Abstracts
Many forested areas have been converted to intensive agricultural use to satisfy food, fiber, and forage production for a growing world population. There is great interest in evaluating forest conversion to cultivated land because this conversion adversely affects several soil properties. We examined soil microbial, physical, and chemical properties in an Oxisol (Latossolo Vermelho distrófico) of southern Brazil 24 years after forest conversion to a perennial crop with coffee or annual grain crops (maize and soybeans) in conventional tillage or no-tillage. One goal was to determine which soil quality parameters seemed most sensitive to change. A second goal was to test the hypothesis that no-tillage optimized preservation of soil quality indicators in annual cropping systems on converted land. Land use significantly affected microbial biomass and its activity, C and N mineralization, and aggregate stability by depth. Cultivated sites had lower microbial biomass and mineralizable C and N than a forest used as control. The forest and no-tillage sites had higher microbial biomass and mineralizable C and N than the conventional tillage site, and the metabolic quotient was 65 and 43 % lower, respectively. Multivariate analysis of soil microbial properties showed a clear separation among treatments, displaying a gradient from conventional tillage to forest. Although the soil at the coffee site was less disturbed and had a high organic C content, the microbial activity was low, probably due to greater soil acidity and Al toxicity. Under annual cropping, microbial activity in no-tillage was double that of the conventional tillage management. The greater microbial activity in forest and no-tillage sites may be attributed, at least partially, to lower soil disturbance. Reducing soil disturbance is important for soil C sequestration and microbial activity, although control of soil pH and Al toxicity are also essential to maintain the soil microbial activity high.
land use; tillage; coffee; biological activity; potential mineralization; aggregate stability
Muitas áreas de mata nativas têm sido convertidas para o uso agrícola intensivo para satisfazer a produção de alimentos, fibras e forragem em razão do crescimento da população mundial. Assim, existe grande interesse em avaliar o efeito da conversão de florestas em áreas de cultivo agrícola, porque essa conversão interfere em várias propriedades do solo. Foram estudados indicadores microbiológicos, químicos e físicos em um Latossolo Vermelho distrófico (LVd) no sul do Brasil 24 anos após a conversão da mata nativa em área com cultura perene (cafeeiro) ou culturas anuais de grãos (milho/soja) em preparo convencional (PC) ou plantio direto (PD). O tipo de uso do solo influenciou significativamente a biomassa microbiana e sua atividade, a mineralização do C e N e a estabilidade de agregados por causa da profundidade do solo. Áreas cultivadas apresentaram menor biomassa microbiana e mineralização do C e N do que a área de controle (Mata Nativa). Apesar de a mata nativa e de o PD terem apresentado maior biomassa microbiana e mineralização do C e N do que o PC, o quociente metabólico foi de 65 e 43 % inferiores, respectivamente. A análise multivariada das variáveis microbiológicas do solo evidenciou clara separação entre os tratamentos, apresentando um gradiente do tratamento PC para a mata nativa. Apesar de o solo no cafeeiro não ser revolvido e apresentar alto teor de C orgânico demonstrou baixa atividade microbiana, provavelmente por causa da maior acidez e toxidez de Al do solo. A atividade microbiana no PD foi o dobro da observada no PC. A maior atividade microbiana na área da mata e de PD pode ser atribuída, pelo menos parcialmente, à maior estabilidade de agregados. A redução do revolvimento do solo é importante para o incremento do sequestro de C e da atividade microbiana; entretanto, o controle do pH e o da toxidez por Al do solo também são importantes para manter a atividade microbiana do solo elevada.
uso do solo; preparo do solo; cafeeiro; atividade biológica; mineralização potencial; estabilidade de agregados
INTRODUCTION
An increasing world population demands more food, fiber, and forage production. To satisfy this need, many native vegetation areas have been converted to intensive cultivation. Of the more than 350 million hectares used for agriculture in Brazil, 50 % are pastures. There are also extensive areas, used for decades for perennial crops such as coffee (Coffea arabica L.) (about 2.4 million hectares) or annual crops such as maize (Zea mays L.) (9.0 million hectares) or soybean (Glycine max L.) (22.9 million hectares) (IBGE, 2014Instituto Brasileiro de Geografia e Estatística - IBGE. [Accessed: Jul 20, 2014]. Available: http://www.ibge.gov.br.
http://www.ibge.gov.br...
).
Forest conversion to cultivated land leads to several changes in soil properties. Intense management promotes soil erosion and depletes organic matter, which decreases soil quality. Conventional or plow tillage (CT), uses traditional practices of plowing and disking to prepare the land for sowing. Tillage alters the soil structural stability (Six et al., 2004Six J, Bossuyt H, Degryze S, Denef K. A history of research on the link between (micro) aggregates, soil biota, and soil organic matter dynamics. Soil Till Res. 2004;79:7-31.;Caesar-Tonthat et al., 2011Caesar-Tonthat TC, Sinju UM, Wright SF, Shelver WL, Kolberg R, West M. Long-term tillage and cropping effects on microbiological properties associated with aggregation in a semi-arid soil. Biol Fertil Soils. 2011;47:157-65.) resulting in losses of soil organic matter (Franzluebbers, 2009Franzluebbers AJ. Linking soil organic carbon and environmental quality through conservation tillage and residue management. In: Lal R, Follet RL, editors. Soil carbon sequestration and the greenhouse effect. 2nd.ed. Madison: SSSA; 2009. p.263-89. (Special publication, 57).) and microbial biomass (Islam and Weil, 2000Islam KR, Weil RR. Land use effects on soil quality in a tropical forest ecosystem of Bangladesh. Agric Ecosyst Environ. 2000;79:9-16.; Hungria et al., 2009Hungria M, Franchini JC, Brandão Junior O, Kaschuk G, Souza RA. Soil microbial activity and crop sustainability in a long-term experiment with three soil-tillage and two crop-rotation systems. Appl Soil Ecol. 2009;42:288-96.;Frazão et al., 2010Frazão LA, Piccolo MC, Feigl BJ, Cerri CC, Cerri CEP. Inorganic nitrogen, microbial biomass and microbial activity of a sandy Brazilian Cerrado soil under different land uses. Agric Ecosyst Environ. 2010;135:131-7.; Balota and Auler, 2011Balota EL, Auler, PAM. Soil microbial biomass under different management and tillage systems of permanent intercropped cover species in an orange orchard. R Bras Ci Solo. 2011;35:1873-83.).
There is great interest in agricultural practices that reduce soil degradation and increase agricultural sustainability and soil conservation. A key point in sustainable use of agricultural soil is increasing the soil C status and the processes relating to, or at least maintaining, levels close to the original ones. One of these practices is no-tillage (NT), which corresponds to sowing directly through mulch and litter with minimal soil disturbance. The area devoted to NT systems in Brazil (31 million hectares) has increased almost 10-fold in the last 10 years, corresponding to 40 % of the annual cropland (Febrapdp, 2014Federação Brasileira de Plantio Direto na Palha - Febrapdp. [Accessed: Jul 24, 2014]. Available: http://www.febrapdp.org.br/.
http://www.febrapdp.org.br/...
).
Soil and crop management directly influence physical, chemical and biological soil properties, which are used to measure soil quality, which means the capacity of soil to serve a desired function, such as sustainably provide food, fiber and forage (Doran and Parkin, 1994Doran JW, Parkin TB. Defining and assessing soil quality. In: Doran JW, Coleman DC, Bezdicek DF, Stewart BA, editors. Defining soil quality for a sustainable environment. Madison, Wisconsin, [USA]: Soil Science Society of America; 1994. p.3-21. (Special publication, 35).). Microbial parameters have been successfully used to evaluate soil quality (Frazão et al., 2010Frazão LA, Piccolo MC, Feigl BJ, Cerri CC, Cerri CEP. Inorganic nitrogen, microbial biomass and microbial activity of a sandy Brazilian Cerrado soil under different land uses. Agric Ecosyst Environ. 2010;135:131-7.; Kaschuk et al., 2010Kaschuk G, Alberton O, Hungria M. Three decades of soil microbial biomass studies in Brazilian ecosystems: Lessons learned about soil quality and indicators for improving sustainability. Soil Biol Biochem. 2010;42:1-13.). For example, soil microbial biomass is a source of labile nutrients and an agent for transformation and cycling of organic matter and plant nutrients. Microbial biomass and activity can be used as sensitive indicators of agro-ecosystem stress or changes in soil quality. Microorganisms have a relatively high rate of turnover and quick response compared to physical/chemical properties (Brookes, 2001Brookes P. The soil microbial biomass: Concept, measurement and applications in soil ecosystem research. Microbes Environ. 2001;16:131-40.; Wonprasaid, 2003Wonprasaid S. Sensitivity of soil quality indicators to soil management [These]. Lexington [KY]: University of Kentucky; 2003.; Kaschuk et al., 2010Kaschuk G, Alberton O, Hungria M. Three decades of soil microbial biomass studies in Brazilian ecosystems: Lessons learned about soil quality and indicators for improving sustainability. Soil Biol Biochem. 2010;42:1-13.). Aggregates function as a habitat for microorganisms (Six et al., 2004Six J, Bossuyt H, Degryze S, Denef K. A history of research on the link between (micro) aggregates, soil biota, and soil organic matter dynamics. Soil Till Res. 2004;79:7-31.; Caesar-Tonthat et al., 2011Caesar-Tonthat TC, Sinju UM, Wright SF, Shelver WL, Kolberg R, West M. Long-term tillage and cropping effects on microbiological properties associated with aggregation in a semi-arid soil. Biol Fertil Soils. 2011;47:157-65.; Guillou et al., 2012Guillou CL, Angers DA, Maron PA, Leterme P, Menasseri-Aubry S. Linking microbial community to soil water-stable aggregation during crop residue decomposition. Soil Biol Biochem. 2012;50:126-33.) and have a strong relationship to microbial population and activity.
This study evaluated the effect of land use and management on microbial (C and N microbial biomass and mineralization), physical (aggregate stability and mean weight diameter) and chemical (organic carbon content, pH and CEC) indicators of soil quality 24 years after forest conversion to intensive perennial or annual crops cultivation. Our hypothesis was that the beneficial aspects of no-tillage management would partially offset the transition from forest to cultivated soil in terms of the effect on measured soil quality properties. The organic C content, pH and CEC were measured because these parameters are affected by soil management and influence plant nutrition and microbial activity. Aggregate stability was measured because this physical parameter is affected by the degree of soil disturbance and microbial activity.
MATERIAL AND METHODS
Sites and soil description
The study was conducted at the Experimental Station of the Agronomic Institute of Paraná State (IAPAR), district of Londrina, State of Paraná, in southern Brazil (23° 22’ S, 51° 10’ W and 585 m altitude). The soil is an Oxisol,Latossolo Vermelho distrófico according to the Brazilian Classification System (Bhering and Santos, 2008Bhering SB, Santos HG. Mapa de solos do estado do Paraná: Legenda atualizada. Rio de Janeiro: Embrapa Florestas/Embrapa Solos/Instituto Agronômico do Paraná; 2008.), with 760 g kg-1 clay, 130 g kg-1 silt, 110 g kg-1 sand and slope of about 6 %.
The climate is a humid subtropical, of the Cfa type according to the Köppen classification, characterized by a hot summer with rain in all seasons, but with a tendency to rain mainly in summer. Mean annual precipitation was about 1,600 mm for the period 1976-2011. The rainfall in January (219 mm) is four times greater than in July (53 mm). The relative humidity is about 62 % in the winter and 77 % in the summer. Typical evaporation is from 81 mm in June to 156 mm in October. The mean air temperature is 24 oC in January (from 11 to 36oC) and 17 oC in July (from -1 to 31 oC). Sunshine occurs for 191 h in February to 238 h in August (Iapar, 2014Instituto Agronômico do Paraná - Iapar. [Accessed: Jul 25, 2014]. Available: http://www.iapar.br.
http://www.iapar.br...
).
The agricultural areas adjacent to the forest were selected due to their similarity of soil physical and chemical characteristics according to soil profile description (Table 1) (Sidiras et al., 1983Sidiras N, Derpsch R, Mondardo A. Influência de diferentes sistemas de preparo do solo na variação da umidade e rendimento da soja, em Latossolo Roxo distrófico (Oxisol). R Bras Ci Solo. 1983;7:103-6.; Castro Filho, 1988Castro Filho C. Effects of liming on characteristics of a Brazilian Oxisol at three levels of organic matter as related to erosion [These]. Columbus, [USA]: The Ohio State University; 1988.). According to the records of the Experimental Station, the soil environment was homogeneous at the beginning of land use transition. So these agricultural sites are good representatives of long-term land use due to their initial homogeneity and proximity to undisturbed forest. The perennial crop, represented by coffee, is about 100 m from the forest site, while the conventional tillage and no-tillage sites with annual crops are about 400 m away.
Conventional Tillage (CT): area used in an experiment for 24 years (since 1976) with a 2-year crop-rotation with wheat (Triticum aestivum L.) during winter and soybean rotation with maize each year as summer crop. The experiment consisted of plots (8 × 25 m), separated by 2 m rows, with three replications. Tillage consisted of one disk plowing and two diskings with a light harrow to level the soil and prepare the seedbed. Crop stubble is tilled conventionally (plowed to a depth of 20 cm) after harvest, in fall and spring every year. Fertilizers were regularly distributed in the tillage treatments before each crop cycle, consisting of: N 95, P 55, and K 42 kg ha-1 yr-1, for the last 24 years. Nitrogen fertilizer was never applied to soybean.
No-Tillage (NT): same experimental area described for the CT site. No-tillage consisted of sowing on undisturbed soil into a narrow opened trench. Crop stubble was mostly left on the surface after harvest in fall and spring every year.
Coffee (CO): coffee plantation (about 4,500 m2) of cultivar “Mundo Novo”, with 2200 trees ha-1 (spacing 4.0 × 2.0 m), since 1976. Fertilization according to soil analysis before/during the growth season (Nov-Feb), on average 150 kg ha-1 yr-1 N, 40 kg ha-1 yr-1 P2O5, and 80 kg ha-1 yr-1 K2O for the last 24 years. Before planting and every three years, dolomitic lime was applied to the whole area.
Forest (FO): The forest site (4.5 ha) is a remnant of primary tropical semi-deciduous forest that used to cover the north of Parana State. Leguminosae, Myrtaceae, Euphorbiaceae and Lauraceae are the main families of flowering plants at the site and the forest canopy height can reach 15 m.
Soil sampling
Composite soil samples (consisting of five sub-samples each) were taken from each replicate of the NT and CT sites from three layers (0-5, 5-10 and 10-20 cm) in March 2000, at the end of the summer growing season (maize). The FO and CO sites were divided into three parts according to slope (high, medium and low). From each part, a central area (50 × 70 m for FO and 20 × 60 m for CO) was considered a sampling unit from which 10 soil sub-samples (about 150 g each) were taken in a zigzag pattern, at 10 m intervals, to compose one replicate composite for each layer (0-5, 5-10 and 10-20 cm depth). In the CO site the sub-samples were evenly divided between samples taken near the edge of the tree canopy, in the planting row, and in the inter row.
The fresh soil samples were sieved (<4 mm mesh) and the large plant material removed. All determinations for each replicate were made in triplicate and expressed on a dry-weight basis. The moisture was adjusted to 55-60 % of water-holding capacity, for microbial and mineralization analysis. For physical analyses, undisturbed soil samples were taken with a shovel. Care was taken to avoid soil smearing or compaction during transport to the laboratory. At the laboratory, the soil was spread in a room at 22 °C to air-dry. During drying the larger clods/aggregates were gently broken into smaller pieces. Then soil samples were subjected to physical analysis.
Soil chemical analyses
Chemical analyses were made as described for routine analyses adapted for IAPAR by Pavan et al. (1992)Pavan MA, Bloch MF, Zempulski HC, Miyazawa M, Zocoler DC. Manual de análise química de solo e controle de qualidade. Londrina: Iapar: 1992. (Circular técnica, 76).. Organic C concentration (Corg) was measured by the Walkley-Black potassium dichromate sulfuric acid oxidation procedure and total N (Ntot) by Kjeldahl digestion with sulfuric acid.
Soil physical analyses
Aggregate stability was determined by wet sieving to estimate size distribution and aggregate stability in water (Castro Filho et al., 2002Castro Filho C, Lourenço A, Guimarães MF, Fonseca ICB. Aggregate stability under different soil management systems in a Red Latosol in the state of Parana, Brazil. Soil Till Res. 2002;65:45-51.). The aggregate indices: Mean Weight Diameter of soil particle (MWD) and Aggregate Stability Index (ASI) were calculated according to the following equations 1 and 2:
where yi is the proportion of each size class with respect to the total sample and xi is the mean diameter of the size classes (mm).
Soil microbial analyses
Microbial biomass-C (MB-C) was determined by fumigation-extraction (Vance et al., 1987Vance ED, Brookes PC, Jenkinson DS. An extraction method for measuring soil microbial biomass carbon. Soil Biol Biochem. 1987;19:703-7.) and calculated using a correction factor (kc) of 0.33. Microbial biomass-N (MB-N) was determined by the method of Brookes et al. (1985)Brookes PC, Landman A, Pruden G, Jenkinson DS. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem. 1985;17:837-42., with 0.54 as correction factor. Basal respiration (BR) was analyzed in 20 g of soil (fresh weight) incubated in gas-tight jars containing NaOH to trap the released CO2. The CO2 released in 10 days was determined by Flow Injection Analysis for differences in conductivity of the solution. Carbon and N mineralization (CMIN and NMIN) were determined by incubating 30 g of soil sample as described by Balota et al. (2004)Balota EL, Colozzi-Filho A, Andrade DA, Dick RP. Long-term tillage and crop rotation effects on microbial biomass and C and N mineralization in a Brazilian Oxisol. Soil Till Res. 2004;77:137-45.. For CMIN,soil was incubated in a sealed jar with NaOH trap in a dark chamber. The concentration of CO2 released in the period was determined by flow injection analysis of conductivity and NMIN was assessed by the nitrate concentration in soil before and after the 24-d incubation period.
Statistical analyses
The data were subjected to tests of normality of the variables and of homogeneity of variances, according to Bartlett’s test. After all assumptions required for the analysis of variance were verified. ANOVA was conducted for each experimental area and in the forest to check the equality of variance of each variable. Factorial was the procedure used to evaluate land use and sampling layer by F-test (p<0.05). The Minimum Significant Difference (MSD) by Tukey’s test (p<0.05) was used to identify significant differences between the means. These procedures were analyzed by SAS Statistical Package (SAS, 2009SAS Institute. SAS/STAT: user’s Guide. Version 9.2. Cary: 2009.).
Multivariate statistical analyses were used to evaluate the effects of management type and soil depth on soil microbial quality as described by the simultaneous analysis of five variables (MB-C, MB-N, BR, CMIN, and NMIN). For this test, the data were analyzed using multivariate ordination “non-metric multidimensional scaling” - NMS (Sokal, 1979Sokal RR. Testing statistical significance of geographic variation patterns. Syst Zool. 1979;28:227-32.), with Sorensen distances. Prior to analysis, data were standardized by totals within each variable, to eliminate the differences in the variable units. The ordination was performed in the autopilot mode, “slow and thorough” option in the program PC-ORD v. 6.0 (McCune and Mefford, 2011Mccune B, Mefford MJ. PC-ORD. Multivariate analysis of ecological data. Version 6.0. MjM Software. Gleneden Beach, Oregon [US]: 2011.). The number of dimensions to be interpreted was selected considering the criteria of stress and stability of the graphical solutions. The effect of soil management type and soil depth on soil microbial quality was analyzed using PERMANOVA in the program PC-ORD v. 6.0 (McCune and Mefford, 2011Mccune B, Mefford MJ. PC-ORD. Multivariate analysis of ecological data. Version 6.0. MjM Software. Gleneden Beach, Oregon [US]: 2011.). Significance of differences in soil microbial condition between treatments was also determined by PERMANOVA. The DistLM procedure was used to check for the existence of significant relationship between soil microbial data and soil physical and chemical properties by the program DISTLM v.5 (Anderson, 2004Anderson MJ. DISTLM v.5: A FORTRAN computer program to calculate a distance-based multivariate analysis for a linear model. Auckland: [NZ]: Department of Statistics, University of Auckland, 2004.). In addition, this procedure was used to assess the contribution of the most important variables (soil microbial, physical and chemical properties) accounting for the variability among the treatments with DISTLM forward (Anderson, 2003Anderson, M.J. DISTLM forward: a FORTRAN computer program to calculate a distance-based multivariate analysis for a linear model using forward selection. Auckland: [NZ]: Department of Statistics, University of Auckland; 2003.). Pearson’s correlation between soil microbial, physical and chemical properties were performed using the SAS Statistical Package (SAS, 2009SAS Institute. SAS/STAT: user’s Guide. Version 9.2. Cary: 2009.).
RESULTS
Chemical properties
Chemical properties of soils under the different land uses are in table 2. Layer was always a significant factor except for Al content; Land Use was always a significant factor except for CEC. Soil pH values ranged from 4.10 to 4.63. Aluminum content in CO at each layer (1.59-2.09 cmolc kg-1) was routinely twice as high as Al content in any other treatment.
Compared to the forest (FO), Corg always decreased with transition to cultivation in all layers. Overall declines for FO were 20 % in CO, 33 % in NT and 48 % in CT. Total N (Ntot) also declined with depth in all treatments. In the 0-5 cm layer Ntot values declined relative to the FO site in the same order and approximately the same amount as Corg(CO = 24 %, NT = 35 % and CT = 48 %).
Physical properties
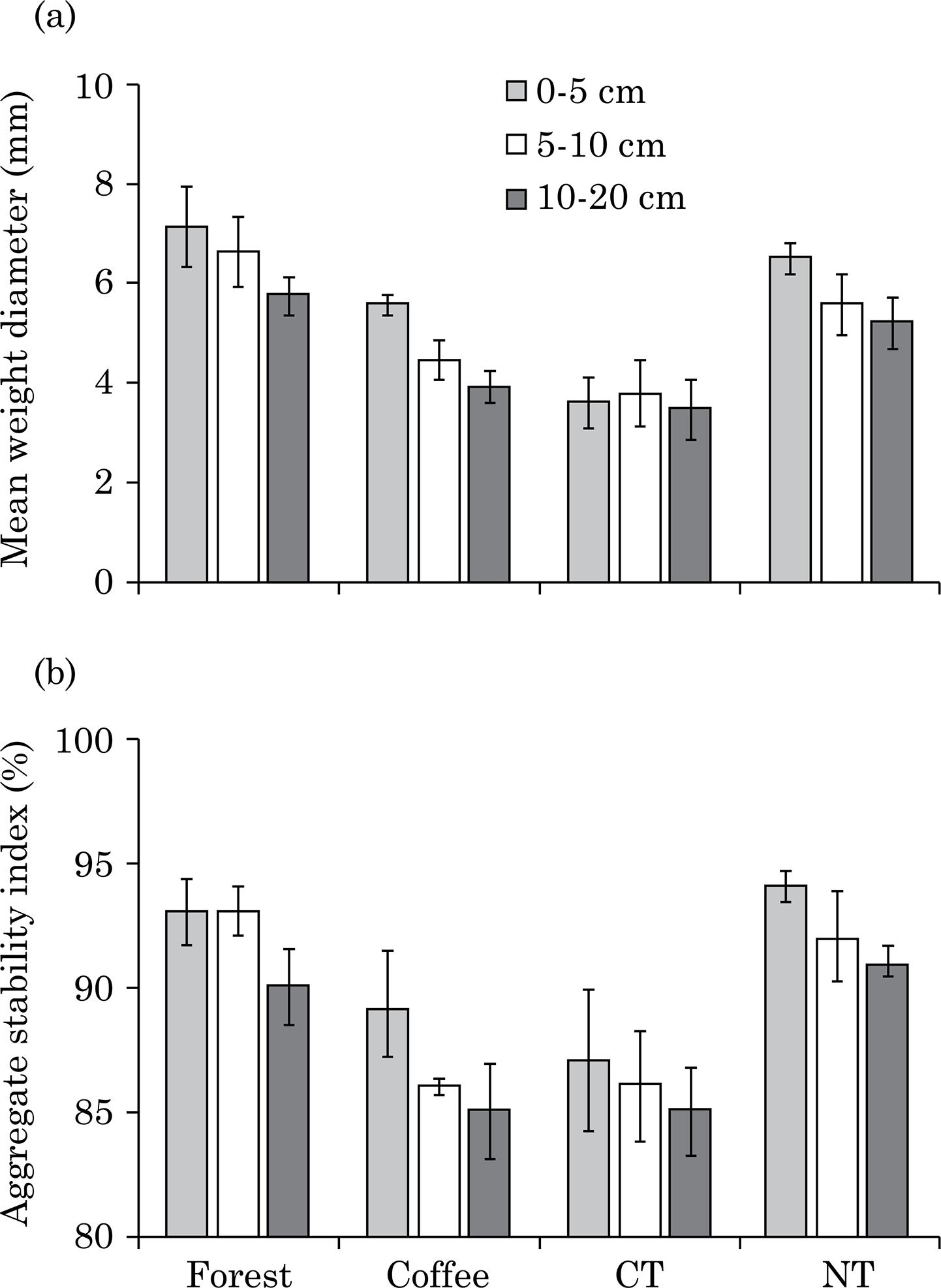
Land use and management had a significant effect on aggregate mean weight diameter (MWD) and aggregate stability index (ASI) (p≤0.001), although depth within each treatment was not significant for either soil property (p≥0.05). The MWD values were highest in FO followed by NT in all layers, even when not significant (Figure 1a). Likewise, ASI was routinely higher in FO and NT compared to CO and CT for each layer (Figure 1b).
Mean weight diameter (a) and aggregate stability index (b) of a Brazilian Oxisol 24 years after forest conversion to a perennial crop with coffee or annual grain crops (maize and soybeans) in conventional tillage or no-tillage from three layers (0-5, 5-10 and 10-20 cm). Average of three replications. Bars indicate standard deviation.
Microbial properties
Microbial biomass-Carbon (MB-C) and microbial biomass-Nitrogen (MB-N) varied significantly for each land use (p≤0.001) and layer (p≤0.05). About 40 % of the total MB-C observed in the 0-20 layer for FO, CO and CT was concentrated in the 0-5 cm layer, and a similar proportion was observed in NT (45 %). For the total MB-N in the 0-20 layer, about 39 % was concentrated in the 0-5 cm layer for FO and CT, 30 % for CO and 44 % for NT. The FO site had the highest MB-C in all layers evaluated, while the lowest values were observed in CT (Figure 2a). The same pattern was evident in terms of MB-N (Figure 2b).
Microbial biomass C (MB-C) (a), and N (MB-N) (b) and C:N microbial biomass ratio (c) of a Brazilian Oxisol 24 years after forest conversion to a perennial crop with coffee or annual grain crops (maize and soybeans) in conventional tillage or no-tillage from three layers (0-5, 5-10 and 10-20 cm). Average of three replications. Bars indicate standard deviation.
Different land use/management had a significant effect on microbial biomass C:N ratio (C:N MB) (p≤0.004), contrary to soil layer. The highest C:N MB ratios occurred in NT (Figure 2c). The C:N MB ratio declined with depth (from 0-5 to 10-20 cm) in FO and CO about 20 and 28 %, respectively.
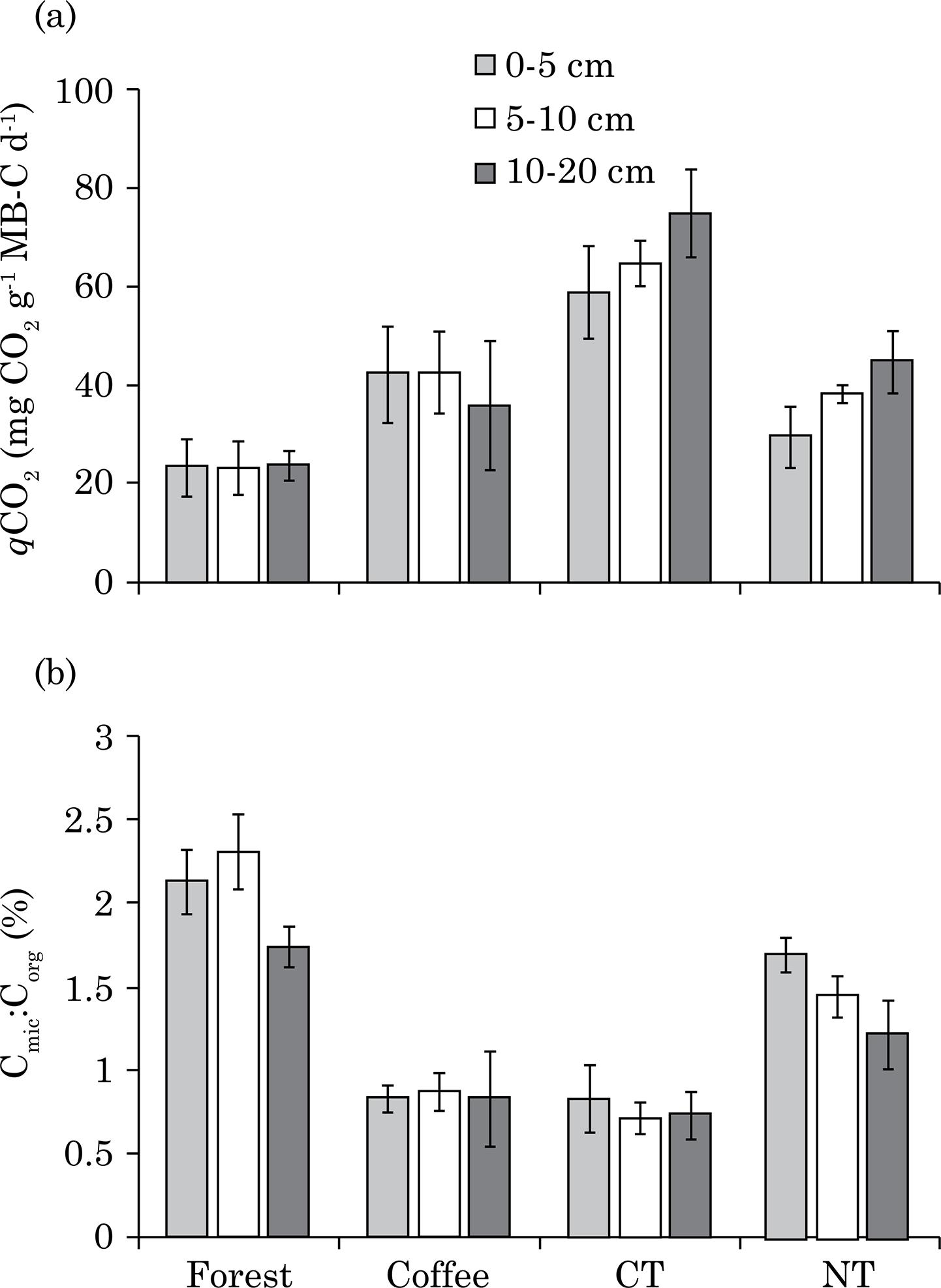
Metabolic quotient (qCO2), which represents the ratio of soil respiration per unit of MB-C, was only significantly affected by soil layer (p≤0.006) (Figure 3a). It approached significance (p≤0.07) with respect to land use. Metabolic quotient decreased (about 14 %) with depth in CO, but increased with depth in CT and NT (27 and 52 %, respectively). Averaged across depths, qCO2increased 59 % for NT, 71 % for CO and 182 % for CT compared to FO.
Metabolic quotient (qCO2) (a) and microbial carbon to organic carbon (Cmic:Corg) ratio (b) of a Brazilian Oxisol after forest conversion to a perennial crop with coffee or annual grain crops (maize and soybeans) in conventional tillage or no-tillage from three layers (0-5, 5-10 and 10-20 cm). Average of three replications. Bars indicate standard deviation.
The ratio of microbial C to organic C (Cmic:Corg) was affected by soil management (p≤0.001) and layer (p≤0.002) (Figure 3b). Forest soil had a higher Cmic:Corg ratio than other uses/managements, while the lowest values occurred in CO and CT. Averaged across land use/management treatment, the Cmic:Corg decreased with depth.
Carbon mineralization (CMIN) (Figure 4a) and nitrogen mineralization (NMIN) (Figure 4b) varied significantly at each site for land use (p<0.001) and layer (p<0.05). About 48 % of the CMIN observed in the 0-20 cm layer for FO, CO and NT was concentrated in the 0-5 cm layer, while about 28 % of the CMIN in CT occurred in the 0-5 cm layer. For NMIN in the 0-20 cm layer, about 40 % was concentrated in the 0-5 cm layer for FO, CO and NT, while about 31 % of NMIN in CT occurred in the 0-5 cm layer. The C:N mineralization ratios varied from 8 to 25 (Figure 4c). Land use/management (p<0.001) but not layer (p<0.825) had a significant effect on this property.
Mineralization of carbon (a) and nitrogen (b) during 24 days, and C:N mineralization ratio (c) of a Brazilian Oxisol after forest conversion to a perennial crop with coffee or annual grain crops (maize and soybeans) in conventional tillage or no-tillage from three layers (0-5, 5-10 and 10-20 cm). Average of three replications. Bars indicate standard deviation.
Multivariate analyses
The changes in soil microbial properties in response to different land use/management and soil layer, according to the NMS ordination method (Figure 5). Most of this variability was associated with Axis 1 (88.6 %), which was positively correlated with MB-C, MB-N, BR, CMIN, and NMIN (Table 3). Axis 2 accounted for about 9 % of the variability in the data from five microbial properties that correlated with only MBN and NMIN. NMS Axis 1 of soil microbial properties shows a clear separation among the treatments, displaying a gradient from CT to FO (Figure 5a). The NMS Axis 1 representation of five variables related to soil microbiota also showed a gradient increase from deepest (10-20 cm) to shallowest (0-5 cm) layers.
Changes in soil microbial properties after forest conversion to a perennial crop with coffee or annual grain crops (maize and soybeans) in conventional tillage or no-tillage (a) and soil layer (b), according to NMS ordination method. The symbols represent the geometric mean of soil microbial properties in each treatment, while the vertical and horizontal lines indicate ±1SD. 2D stress = 6.96. FO: Forest; CO: Coffee; CT: Conventional Tillage; NT: No-Tillage.
When all treatments and depths were analyzed, PERMANOVA showed that soil microbial properties were significantly affected by land use/management (F = 25.6, p=0.001) and soil layer (F = 7.59, p=0.003). The pairwise comparisons between land use/management and soil layer were all significant (p<0.05) except for the comparisons between land use/managements CO vs. CT and 0-5 vs. 5-10 cm layers.
The DistLM analysis showed that soil microbial variables were significantly related to soil physical and chemical properties (F = 10.79, p=0.001). MB-C and MB-N together contributed with 77 % of the observed data variability among the treatments in terms of microbial variables (Table 4). Among the soil physical and chemical properties, Corg, MWD and Ntot together accounted for 85 % of the land use/management variability (Table 4). Overall, significant positive correlations were found between the most important soil microbial (MB-C and MB-N) and soil physical and chemical (MWD, Corg, MWD and Ntot) variables (Table 5).
DISCUSSION
The soil pH at the FO site remained relatively unchanged throughout this period. Soil acidification in CO is greater than at the other cultivated sites, due to various factors: the high amount of fertilizer applied, leaching of base cations and coffee physiology, which absorbs more cations than anions. In addition, the soil was sampled near the edge of the tree canopy, where the fertilizers were broadcasted, most coffee roots are found and the acidification processes are more intense. Although the CEC of the CO site was initially lower than at the others, by the time of sampling it was numerically higher. This is most likely a consequence of continuous lime application to buffer the pH in relation to the FO site, as well as of higher Corg than at the other cultivated sites.
The higher values of MWD and ASI in FO observed in this study are consistent with those observed by Castro Filho et al. (2002)Castro Filho C, Lourenço A, Guimarães MF, Fonseca ICB. Aggregate stability under different soil management systems in a Red Latosol in the state of Parana, Brazil. Soil Till Res. 2002;65:45-51.for the same cultivated area. Greater MWD and ASI in FO and NT were expected because there are conditions to increase addition of Corg and, consequently, increasing microbial activity in these land uses, which would produce more organic binding agents and microbial biomass that contribute to the formation and stability of soil aggregates (Six et al., 2004Six J, Bossuyt H, Degryze S, Denef K. A history of research on the link between (micro) aggregates, soil biota, and soil organic matter dynamics. Soil Till Res. 2004;79:7-31.; Green et al., 2007Green VS, Stott DE, Cruz JC, Curi N. Tillage impact on soil biological activity and aggregation in a Brazilian Cerrado Oxisol. Soil Till Res. 2007;92:114-21.; Caesar-TonThat et al., 2011Caesar-Tonthat TC, Sinju UM, Wright SF, Shelver WL, Kolberg R, West M. Long-term tillage and cropping effects on microbiological properties associated with aggregation in a semi-arid soil. Biol Fertil Soils. 2011;47:157-65.; Guillou et al., 2012Guillou CL, Angers DA, Maron PA, Leterme P, Menasseri-Aubry S. Linking microbial community to soil water-stable aggregation during crop residue decomposition. Soil Biol Biochem. 2012;50:126-33.). The proportion of labile Corg that is physically protected from microbial decomposition may be increased in macroaggregates, thus enhancing microhabitats for microorganisms (Caesar-TonThat et al., 2011Caesar-Tonthat TC, Sinju UM, Wright SF, Shelver WL, Kolberg R, West M. Long-term tillage and cropping effects on microbiological properties associated with aggregation in a semi-arid soil. Biol Fertil Soils. 2011;47:157-65.; Guillou et al., 2012Guillou CL, Angers DA, Maron PA, Leterme P, Menasseri-Aubry S. Linking microbial community to soil water-stable aggregation during crop residue decomposition. Soil Biol Biochem. 2012;50:126-33.). Aggregate stability, which expresses the resistance of soil structural aggregates to dispersion when wetted, is an important soil quality property, because it influences many soil functions and reflects an interrelationship among biological, chemical and physical soil properties (Islam and Weil, 2000Islam KR, Weil RR. Land use effects on soil quality in a tropical forest ecosystem of Bangladesh. Agric Ecosyst Environ. 2000;79:9-16.).
Our results agree with previous studies that soil management affects significant soil properties such as microbial mass and activity (Islam and Weil, 2000Islam KR, Weil RR. Land use effects on soil quality in a tropical forest ecosystem of Bangladesh. Agric Ecosyst Environ. 2000;79:9-16.; Hungria et al., 2009Hungria M, Franchini JC, Brandão Junior O, Kaschuk G, Souza RA. Soil microbial activity and crop sustainability in a long-term experiment with three soil-tillage and two crop-rotation systems. Appl Soil Ecol. 2009;42:288-96.; Frazão et al., 2010Frazão LA, Piccolo MC, Feigl BJ, Cerri CC, Cerri CEP. Inorganic nitrogen, microbial biomass and microbial activity of a sandy Brazilian Cerrado soil under different land uses. Agric Ecosyst Environ. 2010;135:131-7.; Balota and Auler, 2011Balota EL, Auler, PAM. Soil microbial biomass under different management and tillage systems of permanent intercropped cover species in an orange orchard. R Bras Ci Solo. 2011;35:1873-83.). The microbial biomass measured in FO and NT indicated the same trend as other studies, which show a significant positive effect of decreasing soil disturbance on microbial biomass (Balota et al., 2004Balota EL, Colozzi-Filho A, Andrade DA, Dick RP. Long-term tillage and crop rotation effects on microbial biomass and C and N mineralization in a Brazilian Oxisol. Soil Till Res. 2004;77:137-45.; Frazão et al., 2010Frazão LA, Piccolo MC, Feigl BJ, Cerri CC, Cerri CEP. Inorganic nitrogen, microbial biomass and microbial activity of a sandy Brazilian Cerrado soil under different land uses. Agric Ecosyst Environ. 2010;135:131-7.; Kaschuk et al., 2010Kaschuk G, Alberton O, Hungria M. Three decades of soil microbial biomass studies in Brazilian ecosystems: Lessons learned about soil quality and indicators for improving sustainability. Soil Biol Biochem. 2010;42:1-13.). The generally higher microbial biomass under FO and NT compared to CT, especially in tropical/subtropical climates, could be attributed to several factors, such as lower temperature, higher moisture content, greater aggregation and higher C content. The lack of a major disturbance event under FO likely favors the steady supply of Corg to support the microbial community, while in CT, the temporary flush of microbial activity with each tillage event results in large losses of C as CO2.
Although CO had higher Corg compared to NT and CT, the microbial biomass was low, as previously observed by Marchiori Junior and Melo (2002)Marchiori Junior M, Melo WJ. Alterações na matéria orgânica e na biomassa microbiana em solo de mata natural submetido a diferentes manejos. Pesq Agropec Bras. 2002;35:117-82. in which a coffee site had half the MB-C of forest and maize sites and about 30 % of that present in sugarcane. The low value of MB-C under CO could be due to higher soil acidity and Al toxicity. Repeated applications of Cu fungicides for a long period cause Cu accumulation in soil (Nachtigall et al., 2007Nachtigall GR, Nogueirol RC, Alleoni LRF. Formas de cobre em solos de vinhedos em função do pH e da adição de cama-de-frango. Pesq Agropec Bras. 2007;42:427-34.) which may decrease microbial activity. In CO, long-term fertilizer use can improve soil nutrient status, but can also increase soil acidification, by repeated N fertilizer applications (Aciego Pietri and Brookes, 2008Aciego Pietri JC, Brookes PC. Relationships between soil pH and microbial properties in a UK arable soil. Soil Biol Biochem. 2008;40:1856-61.). According to the same authors, soil acidity combined with high Al content may also limit microbial growth, in a mechanism similar to that caused by heavy metals, which decrease the efficiency of C residue conversion into microbial biomass C.
A change in the microbial biomass C/N ratio due to different land use/management may reflect a change in the pattern of microbial nutrient immobilization. Soil microbial biomass is a composite of several groups of organisms. Each microbial group has a different C/N ratio and the predominance of one group may result in the prevalence of a particular ratio, e.g. a range of 4.5-15 for fungi vs. three to six for bacteria. Therefore, a change in microbial biomass C/N ratio may occur as a result of changes in microbial populations during the decomposition of crop residue.
Litter incorporation due to tillage may reduce the microbial biomass C/N ratio as observed in the present study in CT. On the other hand, the wider microbial biomass C/N ratio in FO, and particularly NT, suggests that these sites might have a greater proportion of fungi than bacteria compared to CO and CT (Frey et al., 1999Frey SD, Elliot ET, Paustian K. Bacterial and fungal abundance and biomass in conventional and no-tillage agroecosystems along two climatic gradients. Soil Biol Biochem. 1999;31:573-85.). Reduced soil disturbance may also favor establishment and preservation of fungal hyphal networks with higher C/N ratio. Although the fungi to bacteria dominance under NT system is broadly accepted by the scientific community, Strickland and Rousk (2010)Strickland MS, Rousk J. Considering fungal: Bacterial dominance in soils - Methods, control, and ecosystem implications. Soil Biol Biochem. 2010;42:1385-95. have suggested research addressing the mechanism by which the eventual shift may occur, because recent studies have shown that NT enhances both fungal and bacterial biomass. For example, a metagenomic study comparing NT and CT in a similar soil of Londrina, the dominance of bacteria in CT was not confirmed (Souza et al., 2013Souza RC, Cantão ME, Vasconcelos ATR, Nogueira MA, Hungria M. Soil metagenomics reveals differences under conventional and no-tillage with crop rotation or succession. Appl Soil Ecol. 2013;72:49-61.). Independent of shifts in the fungi-to-bacteria ratio, the changes in the microbial biomass C/N ratio may also affect the rate of immobilization and mineralization of soil nutrients, as more nutrients are stored in microbial biomass.
Although FO and NT had higher microbial biomass than CO and CT, theqCO2 was lower. The inverse relationship between MB-C and qCO2 is commonly observed in reduced tillage systems. For example, Balota et al. (2004)Balota EL, Colozzi-Filho A, Andrade DA, Dick RP. Long-term tillage and crop rotation effects on microbial biomass and C and N mineralization in a Brazilian Oxisol. Soil Till Res. 2004;77:137-45., studying different crop rotations and sampling seasons in the same area, observed that no-tillage had 56 % higher MB-C and 68 % lower qCO2than conventional tillage. These differences may be due to differences of microbial accessibility to C substrates, changes in the metabolic rates, changes in microbial community composition and proportion of recalcitrant material (Islam and Weil, 2000Islam KR, Weil RR. Land use effects on soil quality in a tropical forest ecosystem of Bangladesh. Agric Ecosyst Environ. 2000;79:9-16.). Environmental variation determines changes in microbial composition and physiological status. No tillage may also have a greater proportion of larger aggregates, which was observed in our study, which protect microorganisms from adverse conditions (Green et al., 2007Green VS, Stott DE, Cruz JC, Curi N. Tillage impact on soil biological activity and aggregation in a Brazilian Cerrado Oxisol. Soil Till Res. 2007;92:114-21.; Caesar-TonThat et al., 2011Caesar-Tonthat TC, Sinju UM, Wright SF, Shelver WL, Kolberg R, West M. Long-term tillage and cropping effects on microbiological properties associated with aggregation in a semi-arid soil. Biol Fertil Soils. 2011;47:157-65.) and permit more efficient C use.
Generally, qCO2 decreases in more stable systems, suggesting more efficient C use by the microbial community (Anderson and Domsch, 2010Anderson T-H, Domsch KH. Soil microbial biomass: The eco-physiological approach. Soil Biol Biochem. 2010;42:2039-43.). The lowerqCO2 values may indicate that FO and NT are more stable systems, encouraging a shift toward fungal-dominated microbial communities. Therefore, qCO2 may be expected to decline, as more C-efficient fungi come to dominate soil communities in certain soil conservation managements. In this context, the high value of qCO2 for CT suggests that soil practices such as tillage decrease the efficiency of C substrate use by the soil microbial community. The same effect under CO may be due to low pH and higher Al content. Greater acidity and potential Al toxicity requires greater effort by the microbial community to repair damage due to stress (Anderson and Domsch, 2010Anderson T-H, Domsch KH. Soil microbial biomass: The eco-physiological approach. Soil Biol Biochem. 2010;42:2039-43.). Under field conditions, when liming reduces acidity, a more diverse and efficient microbial community can be established (Aciego Pietri and Brookes, 2008Aciego Pietri JC, Brookes PC. Relationships between soil pH and microbial properties in a UK arable soil. Soil Biol Biochem. 2008;40:1856-61.). The increase of qCO2 with depth (at both CT and NT sites) may also be due to an increasing proportion of recalcitrant organic substrate, as suggested by Islam and Weil (2000)Islam KR, Weil RR. Land use effects on soil quality in a tropical forest ecosystem of Bangladesh. Agric Ecosyst Environ. 2000;79:9-16..
Microbial C pools typically decline at a faster rate than soil organic matter, and the Cmic:Corg percentage will decrease during soil degradation (Islam and Weil, 2000Islam KR, Weil RR. Land use effects on soil quality in a tropical forest ecosystem of Bangladesh. Agric Ecosyst Environ. 2000;79:9-16.; Anderson and Domsch, 2010Anderson T-H, Domsch KH. Soil microbial biomass: The eco-physiological approach. Soil Biol Biochem. 2010;42:2039-43.; Balota and Auler, 2011Balota EL, Auler, PAM. Soil microbial biomass under different management and tillage systems of permanent intercropped cover species in an orange orchard. R Bras Ci Solo. 2011;35:1873-83.), a trend observed in our study. This may allow for a calibrated early soil quality indicator to predict whether soils are accumulating or losing soil C. This relationship also allows comparisons across soils with different organic matter contents. The Cmic:Corgratio could be an indicator of conversion efficiency of Corg into Cmic and losses of soil C during decomposition. This indicator may reveal the microbial community capacity to enrich the microbial C pool under conservation management (Islam and Weil, 2000Islam KR, Weil RR. Land use effects on soil quality in a tropical forest ecosystem of Bangladesh. Agric Ecosyst Environ. 2000;79:9-16.; Balota et al., 2004Balota EL, Colozzi-Filho A, Andrade DA, Dick RP. Long-term tillage and crop rotation effects on microbial biomass and C and N mineralization in a Brazilian Oxisol. Soil Till Res. 2004;77:137-45.; Frazão et al., 2010Frazão LA, Piccolo MC, Feigl BJ, Cerri CC, Cerri CEP. Inorganic nitrogen, microbial biomass and microbial activity of a sandy Brazilian Cerrado soil under different land uses. Agric Ecosyst Environ. 2010;135:131-7.; Kaschuk et al., 2010Kaschuk G, Alberton O, Hungria M. Three decades of soil microbial biomass studies in Brazilian ecosystems: Lessons learned about soil quality and indicators for improving sustainability. Soil Biol Biochem. 2010;42:1-13.; Balota and Auler, 2011Balota EL, Auler, PAM. Soil microbial biomass under different management and tillage systems of permanent intercropped cover species in an orange orchard. R Bras Ci Solo. 2011;35:1873-83.; Pellegrino et al., 2011Pellegrino E, Di Bene C, Tozzini C, Bonari E. Impact on soil quality of a 10-year-old short-rotation coppice poplar stand compared with intensive agricultural and uncultivated systems in a Mediterranean area. Agric Ecosyst Environ. 2011;140:245-54.). The Cmic:Corg ratio was higher in FO followed by NT presumably because it maintained more labile organic substrates in the soil, which allowed higher MB-C per unit of soil Corg. The result observed in NT indicates a shift in Corg equilibrium toward less loss of labile soil organic C than in CO and CT. As a result, this indicates higher quality soil in NT, closer to that of FO, which is more biologically active than in CO and CT, which are therefore considered inferior soils.
In FO and NT, CMIN and NMIN were, respectively, more than 200 % and about 64 % higher than at the CT site. The great difference in CMINand NMIN among sites suggested differences in labile soil Corgand N accumulated, due to soil management and crop species, as previously observed (Franzluebbers and Stuedemann, 2008Franzluebbers AJ, Stuedemann JA. Early response of soil organic fractions to tillage and integrated crop-livestock production. Soil Sci Soc Am J. 2008;72:613-25.;Balota and Chaves, 2010Balota EL, Chaves JCD. Enzymatic activity and mineralization of carbon and nitrogen in soil cultivated with coffee and green manures. R Bras Ci Solo. 2010;34:1573-83.). A broad range of CMIN are reported in the literature, ranging from 2 to 70 μg C g-1 d-1 according to Franzluebbers (2009)Franzluebbers AJ. Linking soil organic carbon and environmental quality through conservation tillage and residue management. In: Lal R, Follet RL, editors. Soil carbon sequestration and the greenhouse effect. 2nd.ed. Madison: SSSA; 2009. p.263-89. (Special publication, 57). who compiled data for 33 published studies. Although in some studies CT has higher CMIN than NT, the conservation system has, on average, 50 % higher CMIN. These results suggest that soil use/management causes different decomposition rates of organic matter and variation in the microbial biomass and activity, with consequent differences in substrate availability.
The C:N mineralization ratio showed significant effects due to land use. There was a wider C:N mineralization ratio in FO and NT than CO and CT, which may have been due to microbial populations using organic matter with wide C:N ratios. A low soil mineralizable C:N ratio may suggest that the flow of C and N through the mineralizable fraction has become less stable - more dependent on the microbial biomass turnover - which may decrease conservation of C and N in the long term. The C:N mineralization ratio can be used as an index of labile substrate availability. Our results are in agreement with previous study (Balota and Chaves, 2010Balota EL, Chaves JCD. Enzymatic activity and mineralization of carbon and nitrogen in soil cultivated with coffee and green manures. R Bras Ci Solo. 2010;34:1573-83.), that demonstrate the sensitivity of soil organic quality (potential mineralization) to changes in soil use/management.
Five microbial properties were selected for multivariate analysis. Each was highly correlated with the principle NMS ordination axis and in sum they clearly distinguished the different land uses. Soil depth had apparent but much less separation, probably because the depth increments were small. Despite being a master variable, soil pH was poorly correlated with the microbial soil quality parameters, probably because the pH range at the sites was relatively small. Other physical and chemical parameters were better correlated with the microbial measures.
The results of the DistLM analysis in the partitioning of microbial, chemical and physical soil quality parameters to separate land use types were extraordinary. Three physical and chemical properties (Corg, MWD and total N) contributed with 85 % of the variability while only four microbial variables (microbial biomass C and N and mineralizable C and N) contributed with 88 % of the variability. The implication of the results, in agreement with the evidence fromqCO2 microbial biomass C/N ratios, and distribution of Corg in soil aggregates suggests that improvements of the physical environment for microbial population are critical to enhance soil quality. Conservation soil management practices such as no-tillage enhance each of these properties for annual crop production in conventional tillage systems. Consequently, C and N are more labile and have higher contents, are contained in larger more stable aggregates, and ultimately make the soil more similar to the original forest soil environment.
CONCLUSIONS
Different land use/management had significant effects on soil biological, physical and chemical properties.
The reduction of soil disturbance is important for soil carbon content and microbial activity, but the control of soil pH and aluminum toxicity is also important to maintain the soil microbial activity high.
Multivariate analysis showed that land use/management could be separated based on five readily measured soil quality properties (microbial biomass C and N, mineralizable C and N and basal respiration).
These microbial measures showed no-tillage management more closely associated with the original forest soil than more intensive cropping systems such as perennial coffee cultivation or annual crop managed in conventional tillage.
ACKNOWLEDGEMENTS
The authors are grateful to Dr. M. A. P. for his helpful suggestions. E. L. B. acknowledges CNPq - Conselho Nacional de Desenvolvimento Científico e Tecnológico (National Counsel of Technological and Scientific Development) and the Program Science without Borders, for a postdoctoral fellowship at the University of Kentucky, United States (Process 201930/2012-9).
REFERENCES
- Aciego Pietri JC, Brookes PC. Relationships between soil pH and microbial properties in a UK arable soil. Soil Biol Biochem. 2008;40:1856-61.
- Anderson, M.J. DISTLM forward: a FORTRAN computer program to calculate a distance-based multivariate analysis for a linear model using forward selection. Auckland: [NZ]: Department of Statistics, University of Auckland; 2003.
- Anderson MJ. DISTLM v.5: A FORTRAN computer program to calculate a distance-based multivariate analysis for a linear model. Auckland: [NZ]: Department of Statistics, University of Auckland, 2004.
- Anderson T-H, Domsch KH. Soil microbial biomass: The eco-physiological approach. Soil Biol Biochem. 2010;42:2039-43.
- Balota EL, Auler, PAM. Soil microbial biomass under different management and tillage systems of permanent intercropped cover species in an orange orchard. R Bras Ci Solo. 2011;35:1873-83.
- Balota EL, Chaves JCD. Enzymatic activity and mineralization of carbon and nitrogen in soil cultivated with coffee and green manures. R Bras Ci Solo. 2010;34:1573-83.
- Balota EL, Colozzi-Filho A, Andrade DA, Dick RP. Long-term tillage and crop rotation effects on microbial biomass and C and N mineralization in a Brazilian Oxisol. Soil Till Res. 2004;77:137-45.
- Bhering SB, Santos HG. Mapa de solos do estado do Paraná: Legenda atualizada. Rio de Janeiro: Embrapa Florestas/Embrapa Solos/Instituto Agronômico do Paraná; 2008.
- Brookes P. The soil microbial biomass: Concept, measurement and applications in soil ecosystem research. Microbes Environ. 2001;16:131-40.
- Brookes PC, Landman A, Pruden G, Jenkinson DS. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem. 1985;17:837-42.
- Caesar-Tonthat TC, Sinju UM, Wright SF, Shelver WL, Kolberg R, West M. Long-term tillage and cropping effects on microbiological properties associated with aggregation in a semi-arid soil. Biol Fertil Soils. 2011;47:157-65.
- Castro Filho C. Effects of liming on characteristics of a Brazilian Oxisol at three levels of organic matter as related to erosion [These]. Columbus, [USA]: The Ohio State University; 1988.
- Castro Filho C, Lourenço A, Guimarães MF, Fonseca ICB. Aggregate stability under different soil management systems in a Red Latosol in the state of Parana, Brazil. Soil Till Res. 2002;65:45-51.
- Doran JW, Parkin TB. Defining and assessing soil quality. In: Doran JW, Coleman DC, Bezdicek DF, Stewart BA, editors. Defining soil quality for a sustainable environment. Madison, Wisconsin, [USA]: Soil Science Society of America; 1994. p.3-21. (Special publication, 35).
- Federação Brasileira de Plantio Direto na Palha - Febrapdp. [Accessed: Jul 24, 2014]. Available: http://www.febrapdp.org.br/.
» http://www.febrapdp.org.br/ - Franzluebbers AJ. Linking soil organic carbon and environmental quality through conservation tillage and residue management. In: Lal R, Follet RL, editors. Soil carbon sequestration and the greenhouse effect. 2nd.ed. Madison: SSSA; 2009. p.263-89. (Special publication, 57).
- Franzluebbers AJ, Stuedemann JA. Early response of soil organic fractions to tillage and integrated crop-livestock production. Soil Sci Soc Am J. 2008;72:613-25.
- Frazão LA, Piccolo MC, Feigl BJ, Cerri CC, Cerri CEP. Inorganic nitrogen, microbial biomass and microbial activity of a sandy Brazilian Cerrado soil under different land uses. Agric Ecosyst Environ. 2010;135:131-7.
- Frey SD, Elliot ET, Paustian K. Bacterial and fungal abundance and biomass in conventional and no-tillage agroecosystems along two climatic gradients. Soil Biol Biochem. 1999;31:573-85.
- Guillou CL, Angers DA, Maron PA, Leterme P, Menasseri-Aubry S. Linking microbial community to soil water-stable aggregation during crop residue decomposition. Soil Biol Biochem. 2012;50:126-33.
- Green VS, Stott DE, Cruz JC, Curi N. Tillage impact on soil biological activity and aggregation in a Brazilian Cerrado Oxisol. Soil Till Res. 2007;92:114-21.
- Hungria M, Franchini JC, Brandão Junior O, Kaschuk G, Souza RA. Soil microbial activity and crop sustainability in a long-term experiment with three soil-tillage and two crop-rotation systems. Appl Soil Ecol. 2009;42:288-96.
- Instituto Agronômico do Paraná - Iapar. [Accessed: Jul 25, 2014]. Available: http://www.iapar.br.
» http://www.iapar.br - Instituto Brasileiro de Geografia e Estatística - IBGE. [Accessed: Jul 20, 2014]. Available: http://www.ibge.gov.br.
» http://www.ibge.gov.br - Islam KR, Weil RR. Land use effects on soil quality in a tropical forest ecosystem of Bangladesh. Agric Ecosyst Environ. 2000;79:9-16.
- Kaschuk G, Alberton O, Hungria M. Three decades of soil microbial biomass studies in Brazilian ecosystems: Lessons learned about soil quality and indicators for improving sustainability. Soil Biol Biochem. 2010;42:1-13.
- Marchiori Junior M, Melo WJ. Alterações na matéria orgânica e na biomassa microbiana em solo de mata natural submetido a diferentes manejos. Pesq Agropec Bras. 2002;35:117-82.
- Mccune B, Mefford MJ. PC-ORD. Multivariate analysis of ecological data. Version 6.0. MjM Software. Gleneden Beach, Oregon [US]: 2011.
- Nachtigall GR, Nogueirol RC, Alleoni LRF. Formas de cobre em solos de vinhedos em função do pH e da adição de cama-de-frango. Pesq Agropec Bras. 2007;42:427-34.
- Pavan MA, Bloch MF, Zempulski HC, Miyazawa M, Zocoler DC. Manual de análise química de solo e controle de qualidade. Londrina: Iapar: 1992. (Circular técnica, 76).
- Pellegrino E, Di Bene C, Tozzini C, Bonari E. Impact on soil quality of a 10-year-old short-rotation coppice poplar stand compared with intensive agricultural and uncultivated systems in a Mediterranean area. Agric Ecosyst Environ. 2011;140:245-54.
- SAS Institute. SAS/STAT: user’s Guide. Version 9.2. Cary: 2009.
- Sidiras N, Derpsch R, Mondardo A. Influência de diferentes sistemas de preparo do solo na variação da umidade e rendimento da soja, em Latossolo Roxo distrófico (Oxisol). R Bras Ci Solo. 1983;7:103-6.
- Six J, Bossuyt H, Degryze S, Denef K. A history of research on the link between (micro) aggregates, soil biota, and soil organic matter dynamics. Soil Till Res. 2004;79:7-31.
- Sokal RR. Testing statistical significance of geographic variation patterns. Syst Zool. 1979;28:227-32.
- Souza RC, Cantão ME, Vasconcelos ATR, Nogueira MA, Hungria M. Soil metagenomics reveals differences under conventional and no-tillage with crop rotation or succession. Appl Soil Ecol. 2013;72:49-61.
- Strickland MS, Rousk J. Considering fungal: Bacterial dominance in soils - Methods, control, and ecosystem implications. Soil Biol Biochem. 2010;42:1385-95.
- Vance ED, Brookes PC, Jenkinson DS. An extraction method for measuring soil microbial biomass carbon. Soil Biol Biochem. 1987;19:703-7.
- Wonprasaid S. Sensitivity of soil quality indicators to soil management [These]. Lexington [KY]: University of Kentucky; 2003.
Publication Dates
-
Publication in this collection
Jul-Aug 2015
History
-
Received
17 Oct 2014 -
Accepted
19 Feb 2015