ABSTRACT
‘Rubi’ table grape has wide acceptance of the consumer market due to its affordable price and attractive color as a function of the accumulation of anthocyanins. However, environmental conditions do not always favor the suitable accumulation of these pigments, resulting in commercial devaluation and nutritional depreciation of fruits. Thus, the present study aimed to investigate the effect of application of different ethephon concentrations, associated or not with CaCl2 application, on the skin color and quality of ‘Rubi’ grape berries. First, specific traits of berries treated with 0, 200, 400, 600, 800 mg L-1 ethephon associated with 1.5% CaCl2 solution were evaluated and pH, titratable acidity (TA), soluble solids (SS), berry firmness, total and reducing soluble sugars, and anthocyanin and flavonol levels were analyzed. This first stage of evaluation revealed no changes in total sugar levels, firmness, SS, TA, SS/TA ratio, and flavonol levels, while changes were detected regarding reducing sugar and anthocyanin levels. Thus, in the second stage, the following variables were evaluated: SS, TA, reducing sugar levels, anthocyanin levels, phenylalanine ammonia lyase (PAL) and glutathione S-transferase (GST) activity of ‘Rubi’ grape berries treated with 0, 200, 400, 600, 800 mg L-1 ethephon, associated or not with 1.5% CaCl2 solution. The results of the present study indicate that ethephon associated with CaCl2 can contribute to improve the post-harvest quality of ‘Rubi’ grape, since this association increased the accumulation of anthocyanins due to the higher activity of PAL and GST, related to biosynthesis and storage of antocyanins, respectively, and increased the levels of reducing sugars (at low ethephon concentrations), not changing other quality aspects. Therefore, a single ethephon application from 200 mg L-1 associated with the application of 1.5% CaCl2 at the final ripening stage, when bunches present from 30 to 50% skin color coverage, was sufficient to produce satisfactory results regarding skin color improvement of ‘Rubi’ grape berries.
Index terms
Vitis vinifera L.; ethylene; calcium; anthocyanins; flavonols; sugars; phenylalanine ammonia lyase; glutathione S-transferase
RESUMO
A uva fina de mesa ‘Rubi’ destaca-se pela grande aceitação do mercado consumidor devido ao preço acessível e à sua cor atrativa, consequência do acúmulo de antocianinas. No entanto, nem sempre as condições ambientais favorecem o adequado acúmulo desses pigmentos, acarretando na desvalorização comercial e nutricional desses frutos. Desta forma, o presente estudo objetivou investigar o efeito da aplicação de diferentes concentrações de ethephon, associadas ou não à aplicação de CaCl2, na cor e qualidade de bagas de uva ‘Rubi’. Primeiramente, foram avaliadas as características específicas de bagas tratadas com 0; 200; 400; 600 e 800 mg L d-1e ethephon associado à solução de CaCl2 1,5% - pH, acidez titulável (AT), sólidos solúveis (SS), firmeza das bagas, açúcares solúveis totais e redutores e terores de antocianinas e flavonóis. Esta primeira etapa de avaliação não revelou mudanças nos teores de açúcares totais, firmeza, SS, AT, ratio (SS/AA) e flavonóis, enquanto mudanças foram detectadas para teores de açúcares redutores e antocianinas. Desta forma, numa segunda etapa, foram investigadas (ao longo do tempo) algumas características (SS, AT, teores de açúcares redutores e de antocianinas e atividade das enzimas fenilalanina amônia liase (PAL) e glutationa S-transferase (GST)) de bagas de uva ‘Rubi’ tratadas com 0; 200; 400; 600 e 800 mg L-1 de ethephon associado ou não à solução de CaCl2 1.5%. Os resultados do presente estudo indicam que o ethephon associado ao CaCl2 pode contribuir para uma alta qualidade pós-colheita de uvas ‘Rubi’ em função do maior acúmulo de antocianinas em consequência da maior atividade das enzimas PAL e GST, relacionadas à biossíntese e ao armazenamento de antocianinas, respectivamente, e aos maiores teores de açúcares redutores (sob baixas concentrações de ethephon), sem alterar outros aspectos de qualidade. Portanto, uma única aplicação de ethephon, a partir da concentração de 200 mg L-1, associada à aplicação de CaCl2 1,5% no período final de maturação, quando as bagas apresentam de 30 a 50% de cobertura de cor, é suficiente para produzir resultados satisfatórios na melhoria de cor de bagas da uva ‘Rubi’.
Termos para indexação
Vitis vinifera L.; etileno; cálcio; antocianinas; flavonóis; açúcares; fenilalanina
Introduction
The Brazilian grape industry is concentrated in a few regions, being especially important in the state of Rio Grande do Sul, in the ‘Serra Gaúcha’ region, where almost the entire production is for processing, and it is essentially produced by smallholder family farmers. The production of table grapes is highlighted in the São Francisco River Valley (states of Pernambuco and Bahia) and in the state of São Paulo, generating income for thousands of families. In 2015, the Brazilian grape production was 1,499 million tons, representing an increase of 4.41% in comparison to 2014 (MELLO, 2016 MELLO, L.M.R. Desempenho da vitivinicultura brasileira em 2015. Disponível em: https://www.embrapa.br/busca-de-noticias/-/noticia/9952204/artigo-desempenho-da-vitivinicultura-brasileira-em-2015. Acesso em: 16 set. 2016.
https://www.embrapa.br/busca-de-noticias...
).
Among the varieties of table grapes, ‘Rubi’ (Vitis vinifera L.) has wide acceptance of the consumer market due to its affordable price and attractive color, since its commercial quality is firstly attributed to its typical red color (HIRATSUKA et al., 2001 HIRATSUKA, S.; ONODERA, H.; KAWAI, Y.; KUBO, T.; ITOH, H.; WADA, R. Enzyme activity changes during anthocyanin synthesis in ‘Olympia’ grape berries. Scientia Horticulturae, New York, v.90, p.255-264. 2001. ; LI et al., 2002 LI, Z.; GEMMA, H.; IWAHORI, S. Stimulation of Fuji apple skin color by ethephon and phosphorus-calcium mixed compounds in relation to flavonoid synthesis. Scientia Horticulturae, New York, v.94, p.193-199. 2002. ).Anthocyanins are pigments responsible for such reddish color (ROBERTO et al., 2012 ROBERTO, S.R.; ASSIS, A.M.; YAMAMOTO, L.Y.; MIOTTO, L.C.V.; SATO, A.J.; KOYAMA, R.; GENTA, W. Application timing and concentration of abscisic acid improve color of ‘Benitaka’ table grape. Scientia Horticulturae, New York, v.142, p.44-48, 2012. ; YAMAMOTO et al., 2015 YAMAMOTO, L.Y.; ASSIS, A.M.; ROBERTO, S.R.; BOVOLENTA, Y.R.; NIXDORF, S.L.; GARCÍA-ROMERO, E.; GÓMEZ-ALONSO, S.; HERMOSÍN-GUTIÉRREZ, I. Application of abscisic acid (S-ABA) to cv. Isabel grapes (Vitis vinifera×Vitis labrusca) for color improvement: Effects on color, phenolic composition and antioxidant capacity of their grape juice. Food Research International, Toronto, v.77, p.572-583, 2015. ). They belong to the class of flavonoids and play an important ecological role in several plant species, attracting pollinators to flowers, seed dispersers in fruits, in addition to their synthesis in several other plant organs in response to stresses like prolonged exposure to UV light (KOYAMA et al., 2014 KOYAMA, R.; ASSIS, A.M.; YAMAMOTO, L.Y.; BORGES, W.S.; PRUDENCIO, S.H.; ROBERTO, S.R. Exogenous abscisic acid increases the anthocyanin concentration of berry and juice from 'Isabel' grapes (Vitis labrusca L.). HortScience, Alexandria, v.49, p.460-464, 2014. ).
Furthermore, anthocyanins may exhibit anticancer, anti-inflammatory, antioxidant, pharmacological, and chemoprotective effects (MIAO et al., 2016 MIAO, L.; ZHANG, Y.; YANG, X.; XIAO, J.; ZHANG, H.; ZHANG, Z.; WANG, Y.; JIANG, G. Colored light-quality selective plastic films affect anthocyanin content, enzyme activities, and the expression of flavonoid genes in strawberry (Fragaria x ananassa) fruit. Food Chemistry, New York, v.207, p.93-100, 2016. ).
According to ‘Rubi’ grape producers from different Brazilian regions, such as the northeastern and southeastern regions, little colored grapes can be produced at certain times of the year since the synthesis of anthocyanins is dependent on environmental factors. Thus, the higher the temperature range, the better the grape coloration; in addition, the higher the air temperature, the sweeter the grapes (SPAYD et al., 2002 SPAYD, S.E.; TARARA, J.M.; MEE, D.L.; FERGUSON, J.C. Separation of sunlight and temperature effects on the composition of Vitis vinifera cv. Merlot berries. American Journal of Enology and Viticulture, Davis, v.53, p.171-182, 2002. ). In Brazilian producing regions, reduced synthesis of anthocyanins has been detected at temperatures higher than 35ºC and low temperature range.
For these reasons, there is an increasing interest in obtaining higher accumulation of flavonoid pigments in grape skin, in which they are stored.
Anthocyanins are mainly synthesized at the beginning of berry ripening, and their levels can triplicate in a week, with posterior stabilization (FERNÁNDEZ-LÓPEZ et al., 1998 FERNÁNDEZ-LÓPEZ, J.A.; ALMELA, L.; MUÑOZ, J.A.; HIDALGO, V.; CARREÑO, J. Dependence between colour and individual anthocyanin content in ripening grapes. Food Research International, Toronto, v.31, p.667-672, 1998. ). In this sense, the application of ethephon (2-Chloroethylphosphonic acid) in grape berries at ripening stage is an alternative to obtain higher anthocyanin biosynthesis. Its mechanism of action consists in the release of exogenous ethylene, which triggers the transcription of genes encoding enzymes of the biosynthesis pathway of anthocyanins, such as phenylalanine ammonia lyase (PAL; EC. 4.3.1.5), which initiates the synthesis of phenolic compounds (EL-KEREAMY et al., 2003 EL-KEREAMY, A.; CHERVIN, C.; SOUQUET, J.; MOUTOUNET, M.; MONJE, M.; NEPVEU, F.; MONDIES, H.; FORD, C.M.; VAN-ILEESWIJCK, R.; ROUTAN, J. Exogenous ethylene stimulates the long-term expression of genes related to anthocyanin biosynthesis in grape berries. Physiologia Plantarum, Lund, v.119, p.175-182, 2003. ).
Thus, ethephon has been related to the increase in berry coloration and the number of colored berries, since it is involved in the increased synthesis of pigments such as anthocyanins, leading to higher uniformity in the coloration of berries (MAILHAC; CHERVIN, 2006 MAILHAC, N.; CHERVIN, C. Ethylene and grape berry ripening. Stewart Postharvest Review, London, v.2, p.1-5, 2006. ; ROBERTO et al., 2013 ROBERTO, S.R.; ASSIS, A.M.; YAMAMOTO, L.Y.; MIOTTO, L.C.; KOYAMA, R.; SATO, A.J.; BORGES, R.S. Ethephon use and application timing of abscisic acid for improving color of 'Rubi' table grape. Pesquisa Agropecuária Brasileira, Brasília, DF, v.48, p.797-800, 2013. ; LEÃO et al., 2015 LEÃO, P.C.D.S.; LIMA, M.A.C.; COSTA, J.P.D.; TRINDADE, D.C.G.D. Abscisic acid and ethephon for improving red color and quality of crimson seedless grapes grown in a tropical region. American Journal of Enology and Viticulture, Davis, v.66, p.37-45, 2015. ).
In addition to the action of biosynthesis enzymes, an increase in the activity of glutathione S-transferase (GST; EC. 2.5.1.18) can occur during grape ripening. GST acts on the translocation of anthocyanins into the vacuole, resulting in higher coloration of berries (MARRS, 1996 MARRS, K.A. The functions and regulation of glutathione S-transferases in plants. Annual Review of Plant Physiology and Plant Molecular Biology, Palo Alto, v.47, p.127-158, 1996. ). In Arabidopsis, Sun et al. (2012) SUN, Y.; LI, H.; HUANG, J.R. Arabidopsis TT19 functions as a carrier to transport anthocyanin from the cytosol to tonoplasts. Molecular Plant, Shanghai, v.5, p.387-400, 2012. reported that the GST Transparent Testa 19 (TT19) acts as a carrier to transport cyanidin and/or anthocyanins to the tonoplast.
Calcium (Ca2+) ions have also been considered promoters of anthocyanin accumulation, since they are involved in signal transduction regarding the biosynthesis of these pigments. This fact occurs because Ca2+ induces an increase in the concentration of hexoses in grape cells, leading to the sugar-dependent expression of genes related to the transcription of chalcone synthase (CHS, EC 2.3.1.74), an enzyme involved in the biosynthesis of anthocyanins (VITRAC et al., 2000 VITRAC, X.; LARRONDE, F.; KRISA, S.; DECENDIT, A.; DEFFIEUX, G.; MÉRILLON, J.M. Sugar sensing and Ca2+-calmodulin requirement in Vitis vinifera cells producing anthocyanins. Phytochemistry, Madison, v.53, p.659-665, 2000. ).
Although studies on the isolated effect of ethephon and Ca2+ on anthocyanin accumulation in grapes have been conducted, reports on the combined effect of both on grapes are scarce. Thus, the present study aimed to investigate the effect of application of different ethephon concentrations, associated or not with CaCl2 application on the color of ‘Rubi’ grapes. In this way, the levels of anthocyanins and flavonols, the activity of PAL and GST, and qualityrelated aspects were evaluated, as well the pH, soluble solids (SS), berry firmness, total and reducing soluble sugars, and titratable acidity (TA) of ‘Rubi’ grape berries.
Material and Methods
The first experiment was carried out in order to standardize procedures for the second trial. Thus, in this first stage, specific traits of berries treated with different ethephon concentrations associated with calcium chloride (CaCl2) were evaluated. The experiment was carried out in a ‘Rubi’ vineyard located in Botucatu, SP, Brazil (-22.89º S, -48.45º W, 804 m above sea level, ‘IAC-572’ rootstock, 10-year old plants, trellising system, 4x3 m spacing). During this period, the lowest mean temperature was 17.6ºC, the highest mean temperature was 28.6ºC, 6.8 mm of mean rainfall, 59.4% of mean relative humidity, and 396.9 cal cm-2 day-1 of mean solar radiation.
The experimental design was in randomized blocks, with four replicates, each one represented by one vine. There were two control groups – one sprayed only with water (W) and the other only with 1.5% CaCl2 solution (CC1.5%).
Treatments consisted of 200, 400, 600, and 800 mg L-1 ethephon concentrations + 1.5% CaCl2 solution (ET200+CC1.5%, ET400+CC1.5%, ET600+CC1.5%, and ET800+CC1.5%, respectively).
The only ethephon application was performed when bunches presented 30-50% typical color by using a portable knapsack sprayer. The commercial product used as ethephon source was Ethrel® (240 g L-1 ethephon, Bayer CropScience). Surfactant Natural Oil® (0.5%, Stoller do Brasil Ltda) was added to solutions. CaCl2 was applied 24h after ethephon applications.
At 25 days after ethephon application (DAEA), bunches were randomly sampled and berries were harvested according to method recommended by the Brazilian Ministry of Agriculture, Livestock and Food Supply (BRASIL, 2004 BRASIL. Ministério da Agricultura, Pecuária e Abastecimento. Disponível em: http://www.agricultura.gov.br/sarc/profruta/html/classificacao_indice.htm. Acesso em: 10 jan. 2004.
http://www.agricultura.gov.br/sarc/profr...
) to evaluate pH, TA, SS, berry firmness, total and reducing soluble sugars. In addition, skins were removed, frozen in liquid nitrogen and stored in freezer at -80°C for posterior quantification of anthocyanin and flavonol levels.
pH values were obtained by using portable pH meter. TA was measured through titration with 0.1 N NaOH and expressed as percentage of tartaric acid.
SS levels were expressed as °Brix and obtained by reading in table refractometer. Firmness (g force-1) was obtained in a texturometer containing TA 9/1000 probe tip in which the penetration distance was 15 mm, with 2 mm s-1 speed. Total soluble sugars were measured according to the phenol-sulfuric acid method (DUBOIS et al., 1956 DUBOIS, M.; GILLES, K.A.; HAMILTON, J.K.; REBER, P.A.; SMITH, F. Colorimetric method for determination of sugar and related substances. Analytical Chemistry, Washington, v.2, p.350-356, 1956. ), and reducing sugars were quantified by the Somogyi-Nelson method (NELSON, 1944 NELSON, N. A photometric adaptation of the Somogyi method for the determination of glucose. Journal of Biological Chemistry, Baltimore, v.153, p.375-380, 1944. ), both expressed as mg glucose mL-1 must. The relationship between SS and TA (ratio) was also calculated.
For the quantification of anthocyanin and flavonol levels, skin samples of 5g were previously ground in liquid nitrogen and, then, macerated in 95% ethanol + 1.5 M HCl (85/15 v v-1) solution as much as possible, adopting a dilution factor of 1250. Then, absorbance readings were performed at 374 nm for flavonols, expressed as mg quercetin 100 g-1 skin, and at 535 nm for anthocyanins, expressed as mg cyanidin-3-glucoside 100 g-1 skin. Molar extinction coefficients 21880 mol L-1 cm-1 (quercetin) and 26900 mol L-1 cm-1 (cyanidin-3-glucoside) were used in calculations (LEES; FRANCIS, 1972 LEES, D.H.; FRANCIS, F.J. Standardization of pigment analyses in cranberries. HortScience, Alexandria, v.7, p.83-84, 1972. ).
The results were submitted to analysis of variance (F-test) and means were compared by the Tukey test (p≤0.05).
After this first stage, another trial was carried out at the same experimental site and following the same procedures regarding ethephon and CaCl2 concentrations, source and application, as well as berry sampling. However, in the second trial, plots sprayed only with ethephon and those sprayed with ethephon and CaCl2 were evaluated. Furthermore, the effect of treatments over time was investigated.
Thus, grape berries were sampled every three days, at 3, 6, 9, 12, 15, and 18 DAEA for analysis of SS, TA, and levels of reducing sugars and anthocyanins.
In addition, PAL and GST activities were evaluated in berries sampled at 3, 9 and 15 DAEA.
SS, TA, reducing sugars and anthocyanins were quantified as previously described. For PAL activity assay, extract was obtained according to method described by Hiratsuka et al. (2001) HIRATSUKA, S.; ONODERA, H.; KAWAI, Y.; KUBO, T.; ITOH, H.; WADA, R. Enzyme activity changes during anthocyanin synthesis in ‘Olympia’ grape berries. Scientia Horticulturae, New York, v.90, p.255-264. 2001. , with modifications. Thus, 2g of skin were macerated in 100 mmol L-1 borate buffer pH 8.8, followed by centrifugation at 10,000 g for 30 min at 4ºC. Then, the supernatant was saturated with 70% ammonium sulphate, followed by centrifugation at 10,000 g for 30 min at 4ºC. The resultant pellet was resuspended in the same extraction buffer and used for PAL activity assay, which was performed according to the method of Ferrarese et al. (2000) FERRARESE, M.L.L.; FERRARESE-FILHO, O.; RODRIGUES, J.D. Consuption of fenolic acid by sybean root in nutrient culture. Acta Physiologiae Plantarum, Cracóvia, v.22, p.201-203, 2000. . Thus, the reaction system consisted of 100 mmol L-1 borate buffer pH 8.8, enzymatic extract and 50 mmol L-1 L-phenylalanine, maintained at 40ºC for 60 min and later added of 5M HCl to stop the reaction. The formed cinnamic acid was analyzed in HPLC (C18 250 mm x 4.6 mm x 1/4” microsorb column) and detected according to retention time at 275 nm (UV) after elution in 7% methanol. In this way, the PAL activity was calculated based on the area of peaks obtained, and expressed as μg cinnamate min-1 mg-1 protein. For the GST assay, extracts were obtained according to method described by Ekler et al. (1993) EKLER, Z.; DUTKA, F.; STEPHENSON, G.R. Safener effects on acetochlor toxicity, uptake, metabolism and glutathione S-transferase activity in maize. Weed Research, Doorwerth, v.33, p.311-318, 1993. , with modifications. Skin samples of 1g were ground in 0.2 mol L-1 tris (hydroxymethyl) aminomethanehydrochloric acid (Tris–HCl) cold buffer pH 7.8 containing 1 mmol L-1 ethylenediaminetetraacetic acid (EDTA) and 7.5% (w v-1) polyvinylpolypyrrolidone (PVPP). After centrifugation at 4°C (20 min at 14,000g), the supernatant was collected and stored at -20°C. The GST activity was evaluated according to procedure proposed by Wu et al. (1996) WU, J.; OMOKAWA, H.; HATZIOS, K.K. Glutathione S-transferase activity in unsafened and fenclorim-safened rice (Oryza sativa). Pesticide Biochemistry and Physiology, Amsterdam, v.54, p.220-229, 1996. . The reaction system contained the enzymatic extract, 100 mmol L-1 potassium phosphate buffer pH 6.9, 3.3 mmol L-1 glutathione (GSH), and 30 mmol L-1 1-chloro-2, 4-dinitrobenzene (CDNB), maintained at 25°C for 30 min. Absorbance change due to the formation of GSH-CDNB conjugate was measured in spectrophotometer at 340 nm. Molar extinction coefficient equal to 10 mmol L-1 cm-1 (MANNERVIK; GUTHENBERG, 1981 MANNERVIK, B.; GUTHENBERG, C. Glutathione transferase (human placenta). Methods in Enzymology, New York, v.77, p.231-235, 1981. ) was used to calculate the enzyme specific activity, expressed as nmol GSH-CDNB min-1 mg-1 protein.
The concentration of total soluble proteins in enzymatic extracts was quantified by the method of Bradford (1976) BRADFORD, M.M. A rapid and sensitive method for quantitation of microgram quantities protein utilizing the principle of protein-dye-binding. Analytical Biochemistry, New York, v.72, p.248-254, 1976. , with absorbance readings performed in UV-visible spectrophotometer at 595 nm, using casein as standard.
The results were submitted to analysis of variance (F-test), with posterior elaboration of polynomial regression models (p≤0.05).
Results and Discussion
Regarding the first trial, there were no changes in the levels of total sugars, firmness, SS, TA, SS/TA ratio, and flavonols (Table 1).
Higher pH values were observed in fruits treated with 200 and 400 mg L-1 ethephon + CaCl2 (ET200+CC1.5% and ET400+CC1.5%, respectively) compared to control fruits treated only with water (W) (Table 1). Conversely, Lima et al. (2000) LIMA, M.A.C.; ALVES, R.E.; ASSIS, J.S.; FILGUEIRAS, H.A.C.; COSTA, J.T.A. Qualidade, fenóis e enzimas oxidativas de uva ‘Itália’ sob influência do cálcio, durante a maturação. Pesquisa Agropecuária Brasileira, Brasília, DF, v.35, p.2493-2499, 2000. observed no effect of calcium on the pH of ‘Itália’ grapes.
Higher reducing sugar levels were detected in fruits treated with the highest ethephon concentrations (600 and 800 mg L-1) + CaCl2 (ET600+CC1.5%, and ET800+CC1.5%, respectively), compared to control (Table 1). Similarly, Chervin et al. (2006) CHERVIN, C.; TERRIER, N.; AGEORGES, A.; RIBES, F.A.; KUAPUNYAKOON, T. Influence of ethylene on sucrose accumulation in grape berry. American Journal of Enology and Viticulture, Davis, v.57, p.511-513, 2006. observed higher sugar accumulation during the ripening of ‘Cabernet Sauvignon’ grapes, since released ethylene leads to the expression of genes encoding sucrose transporters, resulting in higher sucrose translocation from biosynthesis sites to the berry vacuole, in which sucrose is enzymatically converted into glucose and fructose (reducing sugars). Such conversion is achieved by the action of acid invertase (EC 3.2.1.26), which activity is controlled by sucrose accumulation (YU et al. 2008 YU, X.; WANG, X.; ZHANG, W.; QIAN, T.; TANG, G.; GUO, Y.; ZHENG, C. Antisense suppression of an acid invertase gene (MAI1) in muskmelon alters plant growth and fruit development. Journal of Experimental Botany, Lancaster, v.59, p.2969-2977, 2008. ). However, the same correlation was not observed among treatments and total sugar levels. At the end of berry ripening, ethylene inhibits the codification of genes related to invertase synthesis, decreasing sucrose conversion (MAILHAC; CHERVIN, 2006 MAILHAC, N.; CHERVIN, C. Ethylene and grape berry ripening. Stewart Postharvest Review, London, v.2, p.1-5, 2006. ).
Higher anthocyanin levels were detected in berries treated with ethephon and CaCl2, tending to stabilize from 400 mg L-1 ethephon associated with 1.5% CaCl2 (Table 1). Such results were similar to those observed in several studies involving different grape cultivars treated with different concentrations of ethylene releasers, such as those of El-Kereamy et al. (2003) EL-KEREAMY, A.; CHERVIN, C.; SOUQUET, J.; MOUTOUNET, M.; MONJE, M.; NEPVEU, F.; MONDIES, H.; FORD, C.M.; VAN-ILEESWIJCK, R.; ROUTAN, J. Exogenous ethylene stimulates the long-term expression of genes related to anthocyanin biosynthesis in grape berries. Physiologia Plantarum, Lund, v.119, p.175-182, 2003. , Mailhac and Chervin (2006) MAILHAC, N.; CHERVIN, C. Ethylene and grape berry ripening. Stewart Postharvest Review, London, v.2, p.1-5, 2006. , and Leão et al. (2015) LEÃO, P.C.D.S.; LIMA, M.A.C.; COSTA, J.P.D.; TRINDADE, D.C.G.D. Abscisic acid and ethephon for improving red color and quality of crimson seedless grapes grown in a tropical region. American Journal of Enology and Viticulture, Davis, v.66, p.37-45, 2015. , who reported higher anthocyanin levels in ethylene-treated grapes.
The increase in anthocyanin levels can be due to the higher ethephon-induced endogenous ethylene production, which leads to the transcription of genes encoding important enzymes from the biosynthetic pathway involving these pigments, such as PAL, chalcone synthase (CHS, EC 2.3.1.74), chalcone isomerase (CHI, EC 5.5.1.6), and UDPglucose: flavonoid 3-O-glucosyltransferase (UFGT, EC 2.4.1.115) (SUDHA; RAVISHANKAR 2003 SUDHA, G.; RAVISHANKAR, G.A. Elicitation of anthocyanin production in callus cultures of Daucus carota and involvemente of calcium channel modulators. Current Science, Bangalore, v.84, p.775-779, 2003. ; CHERVIN et al. 2006 CHERVIN, C.; TERRIER, N.; AGEORGES, A.; RIBES, F.A.; KUAPUNYAKOON, T. Influence of ethylene on sucrose accumulation in grape berry. American Journal of Enology and Viticulture, Davis, v.57, p.511-513, 2006. ). Although grapes treated only with CaCl2 (CC1.5%) showed similar anthocyanin levels compared with those of the control group (W) in the present study (Table 1), Sudha and Ravishankar (2003) SUDHA, G.; RAVISHANKAR, G.A. Elicitation of anthocyanin production in callus cultures of Daucus carota and involvemente of calcium channel modulators. Current Science, Bangalore, v.84, p.775-779, 2003. reported that calcium was essential for anthocyanin production in carrot cell culture. Thus, Vitrac et al. (2000) VITRAC, X.; LARRONDE, F.; KRISA, S.; DECENDIT, A.; DEFFIEUX, G.; MÉRILLON, J.M. Sugar sensing and Ca2+-calmodulin requirement in Vitis vinifera cells producing anthocyanins. Phytochemistry, Madison, v.53, p.659-665, 2000. suggested that Ca2+ involvement in the induction of anthocyanin biosynthesis occurs from CHS gene expression. Such enzyme has extreme importance in the biosynthetic pathway of these flavonoids and its expression is due to the increase in intracellular hexose levels, which are signalized by Ca+2.
This first stage of evaluations indicated no changes in the levels of total sugars, firmness, SS, TA, SS/TA ratio, and flavonols, but remarkable results in the levels of reducing sugars and anthocyanins.
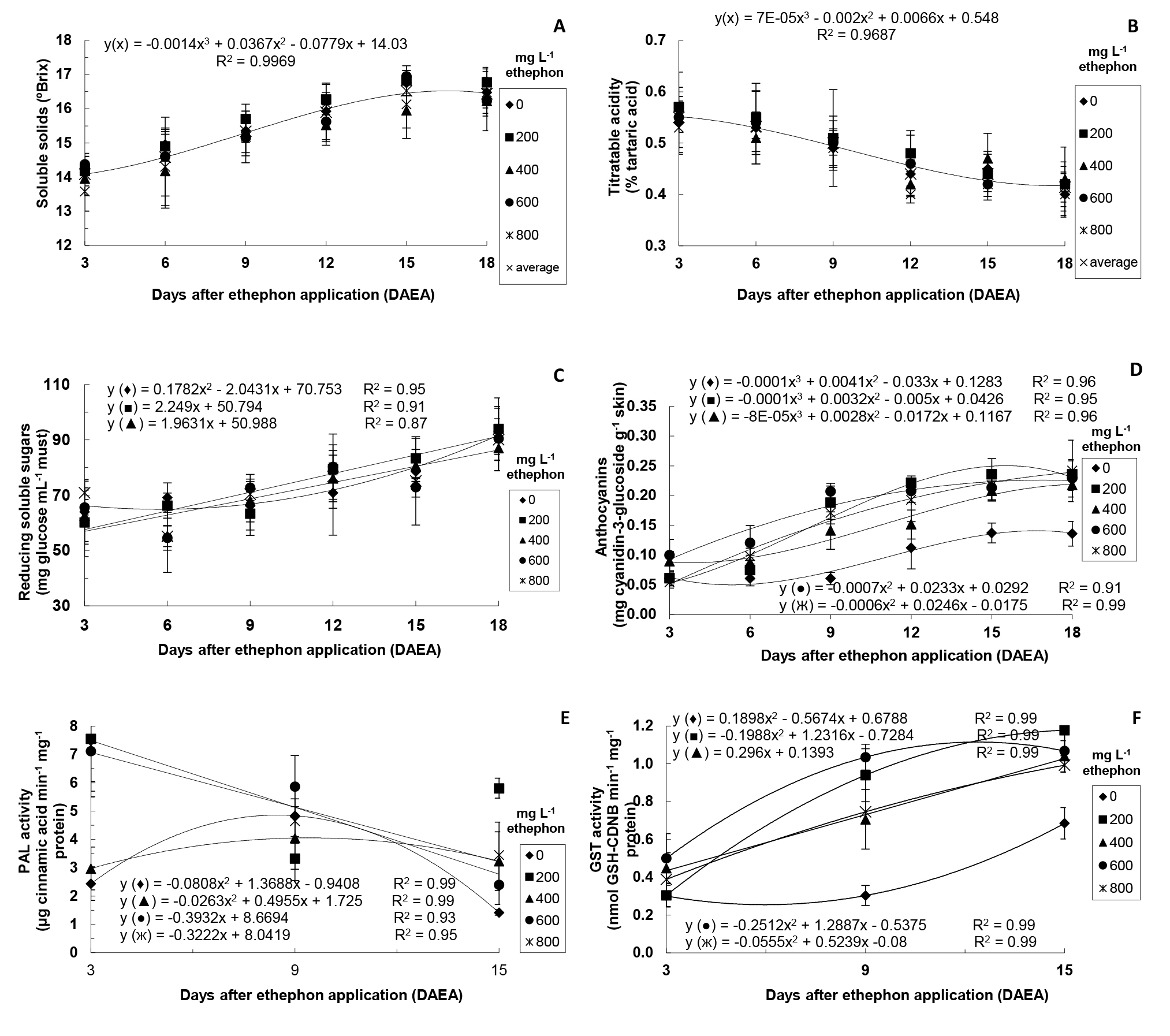
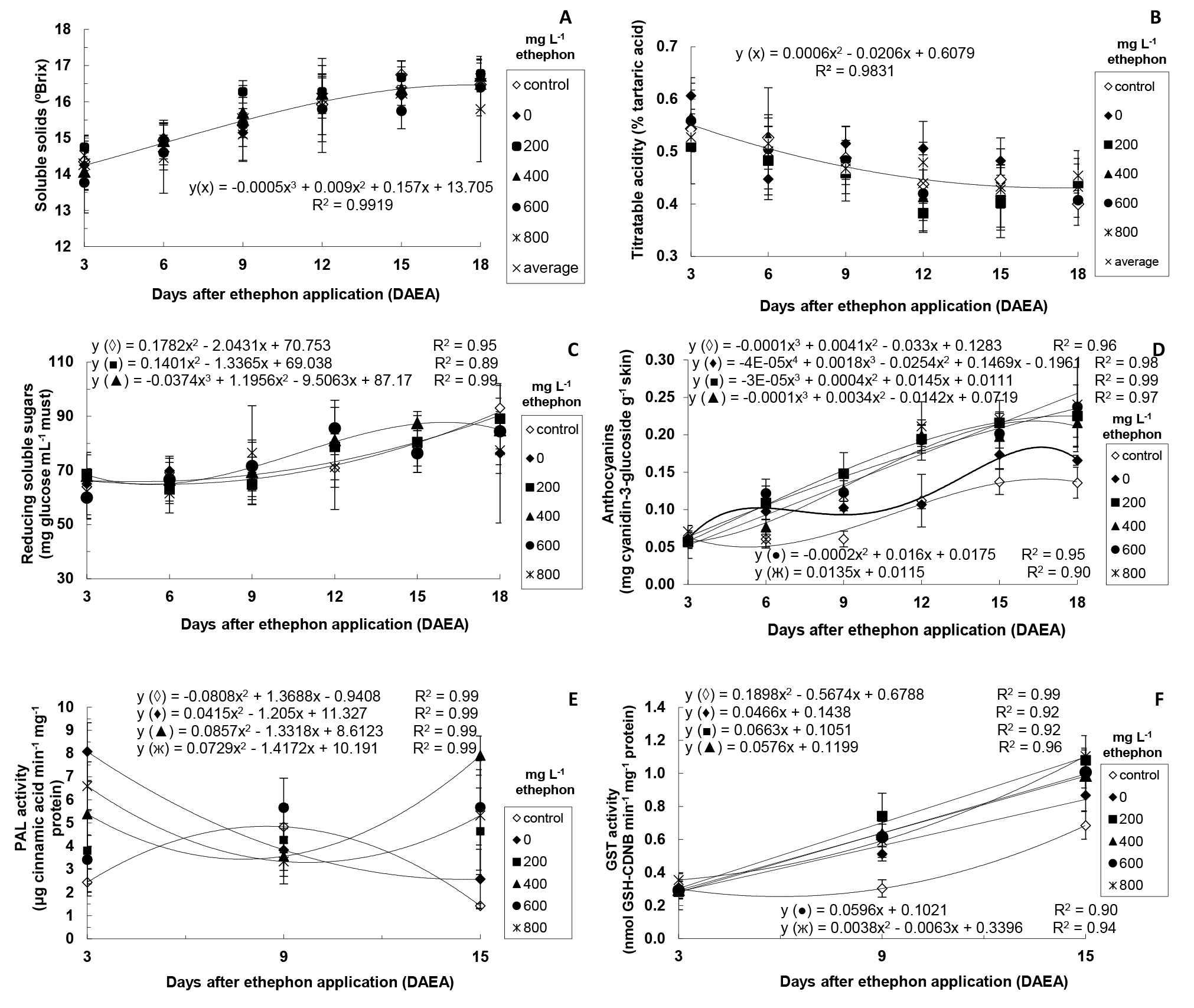
In the second stage, it was considered relevant to investigate (over time) some traits (levels of SS, TA, reducing sugar and anthocyanins, and PAL and GST activity) of ‘Rubi’ grape berries treated only with different ethephon concentrations (Figure 1) and of those submitted to different ethephon concentrations associated with 1.5% CaCl2 solution (Figure 2).
There was no significant interaction between ethephon concentrations and DAEA for SS and TA. Thus, considering the average of all ethephon concentrations in each sampling, there was increase in SS and decrease in TA over time (Figure 1A and Figure 1B). SS levels are the maturity index used as standard for marketing of grapes, and 14ºBrix is the minimum value set by BRASIL (2004) BRASIL. Ministério da Agricultura, Pecuária e Abastecimento. Disponível em: http://www.agricultura.gov.br/sarc/profruta/html/classificacao_indice.htm. Acesso em: 10 jan. 2004.
http://www.agricultura.gov.br/sarc/profr...
for consumption of table grapes. In the present study, however, berries showed mean values of 14.1ºBrix at 3 DAEA, but grapes were not yet suitable for commercialization due to their inadequate color. Conversely, Rodrigues et al. (2010) RODRIGUES, A.; GIRARDI, E.A.; SCARPARE FILHO, J.A. Aplicação de ethephon e qualidade da uva ‘Rubi’ em Porto Feliz-SP. Revista Brasileira de Fruticultura, Jaboticabal, v.32, p.925-930, 2010. observed no effect of different ethephon concentrations (120, 240, 360, 480, 720, and 960 mg L-1) on the SS levels of ‘Rubi’ grapes. With respect to TA, unlike our results, Leão and Assis (1999) LEÃO, P.C.S.; ASSIS, J.S. Efeito do ethephon sobre a coloração e qualidade da uva Red Globe no Vale do São Francisco. Revista Brasileira de Fruticultura, Jaboticabal, v.21, n.1, p.84-87, 1999. observed no influence of ethephon on ‘Red Globe’ grapes when applied at concentrations higher than 200 mg L-1.
There was a significant interaction between ethephon concentrations and DAEA for the levels of reducing sugars, except for the highest ethephon concentrations (600 and 800 mg L-1) (Figure 1C).
Thus, grapes treated with the lowest ethephon concentrations showed a roughly linear increase in the levels of reducing sugars over time. Some studies have reported the positive contribution of ethylene on the development of taste and aroma of grapes (MAILHAC; CHERVIN, 2006 MAILHAC, N.; CHERVIN, C. Ethylene and grape berry ripening. Stewart Postharvest Review, London, v.2, p.1-5, 2006. ), which are traits determined by the relationship between levels of sugars and the pulp acidity.
Regarding the levels of anthocyanins, there was significant interaction between ethephon concentrations and DAEA (Figure 1D).Such levels were higher in ethephon-treated grapes from 6 DAEA. On the last sampling (18 DAEA), these grapes showed similar levels among themselves and considerably higher than those of control plants.
Similar results were obtained by El-Kereamy et al. (2003) EL-KEREAMY, A.; CHERVIN, C.; SOUQUET, J.; MOUTOUNET, M.; MONJE, M.; NEPVEU, F.; MONDIES, H.; FORD, C.M.; VAN-ILEESWIJCK, R.; ROUTAN, J. Exogenous ethylene stimulates the long-term expression of genes related to anthocyanin biosynthesis in grape berries. Physiologia Plantarum, Lund, v.119, p.175-182, 2003. , Mailhac and Chervin (2006) MAILHAC, N.; CHERVIN, C. Ethylene and grape berry ripening. Stewart Postharvest Review, London, v.2, p.1-5, 2006. , and Leão et al. (2015) LEÃO, P.C.D.S.; LIMA, M.A.C.; COSTA, J.P.D.; TRINDADE, D.C.G.D. Abscisic acid and ethephon for improving red color and quality of crimson seedless grapes grown in a tropical region. American Journal of Enology and Viticulture, Davis, v.66, p.37-45, 2015. , who also reported effective action of ethylene precursors leading to color of grapes in different sites and, then, under several weather conditions.
Furthermore, Roberto et al. (2013) ROBERTO, S.R.; ASSIS, A.M.; YAMAMOTO, L.Y.; MIOTTO, L.C.; KOYAMA, R.; SATO, A.J.; BORGES, R.S. Ethephon use and application timing of abscisic acid for improving color of 'Rubi' table grape. Pesquisa Agropecuária Brasileira, Brasília, DF, v.48, p.797-800, 2013. observed higher color intensity in ‘Rubi’ berries submitted to 500 mg L‑1 ethephon compared to the control group. In addition, Rodrigues et al. (2010) RODRIGUES, A.; GIRARDI, E.A.; SCARPARE FILHO, J.A. Aplicação de ethephon e qualidade da uva ‘Rubi’ em Porto Feliz-SP. Revista Brasileira de Fruticultura, Jaboticabal, v.32, p.925-930, 2010. detected a more reddish color in ‘Rubi’ berries after ethephon application, regardless of concentration (120, 240, 360, 480, 720, and 960 mg L-1). El-Kereamy et al. (2003) EL-KEREAMY, A.; CHERVIN, C.; SOUQUET, J.; MOUTOUNET, M.; MONJE, M.; NEPVEU, F.; MONDIES, H.; FORD, C.M.; VAN-ILEESWIJCK, R.; ROUTAN, J. Exogenous ethylene stimulates the long-term expression of genes related to anthocyanin biosynthesis in grape berries. Physiologia Plantarum, Lund, v.119, p.175-182, 2003. reported that the increase in berry coloration after increasing ethylene concentration is related to the transcription of specific anthocyanin biosynthesis genes.
Furthermore, ethylene induces the expression of genes related to the synthesis of enzymes including PAL and CHS, which are essential in the flavonoid biosynthetic pathway (EL-KEREAMY et al., 2003 EL-KEREAMY, A.; CHERVIN, C.; SOUQUET, J.; MOUTOUNET, M.; MONJE, M.; NEPVEU, F.; MONDIES, H.; FORD, C.M.; VAN-ILEESWIJCK, R.; ROUTAN, J. Exogenous ethylene stimulates the long-term expression of genes related to anthocyanin biosynthesis in grape berries. Physiologia Plantarum, Lund, v.119, p.175-182, 2003. ). PAL has great importance in the anthocyanin synthesis by initiating the cascade of reactions of phenylpropanoid biosynthesis (HIRATSUKA et al., 2001 HIRATSUKA, S.; ONODERA, H.; KAWAI, Y.; KUBO, T.; ITOH, H.; WADA, R. Enzyme activity changes during anthocyanin synthesis in ‘Olympia’ grape berries. Scientia Horticulturae, New York, v.90, p.255-264. 2001. ). In the present study, there was significant interaction between ethephon concentrations and DAEA for PAL activity, except at 200 mg L-1 (Figure 1E). Higher PAL activity was observed in grapes submitted to 200, 600, and 800 mg L-1 ethephon at 3 DAEA. Then, a linear decrease was observed over time in grapes treated with the highest ethephon concentrations. On the other hand, control grapes and those treated with 400 mg L-1 showed higher PAL activity at 9 DAEA in comparison to other evaluation periods. Similar results were observed by El-Kereamy et al. (2003) EL-KEREAMY, A.; CHERVIN, C.; SOUQUET, J.; MOUTOUNET, M.; MONJE, M.; NEPVEU, F.; MONDIES, H.; FORD, C.M.; VAN-ILEESWIJCK, R.; ROUTAN, J. Exogenous ethylene stimulates the long-term expression of genes related to anthocyanin biosynthesis in grape berries. Physiologia Plantarum, Lund, v.119, p.175-182, 2003. in ethephon-treated ‘Cabernet Sauvignon’ grapes.
GST enzyme has been found in several organisms and acts on the conjugation of exogenous toxic compounds (xenobiotics) to the tripeptide glutathione (GSH), originating low-toxicity complexes (MARRS, 1996 MARRS, K.A. The functions and regulation of glutathione S-transferases in plants. Annual Review of Plant Physiology and Plant Molecular Biology, Palo Alto, v.47, p.127-158, 1996. ). Furthermore, through similar mechanism, GST facilitates the transportation of anthocyanins into the vacuole, increasing the coloration of grape skins (MOONS, 2005 MOONS, A. Regulatory and functional interactions of plant growth regulators and plant glutathione S-transferases (GSTs). Vitamins &Hormones, San Diego, v.72, p.155-202, 2005. ). In the present study, there was significant interaction between ethephon concentrations and DAEA for GST activity, which gradually increased over time, especially in ethephon-treated grapes from 9 DAEA (Figure 1F). Cinnamic acid and cyanidin-3- glucoside are natural GST substrates (MARRS, 1996 MARRS, K.A. The functions and regulation of glutathione S-transferases in plants. Annual Review of Plant Physiology and Plant Molecular Biology, Palo Alto, v.47, p.127-158, 1996. ; TAULAVUORI et al., 2004 TAULAVUORI, E.; TAHOKOKORPI, M.; TAULAVUORI, K.; LAINE, K. Anthocyanins an glutathione S-transferase activities in response to low temperature an frost hardening in Vaccinium myrtillus L. Journal of Plant Physiology, Jena, v.161, p.903-911, 2004. ), which transport such compounds into the vacuole through conjugation with GSH. Thus, the higher GST activity over time observed in the present study can be related to the higher anthocyanin levels detected and maybe to the high PAL activity previously observed at 3 DAEA.
When the combined effect of different ethephon concentrations and CaCl2 was evaluated over time (Figure 2), almost the same pattern was observed regarding grapes only treated with ethephon. Thus, significant interaction between ethephon concentrations associated with CaCl2 and DAEA was detected, similarly to treatments with no CaCl2 application, in addition to the absence of significant interaction between 0 mg L-1 ethephon + CaCl2 and DAEA for reducing sugars (Figure 2C) and between 600 mg L-1 ethephon + CaCl2 and DAEA for PAL activity (Figure 2E).
Lima et al. (2000) LIMA, M.A.C.; ALVES, R.E.; ASSIS, J.S.; FILGUEIRAS, H.A.C.; COSTA, J.T.A. Qualidade, fenóis e enzimas oxidativas de uva ‘Itália’ sob influência do cálcio, durante a maturação. Pesquisa Agropecuária Brasileira, Brasília, DF, v.35, p.2493-2499, 2000. reported lower SS values in ‘Itália’ grapes submitted to different calcium levels.
According to these authors, Ca2+ limits the diffusion of substrates from the vacuole to the cytosol, decreasing the glycolytic flux, which can decrease the respiration rate. This result was similar to that of the present study only at 15 DAEA, when higher SS values were observed in the control group and at 200 mg L-1 ethephon + CaCl2 (Figure 2A).
Lower TA values were detected over time considering the average of all ethephon concentrations + CaCl2 in each sampling (Figure 2B).Lima et al.(2000) LIMA, M.A.C.; ALVES, R.E.; ASSIS, J.S.; FILGUEIRAS, H.A.C.; COSTA, J.T.A. Qualidade, fenóis e enzimas oxidativas de uva ‘Itália’ sob influência do cálcio, durante a maturação. Pesquisa Agropecuária Brasileira, Brasília, DF, v.35, p.2493-2499, 2000. measured TA in ‘Itália’ grapes submitted to different calcium levels and observed initial increase (from 3.60 to 4.35 g of 100 mL-1 tartaric acid) up to 43 days after fruit formation, followed by a continuous decrease, mainly after 57 days, reaching 0.92 g of 100 mL-1 tartaric acid on the sampling date. According to these authors, such decrease can be due to the diluted concentration of acids considering the increased berry volume, degradation activation, synthesis inhibition, and their transformation into sugars.
In the present study, grapes treated with the lowest ethephon concentrations (200 and 400 mg L-1) associated with CaCl2 showed increase in the levels of reducing sugars over time (Figure 2C).
In fruits treated with 600 and 800 mg L-1 ethephon + CaCl2, higher levels were obtained at 12 and 9 DAEA, respectively. Thus, the application of ethephon concentrations from 600 mg L-1 accelerated the formation of reducing sugars. Glucosides and cyanidins are the final precursors of anthocyanin biosynthesis. In general, these pigments are stored after being associated with a glucose molecule.
This process is important for stabilization of anthocyanins (JU et al., 1995 JU, Z.G.; YUAN, Y.; LIU, C.; XIN, S. Relationships among phenylalanine ammonia lyase activity, simple phenol concentration and anthocyanin accumulation in apple. Scientia Horticulturae, New York, v.61, p.215-226. 1995. ), since the addition of sugars maintains the communication among their different regions (BROUILLARD et al., 2003 BROUILLARD, R.; CHASSAING, S.; FOUGEROUSSE, A. Why are grape/fresh wine anthocyanins so simple and why is it that red wine color lasts so long? Phytochemistry, Oxford, v.64, p.1179 -1186, 2003. ).
Ca2+ acts as a second messenger in the signaling of sugars and ethylene (KWAK; LEE, 1997 KWAK, S.H.; LEE, S.H. The requirements for Ca2+, protein phosphorylation, and dephosphorylation for ethylene signal transduction in Pisum sativum L. Plant &Cell Physiology, Kyoto, v.38, p.1142-1149. 1997. ), being closely involved in the signaling of sugars during anthocyanin biosynthesis in grapes. The regulation of this signaling is related to calmodulin or Ca2+- dependent protein kinases, such as hexokinase (EC 2.7.1.1). According to Vitrac et al. (2000) VITRAC, X.; LARRONDE, F.; KRISA, S.; DECENDIT, A.; DEFFIEUX, G.; MÉRILLON, J.M. Sugar sensing and Ca2+-calmodulin requirement in Vitis vinifera cells producing anthocyanins. Phytochemistry, Madison, v.53, p.659-665, 2000. , hexokinase is considered as a sensor in the signaling of sugars, leading to anthocyanin accumulation in grapes.
In the present study, anthocyanin levels increased linearly over time in the presence of ethephon and CaCl2 (Figure 2D). On the last sampling (18 DAEA), grapes showed very similar levels among themselves and levels considerably higher than those of the control group and of grapes only treated with CaCl2. These latter two groups showed higher values for grapes treated only with CaCl2 at 15 and 18 DAEA, thus highlighting the contribution of Ca2+ to increase anthocyanin levels. Ca2+ induces the activity of ACC oxidase (EC 1.14.17.4), which acts on the biosynthesis of endogenous ethylene (LI et al., 2002 LI, Z.; GEMMA, H.; IWAHORI, S. Stimulation of Fuji apple skin color by ethephon and phosphorus-calcium mixed compounds in relation to flavonoid synthesis. Scientia Horticulturae, New York, v.94, p.193-199. 2002. ; SUDHA; RAVISHANKAR, 2003 SUDHA, G.; RAVISHANKAR, G.A. Elicitation of anthocyanin production in callus cultures of Daucus carota and involvemente of calcium channel modulators. Current Science, Bangalore, v.84, p.775-779, 2003. ), and acts as a second messenger of several elicitors of anthocyanin biosynthesis, such as sugars and ethylene (KWAK; LEE, 1997 KWAK, S.H.; LEE, S.H. The requirements for Ca2+, protein phosphorylation, and dephosphorylation for ethylene signal transduction in Pisum sativum L. Plant &Cell Physiology, Kyoto, v.38, p.1142-1149. 1997. ).
PAL initiates the cascade of reactions in the anthocyanin biosynthesis (HIRATSUKA et al., 2001 HIRATSUKA, S.; ONODERA, H.; KAWAI, Y.; KUBO, T.; ITOH, H.; WADA, R. Enzyme activity changes during anthocyanin synthesis in ‘Olympia’ grape berries. Scientia Horticulturae, New York, v.90, p.255-264. 2001. ). In the present study, PAL activity was higher in grapes treated with ethephon and CaCl2 at 3 and 15 DAEA, with sharp decline at 9 DAEA (Figure 2E). In addition, grapes treated with 400 and 800 mg L-1 ethephon + CaCl2 showed a slight decrease in PAL activity at 9 DAEA, with posterior increase at 15 DAEA. Furthermore, grapes treated only with CaCl2 showed a continuous decrease in PAL activity from 3 DAEA, while those of the control group had a distinguished behavior, with higher PAL activity at 9 DAEA. This pattern regarding PAL activity in grapes treated with ethephon and CaCl2 over time was different from that observed in grapes treated only with ethephon (Figure 1E), which showed higher PAL activity at 3 DAEA, with posterior decrease over time. Thus, data obtained at 15 DAEA allowed inferring that the increase in PAL activity in this period was due to the application of CaCl2. Ca2+ increases the ACC oxidase activity, leading to the synthesis of endogenous ethylene (LI et al., 2002 LI, Z.; GEMMA, H.; IWAHORI, S. Stimulation of Fuji apple skin color by ethephon and phosphorus-calcium mixed compounds in relation to flavonoid synthesis. Scientia Horticulturae, New York, v.94, p.193-199. 2002. ; SUDHA; RAVISHANKAR, 2003 SUDHA, G.; RAVISHANKAR, G.A. Elicitation of anthocyanin production in callus cultures of Daucus carota and involvemente of calcium channel modulators. Current Science, Bangalore, v.84, p.775-779, 2003. ). Furthermore, the ethephon degradation rate decreases in the presence of Ca2+ (BIDDLE et al., 1976 BIDDLE, E.; KERFOOT, D.G.S.; KHO, Y.H.; RUSSEL, K.E. Kinetic studies of the thermal decomposition of 2-chloroethylphosphonic acid in aqueous solution. Plant Physiology, Rockville, v.58, p.700-702, 1976. ). Thus, the increase in PAL activity in the present study at 15 DAEA can be related to the increased concentration of endogenous ethylene, which synthesis was induced by Ca2+.
The GST activity increased linearly over time, especially in grapes treated with ethephon and CaCl2 (Figure 2F). In control grapes, the increase in GST activity was more gradual. Cinnamic acid (product of PAL action) and cyanidin-3-glucoside (main anthocyanin formed) are natural substrates of GST (MARRS, 1996 MARRS, K.A. The functions and regulation of glutathione S-transferases in plants. Annual Review of Plant Physiology and Plant Molecular Biology, Palo Alto, v.47, p.127-158, 1996. ; TAULAVUORI et al., 2004 TAULAVUORI, E.; TAHOKOKORPI, M.; TAULAVUORI, K.; LAINE, K. Anthocyanins an glutathione S-transferase activities in response to low temperature an frost hardening in Vaccinium myrtillus L. Journal of Plant Physiology, Jena, v.161, p.903-911, 2004. ). Thus, the higher GST activity over time can be related to the high levels of anthocyanins observed and the higher PAL activity at 15 DAEA.
Regarding the relationship between Ca and GST activity, Erinle et al. (2016) ERINLE, K.O.; JIANG, Z.; MA, B.; LI, J.; CHEN, Y.; UR-REHMAN, K.; SHAHLA, A.; ZHANG, Y. Exogenous calcium induces tolerance to atrazine stress in Pennisetum seedlings and promotes photosynthetic activity, antioxidant enzymes and psbA gene transcripts. Ecotoxicology and Environmental Safety, New York, v.132, p.403-412, 2016. observed that the GST activity increased with Ca application in Ca-protected Pennisetum seedlings in the presence of atrazine. To our knowledge, this is the first study investigating the effect of Ca on the GST activity in grape skin.
Levels of soluble solids (A), titratable acidity (B), reducing soluble sugars (C), anthocyanins (D), PAL activity (E), and GST activity (F) of ‘Rubi’ grapes after ethephon application. In A and B, there was no significant interaction between ethephon concentrations and DAEA (p=0.05). In this case, the curve indicates the adjustment regarding average values of all ethephon concentrations in each harvest time. In C, no interaction was observed for 600 and 800 mg L-1 – without adjustment. In E, no interaction was observed for 200 mg L-1 – without adjustment. Means of four replicates (except for PAL activity - means of three replicates).
Levels of soluble solids (A), titratable acidity (B), reducing soluble sugars (C), anthocyanins (D), PAL activity (E), and GST activity (F) of ‘Rubi’ grapes after ethephon and 1.5% CaCl2 application. Control represents untreated grapes. In A and B, there was no significant interaction between ethephon concentrations associated with CaCl2 and DAEA (p=0.05). In this case, the curve indicates the adjustment regarding average values of all ethephon concentrations associated with CaCl2 in each harvesting time. In C, no interaction was observed for 0, 600 and 800 mg L-1 ethephon + 1.5% CaCl2 – without adjustment. In E, no interaction was observed for 200 and 600 mg L-1 ethephon + 1.5% CaCl2 – without adjustment. Means of four replicates (except for PAL activity - means of three replicates).
Levels of total and reducing soluble sugars (mg glucose mL-1 must), pH, firmness (g force-1), soluble solids (SS, °Brix), titratable acidity (TA, % tartaric acid), SS/AT ratio, flavonols [mg quercetin (100g skin)-1] and anthocyanins [mg cyanidin-3-glucoside (100 g-1 skin)] levels of ‘Rubi’ grape submitted to ethephon concentrations associated with 1.5% CaCl2 solution.
Conclusion
Ethephon associated with CaCl2 can contribute to increase the post-harvesting quality of ‘Rubi’ grapes, since they increased the accumulation of anthocyanins due to the higher PAL and GST activity related to biosynthesis and storage of antocyanins, respectively, and the levels of reducing sugar and pH, not changing other quality aspects. Therefore, a single ethephon application from 200 mg L-1 associated with the application of 1.5% CaCl2 at the final ripening stage, when bunches present from 30 to 50% coloration, is sufficient to produce positive results in the coloration of ‘Rubi’ grape bunches.
Acknowledgements
We thank Mr. Edson Alves Rosa, technician from the Food Technology Lab, College of Agronomical Sciences, Lageado Experimental Farm, São Paulo State University, for helping in analyses; the Coordination for the Improvement of Higher Education Personnel (CAPES), for financial support; and Dr. Ana Catarina Cataneo and Dr. Elisangela Clarete Camili for helping in the standardization of procedures.
- BIDDLE, E.; KERFOOT, D.G.S.; KHO, Y.H.; RUSSEL, K.E. Kinetic studies of the thermal decomposition of 2-chloroethylphosphonic acid in aqueous solution. Plant Physiology, Rockville, v.58, p.700-702, 1976.
- BRADFORD, M.M. A rapid and sensitive method for quantitation of microgram quantities protein utilizing the principle of protein-dye-binding. Analytical Biochemistry, New York, v.72, p.248-254, 1976.
- BRASIL. Ministério da Agricultura, Pecuária e Abastecimento. Disponível em: http://www.agricultura.gov.br/sarc/profruta/html/classificacao_indice.htm Acesso em: 10 jan. 2004.
» http://www.agricultura.gov.br/sarc/profruta/html/classificacao_indice.htm - BROUILLARD, R.; CHASSAING, S.; FOUGEROUSSE, A. Why are grape/fresh wine anthocyanins so simple and why is it that red wine color lasts so long? Phytochemistry, Oxford, v.64, p.1179 -1186, 2003.
- CHERVIN, C.; TERRIER, N.; AGEORGES, A.; RIBES, F.A.; KUAPUNYAKOON, T. Influence of ethylene on sucrose accumulation in grape berry. American Journal of Enology and Viticulture, Davis, v.57, p.511-513, 2006.
- DUBOIS, M.; GILLES, K.A.; HAMILTON, J.K.; REBER, P.A.; SMITH, F. Colorimetric method for determination of sugar and related substances. Analytical Chemistry, Washington, v.2, p.350-356, 1956.
- EKLER, Z.; DUTKA, F.; STEPHENSON, G.R. Safener effects on acetochlor toxicity, uptake, metabolism and glutathione S-transferase activity in maize. Weed Research, Doorwerth, v.33, p.311-318, 1993.
- EL-KEREAMY, A.; CHERVIN, C.; SOUQUET, J.; MOUTOUNET, M.; MONJE, M.; NEPVEU, F.; MONDIES, H.; FORD, C.M.; VAN-ILEESWIJCK, R.; ROUTAN, J. Exogenous ethylene stimulates the long-term expression of genes related to anthocyanin biosynthesis in grape berries. Physiologia Plantarum, Lund, v.119, p.175-182, 2003.
- ERINLE, K.O.; JIANG, Z.; MA, B.; LI, J.; CHEN, Y.; UR-REHMAN, K.; SHAHLA, A.; ZHANG, Y. Exogenous calcium induces tolerance to atrazine stress in Pennisetum seedlings and promotes photosynthetic activity, antioxidant enzymes and psbA gene transcripts. Ecotoxicology and Environmental Safety, New York, v.132, p.403-412, 2016.
- FERNÁNDEZ-LÓPEZ, J.A.; ALMELA, L.; MUÑOZ, J.A.; HIDALGO, V.; CARREÑO, J. Dependence between colour and individual anthocyanin content in ripening grapes. Food Research International, Toronto, v.31, p.667-672, 1998.
- FERRARESE, M.L.L.; FERRARESE-FILHO, O.; RODRIGUES, J.D. Consuption of fenolic acid by sybean root in nutrient culture. Acta Physiologiae Plantarum, Cracóvia, v.22, p.201-203, 2000.
- HIRATSUKA, S.; ONODERA, H.; KAWAI, Y.; KUBO, T.; ITOH, H.; WADA, R. Enzyme activity changes during anthocyanin synthesis in ‘Olympia’ grape berries. Scientia Horticulturae, New York, v.90, p.255-264. 2001.
- JU, Z.G.; YUAN, Y.; LIU, C.; XIN, S. Relationships among phenylalanine ammonia lyase activity, simple phenol concentration and anthocyanin accumulation in apple. Scientia Horticulturae, New York, v.61, p.215-226. 1995.
- KOYAMA, R.; ASSIS, A.M.; YAMAMOTO, L.Y.; BORGES, W.S.; PRUDENCIO, S.H.; ROBERTO, S.R. Exogenous abscisic acid increases the anthocyanin concentration of berry and juice from 'Isabel' grapes (Vitis labrusca L.). HortScience, Alexandria, v.49, p.460-464, 2014.
- KWAK, S.H.; LEE, S.H. The requirements for Ca2+, protein phosphorylation, and dephosphorylation for ethylene signal transduction in Pisum sativum L. Plant &Cell Physiology, Kyoto, v.38, p.1142-1149. 1997.
- LEÃO, P.C.D.S.; LIMA, M.A.C.; COSTA, J.P.D.; TRINDADE, D.C.G.D. Abscisic acid and ethephon for improving red color and quality of crimson seedless grapes grown in a tropical region. American Journal of Enology and Viticulture, Davis, v.66, p.37-45, 2015.
- LEÃO, P.C.S.; ASSIS, J.S. Efeito do ethephon sobre a coloração e qualidade da uva Red Globe no Vale do São Francisco. Revista Brasileira de Fruticultura, Jaboticabal, v.21, n.1, p.84-87, 1999.
- LEES, D.H.; FRANCIS, F.J. Standardization of pigment analyses in cranberries. HortScience, Alexandria, v.7, p.83-84, 1972.
- LI, Z.; GEMMA, H.; IWAHORI, S. Stimulation of Fuji apple skin color by ethephon and phosphorus-calcium mixed compounds in relation to flavonoid synthesis. Scientia Horticulturae, New York, v.94, p.193-199. 2002.
- LIMA, M.A.C.; ALVES, R.E.; ASSIS, J.S.; FILGUEIRAS, H.A.C.; COSTA, J.T.A. Qualidade, fenóis e enzimas oxidativas de uva ‘Itália’ sob influência do cálcio, durante a maturação. Pesquisa Agropecuária Brasileira, Brasília, DF, v.35, p.2493-2499, 2000.
- MAILHAC, N.; CHERVIN, C. Ethylene and grape berry ripening. Stewart Postharvest Review, London, v.2, p.1-5, 2006.
- MANNERVIK, B.; GUTHENBERG, C. Glutathione transferase (human placenta). Methods in Enzymology, New York, v.77, p.231-235, 1981.
- MARRS, K.A. The functions and regulation of glutathione S-transferases in plants. Annual Review of Plant Physiology and Plant Molecular Biology, Palo Alto, v.47, p.127-158, 1996.
- MELLO, L.M.R. Desempenho da vitivinicultura brasileira em 2015. Disponível em: https://www.embrapa.br/busca-de-noticias/-/noticia/9952204/artigo-desempenho-da-vitivinicultura-brasileira-em-2015 Acesso em: 16 set. 2016.
» https://www.embrapa.br/busca-de-noticias/-/noticia/9952204/artigo-desempenho-da-vitivinicultura-brasileira-em-2015 - MIAO, L.; ZHANG, Y.; YANG, X.; XIAO, J.; ZHANG, H.; ZHANG, Z.; WANG, Y.; JIANG, G. Colored light-quality selective plastic films affect anthocyanin content, enzyme activities, and the expression of flavonoid genes in strawberry (Fragaria x ananassa) fruit. Food Chemistry, New York, v.207, p.93-100, 2016.
- MOONS, A. Regulatory and functional interactions of plant growth regulators and plant glutathione S-transferases (GSTs). Vitamins &Hormones, San Diego, v.72, p.155-202, 2005.
- NELSON, N. A photometric adaptation of the Somogyi method for the determination of glucose. Journal of Biological Chemistry, Baltimore, v.153, p.375-380, 1944.
- ROBERTO, S.R.; ASSIS, A.M.; YAMAMOTO, L.Y.; MIOTTO, L.C.; KOYAMA, R.; SATO, A.J.; BORGES, R.S. Ethephon use and application timing of abscisic acid for improving color of 'Rubi' table grape. Pesquisa Agropecuária Brasileira, Brasília, DF, v.48, p.797-800, 2013.
- ROBERTO, S.R.; ASSIS, A.M.; YAMAMOTO, L.Y.; MIOTTO, L.C.V.; SATO, A.J.; KOYAMA, R.; GENTA, W. Application timing and concentration of abscisic acid improve color of ‘Benitaka’ table grape. Scientia Horticulturae, New York, v.142, p.44-48, 2012.
- RODRIGUES, A.; GIRARDI, E.A.; SCARPARE FILHO, J.A. Aplicação de ethephon e qualidade da uva ‘Rubi’ em Porto Feliz-SP. Revista Brasileira de Fruticultura, Jaboticabal, v.32, p.925-930, 2010.
- SPAYD, S.E.; TARARA, J.M.; MEE, D.L.; FERGUSON, J.C. Separation of sunlight and temperature effects on the composition of Vitis vinifera cv. Merlot berries. American Journal of Enology and Viticulture, Davis, v.53, p.171-182, 2002.
- SUDHA, G.; RAVISHANKAR, G.A. Elicitation of anthocyanin production in callus cultures of Daucus carota and involvemente of calcium channel modulators. Current Science, Bangalore, v.84, p.775-779, 2003.
- SUN, Y.; LI, H.; HUANG, J.R. Arabidopsis TT19 functions as a carrier to transport anthocyanin from the cytosol to tonoplasts. Molecular Plant, Shanghai, v.5, p.387-400, 2012.
- TAULAVUORI, E.; TAHOKOKORPI, M.; TAULAVUORI, K.; LAINE, K. Anthocyanins an glutathione S-transferase activities in response to low temperature an frost hardening in Vaccinium myrtillus L. Journal of Plant Physiology, Jena, v.161, p.903-911, 2004.
- VITRAC, X.; LARRONDE, F.; KRISA, S.; DECENDIT, A.; DEFFIEUX, G.; MÉRILLON, J.M. Sugar sensing and Ca2+-calmodulin requirement in Vitis vinifera cells producing anthocyanins. Phytochemistry, Madison, v.53, p.659-665, 2000.
- WU, J.; OMOKAWA, H.; HATZIOS, K.K. Glutathione S-transferase activity in unsafened and fenclorim-safened rice (Oryza sativa). Pesticide Biochemistry and Physiology, Amsterdam, v.54, p.220-229, 1996.
- YAMAMOTO, L.Y.; ASSIS, A.M.; ROBERTO, S.R.; BOVOLENTA, Y.R.; NIXDORF, S.L.; GARCÍA-ROMERO, E.; GÓMEZ-ALONSO, S.; HERMOSÍN-GUTIÉRREZ, I. Application of abscisic acid (S-ABA) to cv. Isabel grapes (Vitis vinifera×Vitis labrusca) for color improvement: Effects on color, phenolic composition and antioxidant capacity of their grape juice. Food Research International, Toronto, v.77, p.572-583, 2015.
- YU, X.; WANG, X.; ZHANG, W.; QIAN, T.; TANG, G.; GUO, Y.; ZHENG, C. Antisense suppression of an acid invertase gene (MAI1) in muskmelon alters plant growth and fruit development. Journal of Experimental Botany, Lancaster, v.59, p.2969-2977, 2008.
Publication Dates
-
Publication in this collection
2018
History
-
Received
11 Nov 2016 -
Accepted
24 Apr 2017