Abstracts
The use of cover crops has been suggested as an effective method to maintain and/or increase the organic matter content, while maintaining and/or enhancing the soil physical, chemical and biological properties. The fertility of Cerrado soils is low and, consequently, phosphorus levels as well. Phosphorus is required at every metabolic stage of the plant, as it plays a role in the processes of protein and energy synthesis and influences the photosynthetic process. This study evaluated the influence of cover crops and phosphorus rates on soil chemical and biological properties after two consecutive years of common bean. The study analyzed an Oxisol in Selvíria (Mato Grosso do Sul, Brazil), in a randomized block, split plot design, in a total of 24 treatments with three replications. The plot treatments consisted of cover crops (millet, pigeon pea, crotalaria, velvet bean, millet + pigeon pea, millet + crotalaria, and millet + velvet bean) and one plot was left fallow. The subplots were represented by phosphorus rates applied as monoammonium phosphate (0, 60 and 90 kg ha-1 P2O5). In August 2011, the soil chemical properties were evaluated (pH, organic matter, phosphorus, potential acidity, cation exchange capacity, and base saturation) as well as biological variables (carbon of released CO2, microbial carbon, metabolic quotient and microbial quotient). After two years of cover crops in rotation with common bean, the cover crop biomass had not altered the soil chemical properties and barely influenced the microbial activity. The biomass production of millet and crotalaria (monoculture or intercropped) was highest. The biological variables were sensitive and responded to increasing phosphorus rates with increases in microbial carbon and reduction of the metabolic quotient.
microbial biomass carbon; microbial activity; organic matter; cover plants; Cerrado
A utilização de plantas de cobertura é sugerida como método fundamental para manter e, ou, elevar o teor de matéria orgânica no solo, mantendo e, ou, melhorando as condições físicas, químicas e biológicas dele. Os solos do Cerrado apresentam baixa fertilidade, consequentemente baixos teores de fósforo (P), que se relacionam a todas as etapas metabólicas da planta, por essas fazerem parte dos processos de síntese proteica e energética, além de interferir no processo fotossintético. Este trabalho objetivou avaliar a influência de plantas de coberturas e doses de P sobre os atributos químicos e biológicos do solo. O trabalho foi conduzido em Selvíria, MS, em Latossolo Vermelho distrófico, com delineamento de blocos casualizados em esquema de parcelas subdivididas, com plantas de cobertura (milheto, guandu, crotalária, mucuna-preta, milheto + guandu, milheto + crotalária e milheto + mucuna-preta) e pousio nas parcelas e doses de P (0, 60 e 90 kg ha-1 de P2O5, na forma de monoamônio fosfato), nas subparcelas, totalizando 24 tratamentos, com três repetições. Em agosto de 2011, foram avaliados os atributos químicos (pH, matéria orgânica, fósforo, acidez potencial, capacidade de troca catiônica e saturação por bases) e biológicos do solo (carbono do CO2 liberado, carbono microbiano, quociente metabólico e quociente microbiano do solo). Após dois anos de cultivo das plantas de cobertura em rotação com o feijoeiro, verificou-se que a biomassa das plantas de cobertura não alterou as características químicas do solo e pouco influenciou sobre a atividade microbiana. As maiores respostas foram verificadas para milheto e crotalária, isoladas ou em consórcio. As variáveis biológicas foram sensíveis e responderam às doses crescentes de adubação fosfatada, sendo os aumentos do carbono microbiano e a redução do quociente metabólico obtidos com as maiores doses de P.
carbono microbiano; atividade microbiana; matéria orgânica; adubação verde; cerrado
DIVISÃO 2 - PROCESSOS E PROPRIEDADES DO SOLO
COMISSÃO 2.1 - BIOLOGIA DO SOLO
Chemical and biological properties of phosphorus-fertilized soil under legume and grass cover (Cerrado region, Brazil)1 1 Part of the Master’s Dissertation of the first author, Post-Graduation Course in Crop Production, Universidade Estadual Paulista - UNESP, Campus Ilha Solteira (SP), Brazil.
Atributos químicos e biológicos do solo com cobertura de leguminosas, gramínea e doses de fósforo, em região de Cerrado, Brasil
Marcelo Fernando Pereira SouzaI; Mariana Pina da SilvaI; Orivaldo ArfII; Ana Maria Rodrigues CassiolatoII
IPost-Graduation student in Crop Production, Agronomy Department, UNESP, Campus Ilha Solteira. Av. Brasil, 56. Caixa Postal 31. CEP 15385-000 Ilha Solteira (SP), Brazil. E-mail: celonando@hotmail.com, mari_agro@hotmail.com
IIProfessor, UNESP, Campus Ilha Solteira. E-mail: arf@agr.feis.unesp.br, anamaria@bio.feis.unesp.br
SUMMARY
The use of cover crops has been suggested as an effective method to maintain and/or increase the organic matter content, while maintaining and/or enhancing the soil physical, chemical and biological properties. The fertility of Cerrado soils is low and, consequently, phosphorus levels as well. Phosphorus is required at every metabolic stage of the plant, as it plays a role in the processes of protein and energy synthesis and influences the photosynthetic process. This study evaluated the influence of cover crops and phosphorus rates on soil chemical and biological properties after two consecutive years of common bean. The study analyzed an Oxisol in Selvíria (Mato Grosso do Sul, Brazil), in a randomized block, split plot design, in a total of 24 treatments with three replications. The plot treatments consisted of cover crops (millet, pigeon pea, crotalaria, velvet bean, millet + pigeon pea, millet + crotalaria, and millet + velvet bean) and one plot was left fallow. The subplots were represented by phosphorus rates applied as monoammonium phosphate (0, 60 and 90 kg ha-1 P2O5). In August 2011, the soil chemical properties were evaluated (pH, organic matter, phosphorus, potential acidity, cation exchange capacity, and base saturation) as well as biological variables (carbon of released CO2, microbial carbon, metabolic quotient and microbial quotient). After two years of cover crops in rotation with common bean, the cover crop biomass had not altered the soil chemical properties and barely influenced the microbial activity. The biomass production of millet and crotalaria (monoculture or intercropped) was highest. The biological variables were sensitive and responded to increasing phosphorus rates with increases in microbial carbon and reduction of the metabolic quotient.
Index terms: microbial biomass carbon, microbial activity, organic matter, cover plants, Cerrado.
RESUMO
A utilização de plantas de cobertura é sugerida como método fundamental para manter e, ou, elevar o teor de matéria orgânica no solo, mantendo e, ou, melhorando as condições físicas, químicas e biológicas dele. Os solos do Cerrado apresentam baixa fertilidade, consequentemente baixos teores de fósforo (P), que se relacionam a todas as etapas metabólicas da planta, por essas fazerem parte dos processos de síntese proteica e energética, além de interferir no processo fotossintético. Este trabalho objetivou avaliar a influência de plantas de coberturas e doses de P sobre os atributos químicos e biológicos do solo. O trabalho foi conduzido em Selvíria, MS, em Latossolo Vermelho distrófico, com delineamento de blocos casualizados em esquema de parcelas subdivididas, com plantas de cobertura (milheto, guandu, crotalária, mucuna-preta, milheto + guandu, milheto + crotalária e milheto + mucuna-preta) e pousio nas parcelas e doses de P (0, 60 e 90 kg ha-1 de P2O5, na forma de monoamônio fosfato), nas subparcelas, totalizando 24 tratamentos, com três repetições. Em agosto de 2011, foram avaliados os atributos químicos (pH, matéria orgânica, fósforo, acidez potencial, capacidade de troca catiônica e saturação por bases) e biológicos do solo (carbono do CO2 liberado, carbono microbiano, quociente metabólico e quociente microbiano do solo). Após dois anos de cultivo das plantas de cobertura em rotação com o feijoeiro, verificou-se que a biomassa das plantas de cobertura não alterou as características químicas do solo e pouco influenciou sobre a atividade microbiana. As maiores respostas foram verificadas para milheto e crotalária, isoladas ou em consórcio. As variáveis biológicas foram sensíveis e responderam às doses crescentes de adubação fosfatada, sendo os aumentos do carbono microbiano e a redução do quociente metabólico obtidos com as maiores doses de P.
Termos de indexação: carbono microbiano, atividade microbiana, matéria orgânica, adubação verde, cerrado.
INTRODUCTION
The Cerrado represents the predominant vegetation in Central Brazil, where the topography and landscape conditions are well-suited, which has led to the conversion of this biome into the main grain-producing region of the country. The replacement of native vegetation and use for pasture or grain production induces changes in the chemical and biological properties of the soil (Costa et al., 2006; Carneiro et al., 2009). Typical characteristics of these soils are low fertility, acidity and Al toxicity, and low nutrient availability, including phosphorus (P). Phosphorus is required at every metabolic stage, as it plays a role in the protein and energy synthesis processes and in the translocation and formation of fatty acids, as well as influencing the photosynthetic process directly (Marschner, 1995). In tropical soils, plant-available P is found in small quantities due to the adsorption to Al and Fe oxides.
The no-tillage management can reduce negative impacts caused by conventional systems, and maintain and/or improve soil fertility, inducing higher crop yields (Pelá et al., 2010). This system reduces soil losses by erosion, improving the soil chemical, physical and biological conditions and directly affecting fertility (Carvalho et al., 2004).
However, the success of no-tillage systems is closely associated with the maintenance of a soil cover in a crop rotation (Carvalho et al., 2007). The choice of cover crops with high biomass yield that protect the soil against erosion and nutrient loss is an important factor in the maximization of the benefits of no-tillage (Suzuki & Alves, 2006), for improving the nutrient availability for subsequent crops and, in certain situations, contributing to soil nitrogen input by legumes (Perin et al., 2004).
The soil quality has been mainly associated with soil productivity (Hornik, 1992). Doran & Parkin (1994) however defined an expanded concept of soil quality based on the capacity of a soil to sustain the biological productivity, maintain environmental quality, and promote plant and animal health within the boundaries of an ecosystem. Soil quality indicators have proved useful and fundamental to assess not only the capacity of self-sustainability of the soil, but also of the whole ecological system (Doran & Zeiss, 2000).
Soil organisms are dynamic, but vulnerable to physical and chemical changes as well as to the effects of management practices and crop systems (Venzke Filho et al., 2008). The soil microbial biomass is correlated with organic matter (Gonçalves et al., 2007) and is essential to evaluate the processes occurring in the soil; studies of this biological property and activity can provide critical information for soil use and management planning (D’Andréa et al., 2002).
The methods to assess the magnitude of the microbial biomass are based on the amount of soil carbon released as CO2 (C-CO2 release), the determination of the metabolic quotient (qCO2) and the ratio amount of C-CO2 released per unit of microbial biomass carbon (MBC) over time (Anderson & Domsch, 1993). However, the microbial quotient (qCmic), i.e. the ratio of MBC in relation to the soil organic carbon allows a more immediate monitoring of variations in the total organic matter. Therefore, the objective of this study was to evaluate the influence of cover crops and phosphorus fertilization on the soil chemical and biological properties.
MATERIAL AND METHODS
The experiment was conducted in an experimental area of the Fazenda de Ensino, Pesquisa e Extensão of the Universidade Estadual Paulista (UNESP), Faculdade de Engenharia, Campus Ilha Solteira (Selvíria, Mato Grosso do Sul, Brazil) (20o 22’ S, 51o 22’ W; 335 m asl). The regional climate is tropical and humid with rainy summers and dry winters. The average annual rainfall is 1,232 mm and temperature 24 oC. The vegetation of the area is Cerrado sensu stricto. The relief is moderately flat, and the soil a dystrophic Red Latossol (Oxisol) (Embrapa, 2006).
Prior to these experiments, the studied area had been cultivated with corn (Zea mays L.) under no-tillage for 10 years. The soil was chemically analyzed before the area was prepared for the experiment. One composite sample, composed of 20 single samples, was collected in the experimental area from the 0-0.20 m layer and analyzed according to the methods proposed by Raij et al. (2001), with the following results: pH = 4.9; organic matter (OM) = 13 g dm-3, P = 12 mg dm-3; K, Ca and Mg = 1.1, 18 and 15 mmolc dm-3, respectively; potential acidity (H+Al) = 15 mmolc dm-3, and a base saturation of (BS) 70 %.
The experiment was arranged in a randomized block, split-plot design. The plot treatments consisted of cover crops (in monoculture or intercropped): millet (Pennisetum glaucum L.), crotalaria (Crotalaria juncea L.), velvet bean (Mucuna aterrima Piper & Tracy), pigeon pea (Cajanas cajan L.), and the intercrops millet + pigeon pea, millet + crotalaria and millet + velvet bean, plus one plot left fallow, with weed growth, mostly goosegrass (Eleusine indica L.). The subplots were represented by P fertilization (0, 60 and 90 kg ha-1 P2O5) in the form of monoammonium phosphate (MAP), containing 9 % N and 48 % P2O5, applied at a depth of 0.05 m in furrows destined for common bean sowing, after the cover crops. The 24 treatments were replicated three times on a total area of 20 m2, and each subplot consisted of seven 5-m long rows, spaced 0.45 m apart.
The cover crops were planted without fertilizer in February 2010 and again in February 2011, repeating the process of the previous year, i.e., the plots and subplots were installed at the same location. The cover crops were planted after no-tillage corn, in crop succession cycles of: corn, cover crop and common bean in both study years, i.e., initiating the rotation/succession with maize and ending with common bean.
The following cover crops were sown in rows spaced 0.45 m apart: a) millet, totaling 20 kg seeds per ha, b) velvet bean, at a density of 10 seeds per meter c) crotalaria, with 35 seeds per meter and d) pigeon pea with 15 seeds per meter. For the intercrops (millet + pigeon pea, millet + crotalaria and millet + velvet bean), sown in interspersed rows, the sowing density was the same as for monoculture. The cover crops and common bean were irrigated with a conventional fixed sprinkler system, at a flow rate of 3.3 mm h-1.
Seventy days after cover crop sowing, the plants were desiccated with glyphosate (N-(phosphonomethyl) glycine), at the prescribed dose of 1 L ha-1. A mechanical chopper was used to chaff the harvested plants. To evaluate the shoot dry matter production of the cover crops, samples of plant shoots left on the soil surface were collected by the quadrat method (0.50 x 0.50 m frame), at two different points per plot. The collected material was washed to avoid soil contamination and dried in a forced air circulation oven at 65 oC to constant moisture, to determine shoot dry matter production (in kg ha-1).
The winter bean crop was sown in May 2011. According to the soil chemical analysis, uniform N and K fertilization was applied at sowing in all treatments, according to the recommendations of Ambrosano et al. (1997), consisting of 175 kg ha-1 of the N-P-K fertilizer 20-0-20. Since the applied P source (MAP) contains 9 % N, the correction was completed using urea (45 %), so that all treatments received the same amount of N.
The common bean cultivar Pérola, with semi-prostrate growth (type III), was sown mechanically in the no-till system, in rows spaced 0.45 m apart, for a final density of 12 plants per meter. In August 2011, when the plants were at stage R8 (pod filling), one composite sample consisting of five single samples was collected per subplot (depth 0-0.10 m). One part of the soil samples was used for chemical analysis, as described above, and the other for biological and microbiological evaluations.
To quantify the C-CO2 release we used 100 g of soil and titration of the remaining NaOH with HCl, in the presence of the indicator phenolphthalein, following the method proposed by Anderson & Domsch (1989). The incubation time was determined using a calibration curve. The carbon (MBC) released from the microbial biomass was quantified by fumigation using chloroform, and determined by chemical extraction followed by digestion, according to the method proposed by Vance et al. (1987). The qCO2 was determined by the ratio C-CO2 release: MBC (Anderson & Domsch, 1993), while qCmic was calculated by the expression (MBC/Corg)/10 (Sparling, 1992).
The data were subjected to analysis of variance and the means compared by Tukey’s test at 5 %, followed by the Pearson correlation between variables.
RESULTS AND DISCUSSION
Shoot dry matter production of the cover plants
In terms of shoot dry matter production of the cover crops preceding common bean, the yields of the intercropped millet + crotalaria intercrop as well as monoculture crotalaria were highest (Table 1). The cover crops induce increases in density and diversity of soil microorganisms (Carneiro et al., 2004), improvement in soil structure (Andrade et al., 2009), nutrient cycling and, consequently, increase crop yields (Teixeira et al., 2006).
The observed dry matter production for crotalaria and pigeon pea, in this study, was higher than that reported by Torres et al. (2008). Studying the dry matter production of different cover crops, they reported yields of 3,900 and 2,700 kg ha-1 for crotalaria and pigeon pea, respectively. However, a production of 10,000 kg ha-1 was reported for millet, which was higher than the value found in this study (Table 1).
Among the legumes, the dry matter production of pigeon pea was lowest, while yields of velvet bean were high, but did not differ from crotalaria or crotalaria + millet. However, when intercropped with millet, velvet bean produced less dry matter (6,488 kg ha-1) than the other treatments (Table 1).
Chemical properties
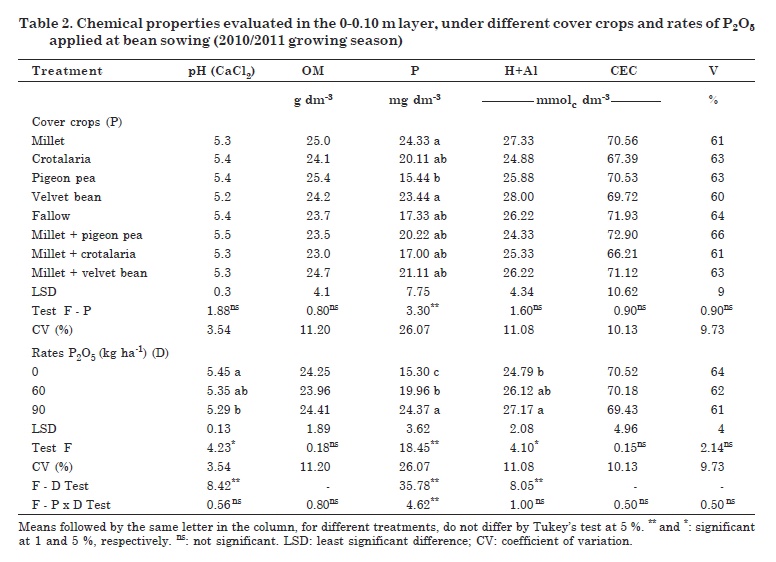
The majority of the studied soil chemical properties (layer 0-0.10 m) showed no significant differences under the different cover crop treatments, in contrast to the soil P levels (Table 2). The P2O5 rates applied at bean sowing induced statistical differences for the properties pH, P content and H+Al. Also, a negative significant correlation was detected between P and pH (-0.288*) and a positive significant correlation between P and H+Al (0.359**).
There were no statistical differences for OM, but there were differences in dry matter production between the different cover crops (Table 2). There was also a significant positive correlation of OM with soil cation exchange capacity (0.611**) and BS (0.281*) in the areas studied. According to Loss et al. (2009), the use of mulch improves the crop development, which in turn results in higher inputs of plant waste into the soil surface layer. Once decomposed, the residues raise the soil OM content.
Our results confirmed those of Steiner et al. (2011), who reported no significant differences for OM between the management systems using cover crops (e.g. no-tillage velvet bean and crotalaria) over three study years. Cunha et al. (2011a), studying the influence of different cover crops on soil chemical properties (e.g. crotalaria and pigeon pea), reported no significant differences either, in the soil OM contents (layers 0-0.10 and 0-0.20 m) in two study years.
The low OM contents can be attributed to the short-term use of cover crops (two years), i.e., the period from installation to evaluation of the experiment. The decomposition rate that alters the soil OM contents is very low, thus several years of no-tillage cover crops are required to induce significant changes in this property (Xavier et al., 2006).
No influence of the cover crops on soil pH was observed. However, changes in this property were detected after MAP application at common bean sowing, with differences between the control and the highest MAP rate (0 and 90 kg ha-1 P2O5, respectively). In other words, pH responded negatively to P fertilization (Table 2).
It was not possible to evaluate the effect of cover plants on Al3+ (data not shown), since the pH values in CaCl2 were above 5.0 in all treatments. Under these conditions, Al3+ is usually precipitated as aluminum hydroxide. However, H+Al showed an opposite trend for pH, with significant but negative correlations between these two properties (-0.823**). The same pattern was observed between H+Al and soil BS (-0.712**).
There were significant differences for H+Al and P content in the soil treated with P2O5 rates (Table 2). However, unlike the trend for pH, both chemical variables showed positive responses to P applications, with differences in P soil contents among treatments. Soil CEC and BS were not affected by the cover crops. There was a significant positive correlation between both properties (0.679**), and between BS and soil pH (0.859**).
The P levels were raised minimally by the cover crops. They were different under millet and velvet bean from pigeon pea but were similar to the results under fallow. This shows that the results were more related to P fertilization applied at common bean sowing in the first year of the experiment, than to the preceding cover crops. These results for soil P agree with those of Moreti et al. (2007) who reported no influence of the cover crops millet and crotalaria on the P contents of an Oxisol, when compared with fallow plots.
The behavior of pigeon pea differed from that described by Carvalho & Amabile (2006), who stated that the plant roots exude organic acids that solubilize P, making it available in the soil (Table 1).
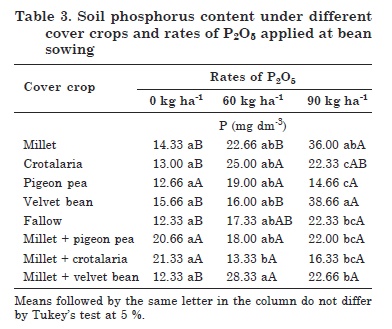
The interaction between cover crops and P rates for soil available P indicated a low contribution of the cover crops to increase P availability in the soil of the phosphate fertilization treatments (Table 3). Highest P levels were found in the velvet bean treatment, followed by millet, by the application of 90 kg ha-1 P2O5.
There were no differences in soil available P between cover crops and fallow when fertilized with 0 (control) and 60 kg ha-1 (Table 3). However, soil P concentrations were highest under velvet bean, followed by millet, but both results were different from the fallow treatment.
A different behavior for crotalaria was observed in this interaction, with a positive response to the application of 60 kg ha-1 P2O5, differing from fallow. Little or no increase in the soil P levels was detected between the treatments 0 and 60 kg ha-1. However, there was an exponential increase in the P levels by the application of 90 kg ha-1 P2O5 (Table 3). This may be related to the fact that the areas with velvet bean were associated with an increase in P levels, due to the amount of applied P. This provided the condition for the development and maintenance of the microbial biomass.
During the decomposition of plant residues, the low content of some nutrients in the soil increases the competition between microorganisms and plants for essential nutrients. These are incorporated into the plant biomass, but returned to the soil after microbial biomass decomposition. Thus, the increase in the soil P content can promote increases in nutrient assimilation and incorporation by the microorganisms. This represents an important energy source for the metabolic activities that can accelerate the decomposition of organic materials in the soil.
In the fallow treatment, P levels increased at higher fertilization rates, differing from the control (Table 3). This may be related to the floristic composition of the treatment, consisting of different plant species, mostly grasses, which may have favored a greater and more rapid decomposition of the crop residues.
Of all cover crop treatments, only millet + velvet bean showed differences in the evaluated property, responding to P fertilization; this intercrop contained the highest average soil P level at both P rates and differed from the control (Table 3).
The fact that the soil P levels did not differ in the other cover crop treatments may be related to greater P absorption and assimilation by the common bean plants, since the soil sampling time coincided with the pod filling stage. This may have contributed to a greater root-shoot translocation of P, and from this region to the sites responsible for grain filling.
Biological properties
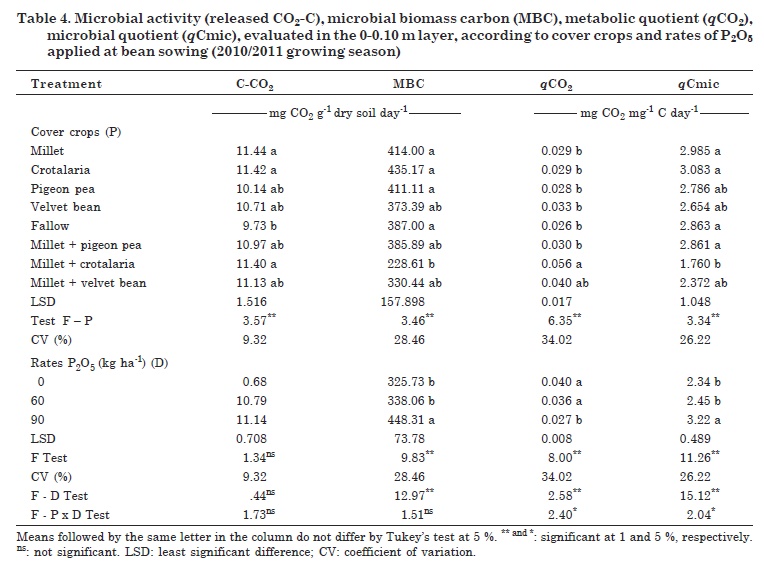
There were significant differences between the cover crop treatments in terms of biological properties (Table 4). Phosphorus application promoted changes in microbial activity, with the exception of CO2-C release, which led to increases in the values of MBC and qCmic and reduction in qCO2. For the interactions cover crop - P rates, differences were found for qCO2 and qCmic.
Only CO2-C release responded to the cover crops, especially to millet, crotalaria and millet + crotalaria, at a 15 % higher rate than in the fallow treatment (Table 4). These results may have been influenced by the fallow flora, composed mostly of grasses, e.g., goosegrass. A significant and positive correlation was observed between CO2-C release and qCO2 (0.233*), OM (0.338**) and P (0.340**), which demonstrates the importance and influence of OM and P contents in the soil microbial activity.
Santos et al. (2004) studied the effect of different management systems, including the use of cover crops in no-tillage systems and native vegetation, on the microbial activity and found no effect of cover crops on CO2-C release (layer 0.05-0.01 m). However, they reported a trend of increased release in soils with natural vegetation, due to the constant residue incorporation. Increases in the amount of organic residues have been reported favoring the OM accumulation, promoting microbial activity, and increasing CO2-C release. This was not observed in the present study, where dry matter production after millet and in the fallow treatment were similar, whereas microbial activity differed.
The cover crops influenced MBC, with lowest and the highest values ranging from 228.61 to 435.17 μg C g-1 dry soil for millet + crotalaria and crotalaria treatments, respectively (Table 4). These results differ from those reported by Fonseca et al. (2007) who investigated cover crops in a corn and bean rotation and observed no effects of the cover plants on MBC.
In a study with irrigated common bean, Silva et al. (2007) compared different cover crops under no-tillage and conventional tillage, and found no influence of cover crops on MBC. These authors also verified differences between the two soil tillage systems. This difference was higher for MBC than for total organic carbon, indicating that MBC is a more sensitive indicator to compare soil tillage. However, low values for MBC, as in the millet + crotalaria intercrop indicate a disturbance in the area or may be related to lower mineralization and slower nutrient release due to the characteristics of the material to be decomposed.
In experiments with common bean after cover crops, Cunha et al. (2011b) reported significant differences for MBC (sampling layer 0-0.10 m). It was observed that pigeon pea and velvet bean plants had a greater influence on MBC than fallow and crotalaria. These results differ from those in the present study (Table 4).
There was no correlation between MBC and OM, possibly because there was no change in OM levels in the cover crop treatments. Similar results were obtained by Silva et al. (2009), who stated that the values were influenced by the soil OM content, behaving as an independent variable. However, these results disagree with Stenberg (1999), who found a close relationship between the amount of MBC and the presence of OM in the soil, i.e., high OM levels indicate high values of MBC, due to the capacity to maintain high decomposition rates.
Changes in MBC values, with a significant increase at the 90 kg P2O5 rate, showed the positive response to P fertilization (Table 4). However, there was no difference between the P2O5 rates 90, 60 or 0 kg ha-1, which may be due to the characteristic effect of the clay soil. This type of soil favors a greater P fixation, and a weaker treatment response.
For qCO2, there were significant differences between millet + crotalaria and the other treatments, except for millet + velvet bean, with qCO2 values ranging from 0.026 to 0.056 mg CO2 mg-1 C day-1 (Table 4). The cover crops had little influence on qCO2, with the exception of millet + crotalaria, which differed from fallow. The highest qCO2 in the millet + crotalaria area may indicate stress or disturbance. Together with the large dry matter production and higher C/N ratio of the intercrop, this resulted in high microbial activity with a consequent increase in carbon release (CO2-C per unit of microbial biomass). There was a significant and negative correlation between qCO2 and MBC (-0.862**).
These results are rather different from those reported by Silva et al. (2007), who studied cover crops in no-tillage and conventional systems and found significant changes in qCO2 in soil under cover crops in rotation with common bean. Similarly, Cunha et al. (2011b) reported that pigeon pea, velvet bean and crotalaria, as cover crops for bean cultivation in no-tillage and conventional systems, had an effect on qCO2. Pigeon pea induced the highest qCO2 values. However, the highest P rate (90 kg ha-1 P2O5), resulted in lower qCO2 values. The variable qCO2 responded negatively to phosphate fertilization (Table 4). These results differed from those reported by Lourente et al. (2011), who evaluated the biological soil properties under no-tillage and conventional systems in the Cerrado region. These authors found a significant and positive correlation between qCO2 and P levels in the soil, indicating that high P levels result in high qCO2 values. Therefore, according to these authors, when P fertilization induces very high P levels in soils, this could cause stress and/or disturbance in agricultural areas.
Lower qCO2 values indicate a more efficient microbial biomass (Gama-Rodrigues, 1999), with less carbon loss (e.g. CO2) through respiration, and significant carbon incorporation into the microbial biomass, resulting in qCO2 decrease (Cunha et al., 2011b). Thus, low qCO2 values indicate stable agro-ecosystems and a better quality of their soil chemical, physical and biological properties.
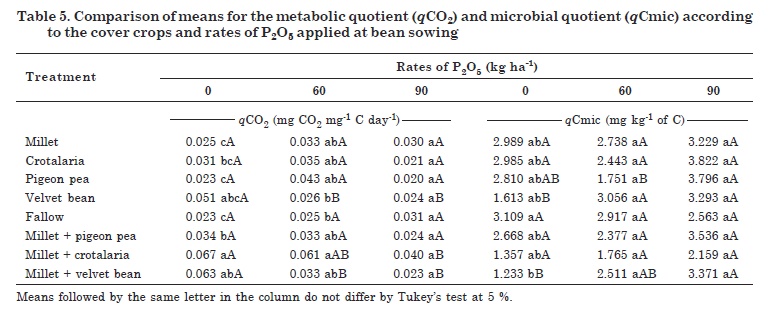
The interactions of cover plants and P rates were significant for qCO2 confirming a negative effect of P fertilization in areas with velvet bean, millet + velvet bean and millet + crotalaria. The performance of millet + crotalaria intercrop was the worst of the cover crops and the response to P application was significant. For the application of 0 and 60 kg ha-1 P2O5, the qCO2 values were lowest in the pigeon pea and fallow treatments. Highest qCO2 values were found in the millet + crotalaria intercrop, characterizing a possible microbial selection in the intercropped areas at 0 and 60 kg ha-1 P2O5 (Table 5).
The qCO2 values in areas with velvet bean, millet + crotalaria and millet + velvet bean differed and responded negatively to the P soil applications (Table 5). This characterizes an ideal and desirable condition with lower qCO2 values, showing a stable system with a more efficient microbial biomass. For millet + crotalaria, the most significant increases were observed at the highest fertilization rate, with a considerable drop in qCO2 by the application of 90 kg ha-1 P2O5. In contrast, the treatments velvet bean and millet + velvet bean showed responses to phosphate fertilization at 60 kg ha-1and higher rates.
For qCmic, there were significant differences between cover crops, P rates and the interaction of cover plants and P rates (Table 4). A significant and positive correlation was observed between qCmic and MBC (0.939**). The variable qCmic, under normal conditions, ranges from 1 to 4 %. Values below 1 % indicate that some factor is limiting the microbial biomass activity (Jakelaitis et al., 2008). In all treatments of this study, qCmic values were higher than 1 % and lower than 4 % (Table 4), indicating a possible influence of cover crops on this property. This could however not be confirmed because the cover crops, except for the intercropped millet + crotalaria, did not differ from the fallow treatment.
The lowest qCmic between treatments was found in the area with millet + crotalaria, characterizing a reduction in levels of microbial carbon, confirmed by the high qCO2 rate in this intercrop (Table 4). The qCmic values in this study differ from those reported by Carneiro et al. (2008), who observed low qCmic in millet and fallow areas, in a study on the effect of cover crops on soil microbial activity under no-tillage.
Phosphate fertilization resulted in increased qCmic rates, and differences were observed at the rate of 90 kg ha-1 P2O5 (Table 4). The results showed the importance of P fertilizer for soil microbial activity, since P can be incorporated in large amounts by the microbial biomass, increasing its activity and efficiency to obtain resources from the system, thus improving soil quality.
For the interactions between cover plants and P rates, qCmic was significant, responding to P application, especially pigeon pea, velvet bean and millet + velvet bean (Table 5). However, the cover crops differed only in the treatment without P application, where fallow differed from intercropped millet + velvet bean. The lower qCmic observed in the millet + velvet bean area may be related to the higher C losses than from the other treatments. This demonstrates the influence, as well as the importance of choosing the right cover crop to improve the soil quality.
The cover crops differed between treatments with or without P application. For pigeon pea, the qCmic differed between the treatments with P application, but not from the control without P. These results did not confirm the tendency observed in the other treatments, where the qCmic rates increased due to phosphate fertilization. These results may be related to the low P levels observed in the areas with pigeon pea (Table 2).
Unlike observed for pigeon pea, in the analysis of velvet bean and millet + velvet bean, qCmic tended to increase in response to P applications (Table 5). Velvet bean showed differences between P fertilized and non-fertilized treatments, with a qCmic increase of approximately 50 % with the application of 60 kg ha-1 P2O5. In the intercropped millet + velvet bean, the increase in qCmic was only possible by the soil application of 90 kg ha-1 P2O5.
According to Sampaio et al. (2008), qCmic is an indicator of OM availability to microorganisms, since an increase in this microbial quotient indicates its greater dynamics in the soil. Velvet bean and the intercropped millet + velvet bean were the plant covers with highest OM input. The influence of P application on qCmic was more evident in the millet + velvet bean area. This crop had the lowest dry matter production, demonstrating that an increase in the amount of soil organic matter only does not necessarily translate into direct gains in soil quality in that area.
CONCLUSIONS
1. After two years, the biomass of cover crops preceding common bean did not affect the soil chemical properties and had little influence on microbial activity. The effects of millet and velvet bean, in monoculture or intercropped, were greatest.
2. The biological variables were sensitive and responded to increasing rates of P fertilizer; higher levels of P induced increases in microbial biomass carbon and reductions in qCO2.
ACKNOWLEDGEMENT
The authors are grateful to CNPq (National Council of Technological and Scientific Development) for the scholarship to the first author and for research awards to the third and fourth authors.
LITERATURE CITED
AMBROSANO, E.J.; WUTKE, E.B.; BULISANI, E.A. & CANTARELLA, H. Feijão. In: RAIJ, B.van; CANTARELLA, H.; QUAGGIO, J.A. & FURLANI, A.M.C., eds. Recomendações de adubação e calagem para o Estado de São Paulo. 2.ed. Campinas, Instituto Agronômico de Campinas, 1997. p.194-195. (Boletim Técnico, 100)
ANDRADE, R.S.; STONE, L.F. & SILVEIRA, P.M. Culturas de cobertura e qualidade física de um Latossolo em plantio direto. R. Bras. Eng. Agríc. Amb., 13:411-418, 2009.
ANDERSON, J.P.E. & DOMSCH, K.H. Ratios of microbial biomass carbon to total organic carbon in arable soils. Soil Biol. Biochem., 21:471-479, 1989.
ANDERSON, T.H. & DOMSCH, K.H. The metabolic quotient for CO2 (qCO2) as a specific activity parameter to assess the effects of environmental conditions, such as pH, on the microbial biomass of forest soils. Soil Biol. Biochem., 25:393-395, 1993.
CARNEIRO, M.A.C.; SOUZA, E.D.; REIS, E.F.; PEREIRA, H.S. & AZEVEDO, W.R. Atributos físicos, químicos e biológicos de solo de Cerrado sob diferentes sistemas de uso e manejo. R. Bras. Ci. Solo, 33:147-157, 2009.
CARNEIRO, M.A.C.; CORDEIRO, M.A.S.; ASSIS, P.C.R.; MORAES, E.S.; PEREIRA, H.S.; PAULINO, H.B. & SOUZA, E.D. Produção de fitomassa de diferentes espécies de cobertura e suas alterações na atividade microbiana de solo de Cerrado. Bragantia, 67:455-462, 2008.
CARNEIRO, R.G.; MENDES, I.C.; LOVATO, P.E.; CARVALHO, A.M. & VIVALDI, L.J. Indicadores biológicos associados ao ciclo de fósforo em solos de Cerrado sob plantio direto e plantio convencional. Pesq. Agropec. Bras., 39:661-669, 2004.
CARVALHO, A.M. & AMABILE, R.F. Plantas condicionadoras de solo: interações edafoclimáticas, uso e manejo. In: CARVALHO, A.M. & AMABILE, R.F., orgs. Cerrado: Adubação verde. Brasília, Embrapa, 2006. p.143-170.
CARVALHO, I.Q.; SILVA, M.J.S.; PISSAIA, A.; PAULETTI, V. & POSSAMAI, J.C. Espécies de cobertura de inverno e nitrogênio na cultura do milho em sistema de plantio direto. Sci. Agric., 8:179-184, 2007.
CARVALHO, M.A.C.; SORATTO, R.P.; ATHAYDE, M.L.F.; ARF, O. & SÁ, M.E. Produtividade do milho em sucessão a adubos verdes. Pesq. Agropec. Bras., 39:47-53, 2004.
COSTA, E.A.; GOEDERT, W.J. & SOUZA, D.M.G. Qualidade de solo submetido a sistemas de cultivo com preparo convencional e plantio direto. Pesq. Agropec. Bras., 41:1185-1191, 2006.
CUNHA, E.Q.; STONE, L.F.; DIDONET, A.D.; FERREIRA, E.P.B.; MOREIRA, J.A.A. & LEANDRO, W.M. Atributos químicos de solo sob produção orgânica influenciados pelo preparo e por plantas de cobertura. R. Bras. Eng. Agríc. Amb., 15:1021-1029, 2011a.
CUNHA, E.Q.; STONE, L.F.; FERREIRA, E.P.B.; DIDONET, A.D.; MOREIRA, J.A.A. & LEANDRO, W.M. Sistema de preparo do solo e culturas de cobertura na produção orgânica de feijão e milho. II - Atributos biológicos do solo. R. Bras. Ci. Solo, 35:603-611, 2011b.
D’ANDRÉA, A.F.; SILVA, M.L.N.; CURI, N.; SIQUEIRA, J.O. & CARNEIRO, M.A.C. Atributos biológicos indicadores da qualidade do solo em sistemas de manejo na região do cerrado no sul do estado de Goiás. R. Bras. Ci. Solo, 26:913-924, 2002.
DORAN, J.W. & PARKIN, T.B. Defining and assessing soil quality. In: DORAN, J.W. & COEMAN, D.C.; BEZDICEK, D.F. & STEWART, B.A., eds. Defining soil quality for sustainable environment. Madison, Soil Science Society of America, 1994. p.3-21. (SSSA Special Publication, 35)
DORAN, J.W. & ZEISS, M.R. Soil health and sustainability: Managing the biotic component of soil quality. Appl. Soil Ecol., 15:3-11, 2000.
EMPRESA BRASILEIRA DE PESQUISA AGROPECUÁRIA - EMBRAPA. Centro Nacional de Pesquisa de Solos. Sistema brasileiro de classificação de solos. Brasília, Embrapa Produção de Informação, 2006. 412 p.
FONSECA, G.C.; CARNEIRO, M.A.C.; COSTA, A.R.; OLIVEIRA, G.C. & BALBINO, L.C. Atributos físicos, químicos e biológicos de Latossolo Vermelho distrófico de Cerrado sob duas rotações de cultura. Pesq. Agropec. Trop., 37:22-30, 2007.
GAMA-RODRIGUES, E.F. Biomassa microbiana e ciclagem de nutrientes. In: SANTOS, G.A. & CAMARGO, F.A.O., eds. Fundamentos da matéria orgânica do solo: Ecossistemas tropicais e subtropicais. Porto Alegre, Gênesis, 1999. p.227-243.
GONÇALVES, A.S.; MONTEIRO, M.T.; GUERRA, J.G.M.; COSTANTINI, A.O. & DE-POLLI, H. Biomassa microbiana em amostras umedecidas após secagem ao ar de solos de toposequência de pastagens. Ci. Suelo, 25:120-129, 2007.
HORNIK, S.B. Factors affecting the nutritional quality of crops. Am. J. Altern. Agric. 7, 63-68, 1992.
JAKELAITIS, A.; SILVA, A.A.; SANTOS, J.B. & VIVIAN, R. Qualidade da camada superficial de solo sob mata, pastagens e áreas cultivadas. Pesq. Agropec. Trop., 38:118- 127, 2008.
LOURENTE, E.R.P.; MERCANTE, F.M.; ALOVISI, A.M.T.; GOMES, C.F.; GASPARINI, A.S. & NUNES, C.M. Atributos microbiológicos, químicos e físicos de solo sob diferentes sistemas de manejo e condições de Cerrado. Pesq. Agropec. Trop., 41:20-28, 2011.
LOSS, A.; PEREIRA, M.G.; TEIXEIRA, M.B.; LIMA, F.M.; OLIVEIRA, A.B. & CRUZ, R.B. Frações orgânicas do solo em áreas sob manejo agroecológico em Capivari, Duque de Caxias, RJ. R. Bras. Ci. Agron., 4:245-251, 2009.
MARSCHNER, H. Mineral nutrition of higher plants. 2. ed. London: Academic, 1995. 889p.
MORETI, D.; ALVES, M.C.; VALÉRIO FILHO, W.V. & CARVALHO, M.P. Atributos químicos de um Latossolo Vermelho sob diferentes sistemas de preparo, adubações e plantas de cobertura. R. Bras. Ci. Solo, 31:167-175, 2007.
PELÁ, A.; SILVA, J.S.; RODRIGUES, E.M. & MELLO, G.P. Plantas de cobertura e adubação com NPK para o milho em plantio direto. Sci. Agric., 11:371-377, 2010.
PERIN, A.; SANTOS, R.H.S.; URQUIAGA, S.; GUERRA, J.G.M. & CECON, P.R. Produção de fitomassa, acúmulo de nutrientes e fixação biológica de nitrogênio por adubos verdes em cultivo isolado e consorciado. Pesq. Agropec. Bras., 39:35-40, 2004.
RAIJ, B.van; ANDRADE, J.C.; CANTARELLA, H. & QUAGGIO, J.A. Análise química para avaliação da fertilidade de solos tropicais. Campinas, Instituto Agronômico, 2001. 285p.
SAMPAIO, D.B.; ARAÚJO, A.S.F. & SANTOS, V.B. Avaliação de indicadores biológicos de qualidade do solo sob sistemas de cultivo convencional e orgânico de frutas. Ci. Agrotec., 32:353-359, 2008.
SANTOS, V.B.; CASTILHOS, D.D.; CASTILHOS, R.M.V.; PAULETTO, E.A.; GOMES, A.S. & SILVA, D.G. Biomassa, atividade microbiana e teores de carbono e nitrogênio totais de um Planossolo sob diferentes sistemas de manejo. R. Agroci., 10:333-338, 2004.
SILVA, L.G.; MENDES, I.C.; REIS JUNIOR, F.B.; FERNANDES, M.F.; MELO, J.T. & KATO, E. Atributos físicos, químicos e biológicos de um Latossolo de Cerrado em plantio de espécies florestais. Pesq. Agropec. Bras., 44:613-620, 2009.
SILVA, M.B.; KLIEMANN, H.J.; SILVEIRA, P.M. & LANNA, A.C. Atributos biológicos do solo sob influência da cobertura vegetal e do sistema de manejo. Pesq. Agropec. Bras., 42:1755-1761, 2007.
SPARLING, G.P. Ratio of microbial biomass carbon to soil organic matter. Aust. J. Soil Res., 30:195-207, 1992.
STEINER, F.; COSTA, M.S.S.M.; COSTA, L.A.M.; PIVETTA, L.A. & CASTOLDI, G. Atributos químicos do solo em diferentes sistemas de culturas e fontes de adubação. Global Sci. Technol., 4:16-28, 2011.
STENBERG, B. Monitoring soil quality of arable land: Microbiological indicators. Soil Plant Sci., 49:1-24, 1999.
SUZUKI, L.E.A.S. & ALVES, M.C. Fitomassa de plantas de cobertura em diferentes sucessões de culturas e sistemas de cultivo. Bragantia, 65:121-127, 2006.
TEIXEIRA, F.C.P.; REINERT, F.; RUMJANEK, N.G. & BODDEY, R.M. Quantification of the contribution of biological nitrogen fixation to Cratylia mollis using the 15N natural abundance technique in the semi-arid caatinga region of Brazil. Soil Biol. Biochem., 38:1989-1993, 2006.
TORRES, J.L.R.; PEREIRA, M.G.; FABIAN, A.J. Produção de fitomassa por plantas de cobertura e mineralização de seus resíduos em plantio direto. Pesq. Agropec. Bras., 43:421-428, 2008.
VANCE, E.D.; BROOKES, P.C. & JENKINSON, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem., 19:773-777, 1987.
VENZKE FILHO, S.; FEIGL, B.J.; PICCOLO, M.C.; SIQUEIRA NETO, M. & CERRI, C.C. Biomassa microbiana do solo em sistema de plantio direto na região de Campos Gerais - Tibagi, PR. R. Bras. Ci. Solo, 32:599-610, 2008.
XAVIER, F.A.S.; MAIA, S.M.F.; OLIVEIRA, T.S. & MENDONÇA, E.S. Biomassa microbiana e matéria orgânica leve em solos sob sistemas agrícolas orgânico e convencional na Chapada da Ibiapaba - CE. R. Bras. Ci. Solo, 30:247-258, 2006.
Received for publication on July 19, 2012 and approved on May 24, 2013.
- AMBROSANO, E.J.; WUTKE, E.B.; BULISANI, E.A. & CANTARELLA, H. Feijão. In: RAIJ, B.van; CANTARELLA, H.; QUAGGIO, J.A. & FURLANI, A.M.C., eds. Recomendações de adubação e calagem para o Estado de São Paulo. 2.ed. Campinas, Instituto Agronômico de Campinas, 1997. p.194-195.
- ANDRADE, R.S.; STONE, L.F. & SILVEIRA, P.M. Culturas de cobertura e qualidade física de um Latossolo em plantio direto. R. Bras. Eng. Agríc. Amb., 13:411-418, 2009.
- ANDERSON, J.P.E. & DOMSCH, K.H. Ratios of microbial biomass carbon to total organic carbon in arable soils. Soil Biol. Biochem., 21:471-479, 1989.
- ANDERSON, T.H. & DOMSCH, K.H. The metabolic quotient for CO2 (qCO2) as a specific activity parameter to assess the effects of environmental conditions, such as pH, on the microbial biomass of forest soils. Soil Biol. Biochem., 25:393-395, 1993.
- CARNEIRO, M.A.C.; SOUZA, E.D.; REIS, E.F.; PEREIRA, H.S. & AZEVEDO, W.R. Atributos físicos, químicos e biológicos de solo de Cerrado sob diferentes sistemas de uso e manejo. R. Bras. Ci. Solo, 33:147-157, 2009.
- CARNEIRO, M.A.C.; CORDEIRO, M.A.S.; ASSIS, P.C.R.; MORAES, E.S.; PEREIRA, H.S.; PAULINO, H.B. & SOUZA, E.D. Produção de fitomassa de diferentes espécies de cobertura e suas alterações na atividade microbiana de solo de Cerrado. Bragantia, 67:455-462, 2008.
- CARNEIRO, R.G.; MENDES, I.C.; LOVATO, P.E.; CARVALHO, A.M. & VIVALDI, L.J. Indicadores biológicos associados ao ciclo de fósforo em solos de Cerrado sob plantio direto e plantio convencional. Pesq. Agropec. Bras., 39:661-669, 2004.
- CARVALHO, A.M. & AMABILE, R.F. Plantas condicionadoras de solo: interações edafoclimáticas, uso e manejo. In: CARVALHO, A.M. & AMABILE, R.F., orgs. Cerrado: Adubação verde. Brasília, Embrapa, 2006. p.143-170.
- CARVALHO, I.Q.; SILVA, M.J.S.; PISSAIA, A.; PAULETTI, V. & POSSAMAI, J.C. Espécies de cobertura de inverno e nitrogênio na cultura do milho em sistema de plantio direto. Sci. Agric., 8:179-184, 2007.
- CARVALHO, M.A.C.; SORATTO, R.P.; ATHAYDE, M.L.F.; ARF, O. & SÁ, M.E. Produtividade do milho em sucessão a adubos verdes. Pesq. Agropec. Bras., 39:47-53, 2004.
- COSTA, E.A.; GOEDERT, W.J. & SOUZA, D.M.G. Qualidade de solo submetido a sistemas de cultivo com preparo convencional e plantio direto. Pesq. Agropec. Bras., 41:1185-1191, 2006.
- CUNHA, E.Q.; STONE, L.F.; DIDONET, A.D.; FERREIRA, E.P.B.; MOREIRA, J.A.A. & LEANDRO, W.M. Atributos químicos de solo sob produção orgânica influenciados pelo preparo e por plantas de cobertura. R. Bras. Eng. Agríc. Amb., 15:1021-1029, 2011a.
- CUNHA, E.Q.; STONE, L.F.; FERREIRA, E.P.B.; DIDONET, A.D.; MOREIRA, J.A.A. & LEANDRO, W.M. Sistema de preparo do solo e culturas de cobertura na produção orgânica de feijão e milho. II - Atributos biológicos do solo. R. Bras. Ci. Solo, 35:603-611, 2011b.
- DORAN, J.W. & PARKIN, T.B. Defining and assessing soil quality. In: DORAN, J.W. & COEMAN, D.C.; BEZDICEK, D.F. & STEWART, B.A., eds. Defining soil quality for sustainable environment. Madison, Soil Science Society of America, 1994. p.3-21.
- DORAN, J.W. & ZEISS, M.R. Soil health and sustainability: Managing the biotic component of soil quality. Appl. Soil Ecol., 15:3-11, 2000.
- EMPRESA BRASILEIRA DE PESQUISA AGROPECUÁRIA - EMBRAPA. Centro Nacional de Pesquisa de Solos. Sistema brasileiro de classificação de solos. Brasília, Embrapa Produção de Informação, 2006. 412 p.
- FONSECA, G.C.; CARNEIRO, M.A.C.; COSTA, A.R.; OLIVEIRA, G.C. & BALBINO, L.C. Atributos físicos, químicos e biológicos de Latossolo Vermelho distrófico de Cerrado sob duas rotações de cultura. Pesq. Agropec. Trop., 37:22-30, 2007.
- GAMA-RODRIGUES, E.F. Biomassa microbiana e ciclagem de nutrientes. In: SANTOS, G.A. & CAMARGO, F.A.O., eds. Fundamentos da matéria orgânica do solo: Ecossistemas tropicais e subtropicais. Porto Alegre, Gênesis, 1999. p.227-243.
- GONÇALVES, A.S.; MONTEIRO, M.T.; GUERRA, J.G.M.; COSTANTINI, A.O. & DE-POLLI, H. Biomassa microbiana em amostras umedecidas após secagem ao ar de solos de toposequência de pastagens. Ci. Suelo, 25:120-129, 2007.
- HORNIK, S.B. Factors affecting the nutritional quality of crops. Am. J. Altern. Agric. 7, 63-68, 1992.
- JAKELAITIS, A.; SILVA, A.A.; SANTOS, J.B. & VIVIAN, R. Qualidade da camada superficial de solo sob mata, pastagens e áreas cultivadas. Pesq. Agropec. Trop., 38:118- 127, 2008.
- LOURENTE, E.R.P.; MERCANTE, F.M.; ALOVISI, A.M.T.; GOMES, C.F.; GASPARINI, A.S. & NUNES, C.M. Atributos microbiológicos, químicos e físicos de solo sob diferentes sistemas de manejo e condições de Cerrado. Pesq. Agropec. Trop., 41:20-28, 2011.
- LOSS, A.; PEREIRA, M.G.; TEIXEIRA, M.B.; LIMA, F.M.; OLIVEIRA, A.B. & CRUZ, R.B. Frações orgânicas do solo em áreas sob manejo agroecológico em Capivari, Duque de Caxias, RJ. R. Bras. Ci. Agron., 4:245-251, 2009.
- MARSCHNER, H. Mineral nutrition of higher plants. 2. ed. London: Academic, 1995. 889p.
- MORETI, D.; ALVES, M.C.; VALÉRIO FILHO, W.V. & CARVALHO, M.P. Atributos químicos de um Latossolo Vermelho sob diferentes sistemas de preparo, adubações e plantas de cobertura. R. Bras. Ci. Solo, 31:167-175, 2007.
- PELÁ, A.; SILVA, J.S.; RODRIGUES, E.M. & MELLO, G.P. Plantas de cobertura e adubação com NPK para o milho em plantio direto. Sci. Agric., 11:371-377, 2010.
- PERIN, A.; SANTOS, R.H.S.; URQUIAGA, S.; GUERRA, J.G.M. & CECON, P.R. Produção de fitomassa, acúmulo de nutrientes e fixação biológica de nitrogênio por adubos verdes em cultivo isolado e consorciado. Pesq. Agropec. Bras., 39:35-40, 2004.
- RAIJ, B.van; ANDRADE, J.C.; CANTARELLA, H. & QUAGGIO, J.A. Análise química para avaliação da fertilidade de solos tropicais. Campinas, Instituto Agronômico, 2001. 285p.
- SAMPAIO, D.B.; ARAÚJO, A.S.F. & SANTOS, V.B. Avaliação de indicadores biológicos de qualidade do solo sob sistemas de cultivo convencional e orgânico de frutas. Ci. Agrotec., 32:353-359, 2008.
- SANTOS, V.B.; CASTILHOS, D.D.; CASTILHOS, R.M.V.; PAULETTO, E.A.; GOMES, A.S. & SILVA, D.G. Biomassa, atividade microbiana e teores de carbono e nitrogênio totais de um Planossolo sob diferentes sistemas de manejo. R. Agroci., 10:333-338, 2004.
- SILVA, L.G.; MENDES, I.C.; REIS JUNIOR, F.B.; FERNANDES, M.F.; MELO, J.T. & KATO, E. Atributos físicos, químicos e biológicos de um Latossolo de Cerrado em plantio de espécies florestais. Pesq. Agropec. Bras., 44:613-620, 2009.
- SILVA, M.B.; KLIEMANN, H.J.; SILVEIRA, P.M. & LANNA, A.C. Atributos biológicos do solo sob influência da cobertura vegetal e do sistema de manejo. Pesq. Agropec. Bras., 42:1755-1761, 2007.
- SPARLING, G.P. Ratio of microbial biomass carbon to soil organic matter. Aust. J. Soil Res., 30:195-207, 1992.
- STEINER, F.; COSTA, M.S.S.M.; COSTA, L.A.M.; PIVETTA, L.A. & CASTOLDI, G. Atributos químicos do solo em diferentes sistemas de culturas e fontes de adubação. Global Sci. Technol., 4:16-28, 2011.
- STENBERG, B. Monitoring soil quality of arable land: Microbiological indicators. Soil Plant Sci., 49:1-24, 1999.
- SUZUKI, L.E.A.S. & ALVES, M.C. Fitomassa de plantas de cobertura em diferentes sucessões de culturas e sistemas de cultivo. Bragantia, 65:121-127, 2006.
- TEIXEIRA, F.C.P.; REINERT, F.; RUMJANEK, N.G. & BODDEY, R.M. Quantification of the contribution of biological nitrogen fixation to Cratylia mollis using the 15N natural abundance technique in the semi-arid caatinga region of Brazil. Soil Biol. Biochem., 38:1989-1993, 2006.
- TORRES, J.L.R.; PEREIRA, M.G.; FABIAN, A.J. Produção de fitomassa por plantas de cobertura e mineralização de seus resíduos em plantio direto. Pesq. Agropec. Bras., 43:421-428, 2008.
- VANCE, E.D.; BROOKES, P.C. & JENKINSON, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem., 19:773-777, 1987.
- VENZKE FILHO, S.; FEIGL, B.J.; PICCOLO, M.C.; SIQUEIRA NETO, M. & CERRI, C.C. Biomassa microbiana do solo em sistema de plantio direto na região de Campos Gerais - Tibagi, PR. R. Bras. Ci. Solo, 32:599-610, 2008.
- XAVIER, F.A.S.; MAIA, S.M.F.; OLIVEIRA, T.S. & MENDONÇA, E.S. Biomassa microbiana e matéria orgânica leve em solos sob sistemas agrícolas orgânico e convencional na Chapada da Ibiapaba - CE. R. Bras. Ci. Solo, 30:247-258, 2006.
Publication Dates
-
Publication in this collection
06 Feb 2014 -
Date of issue
Dec 2013
History
-
Received
19 July 2012 -
Accepted
24 May 2013