Abstracts
Synthetic aluminum-substituted maghemites were characterized by total chemical analysis, powder X-ray diffraction (XRD), Mössbauer spectroscopy (ME), and vibrating sample magnetometry (VSM). The aim was to determine the structural, magnetic, and hyperfine properties of γ-Fe2-xAl xO3 as the Al concentration is varied. The XRD results of the synthetic products were indexed exclusively as maghemite. Increasing Al for Fe substitution decreased the mean crystalline dimension and shifted all diffraction peaks to higher º2θ angles. The a0 dimension of the cubic unit cell decreased with increasing Al according to the equation a o = 0.8385 - 3.63 x 10-5 Al (R²= 0.94). Most Mössbauer spectra were composed of one sextet, but at the highest substitution rate of 142.5 mmol mol-1 Al, both a doublet and sextet were obtained at 300 K. All hyperfine parameters from the sub-spectra were consistent with high-spin Fe3+ (0.2 a 0.7 mms-1) and suggested a strong superparamagnetic component associated with the doublet. The magnetic hyperfine field of the sextets decreased with the amount of Al-substitution [Bhf (T) = 49.751 - 0.1202Al; R² = 0.94] while the linewidth increased linearly. The saturation magnetization also decreased with increasing isomorphous substitution.
isomorphous substitution; hyperfine parameters; spinel; ferromagnetic
Maghemitas sintéticas substituídas com Al foram caracterizadas por meio de análise química total, difratometria de raios-X (DRX), espectroscopia Mössbauer (EM) e magnetometria de amostra vibrante (MAV). Objetivou-se com este trabalho determinar as propriedades estruturais, magnéticas e hiperfinas de γ-Fe2-xAl xO3, conforme a variação da concentração de Al.Os resultados de DRX dos produtos sintéticos foram indexados somente para maghemita. Com o aumento da substituição de Fe por Al, o diâmetro médio do cristalito diminuiu e levou todos os picos de difração para maiores ângulos º2θ. A dimensão a0 da cela unitária cúbica decresceu com o aumento de Al, de acordo com a equação a o = 0,8385 - 3,63 x 10-5 Al (R²= 0,94). A maioria dos espectros Mössbauer foi composta de um sexteto, mas na taxa mais alta de substituição de 142,5 mmol mol-1 Al um doubleto e sexteto foram obtidos a 300 K. Todos os parâmetros hiperfinos do subespectro foram consistentes com o alto spin Fe3+ (0,2 a 0,7 mms-1) e sugeriram um forte componente superparamagnético associado com o dubleto. O campo magnético hiperfino dos sextetos decresceram com a substituição de Al [Bhf (T) = 49,751 -0,1202Al; R² = 0,94], enquanto as larguras da linha aumentaram de forma linear. A magnetização de saturação também diminuiu com o aumento da substituição isomórfica.
substituição isomórfica; parâmetros hiperfinos; espinélio; ferrimagnético
DIVISÃO 2 - PROCESSOS E PROPRIEDADES DO SOLO
COMISSÃO 2.3 - MINERALOGIA DO SOLO
Structural and magnetic characterization of maghemites prepared from Al-substituted magnetites
Caracterização estrutural e magnética de maghemitas preparadas a partir de magnetitas substituídas com Al
Marcelo Augusto BatistaI; Antonio Carlos Saraiva da CostaII; Jerry Marshal BighamIII; Andrea Paesano JuniorIV; Graciele BerndtV; Tadeu Takeyoshi InoueI; Adriele Galeti NonakaVI
IAdjunct Professor, Departament of Agronomy, State University of Maringá - DA/SUM. Av. Colombo, 5790, Bloco J-45. CEP 87020-900 Maringá (PR), Brazil. E-mail: mabatista@uem.br, ttinoue@uem.br
IIAssociate Professor, DA/SUM. E-mail: antoniocscosta@gmail.com
IIIFaculty Emeritus, School of Environment and Natural Resources, 2021 Coffey Road, Ohio State University, Columbus, OH 43210, USA. E-mail: bigham1@osu.edu
IVFull Professor, Departament of Physic, SUM - DP/SUM. E-mail: paesano@wnet.com.br
VDoctoral student, DP/SUM. E-mail:graberndt@gmail.com
VIMaster student in Agronomy, DA/SUM. E-mail: adriele_gn@hotmail.com
SUMMARY
Synthetic aluminum-substituted maghemites were characterized by total chemical analysis, powder X-ray diffraction (XRD), Mössbauer spectroscopy (ME), and vibrating sample magnetometry (VSM). The aim was to determine the structural, magnetic, and hyperfine properties of γ-Fe2-xAlxO3 as the Al concentration is varied. The XRD results of the synthetic products were indexed exclusively as maghemite. Increasing Al for Fe substitution decreased the mean crystalline dimension and shifted all diffraction peaks to higher o2θ angles. The a0 dimension of the cubic unit cell decreased with increasing Al according to the equation ao = 0.8385 - 3.63 x 10-5 Al (R2= 0.94). Most Mössbauer spectra were composed of one sextet, but at the highest substitution rate of 142.5 mmol mol-1 Al, both a doublet and sextet were obtained at 300 K. All hyperfine parameters from the sub-spectra were consistent with high-spin Fe3+ (0.2 a 0.7 mms-1) and suggested a strong superparamagnetic component associated with the doublet. The magnetic hyperfine field of the sextets decreased with the amount of Al-substitution [Bhf (T) = 49.751 - 0.1202Al; R2 = 0.94] while the linewidth increased linearly. The saturation magnetization also decreased with increasing isomorphous substitution.
Index terms: isomorphous substitution, hyperfine parameters, spinel, ferromagnetic.
RESUMO
Maghemitas sintéticas substituídas com Al foram caracterizadas por meio de análise química total, difratometria de raios-X (DRX), espectroscopia Mössbauer (EM) e magnetometria de amostra vibrante (MAV). Objetivou-se com este trabalho determinar as propriedades estruturais, magnéticas e hiperfinas de γ-Fe2-xAlxO3, conforme a variação da concentração de Al.Os resultados de DRX dos produtos sintéticos foram indexados somente para maghemita. Com o aumento da substituição de Fe por Al, o diâmetro médio do cristalito diminuiu e levou todos os picos de difração para maiores ângulos o2θ. A dimensão a0 da cela unitária cúbica decresceu com o aumento de Al, de acordo com a equação ao = 0,8385 - 3,63 x 10-5 Al (R2= 0,94). A maioria dos espectros Mössbauer foi composta de um sexteto, mas na taxa mais alta de substituição de 142,5 mmol mol-1 Al um doubleto e sexteto foram obtidos a 300 K. Todos os parâmetros hiperfinos do subespectro foram consistentes com o alto spin Fe3+ (0,2 a 0,7 mms-1) e sugeriram um forte componente superparamagnético associado com o dubleto. O campo magnético hiperfino dos sextetos decresceram com a substituição de Al [Bhf (T) = 49,751 -0,1202Al; R2 = 0,94], enquanto as larguras da linha aumentaram de forma linear. A magnetização de saturação também diminuiu com o aumento da substituição isomórfica.
Termos de indexação: substituição isomórfica, parâmetros hiperfinos, espinélio, ferrimagnético.
INTRODUCTION
The ferrimagnetic mineral maghemite (γ-Fe2O3) is closely related with metal availability and P adsorption capacity. Magnetization is spontaneous in a significant percentage of Brazilian soils. In the State of Paraná (Brazil) the area of magnetic soils represents nearly 50 % (Costa et al., 1999).
Magnetic soils are common in many parts of the world. These soils show spontaneous magnetization of more than 1 J T-1 kg-1 and, as a result, are attracted to a hand magnet. The minerals that are responsible for this characteristic are magnetite (Fe3O4) and, especially, fine-grained maghemite (γ-Fe2O3). In Brazil, magnetic soils comprise up to 5% of the total land area (Resende et al., 1986) and soil magnetic properties have been utilized in a variety of earth science studies, such as soil genesis, erosion, pollution (Chen et al., 2005), metal availability (Roy & Bhattacharya, 2012), and P adsorption capacity (Vilar et al., 2010). Electromagnetic and magnetic sensors are also frequently used for detection of buried objects (military ordnance, pipelines, etc), but their performance is often compromised in magnetic soils. Hence, it is important for both academic and practical reasons to understand the origin of variations in magnetic soil properties.
Recently, many studies have investigated natural and synthetic maghemites because of their importance in magnetic soils (Souza Junior et al., 2010; Silva et al., 2010; Batista et al., 2010; Batista et al., 2011). Maghemite (γ-Fe2O3 or Fe8/3o1/3O4) is a cation-deficient spinel with Fe3+ ions distributed among both tetrahedral (A) and octahedral (B) sites. The number of B sites is twice that of A. One-third of the total cation sites are vacant (o), and the distribution of these vacancies is some what controversial, as they are reported to occur either exclusively at B sites, or at both A and B sites (Armstrong et al., 1966). Both tetragonal and cubic structures are possible, depending on whether or not all vacancies are at the B sites and ordered (Armstrong et al., 1966).
Pure maghemites are rarely, if ever, found in nature. Isomorphic substitution (IS) of Fe by different elements is common, but the effects of solid solution on the structural and magnetic properties of maghemite are difficult to define in natural samples, where the concentration and physical parameters of the magnetic fraction are seldom known with accuracy. In synthetic samples, these conditions can be controlled (van Oorschot & Dekkersa, 1999).
A variety of techniques is available to analyze iron minerals. In the last three decades, Mössbauer spectroscopy (MS) has become an increasingly important analytical tool for the characterization of synthetic and natural Fe-containing materials, and one of the most rapidly growing application areas of this technique is undoubtedly that of soils and sediments (Ferreira et al., 2003). In soils, Al commonly replaces part of the Fe in maghemite (Schwertmann & Fetcher, 1984). The study of Al-maghemites by MS has been difficult because Al can, in principle, occupy both A and B cation sites, or can show a preference for one over the other (Wolska & Schwertmann, 1989; Bowen et al., 1994). In this work, we characterized aluminum-substituted maghemites synthesized by thermal alteration of co-precipitated Fe/Al magnetites. The aim was to determine the structural, magnetic, and hyperfine properties of γ-Fe2-xAlxO3 as the Al concentration is varied.
MATERIAL AND METHODS
Synthesis method
Precursor magnetites were synthesized following the Schwertmann & Cornell (1991) procedure with some modifications. Aqueous solutions of FeSO4 and Al2(SO4)3 were mixed in different ratios to attain nominal replacement values of Al3+ for Fe3+, ranging from 0.0 to 300.0 mmol mol-1 in the general chemical formula γ-Fe2"xAlxO3. The reaction was conducted at 90 oC under N2 atmospheric conditions. With the addition of an alkali (KOH 1 mol L-1) to the solution, a bluish material precipitated, which evolved to a dark powder (magnetite) in less than 1 h and was easily attracted to a hand magnet. All synthetic materials were washed free of salts with deionized water, frozen under liquid N2, and freeze-dried. The magnetites were transformed to maghemites by heating at 250 oC for 4 h in a forced draft oven. In order to purify the maghemites, poorly crystalline materials were selectively removed one by one, in a 4-h treatment with acid (pH 3.0) ammonium-oxalate (2.0 mol L-1) in the dark, using a sample-to-solution ratio of 1:1000, according to the procedure described in McKeague & Day (1966).
Characterization
The chemical composition of the powdered materials was verified after dissolution with hot (75 oC), concentrated (1:1 v/v) H2SO4. Iron and Al in solution were determined using inductively coupled plasma mass spectrometry (ICP-MS). Powder XRD analysis was carried out on a Shimadzu D6000 equipment, using a Cu-Kα (λ =0.15418 nm) beam and Ni filter. The XRD was conducted with scanning steps of 0.02o (2θ), with 0.6 s for the time of pulse accumulation. The X-ray diffraction patterns were used to evaluate the unit cell parameter, a0, and the mean crystal diameter size (MCD), d, was calculated based on Scherrer’s formula. Mössbauer spectroscopy (MS) in transmission geometry was performed using a conventional Mössbauer spectrometer in constant acceleration mode. The λ -rays were provided by a 57Co (Rh) source. The Mössbauer spectra were analyzed using a non-linear, least-square routine with Lorentzian line shapes and a fixed linewidth (γ). All isomer shift (IS) data given are relative to α-Fe throughout this paper. Magnetization measurements were performed using a vibrating sample magnetometer (VSM) at room temperature. The Vibrating Sample Magnetometer (VSM) provides hysteresis loops for the samples, from which parameters such as coercivity, coercivity of remanence, and saturation magnetization can be determined.
RESULTS AND DISCUSSION
It was possible to synthesize maghemites with different degrees of isomorphous substitution (IS) ranging from 0-142.5 mmol mol-1. However, the IS values observed in the synthesized Al-maghemites were lower than expected if Al was completely miscible with Fe in the maghemite structure (Table 1). The difference between expected and observed values must be associated with the properties of Al, since Batista et al. (2008), studying synthetic Zn-substituted maghemites and using the same synthesis conditions, observed variations smaller than 2% between the expected and observed IS values. Another possibility to explain the differences is the occupancy of site A and B. Costa et al. (2007), working with Zn2+ substituted magnetites, observed Zn2+ substitution only at the A cation sites. On the other hand, some researchers working with Al-substituted maghemites (Wolska & Schwertmann, 1989; Costa et al., 1995) have observed Al mainly in the B sites. Moreover, the synthesis methodology used in this study was first described for pure magnetite synthesis (Schwertmann & Cornell, 1991).
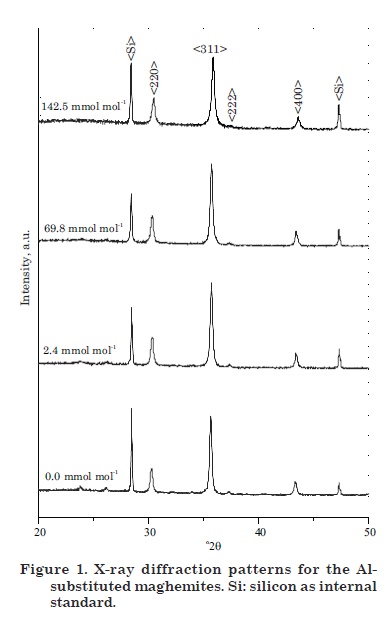
Figure 1 presents the X-ray diffraction patterns for selected samples. Only maghemite diffraction lines were observed (i.e., no impurities were detected). The diffractograms could be indexed to a cubic spinel structure similar to that obtained from the Joint Committee for Powder Diffraction Standards (JCPDS - databasecard # 39-1.346). The diffraction peaks moved to higher 2θ angles with increasing Al-substitution indicating a systematic decrease of the a0 unit cell parameter. This behavior was observed for the six most intense diffraction lines and was related to the fact that Fe3+ has a larger ionic radius (r = 0.064 nm) than Al3+ (r = 0.053 nm). Schwertmann & Fechter (1984) observed that maghemites from highly weathered soils with a high degree of Fe replacement by Al had significantly lower unit cell dimensions than pure maghemites. In addition, a decrease in peak intensities and an increase in full width at half maximum (FWHM) of diagnostic diffraction peaks were observed. These alterations were basically related to a decrease in the crystal size of the maghemites caused by Al entrance into the mineral structure.
Figure 2(a) shows the variation of the mean lattice parameter, a0, calculated and averaged from the six most intense peaks, as a function of the Al content. The mean unit cell parameter varied from 0.8357 nm, close to the a0 value of JCPDS card # 39-1.346 for pure maghemite, to 0.8306 nm for maghemite containing 142.5 mmol mol-1 Al. Schwertmann & Fechter (1984) observed the same negative correlation using natural samples. A detailed inspection of the diffractograms in figure 1 reveals, in addition, changes in the widths of the diffraction peaks with increasing IS. The mean crystalline diameter, calculated from the FWHM of each diffraction peak, decreased linearly with increasing Al for Fe substitution (Pereira et al., 1999; Campbell et al., 2000) (Figure 2b).
Figure 3 shows the room temperature (RT) Mössbauer spectra from end-member samples. For maghemites with 0.0 up to 118.8 mmol mol-1 Al, the best spectral fits were obtained using one discrete sextet (Figure 3a, Table 2). At 142.5 mmol mol-1 Al, the optimum statistical fit required the application of both a sextet and a doublet (Figure 3b). In this specimen, approximately 32 % of the spectral area was attributed to the doublet (Table 2). The hyperfine parameters for both the sextet and doublet were consistent with high-spin Fe3+, and the lack of spectral evidence for Fe2+ or Fe with mixed charge (Fe2+/3+) excluded the presence of residual magnetite in all samples. Mössbauer doublets associated with Fe3+ usually represent either a paramagnetic mineral behaviour, or superparamagnetic iron oxides with very small particle size (Silva et al., 2005). In the present case, superparamagnetism is probably the best explanation for the appearance of a doublet in the sample containing 142.5 mmol mol-1 Al (Figure 3b). The linewidths (γ) of the Mössbauer spectra tended to increase across all samples with increasing Al-substitution (Figure 4a) in a manner similar to that observed with X-ray diffraction peak widths (data not shown). These increases in peak widths indicate reduced crystallinity of the maghemite samples caused by Al substitution.
The Mössbauer data obtained by Costa et al. (1994) at 295 K from aluminous magnetites prepared by different methods (IS =0.32 mms-1 and Bhf = 45-52 T) were similar to the hyperfine parameters from samples in this study (Table 2). Murad & Johnston (1987) observed that increasing Al in the crystalline structure of maghemite decreased Bhf, because of the replacement of ferrimagnetic Fe by paramagnetic Al. Overall, the magnetic hyperfine field values (Bhf) obtained in this study also decreased with increasing isomorphous substitution, except at Al-substitution rates of <20 mmol mol-1 where the Bhf values appeared to increase (Figure 4b). Batista et al. (2010) observed that the mass-specific magnetic susceptibility of these samples also increased with IS. Jesus Filho et al. (1993) calculated the magnetization of synthetic Al- substituted maghemites and concluded that when substitution occurred in the A sites in a collinear condition, the magnetization of the sample would increase considerably. In other cases, where IS occurred in the B sites with collinear configuration or spin canting, the magnetization values should decrease linearly. These observations suggest that the initial increments of Al substituted for Fe in maghemite tend to occupy the A sites, whereas, later increments replace Fe in the octahedral sites, thereby reducing Bhf. Wolska & Schwertmann (1989) also affirmed that maghemite can accommodate up to 2 mol% Al in the A site. According to the results from this study (Figure 6), the maximum Al substitution in maghemite would be approximately 18.3 mol % [Bhf = 49.56 + 0.013 Al - 3.56 x 10-4 Al2 (r2 = 0.98)], which is close to the value estimated by Wolska & Schwertmann (1989).
The magnetization vs. applied field curves obtained for all studied samples are shown in figure 5. These curves are characteristic of systems exhibiting ferrimagnetic behavior. When the magnetizations at 10 kOe are plotted as a function of Al content (Figure 6), an overall decrease in the magnetization was observed as a consequence of the replacement of ferrimagnetic Fe3+ by paramagnetic Al3+ (Batista et al., 2008). However, when the plot is expanded and the first four points are observed in detail there is clearly an increase in magnetization followed by a decrease at higher substitution levels (M = 65.12 + 0.213 Al - 0.0144 Al2, r2 = 0.99). The maximum magnetization occurred with 7.4 mmol mol-1 Al. The same behavior was observed in figure 4(a) with respect to the Bhf data, and in Batista et al. (2010) using mass-specific magnetic susceptibility measurements.
CONCLUSIONS
1. All synthetic samples were identified as maghemites. With the increase of IS the a parameter, MCD, Bhf and magnetization decreases. On the other hand, with the increase of IS an increase of linewidth was observed.
2. The sextets obtained were attributed to maghemite and the doublet was attributed to a small maghemite with superparamagnetic behaviour due to the high value of isomorphous substitution.
3. At low levels of isomorphous substitution, the magnetic behavior (Bhf and magnetization) of the sample increases, while at high levels of the isomorphous substitution the magnetic behavior (Bhf and magnetization) decreases.
LITERATURE CITED
ARMSTRONG, J.R.; MORRISH, A.H. & SAWATZKY, G.A. Mössbauer study of ferric ions in the tetrahedral and octahedral sites of a spinel. Phys. Lett., 23:414-416, 1966.
BATISTA, M.A.; COSTA, A.C.S.; BIGHAM, J.M.; SOUZA JUNIOR, L.G. & JONES, F.S. Acid dissolution kinetics of synthetic aluminum-substituted maghemites (γ-Fe2-xAlxO3). Soil Sci. Soc. Am. J., 75:855-861, 2011.
BATISTA, M.A.; COSTA, A.C.S.; BIGHAM, J.M.; De SANTANA H.; ZAIA, D.A.M. & SOUZA JUNIOR I.G. Mineralogical, chemical, and physical characterization of Al-substituted maghemites (γ-Fe2-xAlxO3). Clays Clay Miner., 58:451-461, 2010.
BATISTA, M.A.; COSTA, A.C.S.; BIGHAM, J.M. & SOUZA JUNIOR, I.G.Crystallochemical characterization of synthetic Zn-substituted maghemites (γ-Fe2-xZnxO3). R. Bras. Ci. Solo, 32:561, 2008.
BOWEN, L.H.; De GRAVE, E. & BRYAN, A.M. Mössbauer studies in an external field of well-crystallized Al-maghemites made from hematite. Hyperf. Interact., 94:1977-1982, 1994.
CAMPBELL, S.J.; KACZMAREK, W.A. & HOFFMANN, M. Mössbauer insight to metallurgy; materials science and engineering. Hyperf. Interact., 126: 175, 2000.
CHEN, T.; XU, H.; XIE, Q.; CHEN, J.; JI, J. & LU, H. Characteristics and genesis of maghemite in Chinese loess and paleosols: mechanism for magnetic susceptibility enhancement in paleosols. Earth Planet. Sci. Lett., 240:790-802, 2005.
COSTA, A.C.S.; SOUZA JUNIOR, I.G.; BATISTA, M.A.; SILVA, K.L.; BELLINI, J.V. & PAESANO JUNIOR, A. Structural, magnetic and hyperfine characterization of zinc-substituted magnetites. Hyperf. Interact., 175:103-111, 2007.
COSTA, G.M.; De GRAVE, E.; BOWEN, L.H.; De BAKKER P.M.A. & VANDENBERGHE, L.H. Variable-temperature Moessbauer spectroscopy of nano-sized maghemite and Al-substituted maghemites. Clays Clay Miner.,43:562-568, 1995.
COSTA, G.M.; De GRAVE, E.; VANDENBERCHE, R.E.; BOWEN, L.H. & De BAKKER, P.M.A.; The center shift in Mössbauer spectra of maghemite and aluminum maghemites. Clays Clay Miner.,42:628-633, 1994.
FERREIRA, B.A.; FABRIS, J.D.; SANTANA, D.P. & CURI, N. Óxidos de ferro das frações areia e silte de um Nitossolo desenvolvido de basalto. R. Bras. Ci. Solo, 27:405-413, 2003.
JESUS FILHO, M.F.; Da NOVA MUSSEL, W.; QI, Q. & COEY, J.M.D. Magnetic properties of aluminum-doped γ-Fe2O3. In: INTERNATIONAL CONFERENCE FERRITES, 6., 1993, Tokyo. Proceedings…Tokyo, 1993. p.126-128.
McKEAGUE, J.A. & DAY, J.H. Dithionite and oxalate-extractable Fe and Al as aids in differentiating various classes of soils. Can. J. Soil Sci., 46:13-22, 1966.
MURAD, E. & JOHNSTON, J.H.Iron oxides and oxyhydroxides. In: LONG, G.I., ed. Mössbauer spectroscopy applied to inorganic chemistry. New York, Spring Street, 1987. v.2. p.507.
PEREIRA, S.L.; PFANNES, H.D.; MENDES FILHO, A.A.; MIRANDA PINTO, L.C.B. & CHÍCARO, M.A. A comparative study of Ni, Zn ferrites modified by the addition of cobalt. Mat. Res., 2:231-234, 1999.
RESENDE, M.; COEY, J.M.D. & ALLAN, J. The magnetic soils of Brazil. Earth Planet. Sci. Lett., 78:322-326, 1986.
ROY, A. & BHATTACHARYA, J. Removal of Cu(II), Zn(II) and Pb(II) from water using microwave-assisted synthesized maghemite nanotubes. Chem. Eng. J., 211/212:493-500, 2012.
SCHWERTMANN, U. & CORNELL, R.M. Iron oxides in the laboratory. Preparation and characterization. New York, Verlag Chemie, 1991.
SCHWERTMANN, U. & FECHTER, H. The influence of aluminum on iron oxides. XI. Aluminum-substituted maghemite in soils and its formation. Soil Sci. Soc. Am. J., 48:1462-1463, 1984.
SILVA, F.D.; COUCEIRO, P.R.C.; FABRIS, J.D.; GOULART, A.T. & KER, J.C. Magnesioferrite and pedogenetic transformation pathway of magnetic iron oxides in two soil profiles developing on tuffite of the Alto Paranaíba region, State of Minas Gerais, Brazil. R. Bras. Ci. Solo, 29:763-775, 2005.
SILVA, A.R.; SOUZA JUNIOR, I.G. & COSTA, A.C.S. Suscetibilidade magnética do horizonte B de solos do Estado do Paraná. R. Bras. Ci. Solo, 34:329-337, 2010.
SOUZA JUNIOR, I.G.; COSTA, A.C.S.; VILAR, C.C. & HOEPERS, A. Mineralogia e suscetibilidade magnética dos óxidos de ferro do horizonte B de solos do Estado do Paraná. Ci. Rural, 40:513-519, 2010.
van OORSCHOT, I.H.M. & DEKKERSA, M.J. Dissolution behaviour of fine-grained magnetite and maghemite in the citrate-bicarbonate-dithionite extraction method. Earth Planet. Sci. Lett., 167:283-295, 1999.
VILAR, C.C.; COSTA, A.C.S.; HOEPERS, A.& SOUZA JUNIOR, I.G. Capacidade máxima de adsorção de fósforo relacionada a formas de ferro e alumínio em solos subtropicais. R. Bras. Ci. Solo, 34:1059-1068, 2010.
WOLSKA, E. & SCHWERTMANN, U. The vacancy ordering and distribution of aluminum ions in γ-(Fe,Al)2O3. Solid State Ionics, 32/33:214-218, 1989.
Received for publication on April 30, 2013 and approved on August 30, 2013.
- ARMSTRONG, J.R.; MORRISH, A.H. & SAWATZKY, G.A. Mössbauer study of ferric ions in the tetrahedral and octahedral sites of a spinel. Phys. Lett., 23:414-416, 1966.
- BOWEN, L.H.; De GRAVE, E. & BRYAN, A.M. Mössbauer studies in an external field of well-crystallized Al-maghemites made from hematite. Hyperf. Interact., 94:1977-1982, 1994.
- CAMPBELL, S.J.; KACZMAREK, W.A. & HOFFMANN, M. Mössbauer insight to metallurgy; materials science and engineering. Hyperf. Interact., 126: 175, 2000.
- CHEN, T.; XU, H.; XIE, Q.; CHEN, J.; JI, J. & LU, H. Characteristics and genesis of maghemite in Chinese loess and paleosols: mechanism for magnetic susceptibility enhancement in paleosols. Earth Planet. Sci. Lett., 240:790-802, 2005.
- COSTA, A.C.S.; SOUZA JUNIOR, I.G.; BATISTA, M.A.; SILVA, K.L.; BELLINI, J.V. & PAESANO JUNIOR, A. Structural, magnetic and hyperfine characterization of zinc-substituted magnetites. Hyperf. Interact., 175:103-111, 2007.
- COSTA, G.M.; De GRAVE, E.; BOWEN, L.H.; De BAKKER P.M.A. & VANDENBERGHE, L.H. Variable-temperature Moessbauer spectroscopy of nano-sized maghemite and Al-substituted maghemites. Clays Clay Miner.,43:562-568, 1995.
- COSTA, G.M.; De GRAVE, E.; VANDENBERCHE, R.E.; BOWEN, L.H. & De BAKKER, P.M.A.; The center shift in Mössbauer spectra of maghemite and aluminum maghemites. Clays Clay Miner.,42:628-633, 1994.
- FERREIRA, B.A.; FABRIS, J.D.; SANTANA, D.P. & CURI, N. Óxidos de ferro das frações areia e silte de um Nitossolo desenvolvido de basalto. R. Bras. Ci. Solo, 27:405-413, 2003.
- McKEAGUE, J.A. & DAY, J.H. Dithionite and oxalate-extractable Fe and Al as aids in differentiating various classes of soils. Can. J. Soil Sci., 46:13-22, 1966.
- MURAD, E. & JOHNSTON, J.H.Iron oxides and oxyhydroxides. In: LONG, G.I., ed. Mössbauer spectroscopy applied to inorganic chemistry. New York, Spring Street, 1987. v.2. p.507.
- PEREIRA, S.L.; PFANNES, H.D.; MENDES FILHO, A.A.; MIRANDA PINTO, L.C.B. & CHÍCARO, M.A. A comparative study of Ni, Zn ferrites modified by the addition of cobalt. Mat. Res., 2:231-234, 1999.
- RESENDE, M.; COEY, J.M.D. & ALLAN, J. The magnetic soils of Brazil. Earth Planet. Sci. Lett., 78:322-326, 1986.
- ROY, A. & BHATTACHARYA, J. Removal of Cu(II), Zn(II) and Pb(II) from water using microwave-assisted synthesized maghemite nanotubes. Chem. Eng. J., 211/212:493-500, 2012.
- SCHWERTMANN, U. & CORNELL, R.M. Iron oxides in the laboratory. Preparation and characterization. New York, Verlag Chemie, 1991.
- SCHWERTMANN, U. & FECHTER, H. The influence of aluminum on iron oxides. XI. Aluminum-substituted maghemite in soils and its formation. Soil Sci. Soc. Am. J., 48:1462-1463, 1984.
- SILVA, F.D.; COUCEIRO, P.R.C.; FABRIS, J.D.; GOULART, A.T. & KER, J.C. Magnesioferrite and pedogenetic transformation pathway of magnetic iron oxides in two soil profiles developing on tuffite of the Alto Paranaíba region, State of Minas Gerais, Brazil. R. Bras. Ci. Solo, 29:763-775, 2005.
- SILVA, A.R.; SOUZA JUNIOR, I.G. & COSTA, A.C.S. Suscetibilidade magnética do horizonte B de solos do Estado do Paraná. R. Bras. Ci. Solo, 34:329-337, 2010.
- SOUZA JUNIOR, I.G.; COSTA, A.C.S.; VILAR, C.C. & HOEPERS, A. Mineralogia e suscetibilidade magnética dos óxidos de ferro do horizonte B de solos do Estado do Paraná. Ci. Rural, 40:513-519, 2010.
- van OORSCHOT, I.H.M. & DEKKERSA, M.J. Dissolution behaviour of fine-grained magnetite and maghemite in the citrate-bicarbonate-dithionite extraction method. Earth Planet. Sci. Lett., 167:283-295, 1999.
- VILAR, C.C.; COSTA, A.C.S.; HOEPERS, A.& SOUZA JUNIOR, I.G. Capacidade máxima de adsorção de fósforo relacionada a formas de ferro e alumínio em solos subtropicais. R. Bras. Ci. Solo, 34:1059-1068, 2010.
Publication Dates
-
Publication in this collection
06 Feb 2014 -
Date of issue
Dec 2013
History
-
Received
30 Apr 2013 -
Accepted
30 Aug 2013