Abstracts
Tropical kudzu (Pueraria phaseoloides (Roxb.) Benth., Leguminosae: Faboideae) is native to the humid Southeastern Asia. Tropical kudzu has potential as a cover crop in regions subjected to dryness. The objective of this paper was to evaluate the effect of soil water depletion on leaflet relative water content (RWC), stomatal conductance (g) and temperature (T L) in tropical kudzu. RWC of waterstressed plants dropped from 96 to 78%, following a reduction in SWC from 0.25 to 0.17 g (H2O).g (dry soil)-1.Stomatal conductance of stressed plants decreased from 221 to 98 mmol.m-2.s-1, following the reduction in soil water content (SWC). The day after re-irrigation, g of water stressed plants was 15% lower than g of unstressed plants. Differences in T L between waterstressed and unstressed plants (deltaT L) rose linearly from 0.1 to 2.2ºC following progressive water deficit. RWC and T L of waterstressed plants paralled RWC and T L of unstressed plants the day after reirrigation. The strong decrease in SWC found in this study only induced moderate water stress in tropical kudzu. In addition, tropical kudzu recover rapidly from the induced water stress after the re-irrigation.
cover crop; ecophysiology; forage; Leguminosae; Pueraria phaseoloides; water deficit
O kudzu (Pueraria phaseoloides (Roxb.) Benth., Leguminosae: Faboideae) é uma espécie nativa do úmido sudeste asiático, com potencial para emprego como cobertura em regiões sujeitas à seca sazonal. Este trabalho objetiva avaliar os efeitos da redução no conteúdo de água do solo (SWC) sobre o conteúdo relativo de água (RWC), condutância estomática (g) e temperatura de folíolos (T L) de plantas de puerária. Redução no RWC, de 96 para 78%, e redução na g, de 221 para 98 mmol.m-2.s-1, se mostraram associadas à diminuição no conteúdo de água do solo (SWC), de 0,25 para 0,17 g (H2O).g (solo seco)-1. No dia seguinte à re-irrigação, a g de plantas estressadas era 15% inferior à g de plantas não submetidas ao estresse hídrico. A diferença em T L entre plantas estressadas e não-estressadas (deltaT L) aumentou linearmente de 0,1 para 2,2ºC com a progressão do déficit hídrico. RWC e T L de plantas estressadas e não-estressadas se igualaram no dia seguinte à re-irrigação. O forte decréscimo em SWC encontrado neste estudo induziu somente um déficit hídrico moderado em kudzu tropical. O déficit hídrico induzido em kudzu tropical pode ser rapidamente superado após a re-irrigação.
cultura de cobertura; ecofisiologia; forragem; Leguminosae; Pueraria phaseoloides; déficit hídrico
FISIOLOGIA VEGETAL
Effects of soil water depletion on the water relations in tropical kudzu
Adaucto Bellarmino de Pereira-Netto2 1 Accepted for publication on June 26, 1998. 2 Biologist, Dr., Dep. de Botânica, S. Ciências Biológicas, UFPR, CaixaPostal19031,CEP81531-970Curitiba,PR,Brazil. E-mail: apereira@bio.ufpr.br 3 Agronomist, Dr., Dep. de Fisiologia Vegetal, Univ. Estadual de Campinas, Caixa Postal 6109, CEP 13083-970 Campinas, SP, Brazil. , Antonio Celso Novaes de Magalhães3 1 Accepted for publication on June 26, 1998. 2 Biologist, Dr., Dep. de Botânica, S. Ciências Biológicas, UFPR, CaixaPostal19031,CEP81531-970Curitiba,PR,Brazil. E-mail: apereira@bio.ufpr.br 3 Agronomist, Dr., Dep. de Fisiologia Vegetal, Univ. Estadual de Campinas, Caixa Postal 6109, CEP 13083-970 Campinas, SP, Brazil. and Hilton Silveira Pinto3 1 Accepted for publication on June 26, 1998. 2 Biologist, Dr., Dep. de Botânica, S. Ciências Biológicas, UFPR, CaixaPostal19031,CEP81531-970Curitiba,PR,Brazil. E-mail: apereira@bio.ufpr.br 3 Agronomist, Dr., Dep. de Fisiologia Vegetal, Univ. Estadual de Campinas, Caixa Postal 6109, CEP 13083-970 Campinas, SP, Brazil.
ABSTRACT - Tropical kudzu (Pueraria phaseoloides (Roxb.) Benth., Leguminosae: Faboideae) is native to the humid Southeastern Asia. Tropical kudzu has potential as a cover crop in regions subjected to dryness. The objective of this paper was to evaluate the effect of soil water depletion on leaflet relative water content (RWC), stomatal conductance (g) and temperature (TL) in tropical kudzu. RWC of waterstressed plants dropped from 96 to 78%, following a reduction in SWC from 0.25 to 0.17 g (H2O).g (dry soil)-1.Stomatal conductance of stressed plants decreased from 221 to 98 mmol.m-2.s-1, following the reduction in soil water content (SWC). The day after re-irrigation, g of water stressed plants was 15% lower than g of unstressed plants. Differences in TL between waterstressed and unstressed plants (DTL) rose linearly from 0.1 to 2.2oC following progressive water deficit. RWC and TL of waterstressed plants paralled RWC and TL of unstressed plants the day after reirrigation. The strong decrease in SWC found in this study only induced moderate water stress in tropical kudzu. In addition, tropical kudzu recover rapidly from the induced water stress after the re-irrigation.
Index terms: cover crop, ecophysiology, forage, Leguminosae, Pueraria phaseoloides, water deficit.
Efeito da deficiência hídrica no solo sobre as relações hídricas em puerária
RESUMO - O kudzu (Pueraria phaseoloides (Roxb.) Benth., Leguminosae: Faboideae) é uma espécie nativa do úmido sudeste asiático, com potencial para emprego como cobertura em regiões sujeitas à seca sazonal. Este trabalho objetiva avaliar os efeitos da redução no conteúdo de água do solo (SWC) sobre o conteúdo relativo de água (RWC), condutância estomática (g) e temperatura de folíolos (TL) de plantas de puerária. Redução no RWC, de 96 para 78%, e redução na g, de 221 para 98 mmol.m-2.s-1, se mostraram associadas à diminuição no conteúdo de água do solo (SWC), de 0,25 para 0,17 g (H2O).g (solo seco)-1. No dia seguinte à re-irrigação, a g de plantas estressadas era 15% inferior à g de plantas não submetidas ao estresse hídrico. A diferença em TL entre plantas estressadas e não-estressadas (DTL) aumentou linearmente de 0,1 para 2,2oC com a progressão do déficit hídrico. RWC e TL de plantas estressadas e não-estressadas se igualaram no dia seguinte à re-irrigação. O forte decréscimo em SWC encontrado neste estudo induziu somente um déficit hídrico moderado em kudzu tropical. O déficit hídrico induzido em kudzu tropical pode ser rapidamente superado após a re-irrigação.
Termos para indexação: cultura de cobertura, ecofisiologia, forragem, Leguminosae, Pueraria phaseoloides, déficit hídrico.
INTRODUCTION
Tropical kudzu (Pueraria phaseoloides Benth) is a fast growing perennial, twining, nitrogen fixing vine, native to southeastern Asia (Bogdan, 1977). Tropical kudzu has been established in other tropical humid areas and is also a commonly used cover plant in rubber tree plantations. As a strategy to reduce damage caused by Microcyclus ulei infection, new rubber tree plantations have been established between tropical and subtropical areas seasonally subjected to fluctuations on soil water content. Tropical kudzu has potential for use as a cover crop in these new rubber tree stands; nevertheless, the seasonal reduction of the soil water content has limited interest in using tropical kudzu in recently established rubber tree plantations.
In most plants the internal water status is controlled by the relative rates of water absorption from the soil water reservoir, and water loss by transpiration to the atmosphere. In contrast, the internal water status of plants growing in dry soil depends primarily on soil water availability and only secondarily on transpiration (Erickson et al., 1991).
The objective of this paper was to describe the impact of limited water supply on leaflet relative water content, stomatal conductance and temperature of greenhouse-grown plants of tropical kudzu.
MATERIAL AND METHODS
Seeds of tropical kudzu, Pueraria phaseoloides (Roxb.) Benth., Leguminosae-Faboideae, were imbibed overnight in pre-heated (75oC) distilled water. Afterwards, the seeds were sown in five-liter plastic pots containing Haplorthox soil and placed in a naturally lit glass-house in the southeastern Brazil (lat 22o 49' S; long 47o 6' W, altitude 669 m). Fifteen days after emergence, 40 plants (measuring about 20 cm in height) were subjected to progressive water deficit (stressed) imposed by withholding irrigation. Another 40 plants (unstressed) were irrigated regularly in order to keep the soil close to its maximal water retention capacity.
Each day, one completely expanded uppermost terminal leaflet from each of three waterstressed and three unstressed plants, were used for measurements of leaflet temperature (DTL) and conductance (g). Immediately after the DTL and g measurements, the leaflet was harvested for relative water content estimation. Sampled plants were randomly selected within each treatment (water-stressed and unstressed) and no plant was selected twice.
Air temperature (Ta) was measured at 13h (solar time) with shaded R. Fuess meteorological thermometers were set over the top of the plants. Immediately after temperature measurements and just before leaflet harvesting, solar radiation flux density (St) perpendicularly incident on the leaflet surface was measured with a thermocouple pyranometer calibrated according to a standard pyranometer (Kipp & Zonen, Delft, Netherlands). During the sampling, air temperature ranged from 27.0 to 34.2oC, and solar radiation flux density from 100 to 450 W.m2.
At 13h (true solar time) leaflet temperature (TL) was measured from a distance of approximately 15 cm from the center of the adaxial surface through a BARNES 14-300 infrared thermometer (Stanford, Connecticut, U.S.A.).
Stomatal conductance (g) of the abaxial surface to water vapor flux was calculated as the reciprocal of the stomatal diffusive resistance (Rs). Rs was measured immediately after the TL measurements through an LI-700 automatic diffusive resistance porometer (LICor, Lincoln, Nebraska, U.S.A.). Rs measurements were taken on the abaxial surface at a mid position along the terminal leaflet (avoiding the main vein).
Leaflet relative water content (RWC) measurements were made by placing a five square centimeter section (avoiding the main vein) from each leaflet sampled for TL and g into a preweighed sealed vial, weighing the leaflet section plus vial, and subtracting the vial weight for the leaflet section fresh weight determination. The sample section was then floated on distilled water for four hours to obtain the saturated weight and oven dried at 80oC for 24 hours to obtain the dry weight. RWC was calculated according to Barrs (1968).
One soil sample was obtained by destructive means from each plastic pot that contained the plants used for TL, g and RWC measurements following RWC sampling. For each pot, the soil and roots were separated, the soil was mixed to ensure moisture uniformity, and a pre-weighed soil moisture can (500 mL) was filled with the soil sample. Soil water content (SWC) was estimated for each sample separately through the gravimetric method (samples were oven dried for two days, at 105oC), according to Kramer (1969). Measurements were expressed as average percent of the maximal SWC.
Water vapor pressure deficit (VPD) was calculated from water vapor pressure data for the leaflet and air temperature. Curves describing the time course change in SWC, RWC, VPD, g, St and DTL during the progressive water deficit were obtained from the entire data set recorded during this trial.
RESULTS AND DISCUSSION
Soil water content
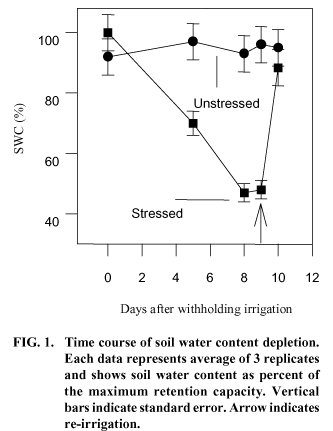
Soil water content (SWC) for the unstressed plants ranged between 92% and 97% of the maximum retention capacity [(0.36 g (H2O).g (dry soil)-1] (Fig. 1). For the water-stressed plants, SWC decreased from 100% of its maximal retention capacity, at the beginning of the experiment, to 48% [0.17 g (H2O). g (dry soil)-1] at the eighth day. Since on the ninth day without irrigation no further reduction on SWC was observed, it was decided to resume irrigation. When soil was reirrigated, SWC of the waterstressed treatment pots recovered to 88% of the soil maximal retention capacity within a day.
Relative water content
RWC remained high and stable for both water-stressed and unstressed plants, ranging between 94% and 97% until the fifth day of progressive water deficit (Fig. 2). During this period, SWC of the water-stressed treatment pots dropped from 100% to 70% of its maximum retention capacity while the deficit of vapor pressure between the leaflet and air dropped from 0.9 to 1.9 kPa (Fig. 3). Between the fifth and the ninth day of the experiment, RWC dropped from 96% to 78% for water-stressed plants, while SWC dropped from 70% to 48%. During the same period, RWC of unstressed plants diminished from 97% to 93%. A rapid and complete recovery in RWC of water-stressed plants followed re-irrigation. The day after re-irrigation both water-stressed and unstressed plants had RWC equal to 97%.
Leaf relative water content measurement characterizes the internal water status of plant tissues and is also a convenient method for following changes in tissue water content without errors caused by continually changing tissue dry weight (Erickson et al., 1991). This makes the technique especially useful for a fast growing plant such as tropical kudzu.
In two wheat genotypes growing in a medium similar in water holding capacity to a high organic-matter soil, early decline in stomatal conductance in response to reduction on SWC was considered to be responsible for the maintenance of maximum leaf RWC (Ritchie et al., 1990). Nevertheless, since no reduction in stomatal conductance was found for tropical kudzu until RWC decreased (Figs. 2 and 3), reduced g may not be responsible for the maintenance of the initial high RWC found in this species.
The maintenance of high and stable RWC, when SWC dropped from 100% to 70% of its maximal retention capacity indicates that tropical kudzu has efficient mechanisms to take water up from the soil and to transport water from the roots to the leaves. Maintenance of high RWC has been considered to be a drought-resistance rather than drought-escape mechanism, and it is a consequence of adaptative characteristics such as osmotic adjustment and/or bulk modulus of elasticity (Ritchie et al., 1990; Grashoff & Ververke, 1991). In any case, the mechanism(s) involved in the control of the tropical kudzu internal water status is an adaptative characteristic important for the successful establishment of this species in areas subjected to periods of dryness. The rapid and complete recovery of RWC after re-irrigation reinforces the idea that kudzu has an efficient mechanism to take up water from the soil and to transport this water to the leaves. This also indicates that no significant damage, such as embolism, is imposed on water transport system as consequence of this level of water deficit.
The strong decrease in SWC found during this trial induced a change in RWC that characterizes a moderate water stress, according to Hsiao (1973). This fact, together with the maintenance of high RWC while SWC decreased from 100% to 70% of the soil maximum retention capacity, and the pattern of RWC recovery after irrigation suggest that tropical kudzu can be established in environments where water supply is lower than in its natural habitat.
Stomatal conductance
Stomatalconductance,likeRWC,was similar for both water-stressed and unstressed plants until the fifth day (Fig. 4). Between the fifth and the eighth day a 50% increase in g of unstressed plants was observed, following a three fold increase in solar radiation flux density from 123 to 307 W.m-2 (Fig. 5). During the same period g of stressed plants decreased 30%. By the ninth day of progressive water deficit, g of water-stressed plants was three times lower than g of unstressed plants. The day after re-irrigation g of water-stressed plants was 15% lower than g of unstressed plants.
A decrease in RWC, similar to the one found in this study, induced larger reductions in g in other species, including a 10 fold decrease in both field-grown Trifolium repens cv. Haifa (Guobin & Kemp, 1992) and greenhouse-grown Triticum aestivum (Ritchie et al., 1990). Stomatal sensitivity to drought is dependent on quantitative changes in tissue abscisic acid content and other metabolic processes such as osmotic adjustment. This sensitivity is also a key point for the control of internal water status, photosynthetic rates and leaf temperature, thus influencing adaptation to temperature and water stress (Ekanayake et al., 1993).
A causal relationship between leaf water status and stomatal conductance has been reported (Ekanayake et al., 1993). However, since no significant change in g was found until substantial change (from 96% to 86%) in RWC was observed, the leaf-derived mechanism controlling g may be more important than an eventual root-derived mechanism for this species.
Reductions in stomatal conductance limit the access of the photosynthetic apparatus to CO2 before the photosynthetic apparatus is damaged by water deficit (Pereira et al., 1992). Nevertheless, reduction in water loss usually is followed by smaller reductions in incoming CO2 (due to the action of the carboxylation enzymes), which results in enhanced water use efficiency (Grashoff & Ververke, 1991; Pereira et al., 1992). In addition, in environments where water is a limiting factor, reduction in water loss can be more important than increase in dry weight, improving competitiveness of the water-saver species.
Leaflet temperature
Fig. 6 shows the time course in the difference of leaflet temperature between water-stressed and unstressed plants (DTL). A linear increase in DTL was found during the progressive depletion of soil water. DTL rose from 0.1oC, at the beginning of the experiment to 2.2oC on the ninth day. The day after re-irrigation of the water-stressed plants, DTL dropped to the same value found at the beginning of the experiment.
Greenhouse-grown plants of Phaseolus vulgaris L. subjected to a water deficit have shown leaf temperatures 4.1oC higher than the leaf temperatures of unstressed plants (Lima Filho, 1983). For field-grown plants of Phaseolus vulgaris the differenceinleaftemperaturebetween water-stressed and unstressed plants (DTL) reached 3.0oC (Walter & Hatfield, 1979). For field-grown Glycine max L., Jung & Scott (1980) have found that maximum DTL reached 5.5oC. Nevertheless, Cox & Jolliff (1987) have found that maximum DTL for field-grown plants of Glycine max was 10.0oC. The differences in the results found for Glycine max must reflect differences such as air temperature and solar radiation flux density between the two study sites rather than differences in the level of water stress between irrigated and non-irrigated plants since the difference in leaf water potential between water-stressed and non water-stressed plants was about the same (0.3 MPa) in both studies.
Higher TL in plants subjected to water deficit, when compared to unstressed plants, has been attributed to an eventual decrease in the transpiration rate due to reduced g in water-stressed plants (Jung & Scott, 1980; Turner & Begg, 1981; Hashimoto et al., 1984; Hatfield et al., 1987). Elevation in leaf temperature increases membrane permeability and ammonia release (from protein degradation), which is considered to be responsible for most of the leaf damage induced by drought (Grace et al., 1980; Henson et al., 1982).
Since tropical kudzu shows a smaller increase in DTL when compared to other crop species submitted to similar water deficits and because crop plants usually do not show special features for protection against excessively high temperature, it would be expected that tropical kudzu would have relatively less heat-damaged tissue than other cover crop species when subjected to the same level of soil water depletion. To conclude, a potentially reduced tissue damage, especially in the photosynthetic organs confer additional advantage in selecting tropical kudzu as the cover crop of choice due to its eventually higher CO2 assimilation rates compared to other cover crops submitted to the same level of water stress.
The results obtained in this study suggest that water supply should not be a major factor limiting the establishment of tropical kudzu in southeastern Brazil.
CONCLUSIONS
1.Strong decrease in soil water content only induce moderate increase in leaflet temperature and moderate reduction on leaflet relative water content and stomatal conductance in tropical kudzu.
2.Values of leaflet relative water content, stomatal conductance and temperature of water-stressed plants were close to the values found for non stressed plants soon after re-irrigation.
ACKNOWLEDGEMENTS
To Dr. J. D. Hay and Dr. O. G. Rocha-Neto for their support and encouragement during this study, and to Dr. David D. Ellis for critically reviewing the manuscript.
REFERENCES
- BARRS, H.D. Determination of water deficits in plant tissues. In: KOZLOWSKI, T.T. (Ed.). Water deficits and plant growth. New York: Academic, 1968. p.235-368.
- BOGDAN, A.V. Tropical pasture and fodder plants: grasses and legumes. London: Longman, 1977. 475p.
- COX, W.J.; JOLLIFF, G.D. Crop water relations of sunflower and soybean under irrigated and dryland conditions. Crop Science , v.27, p.553-557, 1987.
- EKANAYAKE, I.J.; DE DATTA, S.K.; STEPONKUS, P.L. Effect of water deficit stress on diffusive resistance, transpiration,and spikelet desiccation of rice (Oryza sativa L.). Annals of Botany, v.72, p.73-80, 1993.
- ERICKSON, P.I.; KETRING, D.L.; STONE, J.F. Response of internal tissue water balance of peanut to soil water. Agronomy Journal, v.83, p.248-253, 1991.
- GRACE, J.; FASEHUN, F.E.; DIXON, M. Boundary layer conductance of the leaves of some tropical timber trees. Plant Cell and Environment, v.3, p.443-450, 1980.
- GRASHOFF, C.; VERVERKE, D.R. Effect of pattern of water supply on Vicia faba L. 3. Plant water relations, expansive growth and stomatal reactions. Netherlands Journal of Agricultural Science, v.39, p.247-262, 1991.
- GUOBIN, L.; KEMP, D.R. Water stress affects the productivity, growth components, competitiveness and water relations of Phalaris and white clover growing in a mixed pasture. Australian Journal of Agricultural Research, v.43, p.659-672, 1992.
- HASHIMOTO, Y.; INO, T.; KRAMER, P.J.; NAYLOR, A.W.; STRAIN, B.R. Dynamic analysis of water stress of sunflower leaves by means of a thermal image processing system. Plant Physiology, v.76, p.266-269, 1984.
- HATFIELD, J.L.; QUISENBERRY, J.E.; DILBECK, R.E. Use of canopy temperatures to identify water conservation in cotton germplasm. Crop Science , v.27, p.269-273, 1987.
- HENSON, I.E.; ALAGARSWAMY, G.; BIDINGER, F.R.; MAHALAKSHMI, V. Stomatal responses of pearl millet to leaf water status and environmental factors in the field. Plant Cell and Environment, v.5, p.65-74, 1982.
- HSIAO, T.C. Plant responses to water stress. Annual Review of Plant Physiology, v.24, p.519-570, 1973.
- JUNG, P.K.; SCOTT, H.D. Leaf water potential, stomatal resistance and temperature relations in field-grown soybeans. Agronomy Journal, v.72, p.986-990, 1980.
- KRAMER, P.J. Plant and soil water relationships: a modern synthesis. New York: McGraw-Hill, 1969. 482p.
- LIMA FILHO, J.M.P. Temperatura foliar de genótipos de feijão sob duas condições de umidade do solo. Pesquisa Agropecuária Brasileira, Brasília, v.18, n.7, p.703-706, 1983.
- PEREIRA, J.S.; CHAVES, M.M.; FONSECA, F.; ARAÚJO, M.C.; TORRES, F. Photosynthetic capacity of leaves of Eucalyptus globulus (Labill.) growing in the field with different nutrient and water supplies. Tree Physiology, v.11, p.381-389, 1992.
- RITCHIE, S.W.; NGUYEN, H.T.; HOLADAY, A.S. Leaf water content and gas-exchange parameters of two wheat genotypes differing in drought resistance. Crop Science, v.30, p.105-111, 1990.
- TURNER, N.C.; BEGG, J.E. Plant-water relations and adaptation to stress. Plant and Soil, v.58, p.97-131, 1981.
- WALTER, G.K.; HATFIELD, J.L. Test of the stress-degree-day concept using multiple planting dates of red kidney beans. Agronomy Journal, v.71, p.967-971, 1979.
Publication Dates
-
Publication in this collection
14 Oct 2011 -
Date of issue
July 1999
History
-
Received
26 June 1998