Abstracts
The contribution of humic substances of different composts to the synthesis of humin in a tropical soil was evaluated. Increasing doses (0, 13, 26, 52, and 104 Mg ha-1) of five different composts consisting of agroinpowderrial residues were applied to a Red-Yellow Latosol. These composts were chemically characterized and 13C NMR determined and the quantity of the functional alkyl groups of humic acids applied to the soil as compost was estimated. Thirty days after application of the treatments, organic matter samples were collected for fractionation of humic acids (HA), fulvic acids (FA) and humin (HU), from which the ratios HA/FA and (HA + FA)/HU were calculated. The application of the composts based on castor cake resulted in the highest HU levels in the soil; alkyl groups of the HA fraction of the composts were predominant in the organic components added to the HU soil fraction.
humic acids; fulvic acids; 13C NMR; alkyl groups; recalcitrance
Avaliou-se a contribuição de substâncias húmicas provenientes de diferentes compostos orgânicos na síntese de humina em um solo tropical. Para isso, foram adicionadas doses crescentes (0, 13, 26, 52 e 104 Mg ha-1) de cinco diferentes compostos orgânicos formulados com resíduos orgânicos agroindustriais em um Latossolo Vermelho-Amarelo. As substâncias húmicas desses compostos foram caracterizadas quimicamente e por ressonância nuclear magnética do 13C, sendo estimados os quantitativos dos grupos funcionais alquil dos ácidos húmicos aplicados no solo via composto. Trinta dias após aplicação dos tratamentos, coletaram-se amostras de solo para fracionamento da matéria orgânica em ácidos húmicos (AH), ácidos fúlvicos (FA) e humina (HU), a partir dos quais foram calculadas as relações AH/FA e (AH + FA)/HU. A aplicação do composto à base de torta de mamona contribuiu para obtenção de teores mais elevados de HU no solo; a incorporação de componentes orgânicos à fração HU do solo foi regida pelo conteúdo de grupos alquil da fração AH dos compostos.
ácidos húmicos; ácidos fúlvicos; 13C RMN; grupo alquil; recalcitrância
SEÇÃO II - QUÍMICA E MINERALOGIA DO SOLO
Claudivan Costa LimaI; Eduardo de Sá MendonçaII; Asunción RoigIII
IProfessor, Alagoas Federal Institute of Education, Science and Technology, Campus Satuba. CEP 57120-000 Satuba (AL), Brazil. E-mail: claudivanc@yahoo.es
IIProfessor, Soil Science Department, Viçosa Federal University, CEP 36570-000 Viçosa (MG), Brazil. E-mail: esm@ufv.br
IIIResearch scientist, Spanish National Research Council, Center of Soil Science and Biology Applied of the Segura (CSIC/CEBAS), Campus Universitario de Espinardo, Apartado de Correos 164, 30100 Espinardo, Murcia, Espain. E-mail: aroig@cebas.cesic.es
SUMMARY
The contribution of humic substances of different composts to the synthesis of humin in a tropical soil was evaluated. Increasing doses (0, 13, 26, 52, and 104 Mg ha-1) of five different composts consisting of agroinpowderrial residues were applied to a Red-Yellow Latosol. These composts were chemically characterized and 13C NMR determined and the quantity of the functional alkyl groups of humic acids applied to the soil as compost was estimated. Thirty days after application of the treatments, organic matter samples were collected for fractionation of humic acids (HA), fulvic acids (FA) and humin (HU), from which the ratios HA/FA and (HA + FA)/HU were calculated. The application of the composts based on castor cake resulted in the highest HU levels in the soil; alkyl groups of the HA fraction of the composts were predominant in the organic components added to the HU soil fraction.
Index terms: humic acids, fulvic acids, 13C NMR, alkyl groups, recalcitrance.
RESUMO
Avaliou-se a contribuição de substâncias húmicas provenientes de diferentes compostos orgânicos na síntese de humina em um solo tropical. Para isso, foram adicionadas doses crescentes (0, 13, 26, 52 e 104 Mg ha-1) de cinco diferentes compostos orgânicos formulados com resíduos orgânicos agroindustriais em um Latossolo Vermelho-Amarelo. As substâncias húmicas desses compostos foram caracterizadas quimicamente e por ressonância nuclear magnética do 13C, sendo estimados os quantitativos dos grupos funcionais alquil dos ácidos húmicos aplicados no solo via composto. Trinta dias após aplicação dos tratamentos, coletaram-se amostras de solo para fracionamento da matéria orgânica em ácidos húmicos (AH), ácidos fúlvicos (FA) e humina (HU), a partir dos quais foram calculadas as relações AH/FA e (AH + FA)/HU. A aplicação do composto à base de torta de mamona contribuiu para obtenção de teores mais elevados de HU no solo; a incorporação de componentes orgânicos à fração HU do solo foi regida pelo conteúdo de grupos alquil da fração AH dos compostos.
Termos de indexação: ácidos húmicos, ácidos fúlvicos, 13C RMN, grupo alquil, recalcitrância.
INTRODUCTION
In tropical and subtropical soils greatly impacted by weather events, organic matter (OM) is an essential element of the productive capacity, particularly concerning fertility (Bayer & Mielniczuk, 1999), and is believed to account for more than 90 % of the cation exchange capacity (CEC) of soils in the tropical ecosystems (Melo et al., 1997). The maintenance or recovery of the productive capacity of these soils can be obtained with a management system that increases the OM contents, as by using composts.
The mineral nature of soil, as well as the quantitative and qualitative characteristics of the humic acids (HA) and fulvic acids (FA) in compost can affect both the reactivity and stability of these humic substances (HS) in the medium. (Mikutta et al., 2006; Lima et al., 2005, 2006). Carboxylic (-COOH) and phenolic (-OH) functional groups, which are usually found in the periphery of the HS (Fassbender, 1975), are related to their reactivity, contributing to the increase in CEC (Jerzykiewicz et al., 1999; Lead et al., 1999; Hayes & Malcom, 2001), whereas the hydrophobic functional groups are more directly related to the stability of HS in the soil. For being recalcitrant and adsorbed by the soil mineral matrix or occluded intra-aggregates (Mikutta et al., 2006), hydrophobic HS are more resistant to microbial degradation (Piccolo et al., 1999), and have greater residency in the soil. This stability is more effective in poorly crystallized soils containing aluminosilicates, such as allophone, imogolite (Torn et al., 1997) and also montmorillonite minerals, where adsorption apparently occurs within mineral lattices (Khan, 1946, 1950, 1959). Kaolinite, orthoclase, feldspar and microcline are adsorbed at the crystal surface and easily dissolved following alkali treatment (Khan, 1946, 1950, 1959). However, Wiseman & Pütmann (2005) found a relationship between C storage in non-allophanic soils composed of varied phyllosilicates, as well as Fe and Al oxyhydroxides.
The level of alkyl groups (paraffinic C) of HA present in the composts, which is similar to the humin fraction of soil (HU) (Stuermer et al., 1978; Hatcher et al., 1980; Preston & Ripmeester, 1982), may control humin synthesis when these composts are applied, stabilizing OM in the soil. This is probably caused due to the interaction between the functional alkyl groups of HA that exist in the composts with the soil mineral matrix, which confers to these composts the characteristics of a "functional humin". Conceptually, humin is defined as the fraction of humified organic matter insoluble in alkali or acid medium, and which is strongly adsorbed by the soil matrix (Stevenson, 1994). Humin has already been referred to as "anhydrous humic acids" by Odén (1919), as "humic clay-acid complex" by Khan (1945) and later reiterated by Kononova et al. (1966), due to the great similarity between the analytical properties and those of HA in terms of elementary composition, functional groups and infrared spectrum. Moreover, soil treatment with HF, which makes it possible to destroy clays, results in the solubilization of humin in alkali medium (Stevenson, 1994). Despite this fact, Hacher et al. (1985) found significant differences between humin and HA spectra in a 13C RMN analysis, in the same sample, suggesting that humin is not a clay-humic acid complex. Humin differs from HA in the soil in its higher polysaccharide content and lower relative proportion of carboxylic C, but mostly by the higher concentration of paraffinic compounds (alkyl groups) (Hacher et al., 1985).
The purpose of this study was to evaluate the contribution of humic acids of different composts derived from agro-inpowderrial organic residues to the synthesis of humin in a tropical soil.
MATERIAL AND METHODS
The study was carried out in a greenhouse of the Department of Soils of the Federal University of Viçosa, Viçosa, Minas Gerais State, Brazil. A sample of dystrophic medium-textured red-yellow Latosol, from the municipality of João Pinheiro, Minas Gerais, was collected in the 0-20 cm layer and sieved through 2 mm mesh. This soil has the following chemical and physical properties: pH in water: 4.76; P-Mehlich-1: 2.1 mg dm-3; K: 122 mg dm-3; Ca: 0.18 cmolc dm-3; Mg: 0.06 cmolc dm-3; Al: 0.20 cmolc dm-3; H + Al: 3.9 cmolc dm-3; SB: 0.55 cmolc dm-3; t: 0.75 cmolc dm-3; T: 4.45 cmolc dm-3; V %: 12,4; m %: 26.7; MO: 1.43 dag kg-1; HA: 0.09 dag kg-1; FA: 0.16 dag kg-1; HU: 0.47 dag kg-1; P-rem: 26.3 mg L-1; soil density: 1.33 g cm-3; particle density: 2.68 g cm-3; clay content: 37 dag kg-1; texture class: sandy clay loam.
Five composts were added to this soil (Tables 1 and 2). The humic substances (HA and FA contained in the referred composts were extracted and purified for characterization in a 13C NMR spectrum (Figure 1). To perform the 13C NMR analyses in solid state and "magic angle spin" (MAS) of HA and FA samples, a Varian 300 MHz equipment was used with zirconium oxide rotor operating at 75.42 MHz of C, at a rotation rate of 4 KHz, contact time 1.5 ms, acquisition time 35 ms, pulse width 6.7 μs, and the pulse angle 90º. Hexamethylbenzene was used as reference material. The qualitative and semiquantitative interpretation of the RMN spectrum was based on Inbar et al. (1989), Kögel-Knabner (1997) and Ussiri & Johnson (2003). The spectrum was divided into five regions according to the chemical shift, as follows: C alkyl (0-110 ppm), aromatic groups (110-140 ppm), phenolic groups (140-160 ppm), carboxylic group (160-190 ppm) and carbonyl carbon of aldehyde/ketone (190-240 ppm).
Based on the HA contents of the composts (Table 2), for a dose of 13 Mg ha-1; the quantitative HA levels (in kg), applied to the soil with compost were estimated. Also, based on the same criterion, the quantitative levels of alkyl functional groups of HA added to the soil were estimated, from qualitative and semiquantitative interpretations of the data obtained with 13C NMR spectroscopy.
The treatments consisted of five doses (0, 13, 26, 52 and 104 Mg ha-1; dry matter base) of the five composts obtained, which were applied to the above soil, and this mixture was stored in 20 dm3 recipients. The treatments were arranged in a 5 x 5 factorial design, with three replications, and distributed in random blocks.
Soil samples were collected 30 days after compost application for fractioning of the organic matter in humic acids (HA), fulvic acids (FA) and humin (HU), according to the "International Humic Substances Society" - IHSS (Hayes et al., 1989), and the C content of these fractions was determined according to Yeomans & Bremner (1988). From these determinations the HA/FA and (HA + FA)/HU ratios were calculated (Benites et al., 2003).
The data obtained were subjected to descriptive statistics, variance analysis, simple linear correlation (Pearson's Correlation), and the Tukey test at 5 % for the comparison of averages, using the SAEG (System of Statistic and Genetic Analyses) program of the Federal University of Viçosa (FUNARBE, 1993). Regression equations were also adjusted according to the compost doses applied.
RESULTS AND DISCUSSION
Quantitative levels of alkyl functional groups of composts
The nature of the composted material and the mineral enrichment of the compost were found to have quantitative and qualitative influence on HS generation (Table 3), which corroborates results reported by Lima et al. (2005) and Bernal et al. (2009). These authors also stated that the addition of minerals contributes to accelerate the decomposition process of the organic matter of the compost, and at the same time favors the humification of organic matter.
All composts had higher HA than FA contents; the application of FC compost resulted in the highest quantitative levels of FA; and the M-G compost resulted in the highest quantitative HA levels. The composts that led to the greatest amounts of soil humic substances (HA + FA), in decreasing order, were: M + G > FC > SC > SM > AS.
The SC, AS, SM, FC and M-G composts had HA consisting of 64, 56, 57, 56 and 73 % of alkyl functional groups, respectively. Among these composts, the M-G compost is worth mentioning, in which the percentage of alkyl functional groups of HA of 237, 370, 301 was 96 % higher than in SC, AS, SM, and FC composts, respectively.
Humin fraction of the soil
The C contents of the HU fraction were higher than those of the HA and FA fractions in all treatments and tended to increase with increasing compost doses (Figure 2). As the composts do not contain humin, once the humic fraction is conceptually adsorbed in the soil mineral matrix (Stevenson, 1994), other organic fractions added to the soil through the compost may have incorporated functional characteristics of humin. This incorporation of composts to the humin fraction can be determined by the HA fraction of composts applied to the soil (Figure 1), once the HA content of the compost was found to be more correlated to the humin content (r = 0.84**) than to the HA content of the soil (r = 0.45**). This interpretation is reinforced by the observation of the 13C spectra obtained by means of NMR analysis of HA from the studied composts, which are highly cimilar to the humin spectra of the different soils obtained by several authors (Stuermer et al., 1978; Hatcher et al., 1980; Preston & Ripmeester, 1982).
A significant correlation was found between the estimate of the alkyl groups of HA in the composts and the humin content in the soil (r = 0.82**). The humin content of the M-G compost increased most (Figure 2). This result can be attributed to the alkyl groups of HA of the corresponding compost. The quantitative level of this functional group was found to be on average 251 % higher than of the other composts (Table 3). The mineral enrichment of AS and SM composts favored mineralization of the precursors of the alkyl groups (Figure 1), as demonstrated by the reduction of the relative abundance compared to SC (Baldock & Preston, 1995; Gressel et al., 1996; Dai et al., 2001). In the latter, preferential decomposition of other fractions, such as carbohydrates, and preservation of the alkyl groups has probably occurred, reflected in the soil humin contents.
The correlations of the HA alkyl groups of the different composts were evaluated by the estimates obtained for every studied dose. The alkyl groups of HA were positively correlated with soil humin at the doses 13 Mg (r = 0.91**), 26 Mg (r = 0.97**), 52 Mg (r = 0.88**) and 104 Mg ha-1 (r = 0.59**). This synthesis was less significant when higher doses were applied, indicating that the increased level of alkyl groups of HA in the soil affects the effectiveness of the synthesis of the "functional humin". These results differ from the findings reported by Hacher et al. (1985) and are closer to the proposals of several authors, e.g., Odén (1919), Khan (1945) and Kononova et al. (1966) who considered humin to be a "humic clay-acid complex" in the beginning of the 20th century.
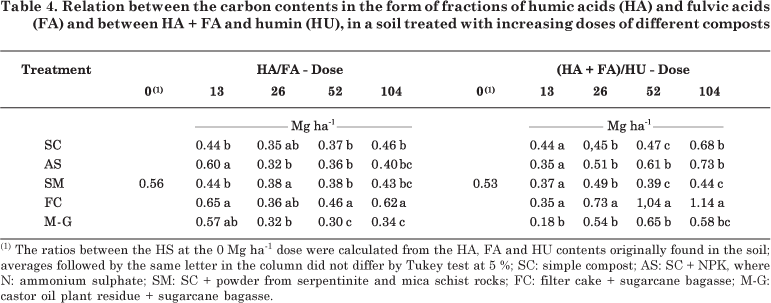
Compost application to the soil has generally promoted alterations in the ratios of the HA, FA and HU fractions, compared to those usually found in the soil (Table 4). These alterations may be caused not only by the quantitative characteristics of HS, but also by the quality of HA contained in the applied composts, which have incorporated the characteristics of functional humin. The HA and FA contents of the soil were found to be similar in all treatments, i.e., FA was predominant over HA (HA/FA < 1). In the FC treatment, at doses of 52 and 104 Mg ha-1; this ratio was greater than in the other treatments, except in the soil without composts, indicating that the relative amounts of HA and FA in this soil type are more balanced. Although all composts have higher HA than FA contents (Table 2), a prevalence of FA was found after compost application to the soil, as observed by Canellas et al. (2001). This predominance can be attributed to the limited condensation reaction (Mendonça & Rowell, 1994). The soil texture and presence of oxyhydroxides may also interfere with the amounts of FA and HA. FA is most predominant in clay (Mendonça & Rowell, 1996) and dystrophic soils (Cunha et al., 2005).
The HA of the composts may interact with clay, forming very stable complexes (Sposito, 1989). This association may occur by means of HA hydrophobic groups, or else, the methylene groups derived from cutin and suberin (Mahieu et al., 1999; Nierop et al., 1999). This association can also be seen when the ionization of the acid functional groups of organic molecules is repressed, that is, when the pH values are in the PCZ (Sposito, 1989). In this case, the HA turns into the humin fraction, and a relative increase in the FA contents occurs, which consequently leads to a reduced HA/FA ratio.
The values of the (HA + FA)/HU ratio were lower than 1 in the SC, AS, SM and M-G treatments, indicating a greater proportion of HA adsorbed in the soil mineral fraction that became the humin fraction. This adsorption was most intense at the dose of 13 Mg ha-1 in the M-G treatment. Only in the FC treatment, from the 52 Mg ha-1 dose upwards, the (HA + FA)/HU ratio was greater than 1, unlike in the other treatments. This indicates that the organic fraction of FC-treated soil contains a higher proportion of the HA + FA than of the HU fraction, or that the quantitative HA and FA levels of this compost caused lower interaction of these fractions with the soil mineral fraction.
CONCLUSIONS
1. Following the application of increasing doses of different composts to the soil, the FA contents in the soil were higher than HA, even though the composts had higher HA than FA contents.
2. The incorporation of the organic compound to the humin fraction was guided by qualitative characteristics of the HA fraction present in the composts, more specifically by the alkyl group content of this fraction.
3. The application of the M-G compost resulted in a higher content of soil humin.
4. Further studies are needed to evaluate the effect of the incubation period of composts in the soil on the synthesis and stability of "functional humin".
ACKNOWLEDGEMENTS
The authors wish to thank the CNPq and FAPEAL for the financial support for this research, as well as Dr. Ana de Godos de Francisco and Diego Martínez Pérez of SACE - Universidad de Murcia - Spain, for their help with the 13C NMR spectroscopy.
LITERATURE CITED
Received for publication in April 2009 and approved in May 2010.
- BALDOCK, J.A. & PRESTON, C.M. Chemistry of carbon decomposition processes in forests as revealed by solid-state carbon-13 nuclear magnetic resonance. In: MCFEE, W.W. & KELLY, J.M., eds. Carbon forms and functions in forest soils. Madison, Soil Science Society of America, 1995. p.89-117.
- BAYER, C. & MIELNICZUK, J. Dinâmica e função da matéria orgânica. In: SANTOS, G.A. & CAMARGO, F.A.O., eds. Fundamentos da matéria orgânica: Ecossistemas tropicais e subtropicais. Porto Alegre, Genesis, 1999. p.9-23.
- BENITES, V.M.; MADARI, B. & MACHADO, P.L.O.A. Extração e fracionamento quantitativo de substâncias húmicas do solo: Um procedimento simplificado de baixo custo. Rio de Janeiro, 2003. 7p. (Comunicado Técnico 16, Embrapa Solos)
- BERNAL, M. P.; ALBUQUERQUE, J. A. & MORAL, R. Composting of animal manures and chemical criteria for compost maturity assessment: A review. Biores. Technol., 100:5444-5453, 2009.
- CANELLAS, L.P.; SANTOS, G.; MORAES, A.A. & RUMJANEK, V. Distribuição da matéria orgânica e características de ácidos húmicos em solos com adição de resíduos de origem urbana. Pesq. Agropec. Bras., 36:1529-1538, 2001.
- CUNHA, T.J.F.; CANELLAS, L.P.; SANTOS, G.A. & RIBEIRO, L.P. Fracionamento da matéria orgânica em solos brasileiros. In: CANELLAS, L.P. & SANTOS, G.A., eds. Humosfera: Tratado preliminar sobre química das substâncias húmicas. Campos dos Goytacazes, 2005. p.54-80.
- DAI, K.H.; JOHNSON, C.E. & DRISCOLL, C.T. Organic matter chemistry in clear-cut and unmanaged hardwood forest ecosystems. Biogeochemistry, 54:51-83, 2001.
- FASSBENDER, H.W. Química de suelos, con énfasis en suelos de América Latina. Turrialba, Instituto Interamericano de Ciencias Agrícolas de la OEA, 1975. 398p.
- FUNARBE. SAEG - Sistema para análises estatísticas - versão 5.0. Viçosa, MG, Fundação Arthur Bernardes. 1993. 80p.
- GRESSEL, N.; McGRATH, A.E.; McCOLL, J.G. & POWERS, R.F. Spectroscopy of aqueous extracts of forest litter. I: Suitability of methods. Soil Sci. Soc. Am. J., 59:1715-1723, 1996.
- HATCHER, P.G.; BREGER, I.A.; MACIEL, G.E. & SZEVERENYI, N.M. Geochemistry of humin. In: AIKEN, G.R.; McKNIGHT, D.M.; WERSHAW, R.L. & MacCARTHY, P., eds. Humic substances in soil, sediments, and water. New York, John Wiley & Sons, 1985. p.275-302.
- HATCHER, P.G.; VANDERHART, D.L. & EARL, W.L. Use of solid-state 13C NMR in structural studies of humic acids and humin from Holocene sediments. Org. Geochem., 2:87-92, 1980.
- HAYES, M.H.; McCARTHY, P.; MALCOLM, R.L. & SWIFT, R.S. Structures of humic substances: The emergence of forms. In: HAYES, M.H.; McCARTHY, P.; MALCOLM, R.L. & SWIFT, R.S., eds. Humic substance II: In search of structure: Setting the scene. New York, John Wiley & Sons, 1989. p.3-31.
- HAYES, M.H. & MALCOLM, R.L. Consideration of composition and aspects of the structures of humic substances, In: HAYES, M.H. & MALCOLM, R.L., eds. Humic substances and chemical contaminants. Madison, Soil Science Society of America, 2001. p.3-39.
- INBAR, Y.; CHEN, Y. & HADAR, Y. Solid-state carbon-13 nuclear magnetic resonance and infrared spectroscopy of composted organic matter. Soil Sci. Soc. Am. J., 53:1695-1701, 1989.
- JERZYKIEWICZ, M.; DROZD, J. & JEZIERSKI, A. Organic radicals and paramagnetic metal complexes in municipal solid waste composts. An EPR and chemical study. Chemosphere, 39:253-268, 1999.
- KHAN, D.V. A method of isolating the insoluble fraction (humin) from podzolic soils. Dokl. Akad. Nauk, 32:7-8, 1945.
- KHAN, D.V. The fixation of the humic acids by various minerals. Dokl. Akad. Nauk, 11:1-2, 1946.
- KHAN, D.V. The absorption of organic matter by soil mineral. Pochvoved., 11:673-678, 1950.
- KHAN, D.V. The composition of humus substances and their link with the mineral part of the soil. Pochvoved., 1:10-18, 1959.
- KÖGEL-KNABNER, I. 13C e 15N NMR spectroscopy as a tool in soil organic matter studies. Geoderma, 80:243-270, 1997.
- KONONOVA, M.M.; NOWAKOWSKI, T.Z. & NEWMAN, A.C.D. Soil organic matter; its nature, its role in soil formation and in soil fertility. 2.ed. London, Pergamon Press, 1966. 544p.
- KORNDÖRFER, G.H.; RIBEIRO, A.C. & ANDRADE, L.A.B. Cana-de-açúcar. In: RIBEIRO, A.C.; GUIMARÃES, P.T.G. & ALVAREZ V., V.H., eds. Recomendações para o uso de corretivos e fertilizantes em Minas Gerais. Viçosa, MG, Comissão de Fertilidade do Solo do Estado de Minas Gerais, 1999. p.285-288.
- LEAD, J.R.; HAMILTON-TAYLOR, J.; DAVISON, W. & HARPER, M. Trace metal sorption by natural particles and coarse colloids. Geochim. Cosmochim. Acta, 63:1661-1670, 1999.
- LIMA, C.C.; MENDONÇA, E.S.; ROIG, A.; SÁCHEZ-MONEDERO, M.A. & PERES, B.H. Effect of mineral enrichment on the humic fraction composition during the composting process. In: EUROPEAN GEOSCIENCES UNION, 2005, Viena. Geophysical Research Abstracts. Viena, 2005. CD-ROM.
- LIMA, C.C.; MENDONÇA, E.S.; SILVA, I.R.; SILVA, L.H.M.; SANCHEZ-MONEDERO, M.A. & ROIG, A. Solid state NMR 13C of humic and fulvic acids from composts prepared with different materials and mineral enrichment In: MEETING OF THE INTERNATIONAL HUMIC SUBSTANCES SOCIETY, 13., Karlsruhe, 2006. Abstract. Karlsruhe, IHSS, 2006. CD ROM.
- MAHIEU, N.; POWLSON, D.S. & RANDALL, E.W. Statistical analysis of published carbon-13 CPMAS NMR spectra of soil organic matter. Soil Sci. Soc. Am. J., 63:307-319, 1999.
- MELO, W.J.; MARQUES, M.O.; SILVA, F.C. & BOARETO, A.E. Uso de resíduos sólidos urbanos na agricultura e impactos ambientais. In: CONGRESSO BRASILEIRO DE CIÊNCIA DO SOLO, 26., Rio de Janeiro, 1997. Anais. Rio de Janeiro, Embrapa/SBCS, 1997. CD ROM.
- MENDONÇA, E.S. & ROWELL, D.L. Dinâmica de alumínio e de diferentes frações orgânicas de um Latossolo argiloso sob cerrado e soja. R. Bras. Ci. Solo, 19:295-303, 1994.
- MENDONÇA, E.S. & ROWELL, D.L. Mineral and organic fractions of two Oxisols and their influence on effective cation-exchange capacity. Soil Sci. Soc. Am. J., 60:1888-1892, 1996.
- MIKUTTA, R.; KLEBER, M.; TORN, M.S. & JAHN, R. Stabilization of soil organic matter: Association with mineral or chemical recalcitrance? Biogeochemistry, 77:25-56, 2006.
- NIEROP, K.G.J.; BUURMAN, P. & LEEUW, J.W. Effect of vegetation on chemical composition of H horizons in insipient podzols as characterized by 13CNMR and pyrolysis-GC/MS. Geoderma, 90:111-129, 1999.
- ODÉN, S. Die Huminsãuren. Kolloidchem. Beih., 11:75-98, 1919.
- PICCOLO, A.; SPACCINI, R.; HABERHAUER, G. & GERZABEK, M.H. Increased sequestration of organic carbon by hydrophobic protection. Naturwiss, 86:496-499, 1999.
- PRESTON, C.M. & RIPMEESTER, J.A. Application of solution and solid-state 13C NMR to four organic soils, their humic acids, fulvic acids, humins, and hydrolysis residues. Can. J. Spectrosc., 27:99-105, 1982.
- SPOSITO, G. The chemistry of soil. New York, Oxford University Press, 1989. 277p.
- STEVENSON, F.J. Humus chemistry: Genesis, composition, reactions. 2.ed. New York, John Wiley & Sons, 1994. 512p.
- STUERMER, D.H.; PETERS, K.E. & KAPLAN, I.R. Source indicators of humic substances and proto-kerogen. Stable isotope ratios, elemental compositions and electron spin resonance spectra. Geochim. Cosmochim. Acta, 42:989-997, 1978.
- TORN, M.S.; TRUMBORE, S.E.; CHADWICK, O.A.; VITOUSEK, P.M. & HENDRICKS, D.M. Mineral control of soil organic carbon storage and turnover. Nature, 389:170-173, 1997.
- USSIRI, D.A.N. & JOHNSON, C.E. Characterization of organic matter in a northern hardwood forest soil by 13C NMR spectroscopy and chemical methods. Geoderma, 111:123-149, 2003.
- WISEMAN, C.L.S. & PÜTTMANN, W. Soil organic carbon and its sorptive preservation in central Germany. Eur. J. Soil Sci., 56:65-76, 2005.
- YEOMANS, J.C. & BREMNER, J.M. A rapid and precise method for routine determination of organic carbon in soil. Comm. Soil Sci. Plant Anal., 19:1467-1476, 1988.
Contribution of humic substances from different composts to the synthesis of humin in a tropical soil
Publication Dates
-
Publication in this collection
13 Oct 2010 -
Date of issue
Aug 2010
History
-
Received
Apr 2009 -
Accepted
May 2010