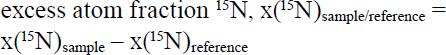Abstract
Brazilian scientists have played a pioneering role in developing and applying stable isotope methodologies, in terms of natural abundance and enriched levels, to trace carbon and nutrient flows in terrestrial ecosystems, including natural and agroecosystems. Significant contributions have been made in the areas of biological N2 fixation, carbon dynamics in soil, synthesis and evaluation of labeled fertilizers, and food science. These contributions have originated from several decentralized units of Embrapa, from research institutions, and from federal or state universities. In order to capitalize the existing Brazilian expertise, it is necessary to provide, at an institutional level, analytical facilities for stable isotope research, aiming to strengthen national capacity and to maintain the international competitiveness of the research.
Index terms:
biological N2 fixation; fertilizer use efficiency; food science; labeled fertilizer; soil carbon; stable isotopes
Resumo
Pesquisadores brasileiros têm tido um papel pioneiro no desenvolvimento e na aplicação das metodologias de isótopos estáveis, em termos de abundância natural e de níveis enriquecidos, para traçar os fluxos de carbono e nutrientes em ecossistemas terrestres, que incluem os naturais e os agroecossistemas. Contribuições significativas têm sido feitas nas áreas de fixação biológica de N2, de dinâmica de carbono nos solos, de síntese e avaliação de fertilizantes marcados, e da tecnologia dos alimentos. Essas contribuições têm se originado de diversas unidades decentralizadas da Embrapa, de institutos de pesquisa e de universidades federais ou estaduais. Para capitalizar a experiência brasileira existente, é necessária a provisão, nas instituições, de instalações analíticas para pesquisa com isótopos estáveis, a fim de fortalecer a capacidade nacional e manter a competitividade internacional das pesquisas.
Termos para indexação:
fixação biológica de N2; eficiência de uso de fertilizantes; tecnologia de alimentos; fertilizante marcado; carbono do solo; isótopos estáveis
Introduction
The Brazilian climate is predominantly tropical and subtropical, in marked contrast to the temperate or colder regions of Europe and North America. Therefore, the soils within the country are, in general, more highly weathered and support a greater variety of both intensive and extensive agricultural enterprises. Despite this, Brazilian soils share a similar set of problems encountered in their northern hemisphere counterparts, namely loss of soil organic matter and declining fertility under arable cropping. Stable isotope tracers have played a crucial role in gaining insight into carbon, nitrogen, and sulfur cycling in agroecosystems, including the estimation of fertilizer use efficiency and the contribution of biological N2 fixation to the N nutrition of legumes and C4 grasses. In the globalization era of agricultural products of plant and animal origin, the public is demanding accountability and authenticity of the external description on food and beverage packaging. In this case, stable isotopes are being used to detect adulteration and to verify geographic origin and mode of production, such as organic vs. conventional systems.
The objective of this review was to highlight contributions that Brazilian science has made and continues to make to the global efforts to trace carbon and nutrient flows in terrestrial ecosystems; synthesize and evaluate the efficiency of labeled fertilizers; and authenticate foods and beverages. It is an overview of the significant accomplishments, seen through a small window or aperçu, from the perspective of an outsider, who, nevertheless, has been fortunate to be involved in Brazilian stable isotope research and review since 1979.
This review addresses key examples, mainly but not exclusively, from agroecosystems, but does not attempt to make an extensive coverage of all relevant Brazilian literature. Recognition has been given to published papers in which the research was conducted and analytical services were provided in the country, irrespectively of the national or international affiliations of the scientists involved. Although considerable expertise exists in Brazil, this review does not cover aquatic environments (except for one example of fish production regime), microbial and plant ecology, plant and animal physiology, and pure and applied sciences, such as entomology, zoology, hydrology, geochemistry, paleontology, and related disciplines.
Absolute (x) and relative (δ) values of isotopic abundance
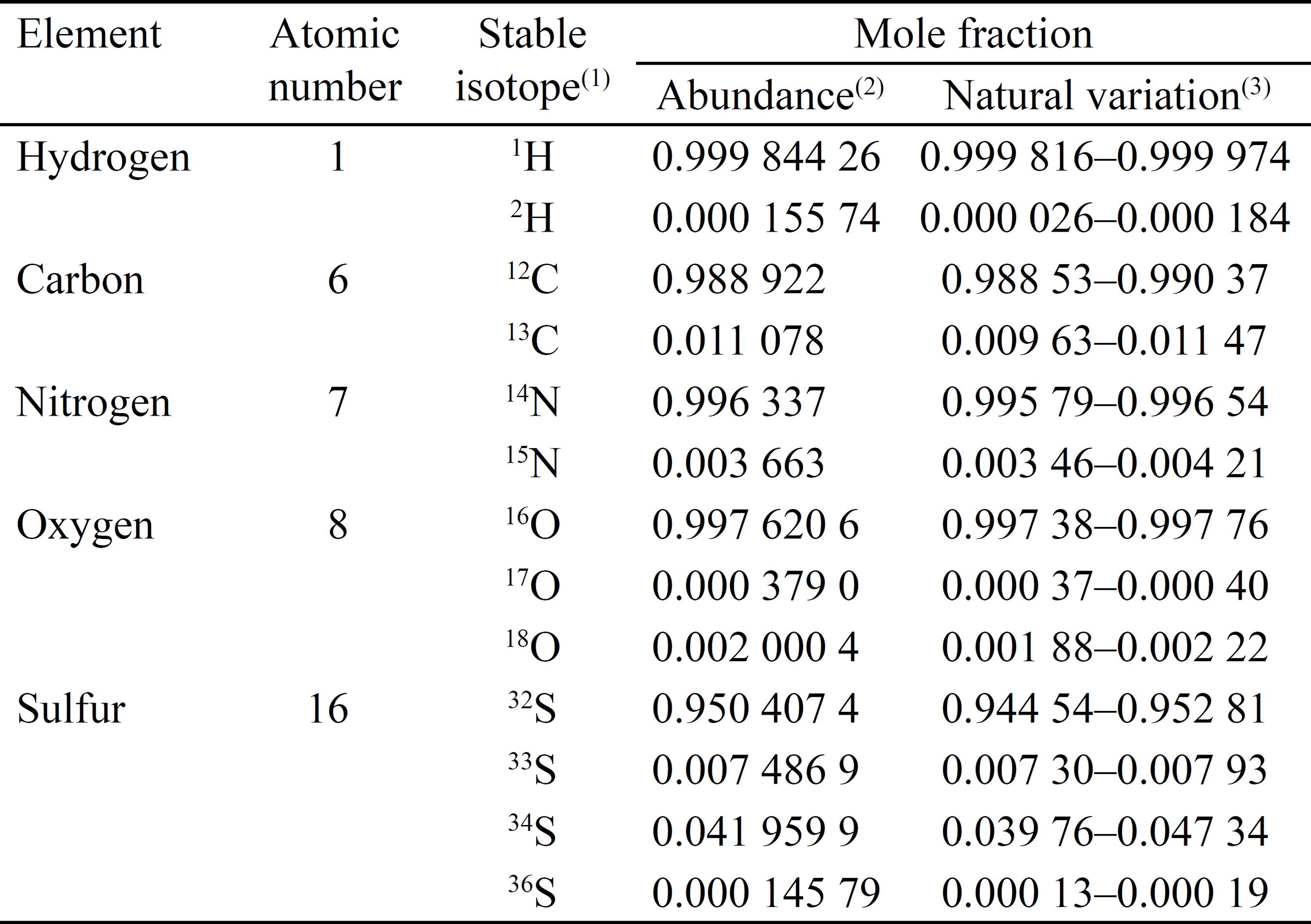
The lighter elements of interest in biological sciences, that is, H, C, N, O, and S, have naturally occurring stable isotopes: two, in the case of H, C, and N; three, in that of O; or four, in that of S (Table 1). The absolute abundance of each isotope, expressed as mole fraction, is given in Table 1. These values are equivalent to the atom fraction of an element, x(iE), which is defined as the amount of a specified atom (isotope) of an element divided by the total amount of atoms of the element within the mixture (Coplen, 2011COPLEN, T.B. Guidelines and recommended terms for expression of stable-isotope-ratio and gas-ratio results. Rapid Communications in Mass Spectrometry, v.25, p.2538-2560, 2011. DOI: 10.1002/rcm.5129.
https://doi.org/10.1002/rcm.5129...
). Therefore, for an element with two stable isotopes, such as N, the absolute value is expressed by the equation:
Isotopic composition of the important elements in biological sciences. Adapted from Berglund & Wieser (2011)BERGLUND, M.; WIESER, M.E. Isotopic compositions of the elements 2009 (IUPAC Technical Report). Pure and Applied Chemistry, v.83, p.397-410, 2011. DOI: 10.1351/PAC-REP-10-06-02.
https://doi.org/10.1351/PAC-REP-10-06-02... .
If a sample is artificially enriched in an isotope, the excess over natural abundance is expressed as an excess atom fraction, x, which is the difference between the mole fraction of an isotope of an element in a substance and that of a reference (Coplen, 2011COPLEN, T.B. Guidelines and recommended terms for expression of stable-isotope-ratio and gas-ratio results. Rapid Communications in Mass Spectrometry, v.25, p.2538-2560, 2011. DOI: 10.1002/rcm.5129.
https://doi.org/10.1002/rcm.5129...
). For N, for instance, the isotopic enrichment is expressed by the equation:
in which the reference is air, which has a 15N mole fraction equal to 0.003 663.
The range of natural variations in isotopic abundances, expressed as mole fraction, in terrestrial materials is also given in Table 1. However, it is more common to express small variations in natural abundance as relative (δ) rather than absolute (x) values. The relative value is the isotope ratio (R) of a sample, relative to the isotope ratio of the international standard for the element, as in the equation: δ(iE) = (Rsample/Rstandard) - 1.
For N, R is the ratio of 15N/14N. The international standard is air, with an R value equal to 0.003 676 47 and, by definition, a δ15N or xE(15N) value equal to zero (Chalk et al., 2015bCHALK, P.M.; INÁCIO, C.T.; CRASWELL, E.T.; CHEN, D. On the usage of absolute (x) and relative (δ) values of 15N abundance. Soil Biology and Biochemistry, v.85, p.51-53, 2015b. DOI: 10.1016/j.soilbio.2015.02.027.
https://doi.org/10.1016/j.soilbio.2015.0...
). In order to avoid fractions, δ values have traditionally been expressed in per mil (‰), by multiplying the right hand side of the equation of the isotope ratio by 1,000. Values of δ15N can be negative or positive depending on whether the sample isotope ratio is, respectively, less than or greater than the isotope ratio of air.
A full description of the source, availability, and properties of primary and secondary international reference materials for isotope-ratio analysis is given in Brand et al. (2014)BRAND, W.A.; COPLEN, T.B.; VOGL, J.; ROSNER, M.; PROHASKA, T. Assessment of international reference materials for isotope-ratio analysis (IUPAC Technical Report). Pure and Applied Chemistry, v.86, p.425-467, 2014. DOI: 10.1515/pac-2013-1023.
https://doi.org/10.1515/pac-2013-1023...
.
Biological N2 fixation (BNF)
Short-term measurements of the ability of legumes or actinorhizal plants to fix atmospheric N2 can be performed by exposure of the whole plant or root system to an atmosphere containing enriched 15N2 (De-Polli et al., 1977DE-POLLI, H.; MATSUI, E.; DÖBEREINER, J.; SALATI, E. Confirmation of nitrogen fixation in two tropical grasses by 15N2 incorporation. Soil Biology and Biochemistry, v.9, p.119-123, 1977. DOI: 10.1016/0038-0717(77)90047-5.
https://doi.org/10.1016/0038-0717(77)900...
). Although the method is direct, it poses many practical difficulties in relation to the containment of the labeled gas and to the maintenance of plants in an enclosure, which is why it is seldom attempted.
Indirect methods, commonly used to estimate legume symbiotic dependence, such as the proportional contribution of biologically-fixed N2 to the N nutrition of the legume, involve paired treatments of a legume and a non-legume reference plant. The latter is required to estimate the ratio of labeled to unlabeled N derived from the soil by the N2-fixing plant.
The 15N enrichment (E) method involves the addition of a fertilizer or organic material artificially enriched in 15N to the soil in the paired plots. The proportional dependence (Patm) of the legume on biologically-fixed N is estimated by isotope dilution, using the following equation: Patm = 1 - (xE(15N)legume/xE(15N)reference plant).
The E methodology was reviewed by Chalk & Ladha (1999)CHALK, P.M.; LADHA, J.K. Estimation of legume symbiotic dependence: an evaluation of techniques based on 15N dilution. Soil Biology and Biochemistry, v.31, p.1901-1917, 1999. DOI: 10.1016/S0038-0717(99)00095-4.
https://doi.org/10.1016/S0038-0717(99)00...
.
The 15N natural abundance (NA) method is based on the same principle, but isotopic discrimination, i.e., the B value, during N2 fixation must be additionally measured, usually by growing the legume in an N-free medium. Symbiotic dependence is estimated according to the equation: Patm = [(δ(15N)reference plant - δ(15N)legume)/δ(15N)reference plant - B] .
The NA methodology was reviewed by Unkovich et al. (2008)UNKOVICH, M.; HERRIDGE, D.; PEOPLES, M.; CADISCH, G.; BODDEY, B.; GILLER, K.; ALVES, B.; CHALK, P. 15N natural abundance method. In: UNKOVICH, M.; HERRIDGE, D.; PEOPLES, M.; CADISCH, G.; BODDEY, R.; GILLER, K.; ALVES, B.; CHALK, P. Measuring plant-associated nitrogen fixation in agricultural systems. Canberra: ACIAR, 2008. p.131-162. (Monograph, 136)..
Endophytic BNF
Brazilian and visiting scientists working with Dr. Johanna Döbereiner, at Embrapa Agrobiologia, were the first to recognize the importance of diazotrophic bacteria in endophytic associations with tropical C4 Gramineae. The first isotope dilution field measurement to quantify endophytic BNF was also carried out at Embrapa Agrobiologia with Paspalum notatum Flugge (Boddey et al., 1983BODDEY, R.M.; CHALK, P.M.; VICTORIA, R.; MATSUI, E. The 15N-isotope dilution technique applied to the estimation of biological nitrogen fixation associated with Paspalum notatum cv. batatais in the field. Soil Biology and Biochemistry, v.15, p.25-32, 1983. DOI: 10.1016/0038-0717(83)90114-1.
https://doi.org/10.1016/0038-0717(83)901...
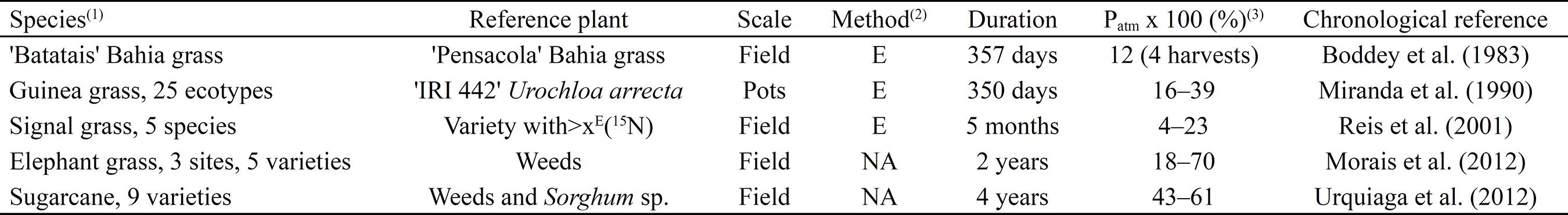
). Since these pioneering studies, endophytic BNF has been quantified by both the E and NA methods in several C4 forage grasses and in sugarcane (Saccharum officinarum L.) (Table 2). This research has unequivocally shown the importance of endophytic BNF to the N nutrition of C4 plants. For example, in a four-year study, Urquiaga et al. (2012)URQUIAGA, S.; XAVIER, R.P.; MORAIS, R.F. de.; BATISTA, R.B.; SCHULTZ, N.; LEITE, J.M.; MAIA e SÁ, J.; BARBOSA, K.P.; RESENDE, A.S. de; ALVES, B.J.R.; BODDEY, R.M. Evidence from field nitrogen balance and 15N natural abundance data for the contribution of biological N2 fixation to Brazilian sugarcane varieties. Plant and Soil, v.356, p.5-21, 2012. DOI: 10.1007/s11104-011-1016-3.
https://doi.org/10.1007/s11104-011-1016-...
found that Patm values varied from 43 to 61% among nine varieties of sugarcane.
Brazilian studies of Embrapa Agrobiologia, using 15N-based methodologies to quantify endophytic N2 fixation in C4 forages and in sugarcane (Saccharum officinarum).
Legume BNF
In Brazil, symbiotic dependence has been estimated for grain and forage legumes in agroecosystems, using the E and NA methods, and for native leguminous shrubs and trees at undisturbed sites in the Cerrado and Caatinga biomes, using the NA method. Some examples are given in Table 3.
Brazilian field studies using 15N dilution to quantify symbiotic N2 fixation in legumes(1) (1) Common bean, Phaseolus vulgaris; soybean, Glycine max; and groundnut, Arachis hypogaea. (2)Wheat, Triticum aestivum; Nn, non-nodulating; Weeds, Digitaria horizontalis, Sorghum arundinaceum, and Cenchrus echinatus; C4 grasses, Urochloa brizantha, U. arrecta, and Panicum maximum; Weeds, Sorghum sp., Zea mays, Cynodon dactylon, Cyperus rotundus, and Sida glaziovii; and Nnlegume, the non-nodulating legumes Bauhinia cheilanta and Caesalpinia pyramidalis. (3)E, 15N enrichment; and NA, 15N natural abundance. (4)Patm, proportional dependence of the legume on biologically-fixed N. (5)B value was assumed to be zero (Ramos et al., 2001), -1.3‰ (Teixeira et al., 2006). (6)Stem tissue, low range is dry season, and high range is wet season. .
Hungria & Vargas (2000)HUNGRIA, M.; VARGAS, M.A.T. Environmental factors affecting N2 fixation in grain legumes in the tropics, with emphasis on Brazil. Field Crops Research, v.65, p.151-164, 2000. DOI: 10.1016/S0378-4290(99)00084-2.
https://doi.org/10.1016/S0378-4290(99)00...
suggested that high temperature, water stress, and soil acidity were the main abiotic constraints to BNF in tropical grain legumes in the country. However, this conclusion was based on an examination of the literature in which qualitative data, such as nodulation parameters or assays on acetylene reduction activity, were reported instead of quantitative data on legume symbiotic dependence. Isotope-based methodologies have played an important role in identifying both biotic and abiotic constraints to legume performance in Brazil. For example, the E methodology was used to identify the critical growth stages of common bean (Phaseolus vulgaris L.) during which water stress had a large negative impact on yield and BNF. Similarly, by reviewing the published studies in which the E methodology was used, Chalk et al. (2010)CHALK, P.M.; ALVES, B.J.R.; BODDEY, R.M.; URQUIAGA, S. Integrated effects of abiotic stresses on inoculant performance, legume growth and symbiotic dependence estimated by 15N dilution. Plant and Soil, v.328, p.1-16, 2010. DOI: 10.1007/s11104-009-0187-7.
https://doi.org/10.1007/s11104-009-0187-...
were able to identify temperature, water, salinity, sodicity, acidity, and mineral nutrition as major abiotic factors affecting legume BNF globally.
Moreover, biotic factors - such as the absence of indigenous rhizobia or arbuscular mycorrhizal fungi (Ibijbijen et al., 1996IBIJBIJEN, J.; URQUIAGA, S.; ISMAILI, M.; ALVES, B.J.R.; BODDEY, R.M. Effect of arbuscular mycorrhizal fungi on growth, mineral nutrition and nitrogen fixation of three varieties of common beans. New Phytologist, v.134, p.353-360, 1996. DOI: 10.1111/j.1469-8137.1996.tb04640.x.
https://doi.org/10.1111/j.1469-8137.1996...
) - and legume genotype can also affect legume symbiotic dependence. A comprehensive review of this subject was published by Chalk et al. (2006)CHALK, P.M.; SOUZA, R. de F.; URQUIAGA, S.; ALVES, B.J.R.; BODDEY, R.M. The role of arbuscular mycorrhiza in legume symbiotic performance. Soil Biology and Biochemistry, v.38, p.2944-2951, 2006. DOI: 10.1016/j.soilbio.2006.05.005.
https://doi.org/10.1016/j.soilbio.2006.0...
.
BNF dynamics
Apart from the quantification of legume BNF, a suite of indirect 15N methodologies has been developed to estimate the transfer of BNF or legume N (LN) to companion non-legume species, as reviewed by Chalk et al. (2014b)CHALK, P.M.; PEOPLES, M.B.; MCNEILL, A.M.; BODDEY, R.M.; UNKOVICH, M.J.; GARDENER, M.J.; SILVA, C.F.; CHEN, D. Methodologies for estimating nitrogen transfer between legumes and companion species in agro-ecosystems: a review of 15N-enriched techniques. Soil Biology and Biochemistry, v.73, p.10-21, 2014b. DOI: 10.1016/j.soilbio.2014.02.005.
https://doi.org/10.1016/j.soilbio.2014.0...
. Other 15N-based methods have been developed to quantify the transfer of LN or BNF to an array of crops in sequential rotations. A frequently used method involves the application of a 15N-labeled crop residue obtained from a particular source to unlabeled plots in a subsequent experiment, which might be separated in space and time from the original source. Primo et al. (2014)PRIMO, D.C.; MENEZES, R.S.C.; SAMPAIO, E.V. de S.B.; GARRIDO, M. DA S.; JÚNIOR, J.C.B.; SOUZA, C.S. Recovery of N applied as 15N-manure or 15N-gliricidia biomass by maize, cotton and cowpea. Nutrient Cycling in Agroecosystems, v.100, p.205-214, 2014. DOI: 10.1007/s10705-014-9638-5.
https://doi.org/10.1007/s10705-014-9638-...
, for example, measured cumulative recoveries of 15N-labeled Gliricidia sepium (Jacq.) Kunth biomass in a pot experiment, in three subsequent crops of corn (Zea mays L.), cotton (Gossypium hirsutum L.), or cowpea [Vigna unguiculata (L.) Walp.], obtaining values of 28, 35, and 41%, respectively. In an attempt to overcome the obvious limitations of such an approach, an in situ method based on 15N natural abundance was proposed by Perin et al. (2006)PERIN, A.; SANTOS, R.H.S.; URQUIAGA, S.S.; CECON, P.R.; GUERRA, J.G.M.; FREITAS, G.B. de. Sunnhemp and millet as green manure for tropical maize production. Scientia Agricola, v.63, p.453-459, 2006. DOI: 10.1590/S0103-90162006000500006.
https://doi.org/10.1590/S0103-9016200600...
, who estimated that corn following sunn hemp (Crotalaria juncea L.) green manure derived 15% of its grain N content from N2 fixed by the preceding sunn hemp crop.
Organic carbon dynamics in soil
For carbon, the relative isotopic abundance is expressed by the isotope ratio equation, in which R is the ratio of 13C/12C. The international standard, in this case, is Vienna Pee Dee Belemnite (VPDB), which has a δ value of -0.030 031.
It is well known that 13C isotopic fractionation occurs during photosynthesis, so that plant organic C is naturally depleted in 13C, when compared with atmospheric CO2 (δ13CO2 = -8‰). Different CO2 assimilation pathways between C3 (the Calvin cycle), C4 (the Hatch-Slack pathway), and CCAM (Crassulacean acid metabolism) plants result in markedly different bulk δ13C signatures of photosynthetic plants, mainly due to diffusion and enzymatic effects. The ranges in the δ13C values for C3, C4, and CCAM plants are -22 to -30‰, -9 to -13‰, and -10 to -20‰, respectively.
In undisturbed natural ecosystems, the δ13C signature of surface soil organic matter is similar to that of the native vegetation from which it was derived. Many of the soils used in agriculture were originally under C3 forest and, therefore, had very negative δ13C values. With the introduction of sugarcane and tropical forage grasses, such as C4 species (Table 2), the above-ground C input into the ecosystem had a much higher δ13C signature, and, over time, the δ13C value of the soil organic matter became less negative (Vitorello et al., 1989VITORELLO, V.A.; CERRI, C.C.; VICTÓRIA, R.L.; ANDREUX, F.; FELLER, C. Organic matter and natural carbon-13 distribution in forested and cultivated Oxisols. Soil Science Society of America Journal, v.53, p.773-7781989DOI: 10.2136/sssaj1989.03615995005300030024x.
https://doi.org/10.2136/sssaj1989.036159...
; Moraes et al., 1996MORAES, J.F.L. de; VOLKOFF, B.; CERRI, C.C.; BERNOUX, M. Soil properties under Amazon forest and changes due to pasture installation in Rondônia, Brazil. Geoderma, v.70, p.63-81, 1996. DOI: 10.1016/0016-7061(95)00072-0.
https://doi.org/10.1016/0016-7061(95)000...
; Pinheiro et al., 2010PINHEIRO, É.F.M.; LIMA, E.; CEDDIA, M.B.; URQUIAGA, S.; ALVES, B.J.R.; BODDEY, R.M. Impact of pre-harvest burning versus trash conservation on soil carbon and nitrogen stocks on a sugarcane plantation in the Brazilian Atlantic forest region. Plant and Soil, v.333, p.71-80, 2010. DOI: 10.1007/s11104-010-0320-7.
https://doi.org/10.1007/s11104-010-0320-...
) (Table 4).
δ13C signatures of Brazilian soils under forest and after a number of years of C4 pasture or sugarcane (Saccharum officinarum) established on cleared forest.
An innovative methodology to estimate the proportions of soil C from the original and introduced vegetation is based on the shift in the δ13C signature of soil C and was developed by Cerri et al. (1985)CERRI, C.C.; FELLER, C.; BALESDENT, J.; VICTORIA, R.; PLENNECASSAGNE, A. Application du traçage isotopique naturel en 13C à l'ètude de la matière organique dans les sols. Comptes Rendus de l'Académie des Sciences Paris, t.300, série II, p.423-428, 1985. at Centro de Energia Nuclear na Agricultura (Cena), the center for nuclear energy in agriculture of Universidade de São Paulo (USP). The proportion of organic C in a soil layer derived from the introduced C4 vegetation (PC4soil) was expressed by the following equation: PC4soil = δ(13C)C4soil - δ(13C)C3soil)/(δ(13C)C4plant - δ(13C)C3soil)], in which δ(13C)C4soil is the δ13C value of a soil layer under C4 vegetation; δ(13C)C3soil is the δ13C value of a soil layer under the original C3 vegetation; and δ(13C)C4plant is the δ13C value of the C4 vegetation. PC4soil increased with increasing years under C4 pasture or sugarcane (Table 4), and Moraes et al. (1996)MORAES, J.F.L. de; VOLKOFF, B.; CERRI, C.C.; BERNOUX, M. Soil properties under Amazon forest and changes due to pasture installation in Rondônia, Brazil. Geoderma, v.70, p.63-81, 1996. DOI: 10.1016/0016-7061(95)00072-0.
https://doi.org/10.1016/0016-7061(95)000...
reported that the original C3 signal in the 0-10-cm soil layer had disappeared after 81 years under C4 pasture.
The concentrations of soil organic C and δ13C values are not constant with soil depth (Sisti et al., 2004SISTI, C.P.J.; SANTOS, H.P. dos; KOHHANN, R.; ALVES, B.J.R.; URQUIAGA, S.; BODDEY, R.M. Change in carbon and nitrogen stocks in soil under 13 years of conventional or zero tillage in southern Brazil. Soil and Tillage Research, v.76, p.39-58, 2004. DOI: 10.1016/j.still.3003.08.007.
https://doi.org/10.1016/j.still.3003.08....
). Organic C concentrations generally decrease with soil depth. However, in C3 forest, soil δ13C values increased with depth, although, in soil under C4 pasture or sugarcane, these values also decreased with depth due to the decreasing effect of the C4 pasture with depth (Vitorello et al., 1989VITORELLO, V.A.; CERRI, C.C.; VICTÓRIA, R.L.; ANDREUX, F.; FELLER, C. Organic matter and natural carbon-13 distribution in forested and cultivated Oxisols. Soil Science Society of America Journal, v.53, p.773-7781989DOI: 10.2136/sssaj1989.03615995005300030024x.
https://doi.org/10.2136/sssaj1989.036159...
; Moraes et al., 1996MORAES, J.F.L. de; VOLKOFF, B.; CERRI, C.C.; BERNOUX, M. Soil properties under Amazon forest and changes due to pasture installation in Rondônia, Brazil. Geoderma, v.70, p.63-81, 1996. DOI: 10.1016/0016-7061(95)00072-0.
https://doi.org/10.1016/0016-7061(95)000...
; Pinheiro et al., 2010PINHEIRO, É.F.M.; LIMA, E.; CEDDIA, M.B.; URQUIAGA, S.; ALVES, B.J.R.; BODDEY, R.M. Impact of pre-harvest burning versus trash conservation on soil carbon and nitrogen stocks on a sugarcane plantation in the Brazilian Atlantic forest region. Plant and Soil, v.333, p.71-80, 2010. DOI: 10.1007/s11104-010-0320-7.
https://doi.org/10.1007/s11104-010-0320-...
).
Management factors can affect the dynamics of C when the system is undergoing interchange between C3- and C4-dominant vegetation. For instance, the tillage system (zero vs. conventional) under different long-term crop rotations was shown to affect the decay of the original C3-derived soil organic C to a depth of 100 cm (Sisti et al., 2004SISTI, C.P.J.; SANTOS, H.P. dos; KOHHANN, R.; ALVES, B.J.R.; URQUIAGA, S.; BODDEY, R.M. Change in carbon and nitrogen stocks in soil under 13 years of conventional or zero tillage in southern Brazil. Soil and Tillage Research, v.76, p.39-58, 2004. DOI: 10.1016/j.still.3003.08.007.
https://doi.org/10.1016/j.still.3003.08....
). Balieiro et al. (2008)BALIEIRO, F. de C.; PEREIRA, M.G.; ALVES, B.J.R.; RESENDE, A.S. de; FRANCO, A.A. Soil carbon and nitrogen in pasture soil reforested with eucalyptus and guachapele. Revista Brasileira de Ciência do Solo, v.32, p.1253-1260, 2008. DOI: 10.1590/S0100-06832008000300033.
https://doi.org/10.1590/S0100-0683200800...
showed that the stocks of soil C and δ13C signatures to 10-cm depth were affected by the reintroduction of two C3-forest species, Eucalyptus grandis W.Hill and Pseudosamanea guachapele Harms, the latter being a N2-fixing tree. The site had formerly supported C4Panicum maximum Jacq. for ten years. The rate of replacement of soil C by new forest C after five years was much faster under a mixed plantation of trees rather than under pure plantations of either species (Balieiro et al., 2008BALIEIRO, F. de C.; PEREIRA, M.G.; ALVES, B.J.R.; RESENDE, A.S. de; FRANCO, A.A. Soil carbon and nitrogen in pasture soil reforested with eucalyptus and guachapele. Revista Brasileira de Ciência do Solo, v.32, p.1253-1260, 2008. DOI: 10.1590/S0100-06832008000300033.
https://doi.org/10.1590/S0100-0683200800...
).
There is an increasing interest worldwide in the use of soil additives, such as biochars, due to their potential to sequester soil C. In a 30-day laboratory incubation study with three biochars derived from C3 oilseed residues added to a sandy soil containing C4 carbon, Rittle et al. (2015)RITTLE, T.F.; NOVOTNY, E.H.; BALIEIRO, F.C.; HOFFLAND, E.; ALVES, B.J.R.; KUYPER, T.W. Negative priming of native soil organic carbon mineralization by oilseed biochars of contrasting quality. European Journal of Soil Science, v.66, p.714-721, 2015. DOI: 10.1111/ejss.12257.
https://doi.org/10.1111/ejss.12257...
found that respired CO2 came principally from the biochars and that these decelerated the mineralization of the indigenous C (negative priming effect). Therefore, there may be both direct and indirect effects of biochar addition on C sequestration.
Synthesis and evaluation of labeled fertilizers
Scientists at Cena of USP have been involved for many years in the synthesis of labeled fertilizers using ion exchange chromatography (Bendassolli et al., 1997BENDASSOLLI, J.A.; TRIVELIN, P.C.O.; CARNEIRO JUNIOR, F. Stable sulfur isotope fractionation by anion exchange chromatography. Production of compounds enriched in 34S. Journal of the Brazilian Chemical Society, v.8, p.13-17, 1997. DOI: 10.1590/S0103-50531997000100004.
https://doi.org/10.1590/S0103-5053199700...
). They have also developed new methods for the isotope-ratio analysis of labeled plants and soils (Carneiro et al., 2008CARNEIRO, J.M.T.; ROSSETE, A.L.R.M.; BENDASSOLLI, J.A. Isotopic determination of silicon by mass spectrometry in plants and soils labeled with 30Si. Analytical Letters, v.41, p.1640-1647, 2008. DOI: 10.1080/00032710802122305.
https://doi.org/10.1080/0003271080212230...
). Some examples of labeled fertilizers synthesized at Cena are given in Table 5. The isotopic enrichment of the product is largely determined either by the isotopic enrichment of the source materials or by the degree of isotopic fractionation during ion exchange chromatography.
Synthesis of labeled fertilizers at Centro de Energia Nuclear na Agricultura of Universidade de São Paulo, as well as fertilizer use efficiency.
By using isotopically-labeled fertilizers, it is possible to determine the recovery in the plant-soil system. The proportion of 15N-enriched fertilizer recovered by a plant, for example, can be calculated according to the equation: proportional N recoveryplant = (mass Nplant× xE(15N)plant/mass Nfertilizer× xE(15N)fertilizer). in which xE(15N)plant/xE(15N)fertilizer is the fraction of plant N derived from the fertilizer. The same procedure is used to determine the recovery of fertilizer in the soil, and fertilizer loss from the plant-soil system can be calculated by mass balance.
Many studies have been undertaken with different crops and strategies of N fertilizer application, in order to estimate N fertilizer efficiency. Recovery by annual crops seldom exceeds 60%, and the cumulative residual value of the N fertilizer in two subsequent crops is usually <10%. Some examples of recoveries of 15N- and 34S-labeled fertilizers in plant tops are given in Table 5.
In addition to studies with labeled synthetic (industrially manufactured) N fertilizers, such as urea and ammonium-based forms, efficiency studies have also been performed with labeled organic sources, including composts (Chalk et al., 2013CHALK, P.M.; MAGALHÃES, A.M.T.; INÁCIO, C.T. Towards an understanding of the dynamics of compost N in the soil-plant-atmosphere system using 15N tracer. Plant and Soil, v.362, p.373-388, 2013. DOI: 10.1007/s11104-012-1358-5.
https://doi.org/10.1007/s11104-012-1358-...
) and animal excreta (Primo et al., 2014PRIMO, D.C.; MENEZES, R.S.C.; SAMPAIO, E.V. de S.B.; GARRIDO, M. DA S.; JÚNIOR, J.C.B.; SOUZA, C.S. Recovery of N applied as 15N-manure or 15N-gliricidia biomass by maize, cotton and cowpea. Nutrient Cycling in Agroecosystems, v.100, p.205-214, 2014. DOI: 10.1007/s10705-014-9638-5.
https://doi.org/10.1007/s10705-014-9638-...
), or with labeled slow- or controlled-release products (Chalk et al., 2015aCHALK, P.M.; CRASWELL, E.T.; POLIDORO, J.C.; CHEN, D. Fate and efficiency of 15N-labelled slow- and controlled-release fertilizers: a review. Nutrient Cycling in Agroecosystems, v.102, p.167-178, 2015a. DOI: 10.1007/s10705-015-9697-2.
https://doi.org/10.1007/s10705-015-9697-...
). It is also possible to trace the dynamics of N from source (fertilizer) to product (food) in agroecosystems using 15N natural abundance (Chalk et al., 2014aCHALK, P.M.; INÁCIO, C.T.; MAGALHÃES, A.M.T. From fertilizer to food: tracing N dynamics in conventional and organic farming systems using 15N natural abundance. In: HENG, L.K.; SAKADEVAN, K.; DERCON, G.; NGUYEN, M.L. (Ed.). International symposium on managing soils for food security and climate change adaptation and mitigation. Rome: Food and Agriculture Organization of the United Nations, 2014a. p.339-348.).
Food and beverage authentication
In the globalization era of agricultural products of plant and animal origin, the public is demanding authenticity and accountability in food and beverage labelling. Stable isotopes are playing an expanding role in detecting adulteration and in verifying geographic origin and production regime. Some examples of research carried out in Brazil are summarized in Table 6, of which several will be outlined in some detail.
Adulteration
Marked differences are observed in the δ13C signatures between sugars derived from C3 and C4 plants. This makes it possible to detect the presence of C4 sugar or C4 ethanol in a product ostensibly derived from a C3 source. Therefore, it allows identifying, for instance, the sources of ethanol in Brazilian brandy (Pissinatto et al., 1999PISSINATTO, L.; MARTINELLI, L.A.; VICTORIA, R.L.; CAMARGO, P.B. de. Stable carbon isotopic analysis and the botanical origin of ethanol in Brazilian brandies. Food Research International, v.32, p.665-668, 1999. DOI: 10.1016/S0963-9969(99)00143-X.
https://doi.org/10.1016/S0963-9969(99)00...
) or sparkling wine (Martinelli et al., 2003MARTINELLI, L.A.; MOREIRA, M.Z.; OMETTO, J.P.H.B.; ALCARDE, A.R.; RIZZON, L.A.; STANGE, E.; EHLERINGER, J.R. Stable carbon isotopic composition of the wine and CO2 bubbles of sparkling wines: detecting C4 sugar additions. Journal of Agricultural and Food Chemistry, v.51, p.2625-2631, 2003. DOI: 10.1021/jf026088c.
https://doi.org/10.1021/jf026088c...
), or whether C4 sugar has been added to freshly-squeezed orange juice (Pupin et al., 1998PUPIN, A.M.; DENNIS, M.J.; PARKER, I.; KELLY, S.; BIGWOOD, T.; TOLEDO, M.C.F. Use of isotopic analyses to determine the authenticity of Brazilian orange juice (Citrus sinensis). Journal of Agricultural and Food Chemistry, v.46, p.1369-1373, 1998. DOI: 10.1021/jf970746p.
https://doi.org/10.1021/jf970746p...
) or to honey (Padovan et al., 2003PADOVAN, G.J.; DE JONG, D.; RODRIGUES, L.P.; MARCHINI, J.S. Detection of adulteration of commercial honey samples by the 13C/12C isotope ratio. Food Chemistry, v.82, p.633-636, 2003. DOI: 10.1016/S0308-8146(02)00504-6.
https://doi.org/10.1016/S0308-8146(02)00...
).
Honey is produced from the nectar collected by bees from flowering plants, mainly from C3 plants, and, to a lesser extent, from C4 and CCAM plants. The δ13C signature of the protein extract of honey will reflect the dominant source from which the honey was derived, e.g. -22 to -33‰ for C3 plants, -10 to -20 for C4 plants, and -11 to -13.5 for CCAM plants (Padovan et al., 2003PADOVAN, G.J.; DE JONG, D.; RODRIGUES, L.P.; MARCHINI, J.S. Detection of adulteration of commercial honey samples by the 13C/12C isotope ratio. Food Chemistry, v.82, p.633-636, 2003. DOI: 10.1016/S0308-8146(02)00504-6.
https://doi.org/10.1016/S0308-8146(02)00...
). Adulteration of pure honey with cheap C4 sugars, such as cane or corn syrup, has become an international problem. It can be detected by δ13C analysis of the bulk honey and of the protein extracted from the honey, although the latter is not affected by the adulteration. A difference of 1 δ indicates 7% of C4 sugar added, which is the limit tolerated worldwide (Padovan et al., 2003PADOVAN, G.J.; DE JONG, D.; RODRIGUES, L.P.; MARCHINI, J.S. Detection of adulteration of commercial honey samples by the 13C/12C isotope ratio. Food Chemistry, v.82, p.633-636, 2003. DOI: 10.1016/S0308-8146(02)00504-6.
https://doi.org/10.1016/S0308-8146(02)00...
).
Geographic origin
The most useful indicators of the geographic origin or provenance of terrestrial plants are the δ18O or δ2H values of stem water. At a given geographic location, the relationship between δ18O and δ2H of meteoric water, i.e., water from precipitation, is linear, referred to as the meteoric water line. δ18O is defined by the isotope ratio equation, in which R is the ratio of 18O/16O. The primary international standard, in this case, is Vienna Standard Mean Ocean Water (VSMOW), which, by definition, has δ18O and δ2H values of zero (Brand et al., 2014BRAND, W.A.; COPLEN, T.B.; VOGL, J.; ROSNER, M.; PROHASKA, T. Assessment of international reference materials for isotope-ratio analysis (IUPAC Technical Report). Pure and Applied Chemistry, v.86, p.425-467, 2014. DOI: 10.1515/pac-2013-1023.
https://doi.org/10.1515/pac-2013-1023...
).
The isotopic composition of meteoric water is affected by physical phenomena such as condensation and evaporation, with temperature being the main variable that affects isotopic fractionation. The δ18O and δ2H composition of meteoric water follows a predictable geographic pattern that is related to latitude, altitude, amount of precipitation, and distance from the ocean. The annual mean δ18O in meteoric water ranges from +2 to -2‰, in equatorial regions, to as low as -22‰ in the north-polar region. It should be noted that there is no isotopic fractionation during water uptake by terrestrial plants and, therefore, stem water has the same signature as the water source, regarding access to deep vs. shallow water.
The three main wine producing regions in the state of Rio Grande do Sul could be differentiated on the basis of δ18O signatures of water in the wines (Table 7) (Dutra et al., 2011DUTRA, S.V.; ADAMI, L.; MARCON, A.R.; CARNIELI, G.J.; ROANI, C.A.; SPINELLI, F.R.; LEONARDELLI, S.; DUCATTI, C.; MOREIRA, M.Z.; VANDERLINDE, R. Determination of the geographical origin of Brazilian wines by isotope and mineral analysis. Analytical and Bioanalytical Chemistry, v.401, p.1571-1576, 2011. DOI: 10.1007/s00216-011-5181-2.
https://doi.org/10.1007/s00216-011-5181-...
), but not in the wine ethanol. These authors speculated that the variation in δ18O among regions may be due to differences in local meteorological conditions, such as rainfall and temperature, but that altitude and distance to the ocean may also play a role. A more intensive data analysis of site-specific and regional climate and geography is necessary to separate these variables.
δ18O in water and δ13C in ethanol in wines from three regions of the state of Rio Grande do Sul, Brazil (Dutra et al., 2011DUTRA, S.V.; ADAMI, L.; MARCON, A.R.; CARNIELI, G.J.; ROANI, C.A.; SPINELLI, F.R.; LEONARDELLI, S.; DUCATTI, C.; MOREIRA, M.Z.; VANDERLINDE, R. Determination of the geographical origin of Brazilian wines by isotope and mineral analysis. Analytical and Bioanalytical Chemistry, v.401, p.1571-1576, 2011. DOI: 10.1007/s00216-011-5181-2.
https://doi.org/10.1007/s00216-011-5181-... )(1) (1) Means followed by equal letters do not differ by Tukey’s test, at 5% probability. .
Geographic origin may also be differentiated by differences in δ13C signatures. For example, a survey of δ13C signatures of Big Mac hamburger patties from outlets in Brazil and Great Britain showed extreme median values of -11.1 and -25.4‰, respectively, indicating the predominant C4 (tropical grasses) or C3 (temperate species) diet of the beef cattle (Martinelli et al., 2011MARTINELLI, L.A.; NARDOTO, G.B.; CHESSON, L.A.; RINALDI, F.D.; OMETTO, J.P.H.B.; CERLING, T.E.; EHLERINGER, J.R. Worldwide stable carbon and nitrogen isotopes of Big Mac® patties: an example of a truly "glocal" food. Food Chemistry, v.127, p.1712-1718, 2011. DOI: 10.1016/j.foodchem.2011.02.046.
https://doi.org/10.1016/j.foodchem.2011....
).
Both δ13C and δ15N signatures have also been successfully used to differentiate the regional production of the illegal drug marijuana (Cannabis sativa L.) in Brazil (Shibuya et al., 2006SHIBUYA, E.K.; SARKIS, J.E.S.; NEGRINI NETO, O.; MOREIRA, M.Z.; VICTORIA, R.L. Sourcing Brazilian marijuana by applying IRMS analysis to seized samples. Forensic Science International, v.160, p.35-43, 2006. DOI: 10.1016/j.forsciint.2005.08.011.
https://doi.org/10.1016/j.forsciint.2005...
). From the known producing areas of the states of Pernambuco and Bahia (dry region) and of Mato Grosso do Sul and Pará (wet region), mean δ15N values of the samples seized from the dry region varied from +1 to +2‰, whereas those from the wet region were higher, varying from +5 to +6.8‰. The mean δ13C values for the dry region were of -26 to -26.5‰, differing from those of the wet region, which varied from -29.2 to -30.2‰. This information provides the basis for the allocation of police resources to intercept shipments from the source to the largest population of consumers in the city of São Paulo, in the state of São Paulo.
Production regime
The δ13C signature of the feces or body tissues of domestic animals is a robust indicator of diet composition. For instance, in another study, the 13C natural abundance of cattle manure was an effective predictor of the proportion of legume in a diet consisting of legume (Desmodium ovalifolium Wall.) + grass [Urochloa dictyoneura (Fig. & de Not.) Veldkamp (Syn. Brachiaria dictyoneura )] forage (Macedo et al., 2010MACEDO, R.; TARRÉ, R.M.; FERREIRA, E.; REZENDE, C. de P.; PEREIRA, J.M.; CADISCH, G.; ROUWS, J.R.C.; ALVES, B.J.R.; URQUIAGA, S.; BODDEY, R.M. Forage intake and botanical composition of feed for cattle fed Brachiaria/legume mixtures. Scientia Agricola, v.67, p.384-392, 2010. DOI: 10.1590/S0103-90162010000400002.
https://doi.org/10.1590/S0103-9016201000...
). Colleta et al. (2012)COLLETA, L.D.; PEREIRA, A.L.; COELHO, A.A.D.; SAVINO, V.J.M.; MENTEN, J.F.M.; CORRER, E.; FRANÇA, L.C.; MARTINELLI, L.A. Barn vs. free-range chickens: differences in their diets determined by stable isotopes. Food Chemistry, v.131, p.155-160, 2012. were able to separate barn vs. free-range chicken according to their diet, in which the latter had more C4 in their diet and higher δ15N values, which the authors attributed to the ingestion of animal protein.
The presence of poultry offal meal, i.e., a product derived from the entrails and internal organs of poultry, in broiler diets is a potential threat to the large Brazilian export market in chicken meat due to concerns regarding contamination with Salmonella or Escherichia coli . Both δ13C and δ15N have shown promise under controlled experimental conditions as a diagnostic tool to identify the presence of offal in broiler diets (Oliveira et al., 2010OLIVEIRA, R.P.; DUCATTI, C.; PEZZATO, A.C.; DENADAI, J.C.; CRUZ, V.C.; SARTORI, J.R.; CARRIJO, A.S.; CALDARA, F.R. Traceability of poultry offal meal in broiler feeding using isotopic analysis (δ13C and δ15N) of different tissues. Revista Brasileira Ciência Avícola, v.12, p.13-20, 2010. DOI: 10.1590/S1516-635X2010000100002.
https://doi.org/10.1590/S1516-635X201000...
), but, by themselves, cannot be used as a conclusive proof. Despite this, δ13C values showed clear differences between a species of farmed and wild freshwater fish (Cachara) from the Alta Floresta region in the Amazon (Sant'Ana et al., 2010SANT'ANA, L.S.; DUCATTI, C.; RAMIRES, D.G. Seasonal variations in chemical composition and stable isotopes of farmed and wild Brazilian freshwater fish. Food Chemistry, v.122, p.74-77, 2010. DOI: 10.1016/j.foodchem.2010.02.016.
https://doi.org/10.1016/j.foodchem.2010....
). This was observed both in the wet and dry seasons, with mean values varying from -23.6 to -24‰ for farmed fish and from -29.1 to -30‰ for wild fish.
Furthermore, δ13C composition is also a good index of the ingredients used in the brewing process of Brazilian beers (Mardegan et al., 2013MARDEGAN, S.F.; ANDRADE, T.M.B.; SOUSA NETO, R. de S.; VASCONCELLOS, E.B. de C.; MARTINS, L.F.B.; MENDONÇA, T.G.; MARTINELLI, L.A. Stable carbon isotopic composition of Brazilian beers - a comparison between large- and small-scale breweries. Journal of Food Composition and Analysis, v.29, p.52-57, 2013. DOI: 10.1016/j.jfca.2012.10.004.
https://doi.org/10.1016/j.jfca.2012.10.0...
). Large breweries use a relatively high proportion of C4 corn resulting in a mean δ13C value of -20‰, whereas small artisanal (boutique) breweries use a higher proportion of C3 cereals, resulting in a mean δ13C value of -25‰. These differences are driven by the need of small brewers to market a product distinctive in quality from mass-produced beer, and also by the need to use fewer ingredients of lower cost, more rapid fermentation, and higher alcohol content, which corn provides.
The use of stable isotopes to differentiate organic vs. conventional plant and animal products was reviewed by Inácio & Chalk (2015)INÁCIO, C.T.; CHALK, P.M. Principles and limitations of stable isotopes in differentiating organic and conventional foodstuffs: 2. Animal products. Critical Reviews in Food Science and Nutrition, online, 2015. DOI: 10.1080/10408398.2014.887056.
https://doi.org/10.1080/10408398.2014.88...
and Inácio et al. (2015a)INÁCIO, C.T.; CHALK, P.M.; MAGALHÃES, A.M.T. Principles and limitations of stable isotopes in differentiating organic and conventional foodstuffs: 1. Plant products. Critical Reviews in Food Science and Nutrition, v.55, p.1206-1218, 2015a. DOI: 10.1080/10408398.2012.689380.
https://doi.org/10.1080/10408398.2012.68...
, respectively. In a study of conventionally and organically produced lettuce (Lactuca sativa L.) in the state of Rio de Janeiro, Inácio et al. (2015b)INÁCIO, C.T.; URQUIAGA, S.; CHALK, P.M.; MATA, M.G.F. ; SOUZA, P.O. Identifying N fertilizer regime and vegetable production system in tropical Brazil using 15N natural abundance. Journal of the Science of Food and Agriculture, v.95, p.3025-3032, 2015b. DOI: 10.1002/jsfa.7177.
https://doi.org/10.1002/jsfa.7177...
concluded that the δ15N signatures of lettuce were an indicator of the principal N inputs (BNF, organic or manufactured fertilizer) instead of a definitive indicator of the production regime.
Brazilian institutions involved in stable isotope applications in agroecosystems
The precise measurement of isotopic abundance of the lighter elements requires an isotope-ratio mass spectrometer (IRMS). Computer-controlled, automated sample processing and analysis is a feature of modern instruments, which are equipped with multiple inlet and collector systems, allowing for the simultaneous measurement of several ions differing in mass-to-charge ratio. IRMS is a mature but somewhat expensive technology, requiring a relatively large initial capital investment and on-going technical support and maintenance costs.
Several Brazilian research institutions have been involved in stable isotope research for many years, and their increasing number testifies to the importance of this technology. The known Brazilian institutions involved in stable isotope research are identified in Table 8.
Brazilian institutions involved in stable isotope research in terrestrial and agroecosystems(1) (1) While endeavoring to be comprehensive, some institutions may have been inadvertently overlooked. (2)IRMS, isotope-ratio mass spectrometer. .
Several educational institutions, including Cena of USP, or institutions affiliated with Universities, such as, Embrapa Agrobiologia and Universidade Federal Rural do Rio de Janeiro, have strong post-graduate research training and teaching programs in stable isotope applications. Therefore, Brazilian scientists are well equipped to participate not only in national programs but also in international ones involving stable isotope applications in soil, plant, food, and animal sciences. Among the opportunities for Brazilian scientists, are possible contributions to the research programs of the Division of Nuclear Techniques in Food and Agriculture of the Joint Food and Agriculture Organization of the United Nations/International Atomic Energy Agency (FAO/IAEA) and to the technical cooperation programs of IAEA, either as project participants or as institutional employees. The programs in food and agriculture cover the areas of plant breeding and genetics, soil and water management and crop nutrition, insect pest control, animal production and health, and food and environmental protection (Iaea, 2015IAEA. INTERNATIONAL ATOMIC ENERGY AGENCY. Joint FAO/IAEA programme: nuclear techniques in food and agriculture. 2015. Available at: <http://www-naweb.iaea.org/nafa/>. Accessed on: Jul. 16 2015.
http://www-naweb.iaea.org/nafa/...
). Participation in such programs raises the international profile of the important work being undertaken in Brazil.
Conclusions
Brazilian scientists have made significant original contributions, through stable isotope tracing, for better understanding of carbon and nutrient cycling in agroecosystems, as well as in food and beverage authentication. There is an increasing awareness of the importance of stable isotope research in many branches of science, for which IRMS is an essential piece of equipment that is best provided at institutional level. The less desirable and often impractical alternative is to rely on expensive fee-for-service laboratories or to collaborate with a sister institution that has IRMS facilities. In this sense, one of the main impediments to a more innovative research in nutrient cycling in natural and agroecosystems in Brazil is the obstacle created by the current paucity of IRMS facilities in the national institutions.
New or emerging technologies in the agricultural sector will require the application of stable isotope techniques to follow the dynamics of C, N, and S in the soil-plant-animal-atmosphere continuum. For example, for the introduction of new legume species or cultivars, as well as of new crop rotations, including legumes or new inoculants, it will be necessary to assess the contribution of BNF to N balance in agroecosystems. Similarly, the efficacy of new slow- or controlled-release fertilizers or chemical inhibitors will need to be evaluated using stable isotope techniques. The effect of innovative agronomic practices, such as the use of biochar, will require the strategic application of stable isotopes to verify the sequestration of C, N, and S. The alternative use of radioactive tracers (e.g. 14C and 35S) is generally precluded due to restrictions imposed by environmental and safety issues, whereas the half-life of 13N of 9.97 min is too short to be of practical value. Stable isotopes have a role in characterizing negative interactions between agriculture and the environment, such as methane emissions from ruminants and rice paddies, and also fertilizer-induced emissions of nitrous oxide. Scientists in Brazil are in a unique position to assess innovative technologies to enhance resource-use efficiency across a wide range of soils and climatic conditions, extending from the humid tropics to temperate regions.
Acknowledgements
To Embrapa colleagues, for constructive comments on the manuscript; to Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (Faperj), for financial support (grant No. 102.515/2009, 101.466/2014); and to University of Melbourne, for assistance with travel expenses.
References
- AMBROSANO, E.J.; TRIVELIN, P.C.O.; CANTARELLA, H.; AMBROSANO, G.M.B.; SCHAMMASS, E.A.; GUIRADO, N.; ROSSI, F.; MENDES, P.C.D.; MURAOKA, T. Utilization of nitrogen from green manure and mineral fertilizer by sugarcane. Scientia Agricola, v.62, p.534-542, 2005. DOI: 10.1590/S0103-90162005000600004.
» https://doi.org/10.1590/S0103-90162005000600004 - BALIEIRO, F. de C.; PEREIRA, M.G.; ALVES, B.J.R.; RESENDE, A.S. de; FRANCO, A.A. Soil carbon and nitrogen in pasture soil reforested with eucalyptus and guachapele. Revista Brasileira de Ciência do Solo, v.32, p.1253-1260, 2008. DOI: 10.1590/S0100-06832008000300033.
» https://doi.org/10.1590/S0100-06832008000300033 - BENDASSOLLI, J.A.; TRIVELIN, P.C.O.; CARNEIRO JUNIOR, F. Stable sulfur isotope fractionation by anion exchange chromatography. Production of compounds enriched in 34S. Journal of the Brazilian Chemical Society, v.8, p.13-17, 1997. DOI: 10.1590/S0103-50531997000100004.
» https://doi.org/10.1590/S0103-50531997000100004 - BERGLUND, M.; WIESER, M.E. Isotopic compositions of the elements 2009 (IUPAC Technical Report). Pure and Applied Chemistry, v.83, p.397-410, 2011. DOI: 10.1351/PAC-REP-10-06-02.
» https://doi.org/10.1351/PAC-REP-10-06-02 - BODDEY, R.M.; CHALK, P.M.; VICTORIA, R.; MATSUI, E. Nitrogen fixation by nodulated soybean under tropical field conditions estimated by the 15N isotope dilution technique. Soil Biology and Biochemistry, v.16, p.583-588, 1984. DOI: 10.1016/0038-0717(84)90076-2.
» https://doi.org/10.1016/0038-0717(84)90076-2 - BODDEY, R.M.; CHALK, P.M.; VICTORIA, R.; MATSUI, E. The 15N-isotope dilution technique applied to the estimation of biological nitrogen fixation associated with Paspalum notatum cv. batatais in the field. Soil Biology and Biochemistry, v.15, p.25-32, 1983. DOI: 10.1016/0038-0717(83)90114-1.
» https://doi.org/10.1016/0038-0717(83)90114-1 - BRAND, W.A.; COPLEN, T.B.; VOGL, J.; ROSNER, M.; PROHASKA, T. Assessment of international reference materials for isotope-ratio analysis (IUPAC Technical Report). Pure and Applied Chemistry, v.86, p.425-467, 2014. DOI: 10.1515/pac-2013-1023.
» https://doi.org/10.1515/pac-2013-1023 - CALVACHE, A.M.; REICHARDT, K. Efeito de épocas de deficiência hídrica na eficiência do uso do nitrogênio da cultura do feijão cv. Imbabello. Scientia Agricola, v.53, p.343-353, 1996. DOI: 10.1590/S0103-90161996000200025.
» https://doi.org/10.1590/S0103-90161996000200025 - CARNEIRO, J.M.T.; ROSSETE, A.L.R.M.; BENDASSOLLI, J.A. Isotopic determination of silicon by mass spectrometry in plants and soils labeled with 30Si. Analytical Letters, v.41, p.1640-1647, 2008. DOI: 10.1080/00032710802122305.
» https://doi.org/10.1080/00032710802122305 - CERRI, C.C.; FELLER, C.; BALESDENT, J.; VICTORIA, R.; PLENNECASSAGNE, A. Application du traçage isotopique naturel en 13C à l'ètude de la matière organique dans les sols. Comptes Rendus de l'Académie des Sciences Paris, t.300, série II, p.423-428, 1985.
- CHALK, P.M.; ALVES, B.J.R.; BODDEY, R.M.; URQUIAGA, S. Integrated effects of abiotic stresses on inoculant performance, legume growth and symbiotic dependence estimated by 15N dilution. Plant and Soil, v.328, p.1-16, 2010. DOI: 10.1007/s11104-009-0187-7.
» https://doi.org/10.1007/s11104-009-0187-7 - CHALK, P.M.; CRASWELL, E.T.; POLIDORO, J.C.; CHEN, D. Fate and efficiency of 15N-labelled slow- and controlled-release fertilizers: a review. Nutrient Cycling in Agroecosystems, v.102, p.167-178, 2015a. DOI: 10.1007/s10705-015-9697-2.
» https://doi.org/10.1007/s10705-015-9697-2 - CHALK, P.M.; INÁCIO, C.T.; CRASWELL, E.T.; CHEN, D. On the usage of absolute (x) and relative (δ) values of 15N abundance. Soil Biology and Biochemistry, v.85, p.51-53, 2015b. DOI: 10.1016/j.soilbio.2015.02.027.
» https://doi.org/10.1016/j.soilbio.2015.02.027 - CHALK, P.M.; INÁCIO, C.T.; MAGALHÃES, A.M.T. From fertilizer to food: tracing N dynamics in conventional and organic farming systems using 15N natural abundance. In: HENG, L.K.; SAKADEVAN, K.; DERCON, G.; NGUYEN, M.L. (Ed.). International symposium on managing soils for food security and climate change adaptation and mitigation Rome: Food and Agriculture Organization of the United Nations, 2014a. p.339-348.
- CHALK, P.M.; LADHA, J.K. Estimation of legume symbiotic dependence: an evaluation of techniques based on 15N dilution. Soil Biology and Biochemistry, v.31, p.1901-1917, 1999. DOI: 10.1016/S0038-0717(99)00095-4.
» https://doi.org/10.1016/S0038-0717(99)00095-4 - CHALK, P.M.; MAGALHÃES, A.M.T.; INÁCIO, C.T. Towards an understanding of the dynamics of compost N in the soil-plant-atmosphere system using 15N tracer. Plant and Soil, v.362, p.373-388, 2013. DOI: 10.1007/s11104-012-1358-5.
» https://doi.org/10.1007/s11104-012-1358-5 - CHALK, P.M.; PEOPLES, M.B.; MCNEILL, A.M.; BODDEY, R.M.; UNKOVICH, M.J.; GARDENER, M.J.; SILVA, C.F.; CHEN, D. Methodologies for estimating nitrogen transfer between legumes and companion species in agro-ecosystems: a review of 15N-enriched techniques. Soil Biology and Biochemistry, v.73, p.10-21, 2014b. DOI: 10.1016/j.soilbio.2014.02.005.
» https://doi.org/10.1016/j.soilbio.2014.02.005 - CHALK, P.M.; SOUZA, R. de F.; URQUIAGA, S.; ALVES, B.J.R.; BODDEY, R.M. The role of arbuscular mycorrhiza in legume symbiotic performance. Soil Biology and Biochemistry, v.38, p.2944-2951, 2006. DOI: 10.1016/j.soilbio.2006.05.005.
» https://doi.org/10.1016/j.soilbio.2006.05.005 - COLLETA, L.D.; PEREIRA, A.L.; COELHO, A.A.D.; SAVINO, V.J.M.; MENTEN, J.F.M.; CORRER, E.; FRANÇA, L.C.; MARTINELLI, L.A. Barn vs. free-range chickens: differences in their diets determined by stable isotopes. Food Chemistry, v.131, p.155-160, 2012.
- COPLEN, T.B. Guidelines and recommended terms for expression of stable-isotope-ratio and gas-ratio results. Rapid Communications in Mass Spectrometry, v.25, p.2538-2560, 2011. DOI: 10.1002/rcm.5129.
» https://doi.org/10.1002/rcm.5129 - DE-POLLI, H.; MATSUI, E.; DÖBEREINER, J.; SALATI, E. Confirmation of nitrogen fixation in two tropical grasses by 15N2 incorporation. Soil Biology and Biochemistry, v.9, p.119-123, 1977. DOI: 10.1016/0038-0717(77)90047-5.
» https://doi.org/10.1016/0038-0717(77)90047-5 - DUTRA, S.V.; ADAMI, L.; MARCON, A.R.; CARNIELI, G.J.; ROANI, C.A.; SPINELLI, F.R.; LEONARDELLI, S.; DUCATTI, C.; MOREIRA, M.Z.; VANDERLINDE, R. Determination of the geographical origin of Brazilian wines by isotope and mineral analysis. Analytical and Bioanalytical Chemistry, v.401, p.1571-1576, 2011. DOI: 10.1007/s00216-011-5181-2.
» https://doi.org/10.1007/s00216-011-5181-2 - FENILLI, T.A.B.; REICHARDT, K.; FAVARIN, J.L.; BACCHI, O.O.S.; SILVA, A.L.; TIMM, L.C. Fertilizer 15N balance in a coffee cropping system: a case study in Brazil. Revista Brasileira de Ciência do Solo, v.32, p.1459-1469, 2008. DOI: 10.1590/S0100-06832008000400010.
» https://doi.org/10.1590/S0100-06832008000400010 - HUNGRIA, M.; VARGAS, M.A.T. Environmental factors affecting N2 fixation in grain legumes in the tropics, with emphasis on Brazil. Field Crops Research, v.65, p.151-164, 2000. DOI: 10.1016/S0378-4290(99)00084-2.
» https://doi.org/10.1016/S0378-4290(99)00084-2 - IAEA. INTERNATIONAL ATOMIC ENERGY AGENCY. Joint FAO/IAEA programme: nuclear techniques in food and agriculture. 2015. Available at: <http://www-naweb.iaea.org/nafa/>. Accessed on: Jul. 16 2015.
» http://www-naweb.iaea.org/nafa/ - IBIJBIJEN, J.; URQUIAGA, S.; ISMAILI, M.; ALVES, B.J.R.; BODDEY, R.M. Effect of arbuscular mycorrhizal fungi on growth, mineral nutrition and nitrogen fixation of three varieties of common beans. New Phytologist, v.134, p.353-360, 1996. DOI: 10.1111/j.1469-8137.1996.tb04640.x.
» https://doi.org/10.1111/j.1469-8137.1996.tb04640.x - INÁCIO, C.T.; CHALK, P.M. Principles and limitations of stable isotopes in differentiating organic and conventional foodstuffs: 2. Animal products. Critical Reviews in Food Science and Nutrition, online, 2015. DOI: 10.1080/10408398.2014.887056.
» https://doi.org/10.1080/10408398.2014.887056 - INÁCIO, C.T.; CHALK, P.M.; MAGALHÃES, A.M.T. Principles and limitations of stable isotopes in differentiating organic and conventional foodstuffs: 1. Plant products. Critical Reviews in Food Science and Nutrition, v.55, p.1206-1218, 2015a. DOI: 10.1080/10408398.2012.689380.
» https://doi.org/10.1080/10408398.2012.689380 - INÁCIO, C.T.; URQUIAGA, S.; CHALK, P.M.; MATA, M.G.F. ; SOUZA, P.O. Identifying N fertilizer regime and vegetable production system in tropical Brazil using 15N natural abundance. Journal of the Science of Food and Agriculture, v.95, p.3025-3032, 2015b. DOI: 10.1002/jsfa.7177.
» https://doi.org/10.1002/jsfa.7177 - MACEDO, R.; TARRÉ, R.M.; FERREIRA, E.; REZENDE, C. de P.; PEREIRA, J.M.; CADISCH, G.; ROUWS, J.R.C.; ALVES, B.J.R.; URQUIAGA, S.; BODDEY, R.M. Forage intake and botanical composition of feed for cattle fed Brachiaria/legume mixtures. Scientia Agricola, v.67, p.384-392, 2010. DOI: 10.1590/S0103-90162010000400002.
» https://doi.org/10.1590/S0103-90162010000400002 - MARDEGAN, S.F.; ANDRADE, T.M.B.; SOUSA NETO, R. de S.; VASCONCELLOS, E.B. de C.; MARTINS, L.F.B.; MENDONÇA, T.G.; MARTINELLI, L.A. Stable carbon isotopic composition of Brazilian beers - a comparison between large- and small-scale breweries. Journal of Food Composition and Analysis, v.29, p.52-57, 2013. DOI: 10.1016/j.jfca.2012.10.004.
» https://doi.org/10.1016/j.jfca.2012.10.004 - MARTINELLI, L.A.; MOREIRA, M.Z.; OMETTO, J.P.H.B.; ALCARDE, A.R.; RIZZON, L.A.; STANGE, E.; EHLERINGER, J.R. Stable carbon isotopic composition of the wine and CO2 bubbles of sparkling wines: detecting C4 sugar additions. Journal of Agricultural and Food Chemistry, v.51, p.2625-2631, 2003. DOI: 10.1021/jf026088c.
» https://doi.org/10.1021/jf026088c - MARTINELLI, L.A.; NARDOTO, G.B.; CHESSON, L.A.; RINALDI, F.D.; OMETTO, J.P.H.B.; CERLING, T.E.; EHLERINGER, J.R. Worldwide stable carbon and nitrogen isotopes of Big Mac® patties: an example of a truly "glocal" food. Food Chemistry, v.127, p.1712-1718, 2011. DOI: 10.1016/j.foodchem.2011.02.046.
» https://doi.org/10.1016/j.foodchem.2011.02.046 - MÁXIMO, E.; SANT ANA FILHO, C.R.; TRIVELIN, P.C.O.; BENDASSOLLI, J.A. Isotope separation of nitrogen by ion exchange chromatography in a cascade system. Solvent Extraction and Ion Exchange, v.31, p.743-762, 2013. DOI: 10.1080/07366299.2013.810912.
» https://doi.org/10.1080/07366299.2013.810912 - MIRANDA, C.H.B.; URQUIAGA, S.; BODDEY, R.M. Selection of ecotypes of Panicum maximum for associated biological nitrogen fixation using the 15N isotope dilution technique. Soil Biology and Biochemistry, v.22, p.657-663, 1990. DOI: 10.1016/0038-0717(90)90012-O.
» https://doi.org/10.1016/0038-0717(90)90012-O - MORAES, J.F.L. de; VOLKOFF, B.; CERRI, C.C.; BERNOUX, M. Soil properties under Amazon forest and changes due to pasture installation in Rondônia, Brazil. Geoderma, v.70, p.63-81, 1996. DOI: 10.1016/0016-7061(95)00072-0.
» https://doi.org/10.1016/0016-7061(95)00072-0 - MORAIS, R.F. de; QUESADA, D.M.; REIS, V.M.; URQUIAGA, S.; ALVES, B.J.R.; BODDEY, R.M. Contribution of biological nitrogen fixation to Elephant grass (Pennisetum purpureum Schum.). Plant and Soil, v.356, p.23-34, 2012. DOI: 10.1007/s11104-011-0944-2.
» https://doi.org/10.1007/s11104-011-0944-2 - OKITO, A.; ALVES, B.J.R.; URQUIAGA, S.; BODDEY, R.M. Nitrogen fixation by groundnut and velvet bean and residual benefit to a subsequent maize crop. Pesquisa Agropecuária Brasileira, v.39, p.1183-1190, 2004. DOI: 10.1590/S0100-204X2004001200004.
» https://doi.org/10.1590/S0100-204X2004001200004 - OLIVEIRA, R.P.; DUCATTI, C.; PEZZATO, A.C.; DENADAI, J.C.; CRUZ, V.C.; SARTORI, J.R.; CARRIJO, A.S.; CALDARA, F.R. Traceability of poultry offal meal in broiler feeding using isotopic analysis (δ13C and δ15N) of different tissues. Revista Brasileira Ciência Avícola, v.12, p.13-20, 2010. DOI: 10.1590/S1516-635X2010000100002.
» https://doi.org/10.1590/S1516-635X2010000100002 - PADOVAN, G.J.; DE JONG, D.; RODRIGUES, L.P.; MARCHINI, J.S. Detection of adulteration of commercial honey samples by the 13C/12C isotope ratio. Food Chemistry, v.82, p.633-636, 2003. DOI: 10.1016/S0308-8146(02)00504-6.
» https://doi.org/10.1016/S0308-8146(02)00504-6 - PERIN, A.; SANTOS, R.H.S.; URQUIAGA, S.S.; CECON, P.R.; GUERRA, J.G.M.; FREITAS, G.B. de. Sunnhemp and millet as green manure for tropical maize production. Scientia Agricola, v.63, p.453-459, 2006. DOI: 10.1590/S0103-90162006000500006.
» https://doi.org/10.1590/S0103-90162006000500006 - PINHEIRO, É.F.M.; LIMA, E.; CEDDIA, M.B.; URQUIAGA, S.; ALVES, B.J.R.; BODDEY, R.M. Impact of pre-harvest burning versus trash conservation on soil carbon and nitrogen stocks on a sugarcane plantation in the Brazilian Atlantic forest region. Plant and Soil, v.333, p.71-80, 2010. DOI: 10.1007/s11104-010-0320-7.
» https://doi.org/10.1007/s11104-010-0320-7 - PISSINATTO, L.; MARTINELLI, L.A.; VICTORIA, R.L.; CAMARGO, P.B. de. Stable carbon isotopic analysis and the botanical origin of ethanol in Brazilian brandies. Food Research International, v.32, p.665-668, 1999. DOI: 10.1016/S0963-9969(99)00143-X.
» https://doi.org/10.1016/S0963-9969(99)00143-X - PRIMO, D.C.; MENEZES, R.S.C.; SAMPAIO, E.V. de S.B.; GARRIDO, M. DA S.; JÚNIOR, J.C.B.; SOUZA, C.S. Recovery of N applied as 15N-manure or 15N-gliricidia biomass by maize, cotton and cowpea. Nutrient Cycling in Agroecosystems, v.100, p.205-214, 2014. DOI: 10.1007/s10705-014-9638-5.
» https://doi.org/10.1007/s10705-014-9638-5 - PUPIN, A.M.; DENNIS, M.J.; PARKER, I.; KELLY, S.; BIGWOOD, T.; TOLEDO, M.C.F. Use of isotopic analyses to determine the authenticity of Brazilian orange juice (Citrus sinensis). Journal of Agricultural and Food Chemistry, v.46, p.1369-1373, 1998. DOI: 10.1021/jf970746p.
» https://doi.org/10.1021/jf970746p - RAMOS, M.G.; VILLATORO, M.A.A.; URQUIAGA, S.; ALVES, B.J.R.; BODDEY, R.M. Quantification of the contribution of biological nitrogen fixation to tropical green manure crops and the residual benefit to a subsequent maize crop using 15N-isotope techniques. Journal of Biotechnology, v.91, p.105-115, 2001. DOI: 10.1016/S0168-1656(01)00335-2.
» https://doi.org/10.1016/S0168-1656(01)00335-2 - REIS JUNIOR, F.B. dos; SIMON, M.F.; GROSS, E.; BODDEY, R.M.; ELLIOTT, G.N.; NETO, N.E.; LOUREIRO, M. de F.; QUEIROZ, L.P. de; SCOTTI, M.R.; CHEN, W.-M.; NORÉN, A.; RUBIO, M.C.; FARIA, S.M. de; BONTEMPS, C.; GOI, S.R.; YOUNG, J.P.W.; SPRENT, J.I.; JAMES, E.K. Nodulation and nitrogen fixation by Mimosa spp. in the Cerrado and Caatinga biomes of Brazil. New Phytologist, v.186, p.934-946, 2010. DOI: 10.1111/j.1469-8137.2010.03267.x.
» https://doi.org/10.1111/j.1469-8137.2010.03267.x - REIS, V.M.; REIS JUNIOR, F.B. dos; QUESADA, D.M.; OLIVEIRA, O.C.A. de; ALVES, B.J.R.; URQUIAGA, S.; BODDEY, R.M. Biological nitrogen fixation associated with tropical pasture grasses. Functional Plant Biology, v.28, p.837-844, 2001. DOI: 10.1071/PP01079.
» https://doi.org/10.1071/PP01079 - RITTLE, T.F.; NOVOTNY, E.H.; BALIEIRO, F.C.; HOFFLAND, E.; ALVES, B.J.R.; KUYPER, T.W. Negative priming of native soil organic carbon mineralization by oilseed biochars of contrasting quality. European Journal of Soil Science, v.66, p.714-721, 2015. DOI: 10.1111/ejss.12257.
» https://doi.org/10.1111/ejss.12257 - ROSSETE, A.L.R.M.; BENDASSOLLI, J.A.; MÁXIMO, E.; SANT ANA FILHO, C.R.; IGNOTO, R. de F. de. Production of 34S-labeled gypsum (Ca34SO42H2O). Scientia Agricola, v.63, p.399-404, 2006. DOI: 10.1590/S0103-90162006000400012.
» https://doi.org/10.1590/S0103-90162006000400012 - RUSCHEL, A.P.; VOSE, P.B.; MATSUI, E.; VICTORIA, R.L.; TSAI SAITO, S.M. Field evaluation of N2-fixation and N-utilization by Phaseolus bean varieties determined by 15N isotope dilution. Plant and Soil, v.65, p.397-407, 1982. DOI: 10.1007/BF02375060.
» https://doi.org/10.1007/BF02375060 - SANT ANA FILHO, C.R.; ROSSETE, A.L.R.M.; TAVARES, C.R.O.; PRESTES, C.V.; BENDASSOLLI, J.A. Synthesis of 15N-enriched urea (CO(NH2)2) from 15NH3, CO, and S in a discontinuous process. Brazilian Journal of Chemical Engineering, v.29, p.795-806, 2012. DOI: 10.1590/S0104-66322012000400011.
» https://doi.org/10.1590/S0104-66322012000400011 - SANT'ANA, L.S.; DUCATTI, C.; RAMIRES, D.G. Seasonal variations in chemical composition and stable isotopes of farmed and wild Brazilian freshwater fish. Food Chemistry, v.122, p.74-77, 2010. DOI: 10.1016/j.foodchem.2010.02.016.
» https://doi.org/10.1016/j.foodchem.2010.02.016 - SHIBUYA, E.K.; SARKIS, J.E.S.; NEGRINI NETO, O.; MOREIRA, M.Z.; VICTORIA, R.L. Sourcing Brazilian marijuana by applying IRMS analysis to seized samples. Forensic Science International, v.160, p.35-43, 2006. DOI: 10.1016/j.forsciint.2005.08.011.
» https://doi.org/10.1016/j.forsciint.2005.08.011 - SISTI, C.P.J.; SANTOS, H.P. dos; KOHHANN, R.; ALVES, B.J.R.; URQUIAGA, S.; BODDEY, R.M. Change in carbon and nitrogen stocks in soil under 13 years of conventional or zero tillage in southern Brazil. Soil and Tillage Research, v.76, p.39-58, 2004. DOI: 10.1016/j.still.3003.08.007.
» https://doi.org/10.1016/j.still.3003.08.007 - TEIXEIRA, F.C.P.; REINERT, F.; RUMJANEK, N.G.; BODDEY, R.M. Quantification of the contribution of biological nitrogen fixation to Cratylia mollis using the 15N natural abundance technique in the semi-arid Caatinga region of Brazil. Soil Biology and Biochemistry, v.38, p.1989-1993, 2006. DOI: 10.1016/j.soilbio.2005.11.013.
» https://doi.org/10.1016/j.soilbio.2005.11.013 - TRIVELIN, P.C.O.; BENDASSOLLI, J.A.; MURAOKA, T.; CARNEIRO JUNIOR, F. Sulfur utilization by rice and Crotalaria juncea from sulphate-34S applied to the soil. Scientia Agricola, v.59, p.205-207, 2002. DOI: 10.1590/S0103-90162002000100030.
» https://doi.org/10.1590/S0103-90162002000100030 - UNKOVICH, M.; HERRIDGE, D.; PEOPLES, M.; CADISCH, G.; BODDEY, B.; GILLER, K.; ALVES, B.; CHALK, P. 15N natural abundance method. In: UNKOVICH, M.; HERRIDGE, D.; PEOPLES, M.; CADISCH, G.; BODDEY, R.; GILLER, K.; ALVES, B.; CHALK, P. Measuring plant-associated nitrogen fixation in agricultural systems Canberra: ACIAR, 2008. p.131-162. (Monograph, 136).
- URQUIAGA, S.; XAVIER, R.P.; MORAIS, R.F. de.; BATISTA, R.B.; SCHULTZ, N.; LEITE, J.M.; MAIA e SÁ, J.; BARBOSA, K.P.; RESENDE, A.S. de; ALVES, B.J.R.; BODDEY, R.M. Evidence from field nitrogen balance and 15N natural abundance data for the contribution of biological N2 fixation to Brazilian sugarcane varieties. Plant and Soil, v.356, p.5-21, 2012. DOI: 10.1007/s11104-011-1016-3.
» https://doi.org/10.1007/s11104-011-1016-3 - VIERA-VARGAS, M.S.; SOUTO, C.M.; URQUIAGA, S.; BODDEY, R.M. Quantification of the contribution of N2 fixation to tropical forage legumes and transfer to associated grass. Soil Biology and Biochemistry, v.27, p.1193-1200, 1995. DOI: 10.1016/0038-0717(95)00022-7.
» https://doi.org/10.1016/0038-0717(95)00022-7 - VITORELLO, V.A.; CERRI, C.C.; VICTÓRIA, R.L.; ANDREUX, F.; FELLER, C. Organic matter and natural carbon-13 distribution in forested and cultivated Oxisols. Soil Science Society of America Journal, v.53, p.773-7781989DOI: 10.2136/sssaj1989.03615995005300030024x.
» https://doi.org/10.2136/sssaj1989.03615995005300030024x
Publication Dates
-
Publication in this collection
Sept 2016
History
-
Received
24 July 2015 -
Accepted
29 Feb 2016