ABSTRACT
Soybean looper (Chrysodeixis includens) and Spodoptera group are important defoliators insects responsible for significant yield losses in soybean, cotton and maize in Brazil. Bioinsecticides and transgenic plants expressing insecticidal proteins from Bacillus thuringiensis are among the most used sustainable control methods for pest control in agriculture, especially for several lepidopteran species. To provide new components for insect control, this study aimed to select and characterize B. thuringiensis strains toxic to C. includens, S. cosmioides, S. eridania, and S. frugiperda. We performed initial bioassays with fifty B. thuringiensis strains for C. includens and selected four strains – 1608A, 726, 773, and 775E – that caused high larval mortality (100%) to be tested against Spodoptera species. These strains harbored cry insecticidal genes, megaplasmids, absence of β-exotoxin and showed two major proteins about 65 and 130 kDa in SDS-page attributed to Cry protein classes toxic to lepidopteran species. The 1608A and 775E strains also showed high toxicity to Spodoptera species that demonstrate great potential for B. thuringiensis-based bioproduct development and as sources of new insecticidal genes for transgenic crops for multiple pest control relevant in the Brazilian agricultural system.
Keywords:
Multiple pest control; Lepidoptera; B. thuringiensis; Molecular approach
Introduction
The soybean looper, Chrysodeixis includens [Walker, 1858] (Lepidoptera: Noctuidae), is a polyphagous pest present in several countries of the American continent, with occurrence from the north of the USA to the south of South America. C. includens causes economic losses in diverse crops (Moscardi et al., 2012Moscardi, F., Bueno, A. D. F., Bueno, R. C. O. D. F., Garcia, A., 2012. Soybean response to different injury levels at early developmental stages. Cienc. Rural 42 (3), 389-394. https://doi.org/10.1590/S0103-84782012000300001.
https://doi.org/10.1590/S0103-8478201200...
; Souza et al., 2019Souza, L. A., Barbosa, J. C., Carvalho, J. H. S., Santos, L. S., Fraga, D. F., Busoli, A. C., 2019. Spatial distribution of Chrysodeixis includens eggs (Walker, 1858)(Lepidoptera: Noctuidae) in soybean crop. Rev. Cienc. Agric. 17 (2), 51-57. https://doi.org/10.28998/rca.v17i2.7124.
https://doi.org/10.28998/rca.v17i2.7124...
) and, it is considered one of the most important pests in soybean crop in Brazil. The Spodoptera genus are composed of important species of defoliator caterpillars, such as S. cosmioides, S. eridania and the fall armyworm, S. frugiperda (JE Smith), responsible for significant losses in maize production in Brazil, African continent, India, and China (Goergen et al., 2016Goergen, G., Kumar, P. L., Sankung, S. B., Togola, A., Tamò, M., 2016. First report of outbreaks of the fall armyworm Spodoptera frugiperda (JE Smith)(Lepidoptera, Noctuidae), a new alien invasive pest in West and Central Africa. PLoS One 11 (10), e0165632. https://doi.org/10.1371/journal.pone.0165632.
https://doi.org/10.1371/journal.pone.016...
; Guo et al., 2018Guo, J., Zhao, J., He, K., Zhang, F., Wang, Z., 2018. Potential invasion of the crop- devastating insect pest fall armyworm Spodoptera frugiperda to China. Plant Protection. 44(6), 1-10. https://doi.org/16688/j.zwbh.2018452.
https://doi.org/16688/j.zwbh.2018452...
; Sharanabasappa et al., 2018Sharanabasappa, K. C. M., Asokan, R., Mahadeva, S. H. M., Maruthi, M. S., Pavithra, H. B., Hegde, K., Goergen, G., 2018. First report of the fall armyworm, Spodoptera frugiperda (J E Smith) (Lepidoptera: Noctuidae), an alien invasive pest on maize in India. Pest Manage. Hortic. Ecosyst. 24 (1), 23-29. Available in: https://hdl.handle.net/10568/103519 (acessed 10 April 2019).
https://hdl.handle.net/10568/103519...
). Control of these insects is mainly achieved using chemical insecticides, and it requires control actions throughout most of the growing crop seasons. Biological control using Bacillus thuringiensis (Berliner) is promising alternative to control these insects.
B. thuringiensis is an aerobic Gram-positive bacterium that produces protein crystalline inclusions named Cry proteins during the stationary phase and encoded by different cry genes (Angus, 1954Angus, T. A., 1954. A bacterial toxin paralysing silkworm larvae. Nature 173 (4403), 545-546. https://doi.org/10.1038/173545a0.
https://doi.org/10.1038/173545a0...
; Bechtel and Bulla, 1976Bechtel, D. B., Bulla, L. A., 1976. Electron microscope study of sporulation and parasporal crystal formation in Bacillus thuringiensis. J. Bacteriol. 127 (3), 1472-1481. https://doi.org/10.1128/JB.127.3.1472-1481.1976.
https://doi.org/10.1128/JB.127.3.1472-14...
). These genes are mainly present in the plasmids that are considered important elements of genetics bacterial, although some these genes may also be present on chromosome (Liu et al., 2010Liu, X. Y., Ruan, L. F., Hu, Z. F., Peng, D. H., Cao, S. Y., Yu, Z. N., Sun, M., 2010. Genome-wide screening reveals the genetic determinants of an antibiotic insecticide in Bacillus thuringiensis. J. Biol. Chem. 285 (50), 39191-39200. https://doi.org/10.1074/jbc.M110.148387.
https://doi.org/10.1074/jbc.M110.148387...
). Crystal proteins are composed of one or more Cry and/or Cyt proteins, cytolytic proteins named delta (δ) endotoxins. These components determine B. thuringiensis pathogenicity (Schnepf et al., 1998Schnepf, E., Crickmore, N. V., Van Rie, J., Lereclus, D., Baum, J., Feitelson, J., Dean, D. H., 1998. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 62 (3), 775-806.). B. thuringiensis strains also produce other types of insecticidal proteins, such as the vegetative insecticidal proteins (Vip) and secreted insecticidal proteins (Sip), synthesized during the vegetative phase growth not forming any crystals. In addition, are capable of producing non-proteinaceous, thermostable and secretable secondary metabolites exhibiting non-specific toxic activity not only against a wide range of insects but also against mammals (Levinson et al., 1990Levinson, B. L., Kasyan, K. J., Chiu, S. S., Currier, T. C., Gonzalez, J. M., 1990. Identification of beta-exotoxin production, plasmids encoding beta-exotoxin, and a new exotoxin in Bacillus thuringiensis by using high-performance liquid chromatography. J. Bacteriol. 172, 3172-3179.). Due to these dangerous characteristics, it is important to detect isolates that produce β-exotoxins to no use them in biopesticide formulation (WHO, 1999World Health Organization - WHO, 1999. Bacillus thuringiensis: environmental Health Criteria, WHO, Geneva, pp. 217.).
The insecticide activities of entomopathogenic microorganisms such as B. thuringiensis have succeeded in promoting the pest control. The damage caused by the high incidence of defoliating caterpillars is constant in each crop. Therefore, is important the search for new isolates with toxic activities for different pest species for production of new agricultural products such as biopesticides, and the development of transgenic plants (Lee et al., 2015Lee, Y. K., Jin, N. Y., Lee, B. R., Seo, M. J., Youn, Y. N., Gill, S. S., Yu, Y. M., 2015. Isolation and characterization of new Bacillus thuringiensis strains with insecticidal activity to difficult to control lepidopteran pests. J. Facul. Agricul. 60 (1), 103-112.; Mukhija and Khanna, 2018Mukhija, B., Khanna, V., 2018. Cry Protein Profiling of Bacillus thuringiensis Isolated from Different Agro-Climate Soils of Punjab. Int. J. Curr. Microbiol. Appl. Sci. 7 (3), 2866-2870. https://doi.org/10.20546/ijcmas.2018.703.330.
https://doi.org/10.20546/ijcmas.2018.703...
). The use of molecular strategies for the characterization of efficient B. thuringiensis isolates is widely applied and efficient to distinguish their properties mainly in relation to the gene content (Carozzi et al., 1991Carozzi, N. B., Kramer, V. C., Warren, G. W., Evola, S., Koziel, M. G., 1991. Prediction of insecticidal activity of Bacillus thuringiensis strains by polymerase chain reaction product profiles. Appl. Environ. Microbiol. 57 (11), 3057-3061. https://doi.org/10.1128/AEM.57.11.3057-3061.1991.
https://doi.org/10.1128/AEM.57.11.3057-3...
; Valicente et al., 2010Valicente, F. H., Picoli, E. A., Vasconcelos, M. J. V., Carneiro, N. P., Carneiro, A. A., Guimarães, C. T., Lana, U. G., 2010. Molecular characterization and distribution of Bacillus thuringiensis cry1 genes from Brazilian strains effective against the fall armyworm, Spodoptera frugiperda. Biol. Control 53 (3), 360-366. https://doi.org/10.1016/j.biocontrol.2010.02.003.
https://doi.org/10.1016/j.biocontrol.201...
, Chandrasekaran et al., 2018Chandrasekaran, R., Revathi, K., Senthil-Nathan, S., Kalaivani, K., Hunter, W. B., Duraipandiyan, V., Esmail, G. A., 2018. Eco-friendly formulation of wild Bacillus thuringiensis secondary metabolites through molecular characterization against the lepidopteran pest. Physiol. Mol. Plant Pathol. 101, 93-104. https://doi.org/10.1016/j.pmpp.2017.09.002.
https://doi.org/10.1016/j.pmpp.2017.09.0...
).
In order to provide new components for insect control, this study aimed to select and characterize B. thuringiensis strains toxic to C. includens and three Spodoptera species (S. cosmioides, S. eridania and S. frugiperda).
Materials and methods
B. thuringiensis strains
Fifty strains of B. thuringiensis from Multifunction Microorganisms Collection of Embrapa Milho e Sorgo (Sete Lagoas, MG, Brazil) were used for bioassays against C. includens. These isolates were previously collected from soil samples and grain dust from different locations in Brazil (Valicente and Barreto, 2003Valicente, F. H., Barreto, M. R., 2003. Bacillus thuringiensis survey in Brazil: geographical distribution and insecticidal activity against Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae). Neotrop. Entomol. 32 (4), 639-644. https://doi.org/10.1590/S1519-566X2003000400014.
https://doi.org/10.1590/S1519-566X200300...
). Each B. thuringiensis strain was inoculated into Petri dishes with Luria Bertani (LB) agar medium enriched with mineral salts (FeSO4, ZnSO4, MnSO4 and MgSO4) and incubated at 29°C for 72 h for complete sporulation (Valicente and Mourão, 2008Valicente, F. H., Mourão, A. H., 2008. Use of by-products rich in carbon and nitrogen as a nutrient source to produce Bacillus thuringiensis (Berliner)-based biopesticide. Neotrop. Entomol. 37 (6), 702-708. https://doi.org/10.1590/S1519- 566X2008000600012.
https://doi.org/10.1590/S1519- 566X20080...
). Bacterial content was collected, diluted in 0.05% (v/v) Tween-20 and the spores counted with a Neubauer chamber.
Bioassays against C. includens
Artificial diet was poured on 128-well bioassay trays (BIO-BA-128, CD International Inc., USA) at a volume of 1 mL per well (Greene et al., 1976Greene, G. L., Leppla, N. C., Dickerson, W. A., 1976. Velvetbean caterpillar: a rearing procedure and artificial medium. J. Econ. Entomol. 69 (4), 487-488. https://doi.org/10.1093/jee/69.4.487.
https://doi.org/10.1093/jee/69.4.487...
; Valicente and Barreto, 2003Valicente, F. H., Barreto, M. R., 2003. Bacillus thuringiensis survey in Brazil: geographical distribution and insecticidal activity against Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae). Neotrop. Entomol. 32 (4), 639-644. https://doi.org/10.1590/S1519-566X2003000400014.
https://doi.org/10.1590/S1519-566X200300...
). After solidification, 50 μL of the mixture of spores and crystals in a concentration of 109 spores/mL was applied on the diet surface. Neonate larvae were placed individually in each cell and sealed with a plastic lid. The bioassay consisted of three replicates with 32 larvae of C. includens for each B. thuringiensis strain, and water was used as negative control. The experimental design was completely randomize. Larvae were maintained at 26 ± 2°C and mortality assessed after three days. Mortality rate was calculated using the number of dead caterpillars / number of surviving caterpillar x 100. Percentage mortality data were transformed before analysis using the Box-Cox transformation (Box and Cox, 1964Box, G. E., Cox, D. R, 1964. An analysis of transformations. J. R. Stat. Soc. 26(2), 211-252.). The mortality was submitted to analysis of variance (ANOVA) and the means compared using Scott-Knott test with 5% of significance using Assistat 7.7 program (Silva and Azevedo, 2016Silva, F. A. A., Azevedo, C. A. V., 2016. The Assistat Software Version 7.7 and its use in the analysis of experimental data. Afr. J. Agric. Res. 11 (39), 3733-3740. https://doi.org/10.5897/AJAR2016.11522.
https://doi.org/10.5897/AJAR2016.11522...
). B. thuringienis strains that caused mortality above 70% were evaluated for the production of β-exotoxins, presence of cry genes, plasmid patterns, protein profiles, and used in bioassays against Spodoptera species.
Detection of cry insecticidal genes
DNA extraction was performed according Shuhaimi et al. (2001)Shuhaimi, M., Ali, A. M., Saleh, N. M., Yazid, A. M., 2001. Utilisation of enterobacterial repetitive intergenic consensus (ERIC) sequence-based PCR to fingerprint the genomes of Bifido bacterium isolates and other probiotic bacteria. Biotechnol. Lett. 23 (9), 731-736. https://doi.org/10.1023/A:1010355003674.
https://doi.org/10.1023/A:1010355003674...
and used as template for PCR with 18 primers designed to amplify the cry1, cry2, and cry9 insecticidal genes (Ceron et al., 1994Ceron, J., Covarrubias, L., Quintero, R., Ortiz, A., Ortiz, M., Aranda, E., Bravo, A., 1994. PCR analysis of the cryI insecticidal crystal family genes from Bacillus thuringiensis. Appl. Environ. Microbiol. 60 (1), 353-356. https://doi.org/10.1128/AEM.60.1.353-356.
https://doi.org/10.1128/AEM.60.1.353-356...
; Ceron et al., 1995Ceron, J., Ortíz, A., Quintero, R., Güereca, L., Bravo, A., 1995. Specific PCR primers directed to identify cryI and cryIII genes within a Bacillus thuringiensis strain collection. Appl. Environ. Microbiol. 61 (11), 3826-3831.; Valicente et al., 2010Valicente, F. H., Picoli, E. A., Vasconcelos, M. J. V., Carneiro, N. P., Carneiro, A. A., Guimarães, C. T., Lana, U. G., 2010. Molecular characterization and distribution of Bacillus thuringiensis cry1 genes from Brazilian strains effective against the fall armyworm, Spodoptera frugiperda. Biol. Control 53 (3), 360-366. https://doi.org/10.1016/j.biocontrol.2010.02.003.
https://doi.org/10.1016/j.biocontrol.201...
) (Table 1). The amplification reactions were carried out in a 25 µL reaction volume using 10 ng of genomic DNA, 2 mM MgCl2, 0,125 mM of dNTP, 0.5 µM of each primer and 2 U of Taq DNA polymerase (KAPA Biosystems, USA). The amplified fragments were electrophoresed on a 1.5% (w/v) agarose gel, stained with GelRed™ (Biotium, USA) and photographed using L-PIX Image transilluminator (Loccus Biotechnology, USA).
Detection of β-exotoxin
The production of thermostable β-exotoxins by B. thurigiensis strains was performed using neonate larvae of S. frugiperda (Hornby and Gardner, 1987Hornby, J. A., Gardner, W. A., 1987. Dosage/mortality response of Spodoptera frugiperda (Lepidoptera: Noctuidae) and other Noctuid larvae to beta-exotoxin of Bacillus thuringiensis. J. Econ. Entomol. 80 (4), 925-929. https://doi.org/10.1093/jee/80.4.925.
https://doi.org/10.1093/jee/80.4.925...
). Each strain was grown in LB medium enriched with mineral salts (Valicente and Mourão, 2008Valicente, F. H., Mourão, A. H., 2008. Use of by-products rich in carbon and nitrogen as a nutrient source to produce Bacillus thuringiensis (Berliner)-based biopesticide. Neotrop. Entomol. 37 (6), 702-708. https://doi.org/10.1590/S1519- 566X2008000600012.
https://doi.org/10.1590/S1519- 566X20080...
) and incubated at 28°C for 144 h at 200 rpm. B. thuringiensis cultures were centrifuged at 10,000 x g for 10 min, and the supernatant autoclaved at 121°C for 20 min. Filtration of the supernatant was performed using TTP brand paper filters with 0.22 μm. For the bioassays, 165 μL of the filtered supernatants were superficially applied in approximately 1 cm3 of artificial diet (Valicente and Barreto, 2003Valicente, F. H., Barreto, M. R., 2003. Bacillus thuringiensis survey in Brazil: geographical distribution and insecticidal activity against Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae). Neotrop. Entomol. 32 (4), 639-644. https://doi.org/10.1590/S1519-566X2003000400014.
https://doi.org/10.1590/S1519-566X200300...
), and placed in plastic recipients sealed with a plastic lid. Each treatment consisted of three replicates with 24 larvae. Water was used as negative control and HD125 strain as a positive control (β-exotoxin producer). β-exotoxins production by B. thuringiensis strains were determined by larvae growth inhibition or high mortality of S. frugiperda after eight days of inoculation (Pinheiro, 2013Pinheiro, H. D. 2013. Interação de proteínas Cry1A com as vesículas da borda escovada da membrana (BBMVs) do intestino médio de Spodoptera frugiperda e Diatraea saccharalis e avaliação do tempo de cultivo sobre a produção de β-exotoxina em isolados de Bacillus thuringiensis. Dissertation, Federal University of Lavras.). The treatment means were submitted to analysis of variance (ANOVA) and the means compared using Scott-Knott test with 5% of significance using Assistat 7.7 program (Silva and Azevedo, 2016Silva, F. A. A., Azevedo, C. A. V., 2016. The Assistat Software Version 7.7 and its use in the analysis of experimental data. Afr. J. Agric. Res. 11 (39), 3733-3740. https://doi.org/10.5897/AJAR2016.11522.
https://doi.org/10.5897/AJAR2016.11522...
). In addition, a PCR analysis performed to detect the presence of thuE gene in B. thuringiensis strains genome, that is strongly associated with the synthesis of type I of β-exotoxin (Sauka et al., 2014Sauka, D. H., Pérez, M. P., López, N. N., Onco, M. I., Berretta, M. F., Benintende, G. B., 2014. PCR-based prediction of type I β-exotoxin production in Bacillus thuringiensis strains. J. Invertebr. Pathol. 122, 28-31. https://doi.org/10.1016/j.jip.2014.08.001.
https://doi.org/10.1016/j.jip.2014.08.00...
).
Plasmidial profile
The strains were submitted to two methods of plasmid extraction. The first extraction method was according to Fagundes et al. (2011)Fagundes, R. B. S., Picoli, E. A. T., Lana, U. G. P., Valicente, F. H., 2011. Plasmid patterns of efficient and inefficient strains of Bacillus thuringiensis against Spodoptera frugiperda (JE Smith) (Lepidoptera: noctuidae). Neotrop. Entomol. 40 (5), 600-606. https://doi.org/10.1590/S1519-566X2011000500012.
https://doi.org/10.1590/S1519-566X201100...
and the second using the commercial Maxi Plasmid Purification kit (Qiagen, USA) according to the manufacturer's recommendations. To analyze the integrity and plasmid profile, the samples were electrophoresed on a 0.5% (w/v) agarose gel at 80 V. The gel was stained with GelRed (Biotium, USA) and photographed using L-PIX Image transilluminator (Loccus Biotechnology, USA).
Protein profile by SDS-PAGE
B. thuringiensis strains were inoculated in LB medium enriched with mineral salts and incubated at 30°C for 96 h at 245 rpm, until complete sporulation. Total proteins were purified (Valicente and Lana, 2008Valicente, F. H., Lana, U. D. P., 2008. Molecular characterization of the Bacillus thuringiensis (Berliner) strains 344 and 1644, efficient against fall armyworm Spodoptera frugiperda (JE Smith). Rev. Bras. Milho Sorgo 7 (3), 195-209. https://doi.org/10.18512/1980-6477/rbms.v7n03p%25p.
https://doi.org/10.18512/1980-6477/rbms....
) and quantified by the Bradford method (Bradford, 1976Bradford, M. M., 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72 (1-2), 248-254. https://doi.org/10.1016/0003-2697(76)90527-3.
https://doi.org/10.1016/0003-2697(76)905...
) in the UV-mini-1240 Spectrophotometer (Shimadzu, USA). Protein samples were treated with trypsin and incubated at 37°C for 2 h (Valicente and Lana, 2008Valicente, F. H., Lana, U. D. P., 2008. Molecular characterization of the Bacillus thuringiensis (Berliner) strains 344 and 1644, efficient against fall armyworm Spodoptera frugiperda (JE Smith). Rev. Bras. Milho Sorgo 7 (3), 195-209. https://doi.org/10.18512/1980-6477/rbms.v7n03p%25p.
https://doi.org/10.18512/1980-6477/rbms....
). The reaction was inactivated with 1 mM PMSF (phenylmethylsulfonyl fluoride), and protein profile of B. thuringiensis strains analyzed by electrophoresis on 12% (w/v) polyacrylamide gel (SDS-PAGE). The gel was stained with coomassie brilliant blue R250 solution and molecular mass of the proteins was estimated by comparison to SeeBlue® Plus2 Pre-Stained Standard marker (Invitrogen, USA).
Bioassays against Spodoptera spp.
Mortality tests were performed with neonate larvae of S. eridania, S. cosmioides, and S. frugiperda with the B. thuringiensis strains that caused mortality above 70% in C. includens. The bioassays and statistical analysis were conducted following the same steps previously described for bioassays to C. includens.
Results and discussion
Although C. includens it is an important pest of many crops as soybean, maize, cotton, wheat and bean, studies about its control with B. thuringiensis are scarce (Isakova et al., 2007Isakova, I. A., Isakov, Y. B., Rymar, S. E., Kordium, V. A., Fuxa, J. R., 2007. Specificity of Ukrainian Bacillus thuringiensis Berliner strains for agricultural pests of the Southeastern United States. J. Entomol. Sci. 42 (2), 272-285. https://doi.org/10.18474/0749-8004-42.2.272.
https://doi.org/10.18474/0749-8004-42.2....
; Barreto, 2012Barreto, M. R., 2012. Prospecção e Caracterização de Genes de Bacillus thuringiensis com Potencial Para o Controle de Insetos-praga da Cultura da Soja. Thesis, Federal University of Paraná.; Specht et al., 2015Specht, A., Paula-Moraes, S. V., Sosa-Gómez, D. R., 2015. Host plants of Chrysodeixis includens (Walker) (Lepidoptera, Noctuidae, Plusiinae). Rev. Bras. Entomol. 59 (4), 343-345. https://doi.org/10.1016/j.rbe.2015.09.002.
https://doi.org/10.1016/j.rbe.2015.09.00...
). To give some insights to these questions, we tested the efficiency of B. thuringiensis strains for C. includens larvae control and the mortality ranged from 4 to 100%, of which 92% of strains caused low mortality, less than 33%. Strains 1608A, 726, 773 and 775E caused high mortality of C. includens (100%) and therefore, were selected for molecular characterization and bioassays against Spodoptera species.
Some B. thuringiensis strains are capable of producing thermostable and secretable secondary metabolites exhibiting non-specific toxic activity, commonly known as β-exotoxins (Liu et al., 2014Liu, X., Ruan, L., Peng, D., Li, L., Sun, M., Yu, Z., 2014. Thuringiensin: a thermostable secondary metabolite from Bacillus thuringiensis with insecticidal activity against a wide range of insects. Toxins (Basel) 6 (8), 2229-2238. https://doi.org/10.3390/toxins6082229.
https://doi.org/10.3390/toxins6082229...
). No β-exotoxins activity was detected in the supernatants of 1608A, 726, 773 and 775E strains, demonstrated by low mortality against S. frugiperda that ranged from 8.3 to 13.0% without significant difference when compared to the negative control that caused low mortality of 8.3%. The positive control (HD-125 strain) caused 29.2% mortality against S. frugiperda differing significantly from the other treatments. We observed differences in larval weight, growth and development of S. frugiperda after eight days of exposure (Fig. 1). Average weight of larvae fed with supernatants of B. thuringiensis ranged from 26.4 to 32.1 mg differing from the positive control that showed an average weight of 0.55 mg, indicating that these strains did not produce β-exotoxins.
Comparison of growth inhibitory symptoms of Spodoptera frugiperda larvae exposed to Bacillus thuringiensis β-exotoxins after eight days of inoculation. a: Positive control (strain HD-125); b: Negative control (water); c: Strain 773.
To confirm these results, a PCR analysis was performed to detect the presence of thuE gene. No amplification product was observed among select strains (Fig. 2). Therefore, the B. thuringiensis strains 1608A, 726, 773 and 775E are safe to be used in large scale fermentation systems and formulations of biopesticides. Absence of β-exotoxins is a requirement for B. thuringiensis formulations used in Europe, the US, Canada, and Brazil since the exposure to these toxins pose a health risk (Glare and O’Callaghan, 2000Glare, T. R., O’Callaghan, M., 2000. Bacillus thuringiensis: Biology, Ecology and Safety, Wiley, Chichester, UK.).
Amplified DNA fragments with the BEF/BER (a) and BEF1/BER1 (b) primers for detection of type I of β-exotoxins in Bacillus thuringiesis strains efficient against Chrysodeixis includens. C+: Positive control (HD-125 strain); C-: Negative controle (water); MM: 1 Kb DNA ladder plus (Invitrogen, USA.
The presence of insecticidal genes detected with specific primers for cry genes is widely used for characterization of B. thuringiensis strains, allowing the screening, classification, and prediction to their insecticidal activities (Jain et al., 2017Jain, D., Sunda, S. D., Sanadhya, S., Nath, D. J., Khandelwal, S. K., 2017. Molecular characterization and PCR-based screening of cry genes from Bacillus thuringiensis strains. 3 Biotech. 7(1), 4. https://doi.org/10.1007/s13205-016-0583-7.
https://doi.org/10.1007/s13205-016-0583-...
). Amplification products with the expected size were observed for target genes, except for cry1Ea/cry1Eb genes. The genes cry1Ab, cry1G, cry2Ab, cry2Ac and cry2Ad were found in all strains tested (Table 2). Cry1 and Cry2 class of proteins are related to toxicity against C. includens (Van Frankenhuyzen, 2009Van Frankenhuyzen, K., 2009. Insecticidal activity of Bacillus thuringiensis crystal proteins. J. Invertebr. Pathol. 101 (1), 1-16. https://doi.org/10.1016/j.jip.2009.02.009.
https://doi.org/10.1016/j.jip.2009.02.00...
; Bel et al., 2017Bel, Y., Sheets, J. J., Tan, S. Y., Narva, K. E., Escriche, B., 2017. Toxicity and binding studies of Bacillus thuringiensis Cry1Ac, Cry1F, Cry1C, and Cry2A proteins in the soybean pests Anticarsia gemmatalis and Chrysodeixis (Pseudoplusia) includens. Appl. Environ. Microbiol. 83 (11), https://doi.org/10.1128/AEM.00326-17.
https://doi.org/10.1128/AEM.00326-17...
, 2019Bel, Y., Zack, M., Narva, K., Escriche, B., 2019. Specific binding of Bacillus thuringiensis Cry1Ea toxin, and Cry1Ac and Cry1Fa competition analyses in Anticarsia gemmatalis and Chrysodeixis includens. Sci. Rep. 9 (1), 1-7. https://doi.org/10.1038/s41598-019- 54850-3.
https://doi.org/10.1038/s41598-019- 5485...
), and for other lepidopteran species, including S. frugiperda (Knaak et al., 2010Knaak, N., Franz, A. R., Santos, G. F., Fiuza, L. M., 2010. Histopathology and the lethal effect of Cry proteins and strains of Bacillus thuringiensis Berliner in Spodoptera frugiperda JE Smith Caterpillars (Lepidoptera, Noctuidae). Braz. J. Biol. 70 (3), 677-684. https://doi.org/10.1590/S1519-69842010000300028.
https://doi.org/10.1590/S1519-6984201000...
). Strain 1608A harbored several insecticidal genes, including cry9 class genes that are promising tools for pest control (Jansens et al., 1997Jansens, S., Van Vliet, A., Dickburt, C., Buysse, L., Piens, C., Saey, B., Peferoen, M., 1997. Transgenic corn expressing a Cry9C insecticidal protein from Bacillus thuringiensis protected from European corn borer damage. Crop Sci. 37 (5), 1616-1624. https://doi.org/10.2135/cropsci1997.0011183X003700050035x.
https://doi.org/10.2135/cropsci1997.0011...
) with toxic activity to several important insects, and a good strategy for delaying development of insect resistance in the field (Kuvshinov et al., 2001Kuvshinov, V., Koivu, K., Kanerva, A., Pehu, E., 2001. Transgenic crop plants expressing synthetic cry9Aa gene are protected against insect damage. Plant Sci. An Int. J. Exp. Plant Biol. 160, 341-353.; Wang et al., 2018Wang, Z., Fang, L., Zhou, Z., Pacheco, S., Gómez, I., Song, F., Soberón, M., Zhang, J., Bravo, A., 2018. Specific binding between Bacillus thuringiensis Cry9Aa and Vip3Aa toxins synergizes their toxicity against Asiatic rice borer (Chilo suppressalis). J. Biol. Chem. 293 (29), 11447-11458. https://doi.org/10.1074/jbc.RA118.003490.
https://doi.org/10.1074/jbc.RA118.003490...
).
The diversity of cry genes in B. thuringiensis genome may occur due to environmental factors of the places and substrates that strains were collected. The investigation of isolates collected from different ecological and geographical sources enables relating gene content results to potential target insects and may be a good strategy for selecting new isolates with broad toxic activity (Djenane et al., 2017Djenane, Z., Nateche, F., Amziane, M., Gomis-Cebolla, J., El-Aichar, F., Khorf, H., Ferré, J., 2017. Assessment of the Antimicrobial Activity and the Entomocidal Potential of Bacillus thuringiensis Isolates from Algeria. Toxins (Basel) 9 (4), 139. https://doi.org/10.3390/toxins9040139.
https://doi.org/10.3390/toxins9040139...
).
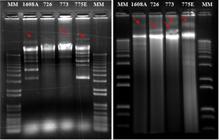
We used two methods of DNA extraction and both allowed to detect extrachromosomal DNA on agarose gel (Fig. 3). A single megaplasmid was observed in three out of four samples analyzed, with differences in their sizes, except for the strain 726, which did not show any megaplasmid when extracted with the method proposed by Fagundes et al. (2011)Fagundes, R. B. S., Picoli, E. A. T., Lana, U. G. P., Valicente, F. H., 2011. Plasmid patterns of efficient and inefficient strains of Bacillus thuringiensis against Spodoptera frugiperda (JE Smith) (Lepidoptera: noctuidae). Neotrop. Entomol. 40 (5), 600-606. https://doi.org/10.1590/S1519-566X2011000500012.
https://doi.org/10.1590/S1519-566X201100...
. In contrast, the use of commercial kit allowed the detection of megaplasmids in all studied B. thuringiensis strains, specially in the strain 773 that showed two megaplasmids with different sizes. Only method reported by Fagundes et al. (2011)Fagundes, R. B. S., Picoli, E. A. T., Lana, U. G. P., Valicente, F. H., 2011. Plasmid patterns of efficient and inefficient strains of Bacillus thuringiensis against Spodoptera frugiperda (JE Smith) (Lepidoptera: noctuidae). Neotrop. Entomol. 40 (5), 600-606. https://doi.org/10.1590/S1519-566X2011000500012.
https://doi.org/10.1590/S1519-566X201100...
was efficient to detect a large number of small plasmids in all strains. Thus, this strategy showed to be better for analysis mainly of the small plasmids, while the use of commercial kit showed better for megaplasmids extraction.
Plasmid profiles of Bacillus thuringiensis efficient strains against Chrysodeixis includens. a: DNA extraction according to Fagundes et al. (2011)Fagundes, R. B. S., Picoli, E. A. T., Lana, U. G. P., Valicente, F. H., 2011. Plasmid patterns of efficient and inefficient strains of Bacillus thuringiensis against Spodoptera frugiperda (JE Smith) (Lepidoptera: noctuidae). Neotrop. Entomol. 40 (5), 600-606. https://doi.org/10.1590/S1519-566X2011000500012.
https://doi.org/10.1590/S1519-566X201100... ; b: DNA extraction using QIAGEN kit (Invitrogen, USA). MM: 1 Kb DNA ladder plus (Invitrogen, USA. The red rows indicate megaplasmids.
The B. thuringiensis showed from 1 to 6 plasmids. Strains 726 and 773 showed similar patterns of small plasmids, both presenting only one small plasmid with sizes of approximately 10,000 bp. Although these strains showed similar plasmid profiles, the DNA sequences of these plasmids may be different, which should be considered in future work (Fagundes et al., 2011Fagundes, R. B. S., Picoli, E. A. T., Lana, U. G. P., Valicente, F. H., 2011. Plasmid patterns of efficient and inefficient strains of Bacillus thuringiensis against Spodoptera frugiperda (JE Smith) (Lepidoptera: noctuidae). Neotrop. Entomol. 40 (5), 600-606. https://doi.org/10.1590/S1519-566X2011000500012.
https://doi.org/10.1590/S1519-566X201100...
). Although some studies have focused on the importance of megaplasmids as host sites for cry genes that encode bioinseticidal delta-endotoxins, small plasmids are also found in B. thuringiensis that contribute to the biology of their host (Li et al., 2014Li, H., Liu, R., Shu, C., Zhang, Q., Zhao, S., Shao, G., Gao, J., 2014. Characterization of one novel cry8 gene from Bacillus thuringiensis strain Q52-7. World J. Microbiol. Biotechnol. 30 (12), 3075-3080. https://doi.org/10.1007/s11274-014-1734-9.
https://doi.org/10.1007/s11274-014-1734-...
). Thus, both molecules are important to be sequenced to detect presence of new insecticidal proteins.
The protein profile of all strains evaluated presented two main bands of pro-toxin of ~ 130 and ~ 65 kDa (Fig. 4). The protein patterns presented by the B. thuringiensis strains are related with the proteins of classes Cry1, Cry2, and Cry9 that are toxic to species of Lepidoptera order, and considered as a good alternative for the management for insect control (Crickmore et al., 2016Crickmore, N., Baum, J., Bravo, A., Lereclus, D., Narva, K., Sampson, K., Schnepf, E., Sun, M., Zeigler, D. R., 2016. Bacillus thuringiensis Toxin Nomenclature. Available in: http://www.btnomenclature.info/ (acessed 10 April 2019).
http://www.btnomenclature.info/...
). In this study the pro-toxins produced by strains were digested using the trypsin protease to simulate in vivo activity. After digestion, all strains showed toxins with 60 kDa size (Fig. 4), frequently associated with activated Cry proteins (Maagd et al., 2003Maagd, R. A., Bravo, A., Berry, C., Crickmore, N., Schnepf, H. E., 2003. Structure, diversity, and evolution of protein toxins from spore-forming entomopathogenic bacteria. Annu. Rev. Genet. 37 (1), 409-433. https://doi.org/10.1146/annurev.genet.37.110801.143042.
https://doi.org/10.1146/annurev.genet.37...
). This step of protein activation is important and crucial in understanding the mechanism of action of these proteins in target insects.
Profile of total and digested trypsin proteins produced by Bacillus thuringiensis strains eficiente against Chrysodeixis includens. (D) Proteins digested with trypsin; MM: SeeBlue® Plus2 Pre-Stained Standard Marker (Invitrogen, USA).
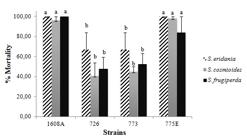
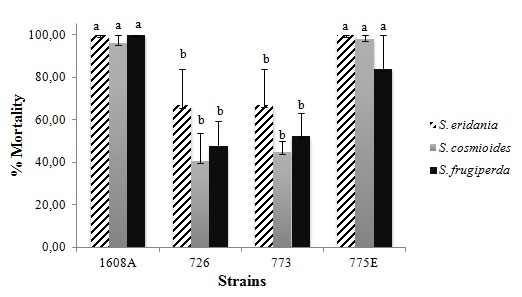
To select B. thuringiensis strains with high activity against multiple insects, we tested the four strains efficient against C. includens in S. eridania, S. cosmioides and S. frugiperda. Strains 1608A and 775E showed mortality rates between 80-100% for the three species (F = 5.724; p < 0.0001; Fig. 5). The highest mortality was caused by 1608A strain, that caused 100% mortality against S. eridania and S. frugiperda, and 775E strain caused 100% mortality against S. eridania. Several lepidopterans species respond differently to the proteins tested, allowing to divide into groups of more permissive or less permissive to the activation of B. thuringiensis toxins (Van Frankenhuyzen, 2009Van Frankenhuyzen, K., 2009. Insecticidal activity of Bacillus thuringiensis crystal proteins. J. Invertebr. Pathol. 101 (1), 1-16. https://doi.org/10.1016/j.jip.2009.02.009.
https://doi.org/10.1016/j.jip.2009.02.00...
). An important aspect is the selection B. thuringiensis strains with high toxic activity for more than one pest and that produce proteins with different modes of action for obtaining new formulations to be used in agriculture (Bobrowski et al., 2001Bobrowski, V. L., Pasquali, G., Bodanese-Zanettini, M. H., Fiuza, L. M., 2001. Detection of cry1 genes in Bacillus thuringiensis isolates from South of Brazil and activity against Anticarsia gemmatalis (Lepidoptera: noctuidae). Braz. J. Microbiol. 32 (2), 105-109. https://doi.org/10.1590/S1517-83822001000200006.
https://doi.org/10.1590/S1517-8382200100...
; Bobrowski et al., 2003Bobrowski, V. L., Fiuza, L. M., Pasquali, G., Bodanese-Zanettini, M. H., 2003. Genes de Bacillus thuringiensis: uma estratégia para conferir resistência a insetos em plantas. Cienc. Rural 33 (5), 843-850. https://doi.org/10.1590/S0103-84782003000500008.
https://doi.org/10.1590/S0103-8478200300...
).
Toxicity of Bacillus thuringiensis strains against three Spodoptera species. Means followed by the same letter do not differ statistically from one another by the Scott-Knott test at the 5% probability level.
Some insects of genus Spodoptera and C. includens are commonly found in soybean fields, making it a concern in the management of these species if infestation occurs in the same period (Marques et al., 2016Marques, L. H., Castro, B. A., Rossetto, J., Silva, O. A. B. N., Moscardini, V. F., Zobiole, L. H. S., Fernandes, O. A., 2016. Efficacy of soybean’s event DAS-81419-2 expressing Cry1F and Cry1Ac to manage key tropical lepidopteran pests under field conditions in Brazil. J. Econ. Entomol. 109 (4), 1922-1928. https://doi.org/10.1093/jee/tow153.
https://doi.org/10.1093/jee/tow153...
; Silva et al., 2017Silva, M. D., Bueno, F. A., Stecca, S. C., Andrade, K., Neves, J. O. M. P., Oliveira, N. M. C., 2017. Biology of Spodoptera eridania and Spodoptera cosmioides (Lepidoptera: Noctuidae) on different host plants. Fla. Entomol. 100 (4), 752-760. https://doi.org/10.1653/024.100.0423.
https://doi.org/10.1653/024.100.0423...
; Sousa et al., 2019Sousa, P. V., Vaz, A. G., Miranda, D. S., da Costa, P. V., Almeida, A. C. S., Araújo, M. S., Jesus, F. G., 2019. Control strategies for Chrysodeixis includens and Spodoptera eridania caterpillars (Lepidoptera: Noctuidae) and selection of resistant cultivars in soybean. Aust. J. Crop Sci. 13(3), 367-371. https://doi: 10.21475/ajcs.19.13.03.p1188.
https://doi.org/https://doi: 10.21475/aj...
). The high insecticidal activity of B. thuringiensis strains described in this work makes them excellent candidates to C. includens, S. eridania, S. cosmioides and S. frugiperda control, a valuable tool in the Integrated Pest Management (IPM). Furthermore, their genome sequencing will contribute to deeper understanding of entomopathogenicity of these B. thuringiensis strains and as sources of new insecticidal genes for transgenic crops development.
Acknowledgments
We are grateful to Embrapa Milho e Sorgo and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
References
- Angus, T. A., 1954. A bacterial toxin paralysing silkworm larvae. Nature 173 (4403), 545-546. https://doi.org/10.1038/173545a0
» https://doi.org/10.1038/173545a0 - Barreto, M. R., 2012. Prospecção e Caracterização de Genes de Bacillus thuringiensis com Potencial Para o Controle de Insetos-praga da Cultura da Soja. Thesis, Federal University of Paraná.
- Bechtel, D. B., Bulla, L. A., 1976. Electron microscope study of sporulation and parasporal crystal formation in Bacillus thuringiensis. J. Bacteriol. 127 (3), 1472-1481. https://doi.org/10.1128/JB.127.3.1472-1481.1976
» https://doi.org/10.1128/JB.127.3.1472-1481.1976 - Bel, Y., Sheets, J. J., Tan, S. Y., Narva, K. E., Escriche, B., 2017. Toxicity and binding studies of Bacillus thuringiensis Cry1Ac, Cry1F, Cry1C, and Cry2A proteins in the soybean pests Anticarsia gemmatalis and Chrysodeixis (Pseudoplusia) includens. Appl. Environ. Microbiol. 83 (11), https://doi.org/10.1128/AEM.00326-17
» https://doi.org/10.1128/AEM.00326-17 - Bel, Y., Zack, M., Narva, K., Escriche, B., 2019. Specific binding of Bacillus thuringiensis Cry1Ea toxin, and Cry1Ac and Cry1Fa competition analyses in Anticarsia gemmatalis and Chrysodeixis includens. Sci. Rep. 9 (1), 1-7. https://doi.org/10.1038/s41598-019- 54850-3
» https://doi.org/10.1038/s41598-019- 54850-3 - Bobrowski, V. L., Fiuza, L. M., Pasquali, G., Bodanese-Zanettini, M. H., 2003. Genes de Bacillus thuringiensis: uma estratégia para conferir resistência a insetos em plantas. Cienc. Rural 33 (5), 843-850. https://doi.org/10.1590/S0103-84782003000500008
» https://doi.org/10.1590/S0103-84782003000500008 - Bobrowski, V. L., Pasquali, G., Bodanese-Zanettini, M. H., Fiuza, L. M., 2001. Detection of cry1 genes in Bacillus thuringiensis isolates from South of Brazil and activity against Anticarsia gemmatalis (Lepidoptera: noctuidae). Braz. J. Microbiol. 32 (2), 105-109. https://doi.org/10.1590/S1517-83822001000200006
» https://doi.org/10.1590/S1517-83822001000200006 - Box, G. E., Cox, D. R, 1964. An analysis of transformations. J. R. Stat. Soc. 26(2), 211-252.
- Bradford, M. M., 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72 (1-2), 248-254. https://doi.org/10.1016/0003-2697(76)90527-3
» https://doi.org/10.1016/0003-2697(76)90527-3 - Carozzi, N. B., Kramer, V. C., Warren, G. W., Evola, S., Koziel, M. G., 1991. Prediction of insecticidal activity of Bacillus thuringiensis strains by polymerase chain reaction product profiles. Appl. Environ. Microbiol. 57 (11), 3057-3061. https://doi.org/10.1128/AEM.57.11.3057-3061.1991
» https://doi.org/10.1128/AEM.57.11.3057-3061.1991 - Ceron, J., Covarrubias, L., Quintero, R., Ortiz, A., Ortiz, M., Aranda, E., Bravo, A., 1994. PCR analysis of the cryI insecticidal crystal family genes from Bacillus thuringiensis. Appl. Environ. Microbiol. 60 (1), 353-356. https://doi.org/10.1128/AEM.60.1.353-356
» https://doi.org/10.1128/AEM.60.1.353-356 - Ceron, J., Ortíz, A., Quintero, R., Güereca, L., Bravo, A., 1995. Specific PCR primers directed to identify cryI and cryIII genes within a Bacillus thuringiensis strain collection. Appl. Environ. Microbiol. 61 (11), 3826-3831.
- Chandrasekaran, R., Revathi, K., Senthil-Nathan, S., Kalaivani, K., Hunter, W. B., Duraipandiyan, V., Esmail, G. A., 2018. Eco-friendly formulation of wild Bacillus thuringiensis secondary metabolites through molecular characterization against the lepidopteran pest. Physiol. Mol. Plant Pathol. 101, 93-104. https://doi.org/10.1016/j.pmpp.2017.09.002
» https://doi.org/10.1016/j.pmpp.2017.09.002 - Crickmore, N., Baum, J., Bravo, A., Lereclus, D., Narva, K., Sampson, K., Schnepf, E., Sun, M., Zeigler, D. R., 2016. Bacillus thuringiensis Toxin Nomenclature. Available in: http://www.btnomenclature.info/ (acessed 10 April 2019).
» http://www.btnomenclature.info/ - Djenane, Z., Nateche, F., Amziane, M., Gomis-Cebolla, J., El-Aichar, F., Khorf, H., Ferré, J., 2017. Assessment of the Antimicrobial Activity and the Entomocidal Potential of Bacillus thuringiensis Isolates from Algeria. Toxins (Basel) 9 (4), 139. https://doi.org/10.3390/toxins9040139
» https://doi.org/10.3390/toxins9040139 - Fagundes, R. B. S., Picoli, E. A. T., Lana, U. G. P., Valicente, F. H., 2011. Plasmid patterns of efficient and inefficient strains of Bacillus thuringiensis against Spodoptera frugiperda (JE Smith) (Lepidoptera: noctuidae). Neotrop. Entomol. 40 (5), 600-606. https://doi.org/10.1590/S1519-566X2011000500012
» https://doi.org/10.1590/S1519-566X2011000500012 - Glare, T. R., O’Callaghan, M., 2000. Bacillus thuringiensis: Biology, Ecology and Safety, Wiley, Chichester, UK.
- Goergen, G., Kumar, P. L., Sankung, S. B., Togola, A., Tamò, M., 2016. First report of outbreaks of the fall armyworm Spodoptera frugiperda (JE Smith)(Lepidoptera, Noctuidae), a new alien invasive pest in West and Central Africa. PLoS One 11 (10), e0165632. https://doi.org/10.1371/journal.pone.0165632
» https://doi.org/10.1371/journal.pone.0165632 - Greene, G. L., Leppla, N. C., Dickerson, W. A., 1976. Velvetbean caterpillar: a rearing procedure and artificial medium. J. Econ. Entomol. 69 (4), 487-488. https://doi.org/10.1093/jee/69.4.487
» https://doi.org/10.1093/jee/69.4.487 - Guo, J., Zhao, J., He, K., Zhang, F., Wang, Z., 2018. Potential invasion of the crop- devastating insect pest fall armyworm Spodoptera frugiperda to China. Plant Protection. 44(6), 1-10. https://doi.org/16688/j.zwbh.2018452
» https://doi.org/16688/j.zwbh.2018452 - Hornby, J. A., Gardner, W. A., 1987. Dosage/mortality response of Spodoptera frugiperda (Lepidoptera: Noctuidae) and other Noctuid larvae to beta-exotoxin of Bacillus thuringiensis. J. Econ. Entomol. 80 (4), 925-929. https://doi.org/10.1093/jee/80.4.925
» https://doi.org/10.1093/jee/80.4.925 - Isakova, I. A., Isakov, Y. B., Rymar, S. E., Kordium, V. A., Fuxa, J. R., 2007. Specificity of Ukrainian Bacillus thuringiensis Berliner strains for agricultural pests of the Southeastern United States. J. Entomol. Sci. 42 (2), 272-285. https://doi.org/10.18474/0749-8004-42.2.272
» https://doi.org/10.18474/0749-8004-42.2.272 - Jain, D., Sunda, S. D., Sanadhya, S., Nath, D. J., Khandelwal, S. K., 2017. Molecular characterization and PCR-based screening of cry genes from Bacillus thuringiensis strains. 3 Biotech. 7(1), 4. https://doi.org/10.1007/s13205-016-0583-7
» https://doi.org/10.1007/s13205-016-0583-7 - Jansens, S., Van Vliet, A., Dickburt, C., Buysse, L., Piens, C., Saey, B., Peferoen, M., 1997. Transgenic corn expressing a Cry9C insecticidal protein from Bacillus thuringiensis protected from European corn borer damage. Crop Sci. 37 (5), 1616-1624. https://doi.org/10.2135/cropsci1997.0011183X003700050035x
» https://doi.org/10.2135/cropsci1997.0011183X003700050035x - Knaak, N., Franz, A. R., Santos, G. F., Fiuza, L. M., 2010. Histopathology and the lethal effect of Cry proteins and strains of Bacillus thuringiensis Berliner in Spodoptera frugiperda JE Smith Caterpillars (Lepidoptera, Noctuidae). Braz. J. Biol. 70 (3), 677-684. https://doi.org/10.1590/S1519-69842010000300028
» https://doi.org/10.1590/S1519-69842010000300028 - Kuvshinov, V., Koivu, K., Kanerva, A., Pehu, E., 2001. Transgenic crop plants expressing synthetic cry9Aa gene are protected against insect damage. Plant Sci. An Int. J. Exp. Plant Biol. 160, 341-353.
- Lee, Y. K., Jin, N. Y., Lee, B. R., Seo, M. J., Youn, Y. N., Gill, S. S., Yu, Y. M., 2015. Isolation and characterization of new Bacillus thuringiensis strains with insecticidal activity to difficult to control lepidopteran pests. J. Facul. Agricul. 60 (1), 103-112.
- Levinson, B. L., Kasyan, K. J., Chiu, S. S., Currier, T. C., Gonzalez, J. M., 1990. Identification of beta-exotoxin production, plasmids encoding beta-exotoxin, and a new exotoxin in Bacillus thuringiensis by using high-performance liquid chromatography. J. Bacteriol. 172, 3172-3179.
- Li, H., Liu, R., Shu, C., Zhang, Q., Zhao, S., Shao, G., Gao, J., 2014. Characterization of one novel cry8 gene from Bacillus thuringiensis strain Q52-7. World J. Microbiol. Biotechnol. 30 (12), 3075-3080. https://doi.org/10.1007/s11274-014-1734-9
» https://doi.org/10.1007/s11274-014-1734-9 - Liu, X. Y., Ruan, L. F., Hu, Z. F., Peng, D. H., Cao, S. Y., Yu, Z. N., Sun, M., 2010. Genome-wide screening reveals the genetic determinants of an antibiotic insecticide in Bacillus thuringiensis. J. Biol. Chem. 285 (50), 39191-39200. https://doi.org/10.1074/jbc.M110.148387
» https://doi.org/10.1074/jbc.M110.148387 - Liu, X., Ruan, L., Peng, D., Li, L., Sun, M., Yu, Z., 2014. Thuringiensin: a thermostable secondary metabolite from Bacillus thuringiensis with insecticidal activity against a wide range of insects. Toxins (Basel) 6 (8), 2229-2238. https://doi.org/10.3390/toxins6082229
» https://doi.org/10.3390/toxins6082229 - Maagd, R. A., Bravo, A., Berry, C., Crickmore, N., Schnepf, H. E., 2003. Structure, diversity, and evolution of protein toxins from spore-forming entomopathogenic bacteria. Annu. Rev. Genet. 37 (1), 409-433. https://doi.org/10.1146/annurev.genet.37.110801.143042
» https://doi.org/10.1146/annurev.genet.37.110801.143042 - Marques, L. H., Castro, B. A., Rossetto, J., Silva, O. A. B. N., Moscardini, V. F., Zobiole, L. H. S., Fernandes, O. A., 2016. Efficacy of soybean’s event DAS-81419-2 expressing Cry1F and Cry1Ac to manage key tropical lepidopteran pests under field conditions in Brazil. J. Econ. Entomol. 109 (4), 1922-1928. https://doi.org/10.1093/jee/tow153
» https://doi.org/10.1093/jee/tow153 - Moscardi, F., Bueno, A. D. F., Bueno, R. C. O. D. F., Garcia, A., 2012. Soybean response to different injury levels at early developmental stages. Cienc. Rural 42 (3), 389-394. https://doi.org/10.1590/S0103-84782012000300001
» https://doi.org/10.1590/S0103-84782012000300001 - Mukhija, B., Khanna, V., 2018. Cry Protein Profiling of Bacillus thuringiensis Isolated from Different Agro-Climate Soils of Punjab. Int. J. Curr. Microbiol. Appl. Sci. 7 (3), 2866-2870. https://doi.org/10.20546/ijcmas.2018.703.330
» https://doi.org/10.20546/ijcmas.2018.703.330 - Pinheiro, H. D. 2013. Interação de proteínas Cry1A com as vesículas da borda escovada da membrana (BBMVs) do intestino médio de Spodoptera frugiperda e Diatraea saccharalis e avaliação do tempo de cultivo sobre a produção de β-exotoxina em isolados de Bacillus thuringiensis Dissertation, Federal University of Lavras.
- Sauka, D. H., Pérez, M. P., López, N. N., Onco, M. I., Berretta, M. F., Benintende, G. B., 2014. PCR-based prediction of type I β-exotoxin production in Bacillus thuringiensis strains. J. Invertebr. Pathol. 122, 28-31. https://doi.org/10.1016/j.jip.2014.08.001
» https://doi.org/10.1016/j.jip.2014.08.001 - Schnepf, E., Crickmore, N. V., Van Rie, J., Lereclus, D., Baum, J., Feitelson, J., Dean, D. H., 1998. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 62 (3), 775-806.
- Sharanabasappa, K. C. M., Asokan, R., Mahadeva, S. H. M., Maruthi, M. S., Pavithra, H. B., Hegde, K., Goergen, G., 2018. First report of the fall armyworm, Spodoptera frugiperda (J E Smith) (Lepidoptera: Noctuidae), an alien invasive pest on maize in India. Pest Manage. Hortic. Ecosyst. 24 (1), 23-29. Available in: https://hdl.handle.net/10568/103519 (acessed 10 April 2019).
» https://hdl.handle.net/10568/103519 - Shuhaimi, M., Ali, A. M., Saleh, N. M., Yazid, A. M., 2001. Utilisation of enterobacterial repetitive intergenic consensus (ERIC) sequence-based PCR to fingerprint the genomes of Bifido bacterium isolates and other probiotic bacteria. Biotechnol. Lett. 23 (9), 731-736. https://doi.org/10.1023/A:1010355003674
» https://doi.org/10.1023/A:1010355003674 - Silva, F. A. A., Azevedo, C. A. V., 2016. The Assistat Software Version 7.7 and its use in the analysis of experimental data. Afr. J. Agric. Res. 11 (39), 3733-3740. https://doi.org/10.5897/AJAR2016.11522
» https://doi.org/10.5897/AJAR2016.11522 - Silva, M. D., Bueno, F. A., Stecca, S. C., Andrade, K., Neves, J. O. M. P., Oliveira, N. M. C., 2017. Biology of Spodoptera eridania and Spodoptera cosmioides (Lepidoptera: Noctuidae) on different host plants. Fla. Entomol. 100 (4), 752-760. https://doi.org/10.1653/024.100.0423
» https://doi.org/10.1653/024.100.0423 - Sousa, P. V., Vaz, A. G., Miranda, D. S., da Costa, P. V., Almeida, A. C. S., Araújo, M. S., Jesus, F. G., 2019. Control strategies for Chrysodeixis includens and Spodoptera eridania caterpillars (Lepidoptera: Noctuidae) and selection of resistant cultivars in soybean. Aust. J. Crop Sci. 13(3), 367-371. https://doi: 10.21475/ajcs.19.13.03.p1188.
» https://doi.org/https://doi: 10.21475/ajcs.19.13.03.p1188 - Souza, L. A., Barbosa, J. C., Carvalho, J. H. S., Santos, L. S., Fraga, D. F., Busoli, A. C., 2019. Spatial distribution of Chrysodeixis includens eggs (Walker, 1858)(Lepidoptera: Noctuidae) in soybean crop. Rev. Cienc. Agric. 17 (2), 51-57. https://doi.org/10.28998/rca.v17i2.7124
» https://doi.org/10.28998/rca.v17i2.7124 - Specht, A., Paula-Moraes, S. V., Sosa-Gómez, D. R., 2015. Host plants of Chrysodeixis includens (Walker) (Lepidoptera, Noctuidae, Plusiinae). Rev. Bras. Entomol. 59 (4), 343-345. https://doi.org/10.1016/j.rbe.2015.09.002
» https://doi.org/10.1016/j.rbe.2015.09.002 - Valicente, F. H., Barreto, M. R., 2003. Bacillus thuringiensis survey in Brazil: geographical distribution and insecticidal activity against Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae). Neotrop. Entomol. 32 (4), 639-644. https://doi.org/10.1590/S1519-566X2003000400014
» https://doi.org/10.1590/S1519-566X2003000400014 - Valicente, F. H., Lana, U. D. P., 2008. Molecular characterization of the Bacillus thuringiensis (Berliner) strains 344 and 1644, efficient against fall armyworm Spodoptera frugiperda (JE Smith). Rev. Bras. Milho Sorgo 7 (3), 195-209. https://doi.org/10.18512/1980-6477/rbms.v7n03p%25p
» https://doi.org/10.18512/1980-6477/rbms.v7n03p%25p - Valicente, F. H., Mourão, A. H., 2008. Use of by-products rich in carbon and nitrogen as a nutrient source to produce Bacillus thuringiensis (Berliner)-based biopesticide. Neotrop. Entomol. 37 (6), 702-708. https://doi.org/10.1590/S1519- 566X2008000600012
» https://doi.org/10.1590/S1519- 566X2008000600012 - Valicente, F. H., Picoli, E. A., Vasconcelos, M. J. V., Carneiro, N. P., Carneiro, A. A., Guimarães, C. T., Lana, U. G., 2010. Molecular characterization and distribution of Bacillus thuringiensis cry1 genes from Brazilian strains effective against the fall armyworm, Spodoptera frugiperda. Biol. Control 53 (3), 360-366. https://doi.org/10.1016/j.biocontrol.2010.02.003
» https://doi.org/10.1016/j.biocontrol.2010.02.003 - Van Frankenhuyzen, K., 2009. Insecticidal activity of Bacillus thuringiensis crystal proteins. J. Invertebr. Pathol. 101 (1), 1-16. https://doi.org/10.1016/j.jip.2009.02.009
» https://doi.org/10.1016/j.jip.2009.02.009 - Wang, Z., Fang, L., Zhou, Z., Pacheco, S., Gómez, I., Song, F., Soberón, M., Zhang, J., Bravo, A., 2018. Specific binding between Bacillus thuringiensis Cry9Aa and Vip3Aa toxins synergizes their toxicity against Asiatic rice borer (Chilo suppressalis). J. Biol. Chem. 293 (29), 11447-11458. https://doi.org/10.1074/jbc.RA118.003490
» https://doi.org/10.1074/jbc.RA118.003490 - World Health Organization - WHO, 1999. Bacillus thuringiensis: environmental Health Criteria, WHO, Geneva, pp. 217.
Edited by
Publication Dates
-
Publication in this collection
16 Dec 2020 -
Date of issue
2020
History
-
Received
11 Aug 2020 -
Accepted
23 Nov 2020