Abstracts
The objective of this work was to verify whether the addition of biochar to the soil affects the degradation of litter and of soil organic matter (SOM). In order to investigate the effect of biochar on the mineralization of barley straw, soil was incubated with 14C-labelled barley straw with or without unlabelled biochar. To investigate the effect of straw on the mineralization of biochar, soil was incubated with 14C-labelled biochar with or without straw. In addition, to investigate the effect of biochar on old SOM, a soil labelled by applying labelled straw 40 years ago was incubated with different levels of biochar. All experiments had a control treatment, without any soil amendment. The effect of biochar on the straw mineralization was small and nonsignificant. Without biochar, 48±0.2% of the straw carbon was mineralized within the 451 days of the experiment. In comparison, 45±1.6% of C was mineralized after biochar addition of 1.5 g kg-1. In the SOM-labelled soil, the organic matter mineralized more slowly with the increasing doses of biochar. Biochar addition at 7.7 g kg-1 reduced SOM mineralization from 6.6 to 6.3%, during the experimental period. The addition of 15.5 g kg-1 of biochar reduced the mineralized SOM to 5.7%. There is no evidence of increased degradation of either litter or SOM due to biochar addition; consequently, there is no evidence of decreased stability of SOM.
anthropogenic dark earth; organic matter stability; priming effect; radiocarbon
O objetivo deste trabalho foi verificar se a adição de "biochar" ao solo afeta a degradação de resíduos orgânicos e da matéria orgânica do solo (MOS). Para investigar o efeito do "biochar" na mineralização de palhada de aveia, um solo foi incubado com palhada de aveia marcada com 14C, com ou sem "biochar" não marcado. Já para investigar o efeito da palhada de aveia na mineralização do "biochar", o solo foi incubado com "biochar" marcado 14C, com ou sem palhada de aveia. Além disso, para investigar o efeito do "biochar" em MOS antiga, um solo marcado pela aplicação de palhada de aveia, marcada há 40 anos, recebeu a incubação de diferentes níveis de "biochar". Todos os experimentos tiveram um tratamento controle, sem qualquer tipo de adição ao solo. O efeito do "biochar" na mineralização da palhada foi pequeno e não significativo. Sem o "biochar", 48±0,2% do carbono na serapilheira foi mineralizado nos 451 dias do experimento. Em comparação, 45±1,6% de C foram mineralizados após a adição de 1,5 g kg-1 de "biochar". No solo com MOS marcada, a matéria orgânica mineralizou-se mais lentamente com o incremento nas doses de "biochar". A adição de "biochar" a 7,7 g kg-1 reduziu a mineralização da matéria orgânica do solo de 6,6 para 6,3%, durante o período experimental. Já a adição de 15,5 g kg-1 de "biochar", reduziu a mineralização da MOS para 5,7%. Não há evidência de aumento na degradação nem de resíduos orgânicos nem da MOS pela adição de "biochar"; consequentemente, não há evidência de redução da estabilidade da MOS.
terra preta de índio; estabilidade da matéria orgânica; efeito "priming"; radiocarbono
SOIL SCIENCE
Biochar effect on the mineralization of soil organic matter
Efeito do "biochar" na mineralização da matéria orgânica do solo
Sander BruunI; Tarek EL-ZeheryII
IUniversity of Copenhagen, Faculty of Life Sciences, Department of Agriculture and Ecology, Plant and Soil Science Laboratory, Denmark. E-mail: sab@life.ku.dk
IIMansoura University, Faculty of Agriculture, Department of Soil Science, El Mansoura, Egypt. E-mail: trk_rgb@yahoo.com
ABSTRACT
The objective of this work was to verify whether the addition of biochar to the soil affects the degradation of litter and of soil organic matter (SOM). In order to investigate the effect of biochar on the mineralization of barley straw, soil was incubated with 14C-labelled barley straw with or without unlabelled biochar. To investigate the effect of straw on the mineralization of biochar, soil was incubated with 14C-labelled biochar with or without straw. In addition, to investigate the effect of biochar on old SOM, a soil labelled by applying labelled straw 40 years ago was incubated with different levels of biochar. All experiments had a control treatment, without any soil amendment. The effect of biochar on the straw mineralization was small and nonsignificant. Without biochar, 48±0.2% of the straw carbon was mineralized within the 451 days of the experiment. In comparison, 45±1.6% of C was mineralized after biochar addition of 1.5 g kg-1. In the SOM-labelled soil, the organic matter mineralized more slowly with the increasing doses of biochar. Biochar addition at 7.7 g kg-1 reduced SOM mineralization from 6.6 to 6.3%, during the experimental period. The addition of 15.5 g kg-1 of biochar reduced the mineralized SOM to 5.7%. There is no evidence of increased degradation of either litter or SOM due to biochar addition; consequently, there is no evidence of decreased stability of SOM.
Index terms: anthropogenic dark earth, organic matter stability, priming effect, radiocarbon.
RESUMO
O objetivo deste trabalho foi verificar se a adição de "biochar" ao solo afeta a degradação de resíduos orgânicos e da matéria orgânica do solo (MOS). Para investigar o efeito do "biochar" na mineralização de palhada de aveia, um solo foi incubado com palhada de aveia marcada com 14C, com ou sem "biochar" não marcado. Já para investigar o efeito da palhada de aveia na mineralização do "biochar", o solo foi incubado com "biochar" marcado 14C, com ou sem palhada de aveia. Além disso, para investigar o efeito do "biochar" em MOS antiga, um solo marcado pela aplicação de palhada de aveia, marcada há 40 anos, recebeu a incubação de diferentes níveis de "biochar". Todos os experimentos tiveram um tratamento controle, sem qualquer tipo de adição ao solo. O efeito do "biochar" na mineralização da palhada foi pequeno e não significativo. Sem o "biochar", 48±0,2% do carbono na serapilheira foi mineralizado nos 451 dias do experimento. Em comparação, 45±1,6% de C foram mineralizados após a adição de 1,5 g kg-1 de "biochar". No solo com MOS marcada, a matéria orgânica mineralizou-se mais lentamente com o incremento nas doses de "biochar". A adição de "biochar" a 7,7 g kg-1 reduziu a mineralização da matéria orgânica do solo de 6,6 para 6,3%, durante o período experimental. Já a adição de 15,5 g kg-1 de "biochar", reduziu a mineralização da MOS para 5,7%. Não há evidência de aumento na degradação nem de resíduos orgânicos nem da MOS pela adição de "biochar"; consequentemente, não há evidência de redução da estabilidade da MOS.
Termos para indexação: terra preta de índio, estabilidade da matéria orgânica, efeito "priming", radiocarbono.
Introduction
Black carbon consists of partially burned plant material, formed in natural fires, and it is often described as a continuum ranging from slightly charred plant material to charcoal, sod and graphite (Seiler & Crutzen, 1980). Black carbon has been shown to be resistant to microbial degradation and, therefore, has a long residence time in soil and in the environment (Schmidt & Noack, 2000). This means that a significant fraction of the organic matter in many soils are black carbon, some soils containing up to 35% black carbon (Schmidt & Noack, 2000; Skjemstad et al., 2002) and, as such, black carbon is an important sink for carbon (Kuhlbusch, 1998). Black carbon also appears to have a range of positive effects on soil properties, such as a high-cation exchange capacity, pH and water holding capacity (Glaser et al., 2002).
"Terra preta de índio" - anthropogenic dark earth - are dark soils found in patches in many locations in South America (Glaser & Birk, 2012). These soils exhibit high-nutrient contents and are much more fertile than the adjacent soils. The occurrence of potsherds indicates that the soils are anthropogenic and probably formed by pre-Columbian Indians, from 500 to 2,500 BP, by addition of fishbone, organic matter and charcoal. The high-quality properties of these soils has to a large extend been ascribed to the addition of charcoal.
Because of the long residence time in soil and the positive effects on soil properties, addition of black carbon or biochar, as it is called in this connection, has been suggested as a way to improve soil quality and sequester carbon from the atmosphere (Lehmann et al., 2006). In order to be an effective way to sequester carbon in soil, a significant fraction of biochar should resist degradation for millennia. Incubation experiments have indicated that only a small frame - usually less than 5% - is degraded within the time fraction of laboratory incubations, depending both on the material from which it has been produced and the processing conditions (Bruun et al., 2008a; Kuzyakov et al., 2009; Nguyen & Lehmann, 2009; Zimmerman, 2010). However, long term stabilization is difficult to assess from incubation studies.
Nguyen et al. (2009) used a chronosequence of soils, which had been burned and converted into agricultural land for different time periods, up to 100 years, and found that about 30% of the black carbon was degraded within the first 30 years, after which no detectable changes existed. Hammes et al. (2008) compared an archived soil profile with a modern soil profile to show that approximately 25% of the black carbon disappeared within 100 years. Higher degradation was observed by Bird et al. (1999), who estimated a loss of 47% within 50 years, based on the content of black carbon in plots protected or unprotected against fire. There is, however, considerable evidence that a significant fraction of the remaining biochar is stable for millennia. Middelburg et al. (1999) estimated that 36% black carbon resisted mineralization in oxidic sediments, based on reduced amounts of black carbon in the oxidized zone of downward-progressing oxidation fronts. However, the most compelling evidence that a significant fraction of black carbon resists oxidation in soil for millennia comes from the presence of black carbon particles with radiocarbon ages of more than 10,000 years, even in tropical soils with good conditions for decomposition (Carcaillet, 1998; Titiz & Sanford Junior, 2007). Finally, mass balances based on stocks and production of black carbon show that it must be considerably more stable than other forms of organic matter (Lehmann et al., 2009)
Nevertheless, an experiment from Sweden has recently indicated that biochar increases the degradation of plant litter (Wardle et al., 2008). If this is the case, CO2 released from the litter, as a consequence of the biochar addition, may offset the carbon sequestered in the biochar. However, a range of methodological problems are associated with this experiment (Lehmann & Sohi, 2008). Furthermore, the experiments conducted by Wardle et al. (2008) do not provide any information of the biochar influence on the degradation of SOM associated with the mineral soil.
Experiments using 14C-labelled have only been used in a few incubation studies of biochar mineralization (Bruun et al., 2008a; Kuzyakov et al., 2009). This technique provide a much improved sensitivity compared with others based on total CO2 evolution or with methods to measure black carbon in soil. Experiments measuring total CO2 evolution from unlabelled biochar in soil are hampered by a large background of CO2 evolution, which has to be subtracted or avoided (Hilscher et al., 2009; Spokas et al., 2009; Zimmerman, 2010).
The objective of this work was to evaluate whether addition of biochar affects the degradation of litter and SOM using 14C-labelling techniques.
Materials and Methods
In 1964, uniformly 14C-labelled (3.5 MBq g-1 C) barley straw was mixed with soil (3.5 mg of carbon per g of soil), placed in a 20 cm layer in two large plastic cylinders (34 cm diameter), and incubated in the open (Sørensen, 1987). In 2004, nine soil cores were sampled at different places in the uppermost 10 cm of the soil and bulked. This bulked sample was taken in order to evaluate the effect of biochar application in the labelled SOM (description follows later). At the same time, another nine soil cores of the same soil, but without the previous incubation of labelled litter, were sampled and bulked. This bulked sample was taken in order to evaluate the effect of biochar application on freshly incubated barley straw (description ahead). All samples were air-dried and stored until the beginning of the experiment. The soil texture was sandy, and it was classified as a Luvisol. Soil characteristics are shown in Table 1.
Uniformly 14C-labelled barley straw, of the same kind used to label SOM, had been saved from the original experiment, while unlabelled barley straw was obtained from a nearby farm. Uniformly 14C-labelled biochar was produced from 14C-labelled barley straw, while unlabelled biochar was produced from unlabelled barley straw. The biochar were produced by separately weighing approximately 0.6 g of labelled straw and 5 g of unlabelled straw, wrapping them thoroughly in several layers of aluminium foil to prevent oxygen exposure, and leaving them in a muffle furnace. The temperature of the oven was adjusted to 400±5°C using a thermometer with a thermocouple to check temperature where the biochar production occurred. Characteristics of the straw and produced biochar are given in Table 2.
The incubations were performed in airtight glass tubes equipped with a scintillation counter vial with 2 mL of 1 mol L-1 NaOH. The tubes were kept in a temperature cabinet at 25±0.5°C. The water content of the soil was adjusted to 15% water. After 2, 4, 7, 11, 18, 25, 32, 46, 60, 127, 162, 205, 316 and 451 days, the vials were sampled and replaced, and the NaOH in the sampled vials were mixed with 8 mL scintillation liquid (Ultima Gold, PerkinElmer, Waltham, MA, USA) and counted for 10 min on a scintillation counter (Winspectral 1414 LSC Liquid Scintillation Counter, Wallac, Turku, Finland). The water content was kept constant during the experiment by regularly weighing the tubes and adding the amount of water necessary to keep the weight at the same value.
Two different incubation experiments were performed. The treatments applied in experiment 1 are shown in Table 3. In this experiment, unlabelled soil was used for incubation of labelled straw, with (U3) or without unlabelled biochar (U2), and of labelled biochar, with (U5) or without unlabelled straw (U4). This was done in order to investigate the effect of biochar on the mineralization of barley straw and vice versa. All treatments were carried out in triplicate.
The treatments applied in incubation experiment 2 are shown in Table 4. In this experiment, the soil which had been labelled by incorporating 14C-labelled barley straw 40 years ago was incubated with different levels of biochar additions, one level of straw addition and a control without any amendment. All treatments were carried out in triplicate.
The data from the experiments were analyzed using the statistical software package R (R Development Core Team, 2010). In experiment 1, the effect of biochar on CO2 evolution from straw was analyzed in a repeated measurement analysis using a mixed-effect model of the mineralization rate of 14C-labelled straw, modelled as a function of biochar addition (either no addition or biochar addition), and using incubation time as fixed variables, and the incubation tube as a random variable. A mixed model is necessary because measurements are done repeatedly on the same samples. If, for example, one tube was leaking CO2, there would be a "tube effect", which would make the measurements of this sample constituently smaller than the other replicates. We could choose to treat the tube effect as a fixed variable, but then we would only model the specific effect of the particular tube. In fact, we are interested in a whole population of tubes. Therefore, it is better to treat the tube as a random variable. In order to ensure stable residuals of the model, incubation time was modelled as a discrete variable, and the mineralization rates were transformed using the natural logarithm. The effect of straw addition on the mineralization of 14C-labelled biochar was tested by a similar model.
The incubation experiment 2, the effect of biochar on the decomposition of SOM was also tested in a repeated measurement analysis employing a mixed-effect model, where the rate of 14C-SOM mineralization was modelled as a function of biochar addition and time as fixed variable, and incubation tube as a random variable. In order to ensure stable residuals for the model, incubation time was modelled as a discrete variable, and the mineralization rates were transformed using the natural logarithm.
Results and Discussion
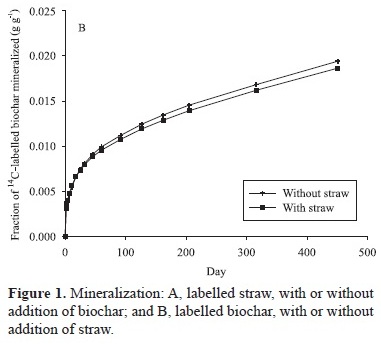
As expected, the straw mineralized much faster than the biochar. During the 451 days of experiment 1, 45 to 47% of the carbon contained in the straw was mineralized (Figure 1 A), while only 1.8 to 1.9% of the carbon contained in the biochar was mineralized (Figure 1 B). In comparison with litter and biochar, SOM from the soil labelled 40 years ago mineralized at intermediate rates. After 451 days of incubation, 6.6% of the SOM was mineralized in the treatment without any addition of straw or biochar (Figure 2). This means that 40-year-old SOM mineralizes much faster than biochar. There is no doubt that the mineralization of the 40-year-old SOM has been enhanced by the disturbance inflicted on the soil during sampling, sieving, drying, and rewetting during the preparation of the experiment, as often observed in incubation experiments (Franzluebbers, 1999; Kristensen et al., 2003). However, the low mineralization rates of freshly made biochar, compared to the 40-year-old SOM still underlines the biochar stability and its inherent ability to resist microbial degradation (Bruun et al., 2008a; Lehmann et al., 2009).
Straw mineralization appears not to have been affected by the presence of biochar (Figure 1 A). The repeated measurement analyses confirmed that there was no significant effect of biochar addition on the mineralization of 14C-labelled straw (p = 0.55). This result contrasts with the ones by Wardle et al. (2008), who observed increased decomposition of humus (i.e. degraded plant litter from the humus layer of the litter layers at the forest floor) in the presence of biochar. In their experiment, biochar, humus, or a mixture of biochar and degraded humus were incubated in litterbags at the forest floor, at three locations. It turned out that the material in the mixture disappeared faster than expected from the losses observed in the other litterbags. There are several potential reasons for the contrasting results between this experiment and ours. First of all, there is a difference in the duration of the experiments. The experiment by Wardle et al. (2008) lasted for 10 years, whereas the current experiment only lasted for 1.2 year. However, the increased loss of biomass in the mixture, in Wardle et al. (2008), was almost exclusively observed during the first year. In addition, the incubation temperature of 25°C in the current study is considerable higher than the average ambient temperature in northern Sweden, where their experiment was conducted. The higher temperature should have promoted an increased mineralization rate, and the effect of biochar addition on litter mineralization should have occurred sooner. Therefore, the differences in the duration of the experiments are unlikely to be the reason for the different results between the two experiments.
One possible mechanism which might explain the discrepancies is water availability. In the current laboratory experiments, water content was adjusted in the beginning of the experiment; then, when biochar is added it may absorb water, thereby decreasing water availability for the microorganisms degrading the straw. In contrast, in Wardle et al. (2008), the biochar in the litterbags could absorb water from rainfall, preventing it from leaching and thereby increasing water availability for the microorganisms degrading the straw. Finally, there is a whole range of possible artifacts associated with the use of litterbags, which include the loss of material from the bags, the lack of contact with the mineral soil, and changed environmental conditions in the bags.
The mineralization of biochar has slightly decreased by the addition of straw (Figure 1 B). The repeated measurement analysis showed that this effect was in fact significant (p = 0.0044), although numerically small, being detectable only because of the high sensitivity of the 14C-labelling method. There are several possible explanations for the decreasing decomposition of biochar with the addition of straw. Again, the addition of straw may decrease water availability and, thus, make the conditions for the microbial degradation less favorable. Another possible mechanism may be the reduced availability of nutrients, due to the immobilization of nutrients by the straw, which has low contents of them, especially of nitrogen (Luxhøi et al., 2006). Reduced nitrogen availability has often been observed to decrease mineralization of other types of organic matter, presumably by creating suboptimal conditions for microbes (Allen & Schlesinger, 2004; Yoshitake et al., 2007), although the effects of nitrogen on the decomposition process is still controversial (Craine et al., 2007). On the other hand, priming effects, where addition of labile organic matter increases the mineralization of biochar, has been shown on several occasions (Kuzyakov et al., 2009). From experiment 2, it appears that biochar has a negative effect on the mineralization rate of SOM, where mineralization decreased with increasing addition of biochar (Figure 2 A). With addition of 0.031 g biochar to 20 g of soil (1.5 g kg-1 of biochar), there was a very small reduction in SOM mineralization. With addition of 1.55 g biochar to 20 g of soil (7.7 g kg-1 of biochar), the percentage of mineralized SOM, during the 451 days, was reduced from 6.6% in the control to 6.3%. Addition of 0.31 g biochar to 20 g of soil (15.5 g kg-1 of biochar) further reduced the percentage of mineralized SOM to 5.7%. The repeated measurement analysis of the data showed that there was a highly significant effect of biochar on the mineralization rates of 40-year-old SOM (p<0.0001), with greater reductions the more biochar was added. Spokas et al. (2009) also observed decreasing mineralization of SOM due to the addition of biochar, after the CO2 evolution from the biochar had been subtracted.
The decreasing rates of SOM mineralization may be caused by a whole range of different factors, including aeration and nitrogen availability. Because of the slow mineralization rates, biochar is not likely to lead to much nitrogen immobilization and, although biochar is able to adsorb ammonia and nitrate (Mizuta et al., 2004), this process is not likely to be able to compete for nitrogen with the microbes. As in the case with the straw, the reduction of the mineralization of SOM may be caused by changes in water availability after the addition of biochar. This is also the explanation offered by Spokas et al. (2009) for the negative effect of biochar on SOM mineralization. As the changes in water availability occurring after biochar addition in the laboratory are not necessarily similar to the changes which would occur under field conditions, it is difficult to know whether biochar would have the same effect on SOM mineralization under field conditions. However, based on the current experiments, there is no evidence that biochar would increase the mineralization of SOM.
The repeated measurement analysis showed that there was a significant effect of straw on the mineralization rates of 40-year-old SOM (p<0.0141). This effect was, however, very small and variable in time, with higher mineralization rates in the beginning and lower rates later in the incubation (Figure 2 A). The initially higher rates of SOM mineralization could be interpreted as a priming effect on the mineralization of SOM due to the straw addition.
Conclusions
1. The mineralization of biochar in the soil occurs much more slowly than that of plant litter and of 40-year-old soil organic matter, confirming its very high inherent stability.
2. There is no evidence of priming effects of biochar, or any other positive effects of its addition, on the mineralization of straw or of soil organic matter, indicating that biochar does not decrease the stability of soil organic matter.
Acknowledgements
To Gluds Legat, for financial support.
Received on December 16, 2010 and accepted on April 2, 2012
- ALLEN, A.S.; SCHLESINGER, W.H. Nutrient limitations to soil microbial biomass and activity in loblolly pine forests. Soil Biology and Biochemistry, v.36, p.581-589, 2004.
- BIRD, M.I.; MOYO, C.; VEENENDAAL, E.M.; LLOYD, J.; FROST, P. Stability of elemental carbon in a savanna soil. Global Biogeochemical Cycles, v.13, p.923-932, 1999.
- BRUUN, S.; JENSEN, E.S.; JENSEN, L.S. Microbial mineralization and assimilation of black carbon: dependency on degree of thermal alteration. Organic Geochemistry, v.39, p.839-845, 2008a.
- BRUUN, S.; THOMSEN, I.K.; CHRISTENSEN, B.T.; JENSEN, L.S. In search of stable soil organic carbon fractions: a comparison of methods applied to soils labelled with 14C for 40 days or 40 years. European Journal of Soil Science, v.59, p.247-256, 2008b.
- CARCAILLET, C. A spatially precise study of Holocene fire history, climate and human impact within the Maurienne valley, North French Alps. Journal of Ecology, v.86, p.384-396, 1998.
- CHRISTENSEN, B.T.; SØRENSEN, L.H. The distribution of native and labelled carbon between soil particle size fractions isolated from long-term incubation experiments. Journal of Soil Science, v.36, p.219-229, 1985.
- CRAINE, J.M.; MORROW, C.; FIERER, N. Microbial nitrogen limitation increases decomposition. Ecology, v.88, p.2105-2113, 2007.
- FRANZLUEBBERS, A.J. Potential C and N mineralization and microbial biomass from intact and increasingly disturbed soils of varying texture. Soil Biology and Biochemistry, v.31, p.1083-1090, 1999.
- GLASER, B.; BIRK, J.J. State of the scientific knowledge on properties and genesis of Anthropogenic Dark Earths in Central Amazonia (terra preta de Índio). Geochimica et Cosmochimica Acta, v.82, p.39-51, 2012.
- GLASER, B.; LEHMANN, J.; ZECH, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal - a review. Biology and Fertility of Soils, v.35, p.219-230, 2002.
- HAMMES, K.; TORN, M.S.; LAPENAS, A.G.; SCHMIDT, M.W.I. Centennial black carbon turnover observed in a Russian steppe soil. Biogeosciences, v.5, p.1339-1350, 2008.
- HILSCHER, A.; HEISTER, K.; SIEWERT, C.; KNICKER, H. Mineralisation and structural changes during the initial phase of microbial degradation of pyrogenic plant residues in soil. Organic Geochemistry, v.40, p.332-342, 2009.
- KRISTENSEN, H.L.; DEBOSZ, K.; MCCARTY, G.W. Short-term effects of tillage on mineralization of nitrogen and carbon in soil. Soil Biology and Biochemistry, v.35, p.979-986, 2003.
- KUHLBUSCH, T.A.J. Black carbon and the carbon cycle. Science, v.280, p.1903-1904, 1998.
- KUZYAKOV, Y.; SUBBOTINA, I.; CHEN, H.Q.; BOGOMOLOVA, I.; XU, X.L. Black carbon decomposition and incorporation into soil microbial biomass estimated by 14C labeling. Soil Biology and Biochemistry, v.41, p.210-219, 2009.
- LEHMANN, J.; CZIMCZIK, C.; LAIRD, D.; SOHI, S. Stability of biochar in soil. In: LEHMANN, J.; JOSEPH, S. (Ed.). Biochar for environmental management London: Earthscan, 2009. p.183-205.
- LEHMANN, J.; GAUNT, J.; RONDON, M. Bio-char sequestration in terrestrial ecosystems - a review. Mitigation and Adaptation Strategies for Global Change, v.11, p.403-427, 2006.
- LEHMANN, J.; SOHI, S. Comment on "fire-derived charcoal causes loss of forest humus". Science, v.321, 2008. DOI: 10.1126/science.1160005.
- LUXHØI, J.; BRUUN, S.; STENBERG, B.; BRELAND, T.A.; JENSEN, L.S. Prediction of gross and net N mineralization-immobilization-turnover from respiration. Soil Science Society of America Journal, v.70, p.1121-1128, 2006.
- MIDDELBURG, J.J.; NIEUWENHUIZE, J.; VAN BREUGEL, P. Black carbon in marine sediments. Marine Chemistry, v.65, p.245-252, 1999.
- MIZUTA, K.; MATSUMOTO, T.; HATATE, Y.; NISHIHARA, K.; NAKANISHI, T. Removal of nitrate-nitrogen from drinking water using bamboo powder charcoal. Bioresource Technology, v.95, p.255-257, 2004.
- NGUYEN, B.T.; LEHMANN, J. Black carbon decomposition under varying water regimes. Organic Geochemistry, v.40, p.846-853, 2009.
- NGUYEN, B.T.; LEHMANN, J.; KINYANGI, J.; SMERNIK, R.; RIHA, S.J.; ENGELHARD, M.H. Long-term black carbon dynamics in cultivated soil. Biogeochemistry, v.92, p.163-176, 2009.
- R DEVELOPMENT CORE TEAM. R: a language and environment for statistical computing. Available at: <http://www.R-project.org>. Accessed on: 24 Nov. 2010.
- SCHMIDT, M.W.I.; NOACK, A.G. Black carbon in soils and sediments: analysis, distribution, implications, and current challenges. Global Biogeochemical Cycles, v.14, p.777-793, 2000.
- SEILER, W.; CRUTZEN, P.J. Estimates of gross and net fluxes of carbon between the biosphere and the atmosphere from biomass burning. Climatic Change, v.2, p.207-247, 1980.
- SKJEMSTAD, J.O.; REICOSKY, D.C.; WILTS, A.R.; MCGOWAN, J.A. Charcoal carbon in US agricultural soils. Soil Science Society of America Journal, v.66, p.1249-1255, 2002.
- SØRENSEN, L.H. Organic matter and microbial biomass in a soil incubated in the field for 20 years with 14C-labeled barley straw. Soil Biology and Biochemistry, v.19, p.39-42, 1987.
- SPOKAS, K.A.; KOSKINEN, W.C.; BAKER, J.M.; REICOSKY, D.C. Impacts of woodchip biochar additions on greenhouse gas production and sorption/degradation of two herbicides in a Minnesota soil. Chemosphere, v.77, p.574-581, 2009.
- TITIZ, B.; SANFORD JUNIOR, R.L. Soil charcoal in old-growth rain forests from sea level to the continental divide. Biotropica, v.39, p.673-682, 2007.
- WARDLE, D.A.; NILSSON, M.C.; ZACKRISSON, O. Fire-derived charcoal causes loss of forest humus. Science, v.320, p.629, 2008. DOI: 10.1126/science.1154960.
- YOSHITAKE, S.; UCHIDA, M.; KOIZUMI, H.; NAKATSUBO, T. Carbon and nitrogen limitation of soil microbial respiration in a High Arctic successional glacier foreland near Ny-Ålesund, Svalbard. Polar Research, v.26, p.22-30, 2007.
- ZIMMERMAN, A.R. Abiotic and microbial oxidation of laboratory-produced black carbon (Biochar). Environmental Science and Technology, v.44, p.1295-1301, 2010.
Publication Dates
-
Publication in this collection
26 June 2012 -
Date of issue
May 2012
History
-
Received
16 Dec 2010 -
Accepted
02 Apr 2012