals of the Patuxent Research Refuge, Maryland. Am Midl Nat. 1952; 48(1): 193-203.). The consumption of insects may increase the risk of contracting the pathogen, which was shown experimentally. Exposed raccoons contracted the pathogen after being fed with infected triatomine bugs; in contrast, raccoons did not acquire the infection upon consumption of meat carrying the parasite (Roellig et al. 2009Roellig DM, Ellis AE, Yabsley MJ. Oral transmission of Trypanosoma cruzi with opposing evidence for the theory of carnivory. J Parasitol. 2009; 95(2): 360-64.). Thus the oral transmission may only occur after raccoons consume the metacyclic trypomastigote stage of T. cruzi when it is present in a triatomine bug or its feces. As bobcats do not usually consume insects, their exposure via the oral route of transmission would be greatly reduced. However, it must be considered that the detection of T. cruzi in bobcats from Georgia was achieved by antibody testing, which may be more sensitive to the detection of current or past exposure to the pathogen.
Infections in opossums and armadillos in Illinois are yet to be documented. These mammals are frequently infected with the pathogen in southern localities; perhaps, a greater sample size may help detecting the prevalence of this parasite in these mammals. It should be considered that the prevalence for T. cruzi in surveys of wild caught opossums varies from 8 to 60% (Bern et al. 2011Bern C, Kjos S, Yabsley MJ, Montgomery SP. Trypanosoma cruzi and Chagas' disease in the United States. Clin Microbiol Rev. 2011; 24(4): 655-81.).
In our attempts to reconstruct the phylogeny of the strains in the United States, we discovered that several sequences used in the phylogenetic reconstruction of strains based on SL are not homologous. All of these sequences do belong to the SL, yet they do not amplify the same region of the gene. In some cases, the resulting sequences appear to go either upstream or downstream relative to the region between 12,300 to 12,400nt of reference T. cruzi 6949 strain CL-Brener (Fig. 2). However, the sequences KM376441 and KM376442, identified as TcIV elsewhere appear to amplify a region not homologous with the rest of the sequences used in this and other reconstructions. Nevertheless, although their use in phylogenetic reconstruction may not be recommended, the set of primers used in the multiplex reaction may remain an option to identify the involved strain in archived host tissues, (i. e, DNA from parasites not grown in culture or isolates), provided their sequences are compared against known references featuring the entire SL, and a second set of primers targeting a different region such as 24Sα rDNA is used (Zingales et al. 2009Zingales B, Andrade SG, Briones MRS, Campbell DA, Chiari E, Fernandes O, et al. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem Inst Oswaldo Cruz. 2009; 104(7): 1051-4., 2012Zingales B, Miles MA, Campbell DA, Tibayrenc M, Macedo AM, Teixeira MM. The revised Trypanosoma cruzi subspecific nomenclature: rationale, epidemiological relevance and research applications. Infect Genet Evol. 2012; 12(2): 240-53.).
Further study may include screening triatomine bugs within Midwestern states for the presence of this parasite, which would bring conclusive evidence of occurrence of the sylvatic cycle in the region. It would also help in better determining the risk of vector-borne transmission of T. cruzi in Illinois and other Midwestern states. As noted by Roellig et al. (2013)Roellig DM, Savage MY, Fujita AW, Barnabe C, Tibayrenc M, Steurer FJ, et al. Genetic variation and exchange in Trypanosoma cruzi isolates from the United States. PLoS ONE. 2013; 8(2): 1-12., there is considerable genetic variability in TcIV across southeastern states, which suggest that this strain has been established in the region for a long time. Thus, it becomes important to compare the genetic diversity of those parasites against those in Midwestern states, which may constitute the northern most limits of distribution of natural populations of the pathogen. Any signals of a recent population expansion would be evident in the form of low genetic diversity, which would be expected near or in the parasite's distribution limits. Determining the diversity, prevalence and geographic distribution of T. cruzi in the United States is key to determine areas of risks of vector-borne transmission within the US. Finally, it would also be advisable to expand the sample size of armadillos screened for the presence of T. cruzi and use more sensitive methods, including quantitative PCR (qPCR), hemoculture and antibody-based assays to identify population prevalence with a greater accuracy. Finally, nine-banded armadillos, are becoming an ubiquitous presence in Illinois (Hofmann 2009Hofmann JE. Records of nine-banded armadillos, Dasypus novemcinctus, in Illinois. Trans Ill State Acad Sci. 2009; 102(1-2): 95-106.); the increased abundance of this insectivore may afford an opportunity to track changes in dynamics and distribution of this parasite in the country as well as the expansion of a strain associated with them into new territories.
-
Financial support: Center for Undergraduate Research and Creative Activities (Southern Illinois University).
ACKNOWLEDGEMENTS
To Dr Rodrigo Carramiñana and Dr Ed Heist (Southern Illinois University) for providing support for professional development to CV and accessing and using of the sequencing facilities of the Conservation Genetics Laboratory at SIU; and Dr Kurt Neubig (Southern Illinois University) who facilitated reagents for completing part of the sequencing.
REFERENCES
- Bern C, Kjos S, Yabsley MJ, Montgomery SP. Trypanosoma cruzi and Chagas' disease in the United States. Clin Microbiol Rev. 2011; 24(4): 655-81.
- Bi LP, Groce C, Davis C. Molecular analysis of Trypanosoma cruzi isolates obtained from raccoons in Warren and Barren counties of Kentucky. BMC Bioinformatics. 2010; 11(Suppl. 4): P3.
- Boyles E, Nielsen CK. PBDEs and dechloranes in raccoons in the Midwestern United States. Bull Environ Contam Toxicol. 2017; 98(6): 758-62.
- Brenière SF, Waleckx E, Barnabê C. Over six thousand Trypanosoma cruzi strains classified into dDiscrete Typing Units (DTUs): attempt at an inventory. PLoS Negl Trop Dis. 2016; 10(8): e19.
- Briones MRS, Souto RP, Stolf BS, Zingales B. The evolution of two Trypanosoma cruzi subgroups inferred from rRNA genes can be correlated with the interchange of American mammalian faunas in the Cenozoic and has implications to pathogenicity and host specificity. Mol Biochem Parasitol. 1999; 104(2): 219-32.
- Brown EL, Roellig DM, Gompper ME, Monello RJ, Wenning KM, Gabriel MW, et al. Seroprevalence of Trypanosoma cruzi among eleven potential reservoir species from six states across the southern United States. Vector Borne Zoonotic Dis. 2010; 10(8): 757-63.
- Cribb P, Tapia E, Diosque P, Serra E. Spliced leader RNA gene promoter sequence heterogeneity in CL-Brener Trypanosoma cruzi reference strain. Infect Genet Evol. 2004; 4(2): 153-7.
- Darriba D, Taboada GL, Doallo R, Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods. 2012; 9(8): 772.
- Dorn PL, Perniciaro L, Yabsley MJ, Roellig DM, Balsamo G, Diaz J, et al. Autochthonous transmission of Trypanosoma cruzi, Louisiana. Emerg Infect Dis. 2007; 13(4): 605-7.
- Fernandes O, Santos SS, Cupolillo E, Mendonca B, Derre R, Junqueira ACV, et al. A mini-exon multiplex polymerase chain reaction to distinguish the major groups of Trypanosoma cruzi and T. rangeli in the Brazilian Amazon. Trans R Soc Trop Med Hyg. 2001; 95(1): 97-9.
- Fernandes O, Sturm NR, Derre R, Campbell DA. The mini-exon gene: a genetic marker for zymodeme III of Trypanosoma cruzi Mol Biochem Parasitol. 1998; 95(1): 129-33.
- Fracker SB. A systematic outline of the Reduviidae of North America. Des Moines: 1913. p. 217-52.
- Garcia MN, Aguilar D, Gorchakov R, Rossmann SN, Montgomery SP, Rivera H, et al. Evidence of autochthonous Chagas disease in southeastern Texas. Am J Trop Med Hyg. 2015; 92(2): 325-30.
- Garcia MN, Burroughs H, Gorchakov R, Gunter SM, Dumonteil E, Murray KO, et al. Molecular identification and genotyping of Trypanosoma cruzi DNA in autochthonous Chagas disease patients from Texas, USA. Infect Genet Evol. 2017; 49: 151-6.
- Hagerty AM, McPherson JE. Survey of the Reduviidae (Heteroptera) of southern Illinois, excluding the Phymatinae, with notes on biology. Great Lakes Entomol. 1999; 32(3): 133-60.
- Herrera CP, Licon MH, Nation CS, Jameson SB, Wesson DM. Genotype diversity of Trypanosoma cruzi in small rodents and Triatoma sanguisuga from a rural area in New Orleans, Louisiana. Parasit Vectors. 2015; 8: 123.
- Hiestand SJ, Nielsen C, Jiménez FA. Epizootic and zoonotic helminths of the bobcat (Lynx rufus) in Illinois and a comparison of helminth component communities across the American Midwest. Parasite. 2014; 21: 4.
- Hodo CL, Goodwin CC, Mayes BC, Mariscal JA, Waldrup KA, Hamer SA. Trypanosome species, including Trypanosoma cruzi, in sylvatic and peridomestic bats of Texas, USA. Acta Trop. 2016; 164: 259-66.
- Hofmann JE. Records of nine-banded armadillos, Dasypus novemcinctus, in Illinois. Trans Ill State Acad Sci. 2009; 102(1-2): 95-106.
- Lima L, Espinosa-Alvarez O, Ortiz PA, Trejo-Varon JA, Carranza JC, Pinto CM, et al. Genetic diversity of Trypanosoma cruzi in bats, and multilocus phylogenetic and phylogeographical analyses supporting Tcbat as an independent DTU (discrete typing unit). Acta Trop. 2015; 151: 166-77.
- Llewellyn LM, Uhler FM. The foods of fur animals of the Patuxent Research Refuge, Maryland. Am Midl Nat. 1952; 48(1): 193-203.
- Marcet PL, Duffy T, Cardinal MV, Burgos JM, Lauricella MA, Levin MJ, et al. PCR-based screening and lineage identification of Trypanosoma cruzi directly from faecal samples of triatomine bugs from northwestern Argentina. Parasitology. 2006; 132(1): 57-65.
- Moser DR, Kirchhoff LV, Donelson JE. Detection of Trypanosoma cruzi by DNA amplification using the Polymerase Chain-Reaction. J Clin Microbiol. 1989; 27(7): 1477-82.
- Porter JA. Triatoma sanguisuga (Leconte 1855) in Illinois. J Parasitol. 1965; 51(4): 500.
- Roellig DM, Ellis AE, Yabsley MJ. Oral transmission of Trypanosoma cruzi with opposing evidence for the theory of carnivory. J Parasitol. 2009; 95(2): 360-64.
- Roellig DM, Savage MY, Fujita AW, Barnabe C, Tibayrenc M, Steurer FJ, et al. Genetic variation and exchange in Trypanosoma cruzi isolates from the United States. PLoS ONE. 2013; 8(2): 1-12.
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Hohna S, et al. MrBayes 3.2: efficient bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 2012; 61(3): 539-42.
- Rosypal AC, Smith T, Alexander A, Weaver M, Stewart R, Houston A. Serologic survey of antibodies to Trypanosoma cruzi in coyotes and red foxes from Pennsylvania and Tennessee. J Zoo Wildl Med. 2014; 45(4): 991-3.
- Souto RP, Fernandes O, Macedo AM, Campbell DA, Zingales B. DNA markers define two major phylogenetic lineages of Trypanosoma cruzi Mol Biochem Parasitol. 1996; 83(2): 141-52.
- Sturm NR, Degrave W, Morel C, Simpson L. Sensitive detection and schizodeme classification of Trypanosoma cruzi cells by amplification of kinetoplast minicircle DNA sequences: use in diagnosis of Chagas' disease. Mol Biochem Parasitol. 1989; 33(3): 205-14.
- Sturm NR, Vargas NS, Westenberger SJ, Zingales B, Campbell DA. Evidence for multiple hybrid groups in Trypanosoma cruzi Int J Parasitol. 2003; 33(3): 269-79.
- Swofford DL. PAUP*. Phylogenetic Analysis Using Parsimony (*and other methods) 4.0b10. Sinauer Associates: Sunderland; 2003.
- Zingales B, Andrade SG, Briones MRS, Campbell DA, Chiari E, Fernandes O, et al. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem Inst Oswaldo Cruz. 2009; 104(7): 1051-4.
- Zingales B, Miles MA, Campbell DA, Tibayrenc M, Macedo AM, Teixeira MM. The revised Trypanosoma cruzi subspecific nomenclature: rationale, epidemiological relevance and research applications. Infect Genet Evol. 2012; 12(2): 240-53.
Publication Dates
-
Publication in this collection
Jan 2018
History
-
Received
12 June 2017 -
Accepted
11 Oct 2017