Abstracts
The objective of this work was to assess the effects of pH and ionic strength upon zinc adsorption, in three highly weathered variable charge soils. Adsorption isotherms were elaborated from batch adsorption experiments, with increasing Zn concentrations (0-80 mg L-1), and adsorption envelopes were constructed through soil samples reactions with 0.01, 0.1 and 1 mol L-1 Ca(NO3)2 solutions containing 5 mg L-1 of Zn, with an increasing pH value from 3 to 8. Driving force of reaction was quantified by Gibbs free energy and separation factor. Isotherms were C-, H- and L-type and experimental results were fitted to nonlinear Langmuir model. Maximum adsorption ranged from 59-810 mg kg-1, and Zn affinity was greater in subsoil (0.13-0.81 L kg-1) than in the topsoil samples (0.01-0.34 L kg-1). Zinc adsorption was favorable and spontaneous, and showed sharply increase (20-90%) in the 4-6 pH range. No effect of ionic strength was observed at pH values below 5, because specific adsorption mechanisms predominated in the 3-5 pH range. Above pH 5, and in subsoil samples, Zn was adsorbed by electrostatic mechanisms, since ionic strength effect was observed. Despite depth and ionic strength effects, Zn adsorption depends mainly on the pH.
pH; adsorption envelope; adsorption isotherm; ionic strength; trace element
O objetivo deste trabalho foi avaliar o efeito do pH e da força iônica sobre a adsorção de zinco, em três solos altamente intemperizados, com predomínio de cargas variáveis. A partir de experimentos tipo " batch" , foram elaboradas isotermas de adsorção, com quantidades crescentes de Zn (0-80 mg L-1), e envelopes de adsorção foram feitos pela reação de amostras de terra com soluções de Ca(NO3)2 0,01, 0,1 e 1 mol L-1 e 5 mg L-1 de Zn, submetidas a variações de pH (3-8). A força direcional da reação de adsorção de Zn foi estimada pela energia livre de Gibbs e pelo fator de separação. As isotermas foram do tipo C, H e L, e os resultados experimentais ajustaram-se ao modelo de Langmuir. A adsorção máxima variou de 59 a 810 mg kg-1, e o coeficiente de afinidade foi maior em subsuperfície (0,13-0,81 L kg-1) do que em superfície (0,01-0,34 L kg-1). A adsorção de Zn foi favorável e espontânea, e mostrou nítido aumento (20-90%) no intervalo de pH entre 4 e 6. Não houve efeito da força iônica nos valores de pH abaixo de 5, o que indica o predomínio de mecanismos de adsorção específica na faixa de pH 3-5. Acima de pH 5 e no subsolo, o Zn foi adsorvido por mecanismos eletrostáticos, já que foi observado efeito da força iônica. Apesar dos efeitos da profundidade e da força iônica, a adsorção de Zn depende principalmente do pH.
pH; envelope de adsorção; isoterma de adsorção; força iônica; elemento-traço
SOIL SCIENCE
Zinc adsorption in highly weathered soils
Adsorção de zinco em solos altamente intemperizados
José Carlos Casagrande; Marcio Roberto Soares; Ernesto Rinaldi Mouta
Universidade Federal de São Carlos, Centro de Ciências Agrárias, Departamento de Recursos Naturais e Proteção Ambiental, Rodovia Anhangüera, Km 174, Caixa Postal 153, CEP 13600-970 Araras, SP, Brazil. E-mail: bighouse@power.ufscar.br, mrsoares@cca.ufscar.br, ermouta@cca.ufscar.br
ABSTRACT
The objective of this work was to assess the effects of pH and ionic strength upon zinc adsorption, in three highly weathered variable charge soils. Adsorption isotherms were elaborated from batch adsorption experiments, with increasing Zn concentrations (080 mg L-1), and adsorption envelopes were constructed through soil samples reactions with 0.01, 0.1 and 1 mol L-1 Ca(NO3)2 solutions containing 5 mg L-1 of Zn, with an increasing pH value from 3 to 8. Driving force of reaction was quantified by Gibbs free energy and separation factor. Isotherms were C-, H- and L-type and experimental results were fitted to nonlinear Langmuir model. Maximum adsorption ranged from 59810 mg kg-1, and Zn affinity was greater in subsoil (0.130.81 L kg-1) than in the topsoil samples (0.010.34 L kg-1). Zinc adsorption was favorable and spontaneous, and showed sharply increase (2090%) in the 46 pH range. No effect of ionic strength was observed at pH values below 5, because specific adsorption mechanisms predominated in the 35 pH range. Above pH 5, and in subsoil samples, Zn was adsorbed by electrostatic mechanisms, since ionic strength effect was observed. Despite depth and ionic strength effects, Zn adsorption depends mainly on the pH.
Index terms: pH, adsorption envelope, adsorption isotherm, ionic strength, trace element.
RESUMO
O objetivo deste trabalho foi avaliar o efeito do pH e da força iônica sobre a adsorção de zinco, em três solos altamente intemperizados, com predomínio de cargas variáveis. A partir de experimentos tipo " batch" , foram elaboradas isotermas de adsorção, com quantidades crescentes de Zn (080 mg L-1), e envelopes de adsorção foram feitos pela reação de amostras de terra com soluções de Ca(NO3)2 0,01, 0,1 e 1 mol L-1 e 5 mg L-1 de Zn, submetidas a variações de pH (38). A força direcional da reação de adsorção de Zn foi estimada pela energia livre de Gibbs e pelo fator de separação. As isotermas foram do tipo C, H e L, e os resultados experimentais ajustaram-se ao modelo de Langmuir. A adsorção máxima variou de 59 a 810 mg kg-1, e o coeficiente de afinidade foi maior em subsuperfície (0,130,81 L kg-1) do que em superfície (0,010,34 L kg-1). A adsorção de Zn foi favorável e espontânea, e mostrou nítido aumento (2090%) no intervalo de pH entre 4 e 6. Não houve efeito da força iônica nos valores de pH abaixo de 5, o que indica o predomínio de mecanismos de adsorção específica na faixa de pH 35. Acima de pH 5 e no subsolo, o Zn foi adsorvido por mecanismos eletrostáticos, já que foi observado efeito da força iônica. Apesar dos efeitos da profundidade e da força iônica, a adsorção de Zn depende principalmente do pH.
Termos para indexação: pH, envelope de adsorção, isoterma de adsorção, força iônica, elemento-traço.
Introduction
Zinc (Zn) is an internal transition heavy metal that enters the environment as a result of both natural and anthropogenic activities. Concentrations of Zn released into the soil by natural pedogenic process are largely related to origin and nature of parent material. Generally, Zn concentration range of noncontaminated soils varies from 10 to 300 mg kg-1 soil (Ohnesorge & Wilhelm, 1991). In highly weathered soils, Zn natural reserve is very low and its deficiency has imposed several limitations on the absolute development of economically important crops in the intertropical zone (Takkar & Walker, 1993). In plants, Zn acts as a catalyzing component of more than 300 enzymes, and it is necessary for triptophane, a precursor amino acid of indoleacetic acid (IAA), a growth promoting plant hormone (Mengel & Kirkby, 2001).
The increase in Zn redistribution rates among the different compartments of the ecosystem results mainly from anthropogenic sources, derived from atmospheric emissions, improper application of agricultural chemicals, or from soil use as the final receiver of sewage sludge and domestic and industrial waste waters. This has created an increase in the quantity of Zn released in the soil, and increased its potential for entrance in the food chain in toxic concentration, either by plant absorption or through leaching to the groundwater (Covelo et al., 2004; Arias et al., 2005).
Interest has recently grown in the phenomena of metallic ion adsorption by surfaces with pH-dependent charges, in order to understand the role of these contaminants in tropical ecosystems, from the agricultural, environmental and public health points of view. In a complex system such as the soil, especially those contaminated by heavy metals, chemical and also mineralogical properties regulate the speciation, mobility and bioavailability of these contaminants (Covelo et al., 2004). Colloidal constituents, typical of soils with variable charges, such as kaolinite, Fe, and Al oxy(hydr)oxides have been indicated as the components responsible for the chemical behavior of Zn in this medium (Düker et al., 1995). These mineral surfaces base several phenomena, especially adsorption, which occurs at the solid-solution interface and is influenced by soil solution properties, such as the pH and ionic strength (I) (Naidu et al., 1994; Casagrande et al., 2004).
The pH is the primary factor that governs adsorption and the heavy metal availability, due to alteration in the metal species in solution and to definition of the net charge in adsorption surface (McBride & Blasiak, 1979; Kuo & Baker, 1980; Harter, 1983; Machado & Pavan, 1987; Casagrande et al., 2004). Studies related to the ionic strength are appropriate to understand the stability, nature and intensity of the metal adsorption phenomenon, because they allow the distinction between specific and electrostatic mechanisms (Shuman, 1986; Naidu et al., 1994; Pierangeli et al., 2003), which are not yet fully understood for Zn.
Adsorption isotherms have been widely used in studies on adsorption phenomena, supplying numerical parameters that inform about the retention capacity and the intensity in which the metal is retained by the soil (Hinz, 2001). Langmuir isotherm has been shown to be suitable for Zn adsorption studies (Pombo & Klamt, 1986; Machado & Pavan, 1987; Cunha et al., 1994; Nascimento & Fontes, 2004; Arias et al., 2005). However, metal adsorption modeling in tropical soils is rarely accompanied by a more detailed approach based on thermodynamics, which can serve as measure of the extension and spontaneity of the adsorption reaction (Silveira et al., 1999; Dias et al., 2003; Soares et al., 2005).
While there have been numerous studies on the nature of Zn interactions with pure mineral systems or with soils from temperate regions (Shuman, 1986; Düker et al., 1995; Covelo et al., 2004), few reports have been devoted to studying these reactions with variable charge soils (Casagrande et al., 2004). Considering the interest in understanding Zn adsorption phenomenon in soils with variable charges, and the growing search for sustainable strategies that respect the multifunctionality of the soil, the objectives of this study were to investigate the influence of pH and ionic strength on Zn adsorption reaction by highly weathered variable charge soils.
Materials and Methods
Topsoil (0.00.2 m) and subsoil (diagnostic B horizon) samples were collected from two acric Oxisols [heavy clayey-textured Anionic " Rhodic" Acrudox (RA) and medium-textured Anionic " Xanthic" Acrudox (XA)], and of an Alfisol [heavy clayey-textured Rhodic Hapludalf (RH)] (Soil Survey Staff, 1999), derived from basalt, in two locations in the State of São Paulo, Brazil (Ribeirão Preto 21°10'S, 47°48'W; Guaíra 20°19'S, 48°18'W).
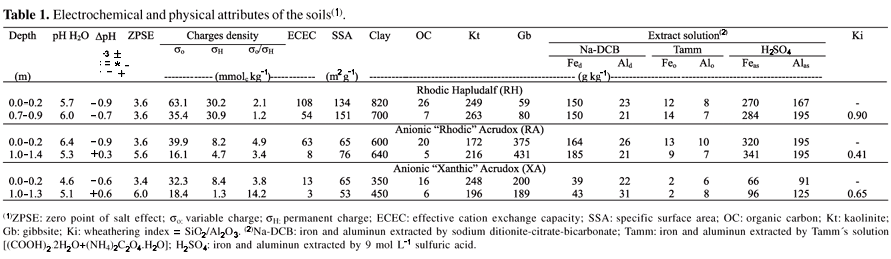
Chemical, physical and mineralogical characterization of the samples was performed according to Camargo et al. (1986) and Raij et al. (2001). Additionally, samples were investigated using the cesium adsorption method, which measures both permanent and variable charges and is based on the preference of Cs+ over Li+ in the siloxane surface sites, and on the lower selectivity of ionizable surface group for the Cs ion (Weber et al., 2005). Some properties of the soil samples are given in Table 1.
Laboratory batch mode adsorption experiments were carried out after adding 20 mL Ca(NO3)2 electrolytic solution, containing different initial concentration of Zn (C0) in the form of hydrated nitrate salt [Zn(NO3)2.4H2O], at 2 g of fine air-dried soil samples (1:10 soil:solution ratio). The soil-solution system was placed in polyethylene flasks, under agitation (150 osc min-1) for 24 hours at 24± 2ºC. The suspension was filtered, and Zn concentration remaining in the supernatant was determined by atomic absorption spectrophotometry. Adsorption isotherms were elaborated for soil samples at natural pH from experiments with increasing Zn concentrations (0, 1, 2, 5, 10, 15, 20, 25, 30, 40 and 80 mg L-1), with 0.01 mol L-1 Ca(NO3)2 as electrolytic support. Under similar experimental conditions, adsorption envelopes were constructed by the %Znads vs. pH plot, after adjusting the pH by adding HCl or NaOH 4 mol L-1 in the suspensions containing 5 mg L-1 Zn in Ca(NO3)2 0.01, 0.1 and 1 mol L-1 electrolyte support, for the simultaneous assessment of the pH and ionic strength effects on Zn adsorption reaction.
The quantity of adsorbed Zn ([Zn]ads) and the adsorption percentage (%Znads) were calculated by the equations [Zn]ads = ([Zn]0 - [Zn]eq)(V/M) and %Znads = 100{([Zn]0 - [Zn]eq)/[Zn]0}, respectively, in which: [Zn]ads is the quantity (mg kg-1) of Zn adsorption after equilibrium; [Zn]0 and [Zn]eq are the initial added and the equilibrium concentrations (mg L-1), respectively; V is the solution volume (mL); M is the mass of soil sample (g). All determinations were run in triplicate.
Adsorption isotherms ([Zn]ads vs. [Zn]eq) were constructed, and the Zn adsorption was compared with that estimated by the nonlinear form of Langmuir isotherm [Zn]ads = Adsmax (KL[Zn]eq/1 + K L[Zn]eq), in which: KL is the parameter related to the soil affinity for Zn (L kg-1); Adsmax is the maximum Zn adsorption capacity (mg kg-1). The Langmuir isotherm was fitted by the Fitfunc and Fitfun.bas softwares (Barrow, 1987), which used the nonlinear optimization of the least squares and did not require the linearization of the isotherm, which avoided both the introduction of changes in the error distribution and acquisition of influenced parameters. Preliminary data concerning the degree of development and spontaneity of the adsorption reaction was obtained from assessing the equilibrium parameter or the KR separation factor, a dimensionless constant that indicates whether the adsorption reaction was favorable by the ratio KR = 1/(1 + KL C0), in which: KL is the affinity constant, estimated by the Langmuir equation (Ho et al., 2002; Singh & Pant, 2004; Soares et al., 2005). The spontaneity of adsorption reactions was also described mathematically by determining the Gibbs free energy DG = RT(logCeq - logC0), in which: DG is the variation in the free energy (J mol-1); R is the universal constant of the gases (8.314 J mol-1 K-1 ); T is the absolute temperature (K) (Silveira et al., 1999; Dias et al., 2003; Soares et al., 2005).
Results and Discussion
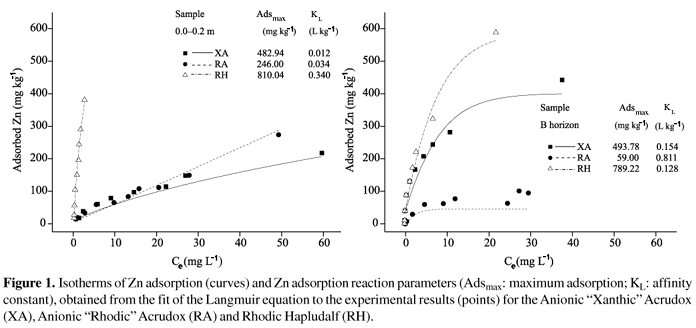
Adsorption isotherms for weathered soils, at natural pH and at constant ionic strength [0.01 mol L-1 Ca(NO3)2], showed increase in Zn adsorption with the increase in the initial concentration (Figure 1). The isotherm shape was characteristic for each sample and exhibited different Zn adsorption pattern, evaluated according to the classification proposed by Giles et al. (1974). Zinc adsorption by the Anionic " Xanthic" Acrudox, and by Anionic " Rhodic" Acrudox topsoil samples was best represented by C-type isotherms (C = constant), with a constant slope along the Zn concentration range. This type of isotherm was not expected, because it shows that the availability of sites for adsorption remained constant, regardless of the initial Zn concentration. A very similar shape was observed for the Rhodic Hapludalf topsoil sample, with strong initial slope characterizing H-type isotherm, as a result of the strong interaction with soil particles. Casagrande et al. (2004) reported that Zn adsorption by topsoil and subsoil samples from a Brazilian acric Oxisol was adequately represented by H-type isotherms, when pH was close to 7. Agbenin & Olojo (2004) reported that Zn adsorption by samples from the topsoil of an Alfisol also produced H-type isotherms.
The L-type isotherm, characterized by decrease in adsorption as the adsorption surface becomes saturated, was more common for subsoil samples. With the increase in the saturation level, the colloidal surface may become completely covered after the formation of a monomolecular layer of the adsorbate, and the isotherm assumes an asymptotic performance, such as that observed for the two Oxisols. In the subsoil, the affinity of Rhodic Hapludalf for the adsorbate decreased after the additions of high initial Zn concentrations, when the isotherm manifested the L-type performance. However, not even the greatest quantity of Zn added could saturate the sites to the point of causing the surface covering and, thus, the asymptote was not observed.
Langmuir equation adequately simulated the experimental Zn adsorption results, as reported previously (Cunha et al., 1994; Casagrande et al., 2004; Nascimento & Fontes, 2004). Experimental results obtained for Rhodic Hapludalf showed greater adherence to the Langmuir isotherm. The Oxisols showed small deviations between the observed and simulated data with the increase in the Zn concentration. The maximum adsorption parameter (Adsmax) and the affinity constant (KL), indicated that Zn adsorption varied according to the soil and to the sampling depth. The lowest and highest Adsmax values were verified for Anionic " Rhodic" Acrudox, in the subsoil, and for Rhodic Hapludalf, in the topsoil samples, respectively. The Adsmax values were from 246 to 810 mg kg-1, for the topsoil samples, and from 59 to 789 mg kg-1, in the subsoil. The results were in line with those reported by Pombo & Klamt (1986) in an Alfisol (698 mg kg-1), but were less than those reported by Casagrande et al. (2004) in an acric Oxisol (1,362 mg kg-1). The affinity constant (KL) indicated that the Zn adsorption reaction proceeded more strongly in the subsoil layers.
There was strong dependence on Zn adsorption in relation to the pH, regardless of the soil or sampling depth. Zinc adsorption increased drastically with the increase in pH, varying from 20 to 90% in a small pH interval (from 4 to 6). In fact, there is a critical interval for solid-solution interfaces, generally less than two pH units, where the metal adsorption percentage can increase clearly from an extremely low value, at low pH, to up to 100%, at high pH values. This critical interval, known as the adsorption edge, is graphically represented in the adsorption envelopes (Sposito, 1984) (Figure 2). Harter (1983) reported that metal adsorption was pH-dependent and that the intensity of the phenomena increased drastically above 7. Rhodic Hapludalf adsorbed more than 80% at pH 4.5 and 50% at pH 3.5, in the topsoil and subsoil samples, respectively. Zinc was adsorbed at lower pH values of the Rhodic Hapludalf samples, probably as a result of the manifestation of permanent charges that did not depend on the pH variation (Table 1). However, Zn adsorption can be reduced at low pH values due to competition with the cations from the support electrolyte for exchange sites (McBride & Blasiak, 1979). The occurrence of Zn adsorption even at pH values below zero point of salt effect (ZPSE) (Table 1) was also attributed to the manifestation of specific adsorption mechanisms, especially in the case of the Oxisols (Kuo & Baker, 1980).
Approximately 20% of Zn was adsorbed immediately at pH 3 in all the subsoil samples. Soil pH is the main factor that determines Zn adsorption, because it is related to the hydrolysis constant of the metal ions (Harter, 1983; Covelo et al., 2004). Practically all the added Zn (5 mg L-1) disappeared from the soil solution, when the pH was about 7, regardless of the soil or depth. Naidu et al. (1994) studied highly weathered soils dominated by sesquioxides, and reported 100% metal adsorption when the pH was greater than 6. This occurred at high pH values, because the value of Zn first hydrolysis constant (pKa1) is around 9.
At the highest pH values obtained in this study, the presence of the ZnOH+ species was suggested, while the Zn(OH)20 species was considered of limited occurrence. The ZnOH+ species concentration increase tenfold at each increase in pH unit and, since ZnOH+ performs as a monovalent ion, the energetic barrier that must be overcome, when it comes closer to the surface of the particle, is smaller than in the case of the Zn2+ ion. Furthermore, the quantity of adsorption sites with greater affinity for Zn increase with the rise in pH (Casagrande et al., 2004).
The proportion of adsorbed metal ions increased uniformly and continuously with the increase in pH, but the shape of the curve and the position of the adsorption edges depended on the soil identity, especially concerning its mineralogical constitution. The adsorption pattern was very similar for the Oxisol samples, as showed by the shape and overlapping positioning of the adsorption edges. The adsorption edges that represented Zn adsorption by the Oxisols presented typical sigmoid shape, with characteristic S-type adsorption curve especially regarding their positioning. In these soils, Zn retention was characterized initially by a small slope in the pH range between 3 and 4, both on the topsoil and at depth, when about 10 to 20% Zn was adsorbed. The atypical shape of the Rhodic Hapludalf adsorption edge suggested that Zn adsorption was qualitatively different, when compared to the Oxisols. The adsorption edge is a function of the affinity of a given type of adsorption surface, and it is normally argued that this affinity is high, when adsorption occurs at low pH values (Harter, 1983), as verified for Rhodic Hapludalf.
The KR values were between 0 and 1 for all the samples (Figure 3). Following a simplified approach (Singh & Pant, 2004; Soares et al., 2005), adsorption is favorable and spontaneous when KR<1, while values of KR>1 indicate absence of spontaneity in the reaction. The KR coefficient can further indicate whether the adsorption isotherm is unfavorable to the process (KR>1), linear (KR = 1), favorable (0<KR<1) or irreversible (KR = 0) (Ho et al., 2002). Thereby, the KR values showed that the adsorption reaction was spontaneous and favorable, regardless of sampling depth. The lower KR values were obtained for the higher Zn quantities added. This indicated that the adsorption reaction was more favorable at the higher initial concentrations (C0), indicating further that all the soils were efficient systems for Zn retention, even with some variations in consequence of depth in the following soil sequence: RH>XA>RA on the topsoil, and RA>XA@RH in the subsoil. High and constant KR values close to 1 indicated that Zn adsorption by the Anionic " Rhodic" Acrudox topsoil samples was less favored or spontaneous. In contrast, the spontaneity of the Zn adsorption reaction was drastically increased in the subsoil samples. This performance indicated that Zn adsorption developed more spontaneously in the absence of organic material, especially in the Oxisols, ratifying data obtained from the affinity constant (KL) (Figure 1). For Rhodic Hapludalf, the separation factor was not a very sensitive parameter for sampling depth, probably because the soil has a more reactive mineralogy, and its electric charge of permanent nature is less influenced by the organic matter.
The Gibbs free energy (DG) is a measure of the extension or force that guides the adsorption reaction (Soares et al., 2005). The DG values were negative and confirmed the feasibility and spontaneity of Zn adsorption (Figure 4), with energy released for conversion of the less stable Zn forms in solution to adsorbed forms with greater stability. Similar results were reported for Cd (Dias et al., 2003) and Cu (Silveira et al., 1999) adsorption by highly weathered soils. Regardless of soil or sampling depth, DG values increased with the pH, indicating that the phenomenon became more thermodynamically spontaneous with this increase. Also with the increase in pH, the DG became more negative implicating an increase in the driving force for the adsorption reaction to take place.
At pH less than 5, Zn adsorption was most favored in the Rhodic Hapludalf topsoil sample. Probably, the higher organic matter and clay contents, combined with a greater specific surface offered by the phyllosilicates of 2:1 type, still present in Rhodic Hapludalf (Table 1), may have optimized the contact between the metal cations present in solution and the surface of the solid phase. In the Oxisols, low pH values (35) did not cause significant increases in DG values. However, a clear increase in the DG of Zn adsorption by Anionic " Rhodic" Acrudox and by Anionic " Xanthic" Acrudox occurred in the pH interval between 5 and 6.5.
Zinc adsorption was also evaluated, regarding alterations in the electrolyte support concentration, using DG as the numerical parameter for the reaction.
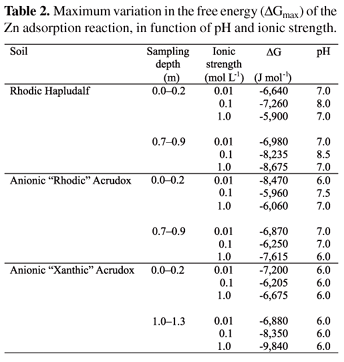
No effect of ionic strength was observed in the DG values for Zn adsorption at pH values below 5. However, starting at this value, its effect depended on the depth. Zinc can be adsorbed either by electrostatic mechanisms or specific mechanisms on Fe and Al oxy(hydr)oxides surfaces (Arias et al., 2005). However, inner-sphere type complex formation has been shown to be the most frequently occurring mechanism, and ions that are specifically adsorbed are less influenced by variation in the ionic strength (Shuman, 1986). The maximum variation in the Zn adsorption free energy (DGmax) also depended on the pH (Table 2). The Zn adsorption reaction by the Oxisols and Alfisol samples, at ionic strength 0.01 mol L-1 Ca(NO3)2, reached a maximum spontaneity close to pH 6 and 7, respectively. The increase in the electrolytic support concentration caused a variation in pH values, where DGmax was reached in the Anionic " Rhodic" Acrudox and Rhodic Hapludalf samples. In the topsoil samples, the DGmax decreased with increase in ionic strength, and the greatest values were between -6,640 (Rhodic Hapludalf) and -8,470 J mol-1 (Anionic " Rhodic" Acrudox) (Table 2), at ionic strength 0.01 mol L-1 Ca(NO3)2. These results corroborated reports by Pierangeli et al. (2003), who attributed the reduction in adsorption with the increase in ionic strength to competition among ions for exchange sites. In contrast, large increases in DGmax would be observed, when ionic strength increased on the subsoil samples. At the electrolytic support concentration of 1 mol L-1, the variation of DGmax values was between -7,615 (Anionic " Rhodic" Acrudox) and -9,840 J mol-1 (Anionic " Xanthic" Acrudox). This fact may have occurred because of the inorganic fraction greater exposure to reactions involving inner sphere-type mechanisms (Düker et al., 1995), that is, of greater energy.
Conclusions
1. Langmuir equation adequately simulates the experimental results of adsorbed Zn, and the model can be used to prognosticate Zn performance in soils with similar attributes to those in this study.
2. Zn adsorption depends, mainly, on the pH.
3. Greater adsorption capacity can be reached in the topsoil layers, but Zn affinity in subsoil is larger, especially in response to alterations in the pH.
4. In the subsoil, Zn adsorption reaction is stronger.
5. At pH values below 5, ionic strength have no effect on Zn adsorption; from pH 5, Zn adsorption decrease, with increase in ionic strength, and this effect allows to distinguish specific and eletrostatic Zn adsorption mechanisms.
Acknowledgements
To Fundação de Amparo à Pesquisa do Estado de São Paulo, for financial support.
Received on October 15, 2007 and accepted on December 13, 2007
- AGBENIN, J.O.; OLOJO, L.A. Competitive adsorption of copper and zinc by a Bt horizon of a savanna Alfisol as affected by pH and selective removal of hydrous oxides and organic matter. Geoderma, v.119, p.85-95, 2004.
- ARIAS, M.; PÉREZ-NOVO, C.P.; OSORIO, F.; LÓPEZ, E.; SOTO, B. Adsorption and desorption of copper and zinc in the surface layer of acid soils. Journal of Colloid and Interface Science, v.288, p.21-29, 2005.
- BARROW, N.J. Reactions with variable-charge soils Dordrecth: Martinus Nijhoff Publishers, 1987. 191p.
- CAMARGO, O.A.; MONIZ, A.C.; JORGE, J.A.; VALADARES, J.M.A.S. Métodos de análise química, mineralógica e física de solos do Instituto Agronômico de Campinas Campinas: IAC, 1986. 94p. (Boletim Técnico, 106).
- CASAGRANDE, J.C.; ALLEONI, L.R.F.; CAMARGO, O.A.; ARNONE, A.D. Effects of pH and ionic strength on zinc sorption by a variable charge soil. Communications in Soil Science and Plant Analysis, v.35, p.2087-2095, 2004.
- COVELO, E.F.; ÁLVAREZ, N.; ANDRADE COUCE, M.L.; VEGA, F.A.; MARCET, P. Zn adsorption by different fractions of Galician soils. Journal of Colloid and Interface Science, v.280, p.343-349, 2004.
- CUNHA, R.C.A.; CAMARGO, O.A.; KINJO, T. Aplicação de três isotermas na adsorção de zinco em Oxissolos, Alfissolos e Ultissolos. Revista Brasileira de Ciência do Solo, v.18, p.15-20, 1994.
- DIAS, N.M.P.; ALLEONI, L.R.F.; CASAGRANDE, CAMARGO, O.A. Energia livre da reação de adsorção de cádmio em Latossolos ácricos. Ciência Rural, v.33, p.829-834, 2003.
- DÜKER, A.; LEDIN, A.; KARLSSON, S.; ALLARD, B. Adsorption of zinc on colloidal (hydr)oxides of Si, Al and Fe in the presence of a fulvic acid. Applied Geochemistry, v.10, p.197-205, 1995.
- GILES, C.H.; SMITH, D.; HUITSON, A. A general treatment and classification of the solute adsorption isotherm. I. Theoretical. Journal of Colloid and Interface Science, v.47, p.755-765, 1974.
- HARTER, R.D. Effect of soil pH on adsorption of lead, copper, zinc, and nickel. Soil Science Society of America Journal, v.47, p.47-51, 1983.
- HINZ, C. Description of sorption data with isotherm equations. Geoderma, v.99, p.225-243, 2001.
- HO, Y.S.; HUANG, C.T.; HUANG, H.W. Equilibrium sorption isotherm for metal ions on tree fern. Process Biochemistry, v.37, p.1421-1430, 2002.
- KUO, S.; BAKER, A.S. Sorption of copper, zinc, and cadmium by some acid soils. Soil Science Society of America Journal, v.44, p.969-974, 1980.
- MACHADO, P.L.O.A.; PAVAN, M.A. Adsorção de zinco por alguns solos do Paraná. Revista Brasileira de Ciência do Solo, v.11, p.353-356, 1987.
- McBRIDE, M.B.; BLASIAK, J.J. Zinc and copper solubility as a function of pH in an acid soil. Soil Science Society of America Journal, v.43, p.866-870, 1979.
- MENGEL, K.; KIRKBY, E.A. Principles of plant nutrition 5th ed. Dordrecht: Kluwer Academic Publishers, 2001. 849p.
- NAIDU, R.; BOLAN, N.S.; KOOKANA, R.S.; TILLER, K.G. Ionic-strength and pH effects on surface charge of Cd sorption characteristics of soils. Journal of Soil Science, v.45, p.419-429, 1994.
- NASCIMENTO, C.W.A.; FONTES, R.L.F. Correlação entre características de Latossolos e parâmetros de equações de adsorção de cobre e zinco. Revista Brasileira de Ciência do Solo, v.28, p.965-971, 2004.
- OHNESORGE, F.K.; WILHELM, M. Zinc. In: MERIAN, E. (Ed.). Metals and their compounds in the environment Weinheim: Wiley-VHC, 1991. p.1309-1342.
- PIERANGELI, M.A.P.; GUILHERME, L.R.G.; OLIVEIRA, L.R.; CURI, N.; SILVA, M.L.N. Efeito da força iônica da solução de equilíbrio na adsorção de cádmio em Latossolos brasileiros. Pesquisa Agropecuária Brasileira, v.38, p.737-745, 2003.
- POMBO, L.C.A.; KLAMT, E. Adsorção de zinco e cobre de dois solos do Estado do Rio Grande do Sul. Revista Brasileira de Ciência do Solo, v.10, p.191-194, 1986. van
- RAIJ, B. van; ANDRADE, J.C.; CANTARELLA, H.; QUAGGIO, J.A. Análise química para avaliação da fertilidade de solos tropicais Campinas: IAC, 2001. 285p.
- SHUMAN, L.M. Effect of ionic strength and anions on zinc adsorption by two soils. Soil Science Society of America Journal, v.50, p.1438-1442, 1986.
- SILVEIRA, M.L.A.; ALLEONI, L.R.F.; CASAGRANDE, J.C.; CAMARGO, O.A. Energia livre da reação da adsorção de cobre em Latossolos ácricos. Scientia Agricola, v.56, p.1117-1122, 1999.
- SINGH, T.S.; PANT, K.K. Equilibrium, kinetics and thermodynamic studies for adsorption of As(III) on activated alumina. Separation and Purification Technology, v.36, p.139-147, 2004.
- SOARES, M.R.; ALLEONI, L.R.F.; CASAGRANDE, J.C. Parâmetros termodinâmicos da reação de adsorção de boro em solos altamente intemperizados. Química Nova, v.28, p.1014-1022, 2005.
- SOIL SURVEY STAFF. Soil taxonomy: a basic system of soil classification for making and interpreting soil surveys. Washington: Government Printing Office, 1999. (Agriculture Handbook, 436).
- SPOSITO, G. The chemistry of soils New York: Oxford, 1984. 277p.
- TAKKAR, P.N.; WALKER, C.D. The distribution and correction of zinc deficiency. In: ROBSON, A.D. (Ed.). Zinc in soils and plants Dordrecht: Kluwer Academic Publishers, 1993. p.151-165.
- WEBER, O.L. dos S.; CHITOLINA, J.C.; CAMARGO, O.A.; ALLEONI, L.R.F. Structural and variable electric charges of highly weathered tropical soils. Revista Brasileira de Ciência do Solo, v.29, p.867-873, 2005.
Publication Dates
-
Publication in this collection
29 Feb 2008 -
Date of issue
Jan 2008
History
-
Accepted
13 Dec 2007 -
Received
15 Oct 2007