Abstracts
The aim of this study was to evaluate the effect of sucrose concentration in the culture medium on growth and on the establishment of mycorrhizas during the acclimatization of pineapple cv. Pérola. The plantlets were micropropagated in MS culture medium with 0, 10, 20 and 30 g L-1 of sucrose and then they were acclimatized during 12 weeks under greenhouse conditions, in a sandy soil - compost mixture, uninoculated or inoculated with a Rhizophagus clarus isolate. Plantlets from the culture medium with 20 g and 30 g of sucrose L-1 showed higher shoot and root biomass than those from sugar-free medium. Mycorrhizal colonization was lower in plantlets micropropagated in sucrose-free medium, but the intensity of arbuscules did not differ among treatments. In the 12-week period of acclimatization, mycorrhizal colonization had no effect on plant biomass.
Rhizophagus clarus; micropropagation; weaning; arbuscular mycorrhiza
O objetivo deste trabalho foi avaliar o efeito da concentração de sacarose no meio de cultura sobre crescimento e o estabelecimento de micorrizas na fase de aclimatização de plantas de abacaxi (cv. Pérola). As plantas foram micropropagadas em meio de cultura MS com 0;10; 20 ou 30 g L-1 de sacarose e depois aclimatizadas por 12 semanas em casa de vegetação, em uma mistura de solo arenoso e composto, inoculada ou não com um isolado de Rhizophagus clarus. Plantas originadas do meio de cultura com 20g e 30 g L-1 de sacarose apresentaram maior massa de parte aérea e raiz que aquelas do meio sem açúcar. A colonização micorrízica foi menor nas plantas micropropagadas no meio de cultura sem sacarose, mas a intensidade de arbúsculos não diferiu entre tratamentos. No período de 12 semanas de aclimatização, a colonização micorrízica não teve efeito sobre a produção de biomassa das plantas.
Rhizophagus clarus; micropropagação; aclimatização; micorrizas arbusculares
COMUNICAÇÃO CIENTÍFICA
PROPAGAÇÃO
Pineapple (Ananas comosus) cv. pérola ex vitro growth and mycorrhizal colonization affected by in vitro sucrose concentration1 1 (Trabalho 125-13).
Crescimento ex vitro e colonização micorrízica de abacaxi (Ananas comosus) cv. pérola afetados pela concentração de sacarose na fase in vitro
Alceu KunzeI; Paulo Emílio LovatoII; Murilo Dalla CostaIII; Lírio Luiz Dal VescoIV
IInstituto Federal Catarinense-Câmpus Araquari, Caixa postal 21, CEP 89245-000, Araquari, SC. E-mail: alceu.kunze@ifc-araquari.edu.br
IIDepto. de Engenharia Rural CCA-UFSC, CP 476, 88040-970 Floriranópolis-SC. E-mail: paulo.lovato@ufsc.br
IIIEpagri, Rua João José Godinho, s/n, 88.506-080 Lages-SC. E-mail: murilodc@epagri.sc.gov.br
IVUFSC/Câmpus de Curitibanos, Cx. Postal 101, CEP. 89.520-000, Curitibanos-SC. E-mail: lirio.luiz@ufsc.br
ABSTRACT
The aim of this study was to evaluate the effect of sucrose concentration in the culture medium on growth and on the establishment of mycorrhizas during the acclimatization of pineapple cv. Pérola. The plantlets were micropropagated in MS culture medium with 0, 10, 20 and 30 g L-1 of sucrose and then they were acclimatized during 12 weeks under greenhouse conditions, in a sandy soil - compost mixture, uninoculated or inoculated with a Rhizophagus clarus isolate. Plantlets from the culture medium with 20 g and 30 g of sucrose L-1 showed higher shoot and root biomass than those from sugar-free medium. Mycorrhizal colonization was lower in plantlets micropropagated in sucrose-free medium, but the intensity of arbuscules did not differ among treatments. In the 12-week period of acclimatization, mycorrhizal colonization had no effect on plant biomass.
Index terms: Rhizophagus clarus, micropropagation, weaning, arbuscular mycorrhiza.
RESUMO
O objetivo deste trabalho foi avaliar o efeito da concentração de sacarose no meio de cultura sobre crescimento e o estabelecimento de micorrizas na fase de aclimatização de plantas de abacaxi (cv. Pérola). As plantas foram micropropagadas em meio de cultura MS com 0;10; 20 ou 30 g L-1 de sacarose e depois aclimatizadas por 12 semanas em casa de vegetação, em uma mistura de solo arenoso e composto, inoculada ou não com um isolado de Rhizophagus clarus. Plantas originadas do meio de cultura com 20g e 30 g L-1 de sacarose apresentaram maior massa de parte aérea e raiz que aquelas do meio sem açúcar. A colonização micorrízica foi menor nas plantas micropropagadas no meio de cultura sem sacarose, mas a intensidade de arbúsculos não diferiu entre tratamentos. No período de 12 semanas de aclimatização, a colonização micorrízica não teve efeito sobre a produção de biomassa das plantas.
Termos para indexação: Rhizophagus clarus, micropropagação, aclimatização, micorrizas arbusculares.
Micropropagation of pineapple has been successfully achieved with the establishment of regeneration protocols for the species through organogenesis in liquid culture medium (DAL VESCO et al., 2001) and use of temporary immersion bioreactor (FIROOZABADY; GUTTERSON, 2003). This allows large-scale clonal multiplication of healthy plantlets of varieties obtained by breeding, and up to 930 thousand plantlets can be obtained and weaned in one year from one single explant (DAL VESCO et al., 2001). Besides maintenance of genetic gains and mass production of plantlets, tissue culture of pineapple helps in controlling pathogens, such as Fusarium subglutinans, which spreads via fieldproduced infected plantlets. The cost of producing pineapple plantlets through micropropagation, around 35% higher than traditional field methods, is a limiting factor for the use of tissue culture in this species (FIROOZABADY; GUTTERSON, 2003). Thus, increased efficiency and reduced production time are amongst the main goals in the development of this technology.
Inoculation of arbuscular mycorrhizal fungi during the weaning stage of micropropagated plants can reduce abiotic stress and increase survival rate in this phase of plant establishment (KAPOOR et al., 2008, KOZAI & KUBOTA, 2005). In the production of the pineapple plants through in vitro tissue culture or by conventional methods (RODRÍGUEZ-ROMERO et al., 2011), this association has positive effects on development and absorption of nutrients. Mycorrhizas have been shown to reduce the incidence of nematodes (GUILLEMIN et al., 1994a) and alleviate the effects of the pathogen Phytophthora cinnamomi on pineapple growth (GUILLEMIN et al. 1994b).
The aim of this study was to evaluate the effect of sucrose concentrations in the in vitro culture medium on growth and establishment of the mycorrhizal symbiosis during the ex vitro stage.
Pineapple microplants (cv. Pérola) were grown in Murashige & Skoog medium with vitamins (MOREL; WETMORE, 1951) and different concentrations of sucrose (0, 10, 20 and 30 g L-1). The culture medium had its pH adjusted to 5.8 and was autoclaved (121º C, 15 minutes). After 45 days of growth, plantlets were weaned in 170-mL tubes containing a 4:1 (v/v) mixture of autoclaved (60 min) sandy soil (Orthic Quartzipsament) and unsterile municipal compost produced with food scraps, grass clippings, and animal bedding with woodshavings (INÁCIO; MILLER, 2009; BRITO et al., 2010). The mix had the following physicochemical attributes: pH (water) 7.1, P> 50 mg dm-3, K 3.8 mmolc dm-3, organic matter 22 g kg-1, Ca 20 mmolc dm-3, Mg 14 mmolc dm-3. Each plant received 3.0 g of Rhizophagus clarus (T.H. Nicolson & N.C. Schenck) C. Walker & A. Sch. Wal (synonymy = Glomus clarum) inoculum, a mixture of Paspalum notatum roots and growth substrate (ZEMKE et al., 2003), placed near the root system. Non-mycorrhizal plants received a filtrate (Whatman n. 2 filter paper) of the inoculum, in order to reestablish the nonmycorrhizal biota. Plants were kept in a greenhouse and after 12 weeks shoot and root biomass were evaluated. Part of the root system was sampled to estimate the rate of mycorrhizal root colonization (TROUVELOT et al., 1986), after clearing with KOH and staining with acidified 0.05% trypan blue glycerol solution (KOSKE; GEMMA, 1989). The experiment had a 4x2 factorial design, corresponding to four concentrations of sucrose (0, 10, 20 e 30 g L-1) in the previous culture medium and two treatments, with or without arbuscular mycorrhizal inoculation during the weaning stage, arranged in complete blocks design with three replications composed of six plants each. Data were submitted to analysis of variance (ANOVA) to check effects of factors and their interaction, and a regression analysis was done with sucrose concentrations as independent variable.
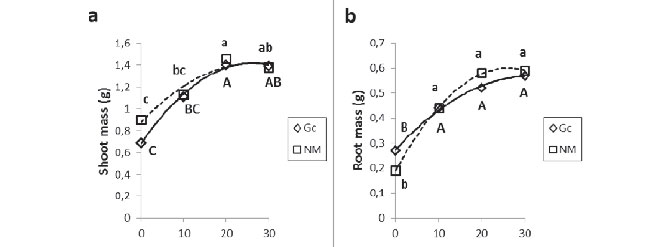
After acclimatization, ANOVA showed that pineapple plantlets propagated in medium with 2% and 3% sucrose had higher shoot and root biomass than those previously grown in medium without sucrose, regardless of inoculation of the mycorrhizal fungus (Figure 1a, 1b). Growth of non mycorrhizal plantlets fitted a linear pattern while mycorrhizal plantlets showed a quadratic response to the sucrose concentration. These results demonstrate the possibility of lowering sucrose concentration in growth media for this species, as shown for ornamental pineapple by Oliveira et al (2010). Although low, pineapple plants showed some growth after being micropropagated in sucrose-free culture medium, suggesting that the in vitro explants were performing some photosynthesis.
The mycorrhizal association was effectively established, as shown by the presence of hyphae and arbuscules in the root cortex (Figures 1c, 1d), although there was no effect of mycorrhizas on shoot or root biomass of the pineapple plantlets in the acclimatization stage. The intensity of cortex colonization (M%, Figure 1c) differed among plants originating from the varying levels of sucrose in the propagation medium. Plantlets which had been propagated in the sucrose-free treatment had lower levels of infection, as compared to the ones from the 1% sucrose treatments, and the plants from 2% and 3% sucrose treatments were in an intermediate position, which resulted in a quadratic response of this variable to the medium previous sucrose concentrations. The levels of mycorrhizal colonization below 50% for most treatments could be due to the use of thermophilic compost in the substrate, with a high concentration of phosphorus, which can inhibit mycorrhizas. The availability to the plant metabolism of high doses of inorganic forms of this nutrient promotes similar growth in mycorrhizal and non-mycorrhizal plants (SCHROEDER; JANOS, 2005). The intensity of arbuscules in the mycorrhizal sections of the roots (a, Figure 1d), according to the ANOVA, did not vary among treatments, suggesting that in all treatments the symbiotic fungus was investing in these structures, used for exchange of photosyntates and nutrients between the plant root and the fungus. However, their behavior fitted a quadratic model, similarly to the behavior of mycorrhizal colonization. That suggests that the intermediate levels of sucrose in the growth media result in plants that are more conducive to the establishment of this mutualistic association. Recent work in Brazil has shown that response of micropropagated pineapple plantlets to mycorrhizal inoculation varies with plant and fungal genotypes (SANTOS et al., 2011), meaning that specific procedures must be tested for each plant genotype. It has also been demonstrated that mycorrhizal inoculation can stimulate root development in micropropagated plants during the weaning stage (LOCATELLI et al., 2002a, 2002b) and that the effects of mycorrhizal inoculation can persist under field conditions (LOVATO et al., 2006). Therefore, in order to optimize a set of procedures for pineapple production through micropropagation, before evaluating field responses, it is necessary to carry out research departing from the in vitro phase.
Recebido em: 15-03-2013.
Aceito para publicação em: 04-02-2014.
- BRITO, F. S.; MILLER, P. R. M.; STADNIK, M. Presença de Trichoderma spp em composto e suas características para o controle de fitopatógenos. Revista Brasileira de Agroecologia, Pelotas, v. 5, p. 43-53, 2010.
- DAL VESCO, L.L.; PINTO, A. de A.; ZAFFARI, G.R.; NODARI, R.O.; REIS, M.S. dos; GUERRA, M.P. Improving pineapple micropropagation protocol through explant size and medium composition manipulation. Fruits, Paris, v. 56, n. 3, p. 143-154, 2001.
- FIROOZABADY, E.; GUTTERSON, N. Costeffective in vitro propagation methods for pineapple. Plant Cell Reports, Berlin, v.21, n.9, p.844-850, 2003.
- GUILLEMIN, J.P.; GIANINAZZI, S.; GIANINAZZI-PEARSON, V.; MARCHAL, J. Contribution of arbuscular mycorrhizas to biological protection of micropropagated pineapple (Ananas comosus (L.) Merr.) against Phytophtora cinnamomi Rands. Agricultural Science in Finland, Jokioinen, v. 3, n. 3, p. 241-251, 1994a.
- GUILLEMIN, J.P.; GIANINAZZI, S.; GIANINAZZI-PEARSON, V.; MARCHAL, J. Control by arbuscular endomycorrhizae of Pratylenchus brachyurus in pineapple microplants. Agricultural Science in Finland, Jokioinen, v. 3, n. 3, p. 253-262, 1994b.
- INÁCIO, C. T.; MILLER, P. R. M. Compostagem: ciência e prática para a gestão de resíduos orgânicos. Rio de Janeiro: Embrapa Solos, 2009.
- KAPOOR, R.; SHARMA, D.; BHATNAGAR, A.K. Arbuscular mycorrhizae in micropropagation systems and their potential applications. Scientia Horticulturae, Amsterdam, v. 116, n. 3, p. 227-239, 2008.
- KOZAI, T.; KUBOTA, C. In vitro root zone environment and their effects on growth and development of plants. In: KOZAI, T.; AFREEN, F.; ZOBAYED, S. M. A. (Ed.). Photoautotrophic (sugar-free medium) micropropagation as a new micropropagation and transplant production system. Dordrecht: Springer, 2005. p 53-60.
- KOSKE, R.E.; GEMMA, J.N. A modified procedure for staining roots to detect VA mycorrhizas. Mycological Research, Cambridge, v.92, n.4, p.486-488, 1989.
- LOCATELLI, L. M.; LOVATO, P. E.; PEDROTTI, E. L Crescimento e desenvolvimento radicular do porta-enxerto Marubakaido (Malus prunifolia) micropropagado submetido à inoculação micorrízica e à poda de raízes, Revista Brasileira de Fruticultura, Jaboticabal, v. 24, n.2, p. 486-490, 2002a.
- LOCATELLI, L. M.; VITOVSKI, C. A.; LOVATO, P. E. Sistema radicular de porta-enxertos micropropagados de macieira colonizados com fungos micorrízicos arbusculares. Pesquisa Agropecuária Brasileira, Brasília, v. 37, n. 9, p. 1239-1245, 2002b.
- LOVATO, P. E.; TROUVELOT, A.; GIANINAZZI-PEARSON, V.; GIANINAZZI, S. Enhanced growth of wild cherry using micropropagated plants and mycorrhizal inoculation. Agronomy for Sustainable Development, Paris, v.26, p.209-213, 2006.
- MOREL, G. M.; WETMORE, R. H. Fern callus tissue culture. American Journal of Botany, Columbus, v. 38, p. 141-143, 1951.
- OLIVEIRA, Y.; ANSELMINI, J. A.; CUQUEL, F. L.; PINTO, F.; QUOIRIN, M. Pré-aclimatização in vitro de abacaxi-ornamental. Ciência e Agrotecnologia, Lavras, v.34, p. 1647-1653, 2010.
- RODRIGUEZ-ROMERO, A.S.; AZCON, R.; JAIZME-VEJA, M.D.C. Early mycorrhization of two tropical crops, papaya (Carica papaya L.) and pineapple [Ananas comosus (L.) Merr.], reduces the necessity of P fertilization during the nursery stage. Fruits, Paris, v. 66, n.1, p 3-10, 2011.
- SANTOS, P. C.; FREITAS, S. J.; FREITAS, M. S. M.; SOUZA, L. B.; CARVALHO, A. J. C. Production of seedlings of type suckers, using crows of three cultivars of pineapple inoculated with mycorrhizal fungi. Revista Brasileira de Fruticultura, Jaboticabal, v. 33, n. 3, p 954 - 961, 2011.
- SCHROEDER, M.S.; JANOS, D.P. Plant growth, phosphorus nutrition, and root morphological responses to arbuscular mycorrhizas, phosphorus fertilization, and intraspecific density. Mycorrhiza, Berlin, v. 15, n. 3, p. 203-216, 2005.
- SILVA, F. A. S. Assistat versão 7.6, 2013. Disponível em: www.assistat.com/indexp.html#down Acesso em 02 de setembro de 2013.
- TROUVELOT, A.; KOUGH, J.L.; GIANINAZZIPEARSON, V. Mesure du taux de mycorhization VA d'un sistème radiculaire: recherche de méthodes d'estimation ayant une signification fonctionnelle. In: GIANINAZZI, S.; GIANINAZZI-PEARSON, V. (Ed.). Mycorhizes: physiologie et genétique. Dijon: Inra, 1986. p. 217-220.
- ZEMKE, J. M.; PEREIRA, F.; Lovato, P. E.; SILVA, A. L. Avaliação de substratos para inoculação micorrízica e aclimatização de dois porta-enxertos de videira micropropagados. Pesquisa Agropecuária Brasileira, Brasília, v. 38, n.11, p. 1309-1315, 2003.
Publication Dates
-
Publication in this collection
19 Nov 2014 -
Date of issue
Sept 2014
History
-
Received
15 Mar 2013 -
Accepted
04 Feb 2014