Abstracts
The metropolitan region of Recife, Brazil is endemic for Dirofilaria immitis and has an environment favorable to the development of Culex quinquefasciatus. The goal of this study was to evaluate the vector competence of the Cx. quinquefasciatus RECIFE population for D. immitis transmission. A total of 2,104 females of Cx. quinquefasciatus RECIFE population were exposed to different densities of D. immitis microfilariae blood meals, ranging from 1,820 to 2,900 mf/ml of blood, in a natural membrane apparatus. The results showed a variation between 92.3% and 98.8% of females fed. The exposure of the Cx. quinquefasciatus RECIFE population to different densities of microfilariae did not influence the mortality of the mosquitoes. Infective larvae from D. immitis were observed in the Malpighian tubules beginning on the 12th day, whereas larvae were observed in the head and proboscis beginning on the 13th day following infection. The vector efficiency index (VEI) presented by the mosquitoes ranged from 7.8 to 56.5. The data demonstrates that the Cx. quinquefasciatus RECIFE population has great potential for the transmission of D. immitis, as it allowed the development of the filarid until the infectious stage at the different densities of microfilariae to which it was exposed.
Biology; experimental infections; mosquitoes
A Região Metropolitana do Recife é endêmica para Dirofilaria immitis e possui ambiente favorável para o desenvolvimento de Culex quinquefasciatus. Neste estudo avaliou-se a competência vetorial de Cx. quinquefasciatus população RECIFE para a transmissão de D. immitis. Para tanto, 2.104 fêmeas de Cx. quinquefasciatus população RECIFE foram expostas a diferentes densidades de microfilárias de D. immitis, variando de 1.820 a 2.900 mf/ml de sangue por meio de membrana natural. Os resultados obtidos demonstraram variação de 92,3% a 98,8% de fêmeas ingurgitadas após a alimentação. A exposição de Cx. quinquefasciatus população RECIFE a diferentes densidades de microfilárias não influenciou na mortalidade dos mosquitos. Larvas infectantes de D. immitis foram observadas nos túbulos de Malpighi a partir do 12º dia, enquanto na cabeça e na probóscide foram observadas a partir do 13º dia após a infecção. Os índices de eficiência vetorial (IEV) apresentados pelo culicídeo variaram de 7,8 a 56,5. Os dados obtidos demonstraram que Cx. quinquefasciatus população RECIFE tem grande potencial para a transmissão de D. immitis, pois permitiu o desenvolvimento do filarídeo até o estágio infectante nas diferentes densidades de microfilárias às quais foi exposto.
Biologia; infecções experimentais; mosquitos
MEDICAL AND VETERINARY ENTOMOLOGY
Vector competence of Culex quinquefasciatus Say, 1823 exposed to different densities of microfilariae of Dirofilaria immitis (Leidy, 1856)
Competência vetorial de Culex quinquefasciatus Say, 1823 exposto a diferentes densidades de microfilárias de Dirofilaria immitis (Leidy, 1856)
Gílcia Aparecida de CarvalhoI; Leucio Câmara AlvesII; Rafael Trindade MaiaIII; Carlos Fernando Salgueirosa de AndradeIV; Rafael Antonio do Nascimento RamosII; Maria Aparecida da Glória FaustinoII
IDepartamento de Parasitologia, Instituto de Biologia, Universidade Estadual de Campinas, 13084-971, Caixa Postal 6109, Campinas-SP, Brasil. gilciasilva@yahoo.com.br
IIDepartamento de Medicina Veterinária, Universidade Federal Rural de Pernambuco, 52171-900, Recife-PE, Brasil. leucioalves@hotmail.com; rafaelanramos@yahoo.com.br; magfaustino@hotmail.com
IIIInstituto Nacional de Pesquisas da Amazônia, 69060-001, Caixa Postal 478, Manaus-AM, Brasil. rafatrinmaia@ig.com.br
IVDepartamento de Zoologia, Instituto de Biologia, Universidade Estadual de Campinas, 13084-971, Caixa Postal 6109, Campinas-SP, Brasil. cfeandra@unicamp.br
ABSTRACT
The metropolitan region of Recife, Brazil is endemic for Dirofilaria immitis and has an environment favorable to the development of Culex quinquefasciatus. The goal of this study was to evaluate the vector competence of the Cx. quinquefasciatus RECIFE population for D. immitis transmission. A total of 2,104 females of Cx. quinquefasciatus RECIFE population were exposed to different densities of D. immitis microfilariae blood meals, ranging from 1,820 to 2,900 mf/ml of blood, in a natural membrane apparatus. The results showed a variation between 92.3% and 98.8% of females fed. The exposure of the Cx. quinquefasciatus RECIFE population to different densities of microfilariae did not influence the mortality of the mosquitoes. Infective larvae from D. immitis were observed in the Malpighian tubules beginning on the 12th day, whereas larvae were observed in the head and proboscis beginning on the 13th day following infection. The vector efficiency index (VEI) presented by the mosquitoes ranged from 7.8 to 56.5. The data demonstrates that the Cx. quinquefasciatus RECIFE population has great potential for the transmission of D. immitis, as it allowed the development of the filarid until the infectious stage at the different densities of microfilariae to which it was exposed.
Keywords: Biology; experimental infections; mosquitoes.
RESUMO
A Região Metropolitana do Recife é endêmica para Dirofilaria immitis e possui ambiente favorável para o desenvolvimento de Culex quinquefasciatus. Neste estudo avaliou-se a competência vetorial de Cx. quinquefasciatus população RECIFE para a transmissão de D. immitis. Para tanto, 2.104 fêmeas de Cx. quinquefasciatus população RECIFE foram expostas a diferentes densidades de microfilárias de D. immitis, variando de 1.820 a 2.900 mf/ml de sangue por meio de membrana natural. Os resultados obtidos demonstraram variação de 92,3% a 98,8% de fêmeas ingurgitadas após a alimentação. A exposição de Cx. quinquefasciatus população RECIFE a diferentes densidades de microfilárias não influenciou na mortalidade dos mosquitos. Larvas infectantes de D. immitis foram observadas nos túbulos de Malpighi a partir do 12º dia, enquanto na cabeça e na probóscide foram observadas a partir do 13º dia após a infecção. Os índices de eficiência vetorial (IEV) apresentados pelo culicídeo variaram de 7,8 a 56,5. Os dados obtidos demonstraram que Cx. quinquefasciatus população RECIFE tem grande potencial para a transmissão de D. immitis, pois permitiu o desenvolvimento do filarídeo até o estágio infectante nas diferentes densidades de microfilárias às quais foi exposto.
Palavras-chave: Biologia; infecções experimentais; mosquitos.
Dirofilaria immitis (Leidy, 1856) is a commonly found parasite in the heart cavities and pulmonary arteries of canines and felines, and can also infect humans (Samarawickrema et al. 1992). Although the transmission of D. immitis is carried out by Culex Linnaeus, 1758, Aedes Meigen, 1818, Anopheles Meigen, 1818, Mansonia Blanchard, 1901, Psorophora Robineau-Desvoidy, 1827 and Coquillettidia Dyar, 1905 species (Ludlam et al. 1970), several studies are being conducted in various regions of the world in order to determine the vector species of this nematode.
In Brazil, the species Cx. quinquefasciatus Say, 1823 is considered a potential vector for D. immitis in the states of Rio de Janeiro, (Lourenço-De-Oliveira & Deane 1995; Labarthe et al. 1998 a,b), Alagoas (Brito et al. 1999) and Maranhão (Ahid & Lourenço-De-Oliveira 1999; Ahid et al. 2000). In the Brazilian Northeast Region, the species Cx. quinquefasciatus is important in locations that are endemic for Wuchereria bancrofti (Cobbold, 1877), as it is the main vector of this filarid (Medeiros et al. 1992), particularly in the metropolitan region of Recife in the state of Pernambuco, where bancroftian (Albuquerque et al. 1999; Lima et al. 2003) and canine filariosis, (Alves et al. 1999) are endemic.
The goal of the present study was to evaluate the vector competence of the Cx. quinquefasciatus RECIFE population artificially exposed to different densities of D. immitis microfilariae.
MATERIAL AND METHODS
Mosquitoes. The mosquitoes used in these experiments were of the species Cx. quinquefasciatus RECIFE population which was established in 2003 from eggs provided by Aggeu Magalhães Research Center (FIOCRUZ), Recife, PE. Maintenance of the colony was performed in a climate-controlled room at a temperature of 28 ± 1ºC with 80 ± 5% relative humidity and a natural photoperiod cycle at the Insect Laboratory for Parasitic Diseases of Domestic Animals of the Department of Veterinary Medicine of the Universidade Federal Rural de Pernambuco (UFRPE), Recife, PE. Canine blood was offered to the females for feeding once a week in an artificial feeder (Rutledge et al. 1964; Mendonça et al. 1998).
D. immitis microfilariae strain. The microfilariae used for the experimental purpose were obtained of a domiciliated dog with natural infection from the Metropolitan Region of Recife, Pernambuco, Brazil.
Blood collection. Blood were collected through venous puncture of the cephalic vein, into a test tube containing Ethylenediaminetetraacetic Acid for blood microfilaria count and also in tube containing heparin destined to the infectious feeding of the female mosquitoes.
Processing of the material. The microfilaremia of the animal was quantified using the modified Knott method (Newton & Wrigth 1956).
Experimental mosquitoes exposure to D. immitis microfilariae. For the first artificial blood meal, a total of 2,104 Cx. quinquefasciatus RECIFE population females between three and seven days of age were used (Rutledge et al. 1964). As the membrane for the artificial meal, a quail skin (Coturnix coturnix (Linnaeus, 1758)) was choose. The time exposure for each meal was two hours. For this procedure, undiluted dog blood containing five densities of D. immitis microfilariae was used. As control group, a total of 1,200 females were fed with non-infected blood (Table I). Prior to exposure, the female mosquitoes were deprived of sugar solution for 24 hours. After the experimental infection, the Cx. quinquefasciatus females were fed with a sugar solution and all the non ingurgitated were discarded.
Dissection of mosquitoes and observation of D. immitis larvae. For a period of 14 days, ten females per day had the Malpighian tubules, head and thorax extracted in a saline solution, macerated by means of compression between the slide and cover slip, and stained using the Giemsa method to facilitate the microscopic analysis of the D. immitis larvae. Observations were made on an optical microscopy and development stages were determined following Taylor (1960).
Analysis of Cx. quinquefasciatus vector competence for transmitting D. immitis. After two hours of blood meal, the percentage of ingurgitated females was recorded, and ten females of each group were dissected and average number of ingested microfilaria was calculated. Mortality of the culicids was observed daily in a cumulative form and expressed in both absolute and relative values. Dead mosquitoes were dissected and examined for D. immitis larvae daily. The infection ratio (IR) was calculated according to procedures described by Ahid et al. (2000) and the vector efficiency index (VEI) was determined according to the procedures described by Kartman (1954).
Statistical analysis. The linear regression test was used to assess the influence of the different densities of D. immitis microfilaria per milliliter (ml) of blood to which the mosquitoes were exposed as well as the proportion of ingurgitation of the females and differences in ingested microfilaria. The Kruskal-Wallis test was used to assess microfilaria ingestion by the Cx. quinquefasciatus RECIFE population at the different densities of D. immitis microfilaria (mf) per ml of blood. ANOVA was used to compare larval development until the infectious stage in the culicid females as well as the mortality variation of the mosquitoes at the different densities of mf/ml. The BioStat program (version 2.0) was employed for the statistical calculations (Ayres et al. 2000).
RESULTS
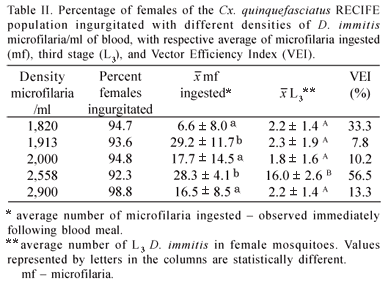
Following the feeding of the Cx. quinquefasciatus RECIFE population with blood containing different densities of microfilaria/ml, a variation between 92.3 and 98.8% of fed females was observed (Table II). On the control group, almost 100% of ingurgitated was observed. The average number of microfilaria ingested per female ranged from 6.6 to 29.2; the average observed at the 1,820 density differed significantly (p<0.05) from averages found for the 1,913 and 2,558 densities.
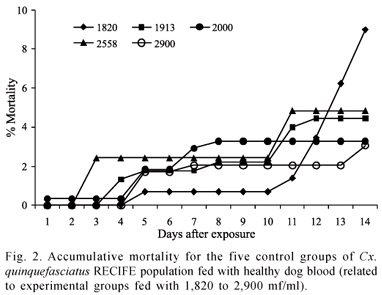
The mortality rate of the Cx. quinquefasciatus RECIFE population did not surpass 36.3%, regardless of the density of D. immitis microfilaria in the blood meal (Fig. 1). For the 2,558 microfilaria/ml density, the culicids exhibited a mortality rate and vector efficiency index of 8.9% and 56.5%, respectively. Similar mortality rates (ANOVA p>0.05) were found among females exposed to densities of 1,913 and 2,558 mf/ml of blood. These rates were lower than those found for the densities of 1,820, 2,000 and 2,900 mf/ml of blood and did not differ significantly from one another (ANOVA p>0.05). The mortality rate among the females of the mosquitoes fed with non-infected blood (control) was lower than that found among the females exposed to D. immitis microfilaria (ANOVA p>0.05) (Fig. 2).
The development time for D. immitis in the Cx. quinquefasciatus RECIFE population was similar for all densities of microfilaria. The sausage stage began to be observed on the third day following infection and persisted until the ninth day. The melanization process occurred in 5.4% (106/1,961) larvae of D. immitis in the culicids; this reaction wasn't observed by the 14th day for 2,558 mf/ml of blood. At densities of 1,913 and 2,900 mf/ml of blood, one and three first stage larvae (L1) with altered morphologies were found, respectively. From the sixth to 11th day, second stage larvae (L2) of D. immitis were observed, whereas third stage larvae (L3) were observed beginning on the 12th day following infection for all densities, except the density of 1,913 mf/ml of blood, which only exhibited infectious larvae on the 13th day.
Culex quinquefasciatus females exhibited infectious larvae (L3) of D. immitis in the Malpighian tubules starting on the 12th day and exhibited infectious larvae in the head and proboscis on the 13th day following infection. The vector efficiency index (VEI) for the Cx. quinquefasciatus RECIFE population was from 7.8 to 56.5% (Table II). Regarding the infection ratio, the highest index was found in the group of females exposed to a density of 1,913 microfilaria/ml (Table III).
DISCUSSION
In regarding of the Cx. quinquefasciatus infection by D. immitis, it is known that Cx. quinquefasciatus can ingest large quantities of microfilaria. Due to physiological and immunological reactions, however, they may limit the number of D. immitis that will develop to the infective stage. This strategy ensures the survival of the culicid, as the development of a large number of nematode larvae destroys the Malpighian tubules of the mosquito, thereby causing high mortality (Palmer et al. 1986). The results regarding the average of microfilaria ingested (28.3) by the Cx. quinquefasciatus RECIFE population at a density of 2,558 mf/ml were higher than those observed by Lai et al. (2000) for the Taiwan population (15.3).
The Cx. quinquefasciatus RECIFE population demonstrated vector efficiency, as the mortality rate of the culicid was not influenced by the exposure of different densities of D. immitis microfilaria (F = 0.0615, p>0.05). According to Scoles (1998), a culicid must resist infection regardless of the parasitic load; survive long enough to permit the development of the larvae until the infectious form for mammals; feed on canine blood; be adapted to the geographic region; be abundant; and exhibit various population peaks throughout the year (Ludlam et al. 1970; Christensen 1977). Thus, the Cx. quinquefasciatus RECIFE population exhibits the necessary characteristics to be considered competent in the transmission of D. immitis.
It is also known that infected mosquitoes are more sensitive to temperature variations, with diminishing survival rates at higher temperatures (Kutz & Dobson 1974). However, the main cause of mortality of infected females is attributed to the development of D. immitis in the mosquito, with peak mortality generally occurring within the first 48 hours after infection, when first stage larvae (L1) of the nematode invade the Malpighian tubules of the females and later, between the 12th and 14th day, break free from these tubules and migrate to the head and proboscis of the culicids (Kartman 1953; Buxton & Mullen 1981).
The development time for D. immitis in the Cx. quinquefasciatus RECIFE population was similar at all microfilaria densities, which is in agreement with data obtained by Ahid et al. (2000) for Cx. quinquefasciatus and is also similar to results obtained by Taylor (1960) and Mendonça et al. (1998) for Ae. aegypti. However, it was observed that melanization occurred in some D. immitis larvae, thereby emphasizing the restrictions that the culicid population placed on the nematode regarding its development. According to McGreevy et al. (1978) and Coluzzi et al. (1982), Cx. quinquefasciatus eliminates a large number of ingested microfilaria by means of imprisonment in the cibarium and later destruction as well as by melanization mechanisms (Christensen & Forton 1986), the rapid coagulation of ingested blood, and the presence of oxyhemoglobin crystals in the intestinal content resulting from the oxidation of the ingested blood (Nayar & Sauerman 1975; Lowrie 1991; Loftin et al. 1995). This allows the development of a supportable number of larvae that do not cause much damage.
The females of Cx. quinquefasciatus exhibited infectious larvae in the Malpighian tubules on the 12th day and in the head and proboscis on the 13th day pos infection. Brito et al. (1999) observed infectious larvae in the Malpighian tubules of the Cx. quinquefasciatus ALAGOAS population on the 14th day, whereas Ahid et al. (2000) found infectious larvae (L3) in the Malpighian tubules of the Cx. quinquefasciatus RECIFE population on the 11th day and in the proboscis only on the 14th day following infection. The detection periods of the larval stage found in the present study are in agreement with reports by Taylor (1960) and Mendonça et al. (1998) for Ae. aegypti Linnaeus, 1762.
The fact that the infection ratio in the Cx. quinquefasciatus RECIFE population was not influenced by the feeding rate of the females (F=3.2937, p>0.05) and did not increase with the increased number of D. immitis microfilaria ingested (F = 2.1952, p>0.05) is similar to observations by Russell & Geary (1996), who found no relationship between the increase in the density of D. immitis microfilaria and the infection ratio of Cx. annulirostris Skuse, 1889. In the present study, the number of D. immitis larvae having developed to the infectious stage in Cx. quinquefasciatus was also not influenced by the different densities of microfilaria (F=0.5912, p>0.05) to which the mosquitoes were exposed, nor by the increase in the number of microfilaria ingested (F=1.3569, p>0.05).
The data obtained demonstrate that the Cx. quinquefasciatus RECIFE population exhibits great potential for the transmission of D. immitis. It withstood the development of the nematode through to the infectious stage at the different densities of microfilaria to which it was exposed. Labarthe et al. (1998a) considers Cx. quinquefasciatus a secondary vector in both Rio de Janeiro as well as São Luis, MA. Ahid et al. (1999) found this species naturally infected by parasite larvae in the infectious stage, suggesting that it is a good D. immitis vector in Northeast Brazil. The results from the present study broaden this possibility. By means of experimental infections, the Cx. quinquefasciatus RECIFE population proved to have competence for the transmission of D. immitis, suggesting that it may be one of the transmission species of this nematode in the metropolitan region of Recife, Brazil.
Received 04/12/2007; accepted 12/09/2008
- Ahid, S. M. M. & R. Lourenço-de-Oliveira. 1999. Mosquitos vetores potenciais de dirofilariose canina na Região Nordeste do Brasil. Revista de Saúde Pública 33: 560565.
- Ahid, S. M. M.; R. Lourenço-de-Oliveira & L. Q. Saraiva. 1999. Dirofilariose canina na Ilha de São Luís, Nordeste do Brasil: uma zoonose potencial. Cadernos de Saúde Pública 15: 405412.
- Ahid, S. M. M.; P. S. S. Vasconcelos & R. Lourenço-de-Oliveira. 2000. Vector competence of Culex quinquefasciatus Say from different regions of Brazil to Dirofilaria immitis Memórias do Instituto Oswaldo Cruz 95: 769775.
- Albuquerque, C. M. R.; V. M. S. Cavalcanti; M. A. V. Melo; P. Verçosa; L. N. Regis & H. Hurd. 1999. Bloodmeal microfilariae density and the uptake and establishment of Wuchereria bancrofti infections in Culex quinquefasciatus and Aedes aegypti Memórias do Instituto Oswaldo Cruz 94: 591596.
- Alves, L. C.; L. V. A. Silva; M. A. G. Faustino; J. W. McCall; P. Supakonderj; N. Labarthe; M. Sanchez & O. Caires. 1999. Survey of canine heartworm in the city of Recife, Pernambuco, Brazil. Memórias do Instituto Oswaldo Cruz 94: 587590.
- Ayres, M.; M. Ayres Jr.; D. L. Ayres & A. L. Santos. 2000. BioEstat 2.0: Aplicações estatísticas nas áreas das ciências biológicas e médicas. Sociedade Civil Mamirauá, CNPq, Brasília, 272 p.
- Brito, A. C.; G. Fontes; E. M. M. Rocha; D. A. M. Rocha & L. Regis. 1999. Development of Dirofilaria immitis (Leidy) in Aedes aegypti (L.) and Culex quinquefasciatus (Say) from Maceió, Alagoas, Brazil. Memórias do Instituto Oswaldo Cruz 94: 575576.
- Buxton, B. A. & G. R. Mullen. 1981. Comparative susceptibility of four strains of Aedes aegypti (Díptera: Culicidae) to infection with Dirofilaria immitis Journal of Medical Entomology 18: 434440.
- Christensen, B. N. & K. F. Forton. 1986. Hemocyte-mediated melanization of microfilariae in Aedes aegypti The Journal of Parasitology 72: 220225.
- Christensen, B. M. 1977. Laboratory studies on the development and transmission of Dirofilaria immitis by Aedes trivittatus Mosquito News 37: 367372.
- Coluzzi, M.; A. Concetti & F. Ascoli. 1982. Effect of cibarial armature of mosquitoes (Diptera: Culicidae) on blood-meal haemolysis. Journal of Insect Physiology 28: 885888.
- Cônsoli, R. A. G. B. & R. L. Lourenço-de-Oliveira. 1994. Principais mosquitos de importância sanitária no Brasil. Ed. Fiocruz, Rio de Janeiro, 228 p.
- Kartman, L. 1953. Factors influencing infection of the mosquito with Dirofilaria immitis (Leidy, 1856). Experimental Parasitolology 2: 2728.
- Kartman, L. 1954. Suggestions concerning an index experimental filaria infection in mosquitoes. American Journal of Tropical Medicine and Hygiene 3: 329337.
- Kutz, F. W. & R. C. Dobson. 1974. Effects of temperature on the development of Dirofilaria immitis (Leidy) in Anopheles quadrimaculatus Say and on vector mortality resulting from this development. Annais of the Entomolology Society of America 67: 325331.
- Labarthe, N.; M. L. Serrão; Y. F. Melo; S. J. Oliveira & R. Lourenço-de-Oliveira. 1998a. Potential vectors of Dirofilaria immitis (Leidy, 1856) in Itacoatiara, oceanic region of Niterói municipality, State of Rio de Janeiro, Brazil. Memórias do Instituto Oswaldo Cruz 93: 425432.
- Labarthe, N.; M. L. Serrão; Y. F. Melo; S. J. Oliveira & R. Lourenço-de-Oliveira.1998b. Mosquito frequency and feeding habits in an enzootic canine dirofilariasis area in Niterói, State of Rio de Janeiro, Brazil. Memórias do Instituto Oswaldo Cruz 93: 145154.
- Lai, C. H.; K. C. Tung; H. K. Ooi & J. S. Wang. 2000. Competence of Aedes albopictus e Culex quinquefasciatus as vector of Dirofilaria immitis after blood meal with different microfilarial density. Veterinary Parasitology 90: 231237.
- Lima, C. A.; W. R. Almeida; H. Hurd & C. M. R. Albuquerque. 2003. Reproductive aspects of the Culex quinquefasciatus (Diptera: Culicidae) infected with Wuchereria bancrofti (Spirurida: Onchocercidae). Memórias do Instituto Oswaldo Cruz 98: 217222.
- Loftin, K. M.; R. L. Byford; M. J. Loftin & M. E. Craig. 1995. Potential mosquito vectors of Dirofilaria immitis in Bernalillo County, New Mexico. Journal of the American Mosquito Control Association 11: 9093.
- Lourenço-de-Oliveira, R. & L. M. Deane. 1995. Presumed Dirofilaria immitis infections in wild-caught Aedes taeniorhynchus and Aedes scapularis in Rio de Janeiro, Brasil. Memórias do Instituto Oswaldo Cruz 90: 387388.
- Lowrie, R. C. 1991. Poor vector efficiency of Culex quinquefasciatus following infection with Dirofilaria immitis Journal of the American Mosquito Control Association 7: 3037.
- Ludlam, K. W.; L. A. Jachowski & G. F. Otto. 1970. Potential vectors of Dirofilaria immitis Journal of the American Veterinary Medical Association 157: 13541359.
- McGrevy, P. B.; J. H. Bryan; P. Oothman & N. Kolstrup. 1978. The lethal effects of the cibarial and pharyngeal armatures of mosquitoes on microfilariae. Transactions of the Royal Society of Tropical Medicine and Hygiene 72: 361368.
- Medeiros, Z.; G. Dreyer & L. D. Andrade. 1992. Wuchereria bancrofti microfilarial density of autochthonous cases and natural Culex infectivity rates in Northeast Brazil. Journal of Tropical Medicine and Hygiene 95: 214217.
- Mendonça, I. L.; L. C. Alves; J. W. McCall; M. G. A. Faustino & P. Supakonderj. 1998. Porcentual de Infecção por Dirofilaria immitis (Leidy, 1856) em Aedes aegypti (Linnaeus) através da utilização de membrana artificial. Ciência Veterinária nos Trópicos 1: 3032.
- Nayar, J. K. & D. M. Sauermann. 1975. Physiological basis of host susceptibility of Florida mosquitoes to Dirofilaria immitis Journal of Insect Physiology 21: 19651975.
- Newton, W. L. & W. H. Wright. 1956. The occurrence of a dog filariid other than Dirofilaria immitis in the United States. The Journal of Parasitology 42: 246258.
- Palmer, C. A.; D. D. Wittrock & B. M. Christensen. 1986. Ultrastructure of malpighian tubules of Aedes aegypti infected with Dirofilaria immitis Journal of Invertebrate Pathology 48: 310317.
- Russell, R. C. & M. J. Geary. 1996. The influence of microfilarial density of dog heartworm Dirofilaria immitis on infection rate and survival of Aedes notoscriptus and Culex annulirostris from Australia. Medical and Veterinary Entomology 10: 2934.
- Rutledge, L. C.; R. A. Ward & D. J. Gould. 1964. Studies on the feeding response of mosquitoes to nutritive solutions in a new membrane feeder. Mosquito News 24: 407419.
- Samarawickema, W. A.; E. Kimura; F. Sones; G. S. Paulson & E. F. Cummings. 1992. Natural infections of Dirofilaria immitis in Aedes polynesiensis and Aedes (Finlaya) samoamus and their implication in human health in Samoa. Transactions of the Royal Society of Tropical Medicine and Hygiene 86: 187188.
- Scoles, G. A. 1998. Vectors of canine heartworm in the United States: a review of the literature including new data from Indiana, Florida and Louisiana. In: Seaward, R.L.& C.H. Courtney. 1998. Recent Advances in Heartworm Disease: Symposium'98. Tampa, FL: American Heartworm Society, 2136.
- Taylor, A. E. R. 1960. The development of Dirofilaria immitis the mosquito Aedes aegypti. Journal of Helminthology 34: 2738.
Publication Dates
-
Publication in this collection
28 Jan 2009 -
Date of issue
2008
History
-
Received
04 Dec 2007 -
Accepted
12 Sept 2008