Abstract
Chagas disease is a major neglected tropical disease caused by Trypanosoma cruzi. It has been treated with the antiparasitic drugs nifurtimox and benzonidazole, which cause several side effects. The market for natural products has considerably grown lately and the use of plants has become an alternative to the development of novel drugs to cure the disease. Therefore, this study aimed at describing the chemical analysis of the essential oil from green fruits of Protium ovatum and evaluating their trypanocidal and cytotoxic potential. The essential oil was obtained by Clevenger hydrodistillation whereas its chemical composition was determined by gas chromatography coupled to mass spectrometry (GC-MS). The major compounds found in the essential oil from green fruits of P. ovatum were ß-myrcene (62.0 %), a-pinene (11.3 %) and limonene (7.3 %). To the best of our knowledge, this was the first time that the chemical composition of the essential oil from green fruits of P. ovatum was described. Results showed that the essential oil had strong trypanocidal activity against trypomastigote forms of theY strain of Trypanosoma cruzi (IC50 = 1.2 µg/mL). In addition, the essential oil from green fruits of P. ovatum did not display cytotoxicity against LLCMK2 adherent epithelial cell at the concentration range under analysis (CC50 = 550.3 µg/mL). As a result, it is an excellent option for the development of novel antiparasitic drugs.
Index terms
Protium ovatum; ß-myrcene; fruits; essential oil; Trypanosoma cruzi; cytotoxic analysis
Resumo
A doença de Chagas é uma das principais doenças tropicais negligenciadas causadas pelo Trypanosoma cruzi, e em seu tratamento utilizam-se medicamentos como o nifurtimox e o benzonidazol, que causam vários efeitos colaterais. O mercado de produtos naturais tem aumentado consideravelmente nos últimos anos, e o uso das plantas continua sendo uma alternativa para o desenvolvimento de novos medicamentos para cura de doenças. Portanto, este estudo aborda a composição química do óleo essencial dos frutos verdes de Protium ovatum e a avaliação de seus potenciais tripanocida e citotóxico. O óleo essencial foi obtido por hidrodestilação, utilizando o aparato do tipo Clevenger. A composição química foi determinada por cromatografia gasosa acoplada ao espectrômetro de massas (CG-EM). Os principais compostos encontrados no óleo essencial dos frutos verdes de P. ovatum foram: ß-mirceno (62,0 %), a-pineno (11,3 %) e limoneno (7,3 %). Este é o primeiro relato da composição química do óleo essencial obtido a partir de frutos verdes de P. ovatum. Os resultados mostraram que o óleo essencial analisado apresenta forte atividade tripanocida contra as formas tripomastigota da cepa Y do Trypanosoma cruzi (IC50 = 1,2 µg/mL). O óleo essencial exibiu ainda moderada citotoxidade frente à linhagem LLCMK2 na concentração avaliada (CC50 = 550,3 µg/mL). Em suma, o óleo essencial dos frutos verdes de P. ovatum pode ser considerado uma fonte alternativa para o desenvolvimento de novos medicamentos antiparasitários.
Termos para indexação
Protium ovatum; ß-mirceno; frutos; óleo essencial; Trypanosoma cruzi; análise citotóxica
Introduction
American trypanosomiasis, also known as Chagas disease (CD), is a neglected tropical disease which is endemic in Latin America. The World Health Organization estimates that approximately 6-7 million Latin Americans have been infected with Trypanosoma cruzi since CD has been mainly found in endemic areas of Latin American countries (PAULA et al. 2015 PAULA, J.C.; DESOTI, V.C.; SAMPIRON, E.G.; MARTINS, S.C.; UEDA-NAKAMURA, T.; RIBEIRO, S.M.; BIANCO, E.M.; SILVA, S.O.; OLIVEIRA, G.G.; NAKAMURA, C.V. Trypanocidal activity of organic extracts from the Brazilian and Spanish marine sponges. Revista Brasileira de Farmacognosia, Curitiba, v.25, n.6, p.651-656, 2015. ).
The disease has been treated with drugs which display either high toxicity against the host’s body or low efficiency against the pathogen (IZUMI et al. 2012 IZUMI, E.; UEDA-NAKAMURA, T.; VEIGA-JUNIOR, V.F.; PINTO, A.C.; NAKAMURA, C.V. Terpenes from Copaifera demonstrated in vitro antiparasitic and synergic activity. Journal of Medicinal Chemistry, Washington, v.55, n.07, p.2994-3001, 2012. ).
So far, no vaccine has been developed for CD and the current treatment has been conducted with nifurtimox (Nf) or benzonidazole (Bz). However, their well-known toxicity, as well as their limited effect on different parasite isolates and on the chronic phase of the disease, has called for the development of new drugs to treat it (SOEIRO; CASTRO, 2011 SOEIRO, M.N.C.; CASTRO, S.L. Screening of potential anti-Trypanosoma Cruzi candidates: in vitro and in vivo studies. Opening Medicinal Chemistry Journal, Sharjah, v.5, p.21-30, 2011. ).
The market for natural products, such as extracts, isolated compounds and essential oils from plants, and the use of plants as an alternative to the development of novel drugs for the treatment of various diseases, including CD, have annually increased in view of the great potential of these compounds (AFFONSO et al. 2012 AFFONSO, R.S.; RENNÓ, M.N.; SLANA, G.B.C.A.; FRANÇA, T.C.C. Aspectos químicos e biológicos do óleo essencial de cravo da índia. Revista Virtual de Quimica, Niterói, v.4, n.2, p.146-161, 2012. ; LEITE et al. 2010 LEITE, A.C.; NETO, A.P.; AMBROZIN, A.R.P.; FERNANDES, J.B.; VIEIRA, P.C.; SILVA, M.F.G.F.; ALBUQUERQUE, S. Trypanocidal activity of flavonoids and limonoids isolated from Myrsinaceae and Meliaceae active plant extracts. Revista Brasileira de Farmacognosia, Curitiba, v.20, n.1, p.1-6, 2010. ). The Cerrado (Brazilian savannah), a natural heritage site owing to its diversity and endemism of biological species, is an important source of novel natural substances with different biological properties (SILVA et al. 2015 SILVA, A.F.; RABELO, M.F.R.; ENOQUE, M.M. Diversidade de angiospermas e espécies medicinais de uma área de Cerrado. Revista Brasileira de Plantas Medicinais, Campinas, v.17, n.4, p.1016-1030, 2015. ).
Essential oils from different plant sources carry out several biological activities, such as antibacterial, anticancer, anti-inflammatory, antimutagenic, antifungal, antioxidant and antiprotozoal ones. As a result, the vast arsenal of bioactive compounds found in essential oils has increasingly attracted researchers’ intense attention in the last years (CARNEIRO et al. 2017 CARNEIRO, N.S.; ALVES, J.M.; ALVES, C.C.F.; ESPERANDIM, V.R.; MIRANDA, M.L.D. Óleo essencial das flores de Eugenia klotzschiana (Myrtaceae): composição química e atividades tripanocida e citotóxica in vitro. Revista Virtual de Química, Niterói, v.9, n.3, p.1381-1392, 2017. ).
The Burseraceae family, for example, comprises 21 genera with 600 species and the genus Protium is its main family member with 135 species. In the literature, species of the Burseraceae family have been described as the ones which are commonly used for treating wounds and ulcers.
Besides, they act as anti-inflamatory and repellent agents.
Triterpenes, mono- and sesquiterpenes from species of the genus Protium are well-known for their significant biological properties and their anti-inflammatory and acaricidal activities (MORAES et al. 2013 MORAES, M.M.; CAMARA, C.A.G.; RAMOS, C.S. Seasonal variation in the essential oil of Protium bahianum Daly (Burseraceae). Journal of Essential Oil Bearing Plants, Abingdon, v.16, p.300-307, 2013. ).
Protium ovatum Engl. is a herbaceous plant found in the Brazilian Cerrado. In the literature, several studies have described the anti-inflammatory, antinociceptive, immunostimulant and anticancer properties of resins (SIANI et al. 2011 SIANI, A.C.; RAMOS, M.F.; MONTEIRO, S.S.; DOS SANTOS, R.R.; SOARES, R.O.A. Essential oils of the oleoresins from Protium heptaphyllum growing in the Brazilian southeastern and their cytotoxicity to neoplasic cell lines. Journal of Essential Oil Bearing Plants, Abingdon, v.14, p.373-378, 2011. ). However, reports have described neither the chemical composition nor the trypanocidal potential and cytotoxicity of the essential oil from specimens of Protium ovatum.
This study aims at describing the chemical composition of the essential oil from green fruits of P. ovatum, its in vitro activities against trypomastigote forms of Trypanosoma cruzi and its cytotoxic activity against LLCMK2 adherent epithelial cells.
Material and Methods
Green fruits of Protium ovatum were collected in Rio Verde, GO, Brazil, in September 2015. The plant was identified by the botanist Erika Amaral (Instituto Federal de Educação, Ciência e Tecnologia Goiano, Campus Rio Verde, GO, Brazil). A voucher specimen (no. HJ 7420) was deposited at the Herbarium Jataiense Professor Germano Guarin Neto, which belongs to the Instituto Federal Goiano, Brazil.
The essential oil was extracted from fresh green fruits of Protium ovatum (100 g) by a modified clevengertype apparatus and hydrodistillation for 2 h. The oil was separated and dried over anhydrous sodium sulfate, stored in hermetically sealed glass containers and kept under refrigeration at 5 °C until analysis and trypanocidal and cytotoxicity assays. Total oil yield was expressed as a percentage value (g/100 g of fresh plant material). All experiments were carried out in triplicate.
Gas chromatography – mass spectrometry (GCMS) analysis was carried out by a Shimadzu QP2010 with an AOC-20i auto-injector and a DB-5MS column (30 m x 0.25 mm, 0.25 mm in thickness). The carrier gas was He with pressure of 57.4 kPa and flow rate of 1.00 mL/ min. The split ratio was 1/30, the injector temperature was 250 °C and the injected volume was 1 μL. Temperature programming was the following : 60 – 240 °C, increasing 3 °C/min. MS were recorded on the electron ionization (EI) mode, with ionization energy of 70 eV (scan time: 2 scans/s). Identification of the constituents was based on the retention indices (the calculation used from C9 to C22 alkanes) and on the comparison of the mass spectra with libraries (Wiley 7 and Nist 62) and references to previously published data (ADAMS, 2007 ADAMS R.P. Identification of essential oil components by gas chromato-graphy/mass spectroscopy. Illinois: Allured Publishing, 2007. ).
1D- 1H- and 13C-NMR spectroscopic data were recorded at room temperature in CDCl3 (Cambridge Isotope Laboratories, Andover, MA, USA) by a Bruker DPX-300 spectrometer (Karlhue, Germany) operating at 300 MHz (1 H)/75 MHz (13C). Standard pulse sequences were used for homo- and heteronuclear correlation experiments. Chemical shifts are reported in ppm, using TMS as an internal standard (δ = 0 ppm) whereas coupling constants (J) are expressed in Hertz.
To obtain the trypomastigotes of T. cruzi, LLCMK2 cells were cultured in RPMI medium supplemented with 2 x 10-6 mol/L L-glutamine, 10-5 mol/L NaHCO3, 100 U/mL penicillin, 100 μg/mL streptomycin and 10 % inactivated fetal bovine serum. The procedure was accomplished in culture bottles at 37 °C, under 5 % ambient CO2 and relative humidity of 95 %. The trypomastigote forms were maintained in RPMI medium and the parasites were transferred to fresh medium every 48 h to furnish free parasite forms. The assay conducted after 24 h was based on the methodology reported by Esperandim et al. (2013) ESPERANDIM, V.R.; FERREIRA, D.S.; RESENDE, K.C.S.; MAGALHÃES, L.G.; SOUZA, J.M.; PAULETTI, P.M.; JANUÁRIO, P.M.; LAURENTZ, R.S.; BASTOS, J.K.; SÍMARO, G.V.; CUNHA, W.R.; SILVA, M.L.A. In vitro antiparasitic activity and chemical composition of the essential oil obtained from the fruits of Piper cubeba. Planta Medica, New York, v.79, p.1653-1655, 2013. .
Approximately 1 x 106 trypomastigotes was added to each well in a 96-well microtiter plate. Then, the essential oil was added at concentrations ranging from 12.5 to 200 μg/mL. After 24 h incubation, the biological activity of the samples was evaluated by the colorimetric MTT tetrazolium salt assay (MTT = 3-(4,5-dimethylthiazol- 2-yl)-2,5-diphenyltetrazolium bromide) (5 mg/mL).
Readings were conducted by a microplate reader at 517 nm wavelength. Positive and negative controls were benzonidazole (from 12.5 to 200 μg/mL) and 0.5 % dimethyl sulfoxide (DMSO), respectively. Assays were performed in triplicate.
LLCMK2 adherent epithelial cells were grown in RPMI 1640 medium supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin and 5 % inactivated fetal calf serum. They were kept at 37 °C in 5 % CO2.
A cell suspension was seeded at a concentration of 1 x 106 cells/mL in a 96-well microplate with RPMI 1640 medium. Thereafter, cells were treated with essential oil at different concentrations (6.25, 12.5, 25, 50, 100, 200 and 400 μg/mL). Plates were incubated at 37 °C for 24 h and the biological activity was evaluated by the MTT colorimetric method [MTT; 3-(4,5-dimethylthiazol-2- yl)-2,5- diphenyltetrazolium bromide] in a microplate reader at 540 nm. RPMI 1640 medium was the positive control whereas DMSO and RPMI 1640 media were the negative ones. All experiments were performed in triplicate. The percentage of cell viability was determined by the following formula: % cell viability = 1 - [(Y-N)/ (N-P)] x 100, where Y = absorbance of wells containing cells and essential oil at different concentrations; N = negative control; and P = positive control (ESPERANDIM et al. 2013 ESPERANDIM, V.R.; FERREIRA, D.S.; RESENDE, K.C.S.; MAGALHÃES, L.G.; SOUZA, J.M.; PAULETTI, P.M.; JANUÁRIO, P.M.; LAURENTZ, R.S.; BASTOS, J.K.; SÍMARO, G.V.; CUNHA, W.R.; SILVA, M.L.A. In vitro antiparasitic activity and chemical composition of the essential oil obtained from the fruits of Piper cubeba. Planta Medica, New York, v.79, p.1653-1655, 2013. ).
Results and Discussion
The hydrodistillation of green fruits of P. ovatum gave colorless oil with a very strong and fragrant odor.
The percentage of oil yielded from the plant was 0.5 % by weight. Altogether, 13 compounds at concentrations above 0.1 % were identified, accounting for 95.0 % of the total oil composition, which was characterized by the predominance of terpenes: β-pinene (5.5 %), sabinene (5.0 %), p-cimene (0.3 %), pirillene (0.4 %), pinocaryone (0.2 %), terpinen-4-ol (0.2 %), borneol (0.2 %), α-copaene (0.3 %), E-caryophyllene (2.0 %) and α-humulene (0.3 %). Their major components were β-myrcene (62.0 %), α-pinene (11.3 %) and limonene (7.3 %) (Table 1).
β-myrcene, α-pinene and limonene (Figure 1), which are the three major components of the essential oil from green fruits of P. ovatum, were found to be among the constituents of the oils from other species of the same genus (ZOGHBI et al. 2005 ZOGHBI, M.G.B.; ANDRADE, E.H.A.; LIMA, M.P.; SILVA, T.M.D.; DALY, D.C. The essential oils of five species of Protium growing in the North of Brazil. Journal of Essential Oil Bearing Plants, Abingdon, v.8, p.312-317, 2005. ). In previous studies of the efficiency of the essential oil from leaves of P. ovatum, the result was 0.10 % and a complex mixture of terpene constituents was determined by 1H NMR, 13C NMR and IR (CASTELO et al. 2010 CASTELO, A.V.M.; MENEZZI, C.H.S.D.; RESCK, I.S. Yield and spectroscopic analysis (1H, 13C NMR; IR) of essential oils from four plants of the Brazilian Savannah. Cerne, Lavras, v.16, n.4, p.573-584, 2010. ). Other species of Protium were analyzed and a complex mixture of monoterpenes and sesquiterpenes were determined in essential oils from resins, foliar rachises, branches and leaves (CARVALHO et al. 2013 CARVALHO, L.E.; MAGALHÃES, L.A.M.; LIMA, M.P.; MARQUES, M.O.M.; FACANALI, R. Essential oils of Protium of the Adolpho Ducke forest reserve: Protium crassipetalum, P. heptaphyllum subs. ulei, P. pilosissimum and P. polybotryum. Journal of Essential Oil Bearing Plants, Abingdon, v.16, n.4, p.551-554, 2013. ;PINTO et al. 2010 PINTO, D.S.; CARVALHO, L.E.; LIMA, M.P.; MARQUES, M.O.M.; FACANALI, R.; RIBEIRO, J.E.L.S. Volatiles of foliar rachis, branches and resin elicited by insects from Protium hebetatum grows wild in Amazon. Journal of Essential Oil Bearing Plants, Abingdon, v.13, p.699-703, 2010. ; CARVALHO et al. 2010 CARVALHO, L.E.; PINTO, D.S.; MAGALHÃES, L.A.M.; LIMA, M.P.; MARQUES, M.O.M.; FACANALI, R. Chemical constituents of essential oil of Protium decandrum (Burseraceae) from western Amazon. Journal of Essential Oil Bearing Plants, Abingdon, v.13, n.2, p.181-184, 2010. ).
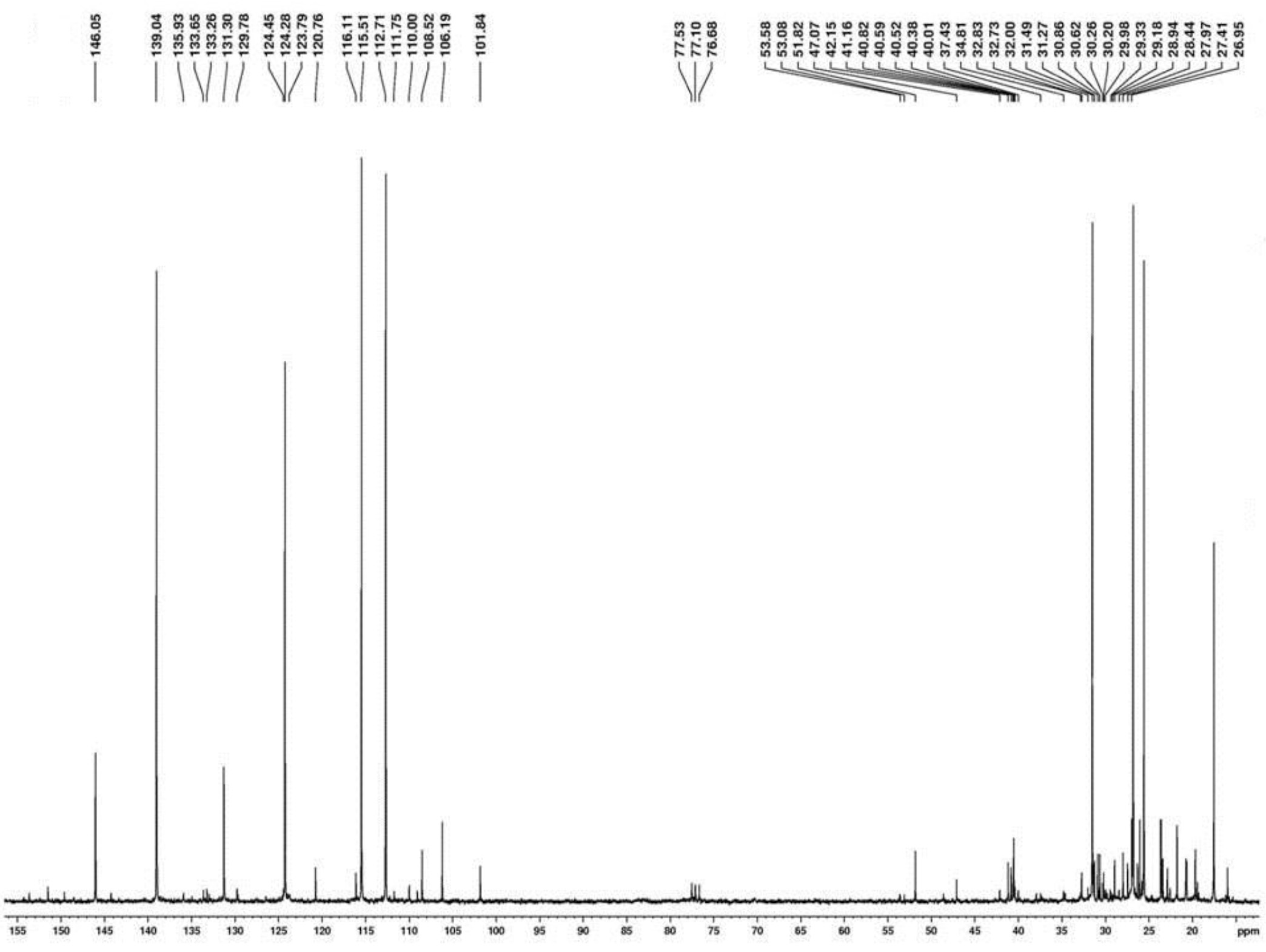
The identification of β-myrcene (62.0 %) in the mixture, the main substance found in the essential oil from fruits of Protium ovatum, was carried out by the analysis of 1H (Figure 2) and 13C (Figure 3) NMR spectra and by comparison with literature data (PESSINI et al. 2005 PESSINI, G.L.; DIAS FILHO, B.P.; NAKAMURA, C.V.; FERREIRA, A.G.; CORTEZ, D.A.G. Neolignanas and the analysis of the essential oil of Piper regnelli (Miq.) C. DC. var. pallescens (C. DC.) Yunck leaves. Revista Brasileira de Farmacognosia, Curitiba, v.15, n.3, p.199-204, 2005. ). The spectroscopic data on β-myrcene are as follows: β-myrcene: NMR 1H (300 MHz, CDCl3): δ 6.35 (dd, J = 10.8 e 17.7 Hz, H-4); 5.43-4.60 (m, H-7, H-9 and H-10); 2.24-2.02 (m, H-5 and H-6); 1.69 (s, H-1); 1.64 (s, H-2). NMR 13C (75 MHz, CDCl3): δ 146.1 (C, C-8); 139.0 (CH, C-9); 131.3 (C, C-3); 124.2 (CH, C-4); 115.5 (CH2, C-7); 112.7 (CH2, C-10); 31.4 (CH2, C-6); 26.9 (CH2, C-5); 25.7 (CH3, C-1) and 17.7 (CH3, C-2).
The essential oil from green fruits of P. ovatum has high trypanocidal activity against trypomastigotes of Trypanosoma cruzi. Increased infeasibility of trypomastigote cells was observed with increasing concentration of essential oil. High activity was obtained at IC50 = 1.2 μg/mL (Table 2) and it was lower than the one of the positive control with benzonidazole, which was IC50 = 9.8 μg/mL.
The literature has reported that samples with trypanocidal activity of IC50 < 10 μg/mL, IC50 > 50 < 100 μg/mL and IC50 > 100 μg/mL are considered highly active, active/moderately active and inactive, respectively (ALVES et al. 2012 ALVES, R.T.; REGASINI, L.O.; FUNARI, C.S.; YOUNG, M.C.M.; RIMOLDI, A.; BOLZANI, V.S.; SILVA, D.H.S.; ALBUQUERQUE, S.; ROSA, J.A. Trypanocidal activity of Brazilian plants against epimastigote forms from Y and Bolivia strains of Trypanosoma cruzi. Revista Brasileira de Farmacognosia, Curitiba, v.22, p.528-533, 2012. ). The trypanocidal properties of the major components of the essential oil from green fruits P. ovatum, β-myrcene (62.0 %), α-pinene (11.3 %) and limonene (7.3 %) were previously reported (SANTOS et al. 2014 SANTOS, N.N.; MENEZES, L.R.; DOS SANTOS, J.A.; MEIRA, C.S.; GUIMARHES, E.T.; SOARES, M.B.; NEPEL, A.; BARISONE, A.; COSTA, E.V. A new source of (R)-limonene and rotundifolone from leaves of Lippia pedunculosa (Verbenaceae) and their trypanocidal properties. Natural Product Communications, Westerville, v.9, p.737-739, 2014. ; SARTORELLI et al. 2012 SARTORELLI, P.; SANTANA, J.S.; GUADAGNIN, R.C.; LAGO, J.H.G.; PINTO, E.G.; TEMPONE, A.G.; STEFANI, H.A.; SOARES, M.G.; SILVA, A.M. In vitro trypanocidal evaluation of pinane derivatives from essential oils of ripe fruits from Schinus terebinthifolius Raddi (Anacardiaceae). Química Nova, São Paulo, v.35, p.743-747, 2012. ; ZENG et al. 2010 ZENG, Q.I.; JIN, H.Z.; QIN, J.J.; FU, J.J.; HU, X.J.; LIU, J.H.; YAN, L.; CHEN, M.; ZHANG, W.D. Chemical constituents of plants from the genus Dracocephalum. Chemistry &Biodiversity, Zurich, v.7, p.1911-1929, 2010. ).
It was proposed that the activity of essential oils against trypanosomatids is mainly due to its terpene composition.
Terpenes are responsible for the hydrophobic character of essential oils, thus allowing their diffusion through the parasite cell membrane and affecting intracellular metabolic pathways and organelles (BORGES et al. 2012 BORGES, A.R.; AIRES, J.R.A.; HIGINO, T.M.M.; MEDEIROS, M.G.F.; CITÓ, A.M.G.L.; LOPES, J.A.D.; FIGUEIREDO, R.C.B.Q. Trypanocidal and cytotoxic activities of essential oils from medicinal plants of northeast of Brazil. Experimental Parasitology, Amsterdam v.132, p.123-128, 2012. ).
This is the first report of the trypanocidal activity of the essential oil from green fruits of Protium ovatum.
Despite great advances made by modern medicine in recent decades, plants are still considered very important in regard to health care (CALIXTO, 2000 CALIXTO, J.B. Efficacy, safety, quality control, marketing and regulatory guidelines for herbal medicines (phytotherapeutic agents). Brazillian Journal of Medical and Biological Research, Ribeirão Preto, v.33, n.2, p.179-189, 2000. ). Several studies of essential oils have shown that some plants have trypanocidal activity against T. cruzi (BALDISSERA et al. 2013 BALDISSERA, M.D.; SILVA, A.S.; OLIVEIRA, C.B.; ZIMMERMANN, C.E.P.; VAUCHER, R.A.; SANTOS, R.C.V.; RECH, V.C.; TONIN, A.A.; GIONGO, J.L.; MATOS, C.B.; KOESTER, L.; SANTURIO, J.M.; MONTEIRO, S.G. Trypanocidal activity of the essential oils in their conventional and nanoemulsion forms: in vitro tests. Experimental Parasitology, Amsterdam, v.134, n.3, p.356-361, 2013. ; ESCOBAR et al. 2010 ESCOBAR, P.; LEAL, S.M.; HERRERA, L.V.; MARTINEZ, J.R.; STASHENKO, E. Chemical composition and antiprotozoal activities of Colombian Lippia spp essential oils and their major components. Memórias do Instituto Oswaldo Cruz, Rio de Janeiro, v.105, p.184-190, 2010. ).
Cultures of LLCMK2 adherent epithelial cells were treated with essential oil at concentrations of 6.25, 12.5, 25.0, 50.0, 100, 200 and 400 μg/mL for 24 h. Results showed that the essential from green fruits did not have toxicity at the concentration evaluated with CC50 550.3 μg/ mL (Table 3), by comparison with benzonidazole positive control with CC50 147.3 μg/mL.
It is important to point out that the essential oil from green fruits of P. ovatum did not display cytotoxicity against adherent epithelial cells at the concentration range under analysis. There is evidence that, owing to their lipid solubility, essential oils have low density and rapid diffusion across cell membranes. As a result, they could damage the parasite cell membrane structure and lead to cellular lysis (ANTHONY et al. 2005 ANTHONY, J.P.; FYFE, L.; SMITH, H. Plant active components – a resource for antiparasitic agents? Trends Parasitology, Cambridge, v.21, n.10, p.462-468, 2005. ). In addition, there could be synergistic and/or additive effects from constituents of the essential oil (CARNEIRO et al. 2017 CARNEIRO, N.S.; ALVES, J.M.; ALVES, C.C.F.; ESPERANDIM, V.R.; MIRANDA, M.L.D. Óleo essencial das flores de Eugenia klotzschiana (Myrtaceae): composição química e atividades tripanocida e citotóxica in vitro. Revista Virtual de Química, Niterói, v.9, n.3, p.1381-1392, 2017. ).
Structures of main constituents of the essential oil from green fruits of P. ovatum: (1) ß-myrcene; (2) a-pinene and (3) limonene.
Conclusion
In summary, the results of this study showed that the essential oil from green fruits Protium ovatum found in the Brazilian Cerrado, which is located in the central-west region of the country, has promising antiparasitic potential with no cytotoxicity towards LLCMK2 adherent epithelial cells. The high concentration of β-myrcene (62.0 %) in the essential oil from green fruits investigated by this study is a prospect of a new source of this secondary metabolite as a raw material in the synthesis of new medication.
Further studies with in vivo and field experiments must be carried out to ascertain its efficiency. However, to the best of our knowledge, this was the first time that the chemical composition of the essential oil from green fruits of P. ovatum was described. It is very important to the knowledge of this botanical species.
Acknowledgments
The authors would like to thank FAPEG, CNPq, IFGOIANO – Campus Rio Verde and CAPES for the financial support.
- ADAMS R.P. Identification of essential oil components by gas chromato-graphy/mass spectroscopy. Illinois: Allured Publishing, 2007.
- AFFONSO, R.S.; RENNÓ, M.N.; SLANA, G.B.C.A.; FRANÇA, T.C.C. Aspectos químicos e biológicos do óleo essencial de cravo da índia. Revista Virtual de Quimica, Niterói, v.4, n.2, p.146-161, 2012.
- ALVES, R.T.; REGASINI, L.O.; FUNARI, C.S.; YOUNG, M.C.M.; RIMOLDI, A.; BOLZANI, V.S.; SILVA, D.H.S.; ALBUQUERQUE, S.; ROSA, J.A. Trypanocidal activity of Brazilian plants against epimastigote forms from Y and Bolivia strains of Trypanosoma cruzi. Revista Brasileira de Farmacognosia, Curitiba, v.22, p.528-533, 2012.
- ANTHONY, J.P.; FYFE, L.; SMITH, H. Plant active components – a resource for antiparasitic agents? Trends Parasitology, Cambridge, v.21, n.10, p.462-468, 2005.
- BALDISSERA, M.D.; SILVA, A.S.; OLIVEIRA, C.B.; ZIMMERMANN, C.E.P.; VAUCHER, R.A.; SANTOS, R.C.V.; RECH, V.C.; TONIN, A.A.; GIONGO, J.L.; MATOS, C.B.; KOESTER, L.; SANTURIO, J.M.; MONTEIRO, S.G. Trypanocidal activity of the essential oils in their conventional and nanoemulsion forms: in vitro tests. Experimental Parasitology, Amsterdam, v.134, n.3, p.356-361, 2013.
- BORGES, A.R.; AIRES, J.R.A.; HIGINO, T.M.M.; MEDEIROS, M.G.F.; CITÓ, A.M.G.L.; LOPES, J.A.D.; FIGUEIREDO, R.C.B.Q. Trypanocidal and cytotoxic activities of essential oils from medicinal plants of northeast of Brazil. Experimental Parasitology, Amsterdam v.132, p.123-128, 2012.
- CALIXTO, J.B. Efficacy, safety, quality control, marketing and regulatory guidelines for herbal medicines (phytotherapeutic agents). Brazillian Journal of Medical and Biological Research, Ribeirão Preto, v.33, n.2, p.179-189, 2000.
- CARNEIRO, N.S.; ALVES, J.M.; ALVES, C.C.F.; ESPERANDIM, V.R.; MIRANDA, M.L.D. Óleo essencial das flores de Eugenia klotzschiana (Myrtaceae): composição química e atividades tripanocida e citotóxica in vitro. Revista Virtual de Química, Niterói, v.9, n.3, p.1381-1392, 2017.
- CARVALHO, L.E.; MAGALHÃES, L.A.M.; LIMA, M.P.; MARQUES, M.O.M.; FACANALI, R. Essential oils of Protium of the Adolpho Ducke forest reserve: Protium crassipetalum, P. heptaphyllum subs. ulei, P. pilosissimum and P. polybotryum. Journal of Essential Oil Bearing Plants, Abingdon, v.16, n.4, p.551-554, 2013.
- CARVALHO, L.E.; PINTO, D.S.; MAGALHÃES, L.A.M.; LIMA, M.P.; MARQUES, M.O.M.; FACANALI, R. Chemical constituents of essential oil of Protium decandrum (Burseraceae) from western Amazon. Journal of Essential Oil Bearing Plants, Abingdon, v.13, n.2, p.181-184, 2010.
- CASTELO, A.V.M.; MENEZZI, C.H.S.D.; RESCK, I.S. Yield and spectroscopic analysis (1H, 13C NMR; IR) of essential oils from four plants of the Brazilian Savannah. Cerne, Lavras, v.16, n.4, p.573-584, 2010.
- ESCOBAR, P.; LEAL, S.M.; HERRERA, L.V.; MARTINEZ, J.R.; STASHENKO, E. Chemical composition and antiprotozoal activities of Colombian Lippia spp essential oils and their major components. Memórias do Instituto Oswaldo Cruz, Rio de Janeiro, v.105, p.184-190, 2010.
- ESPERANDIM, V.R.; FERREIRA, D.S.; RESENDE, K.C.S.; MAGALHÃES, L.G.; SOUZA, J.M.; PAULETTI, P.M.; JANUÁRIO, P.M.; LAURENTZ, R.S.; BASTOS, J.K.; SÍMARO, G.V.; CUNHA, W.R.; SILVA, M.L.A. In vitro antiparasitic activity and chemical composition of the essential oil obtained from the fruits of Piper cubeba. Planta Medica, New York, v.79, p.1653-1655, 2013.
- IZUMI, E.; UEDA-NAKAMURA, T.; VEIGA-JUNIOR, V.F.; PINTO, A.C.; NAKAMURA, C.V. Terpenes from Copaifera demonstrated in vitro antiparasitic and synergic activity. Journal of Medicinal Chemistry, Washington, v.55, n.07, p.2994-3001, 2012.
- LEITE, A.C.; NETO, A.P.; AMBROZIN, A.R.P.; FERNANDES, J.B.; VIEIRA, P.C.; SILVA, M.F.G.F.; ALBUQUERQUE, S. Trypanocidal activity of flavonoids and limonoids isolated from Myrsinaceae and Meliaceae active plant extracts. Revista Brasileira de Farmacognosia, Curitiba, v.20, n.1, p.1-6, 2010.
- MORAES, M.M.; CAMARA, C.A.G.; RAMOS, C.S. Seasonal variation in the essential oil of Protium bahianum Daly (Burseraceae). Journal of Essential Oil Bearing Plants, Abingdon, v.16, p.300-307, 2013.
- PAULA, J.C.; DESOTI, V.C.; SAMPIRON, E.G.; MARTINS, S.C.; UEDA-NAKAMURA, T.; RIBEIRO, S.M.; BIANCO, E.M.; SILVA, S.O.; OLIVEIRA, G.G.; NAKAMURA, C.V. Trypanocidal activity of organic extracts from the Brazilian and Spanish marine sponges. Revista Brasileira de Farmacognosia, Curitiba, v.25, n.6, p.651-656, 2015.
- PESSINI, G.L.; DIAS FILHO, B.P.; NAKAMURA, C.V.; FERREIRA, A.G.; CORTEZ, D.A.G. Neolignanas and the analysis of the essential oil of Piper regnelli (Miq.) C. DC. var. pallescens (C. DC.) Yunck leaves. Revista Brasileira de Farmacognosia, Curitiba, v.15, n.3, p.199-204, 2005.
- PINTO, D.S.; CARVALHO, L.E.; LIMA, M.P.; MARQUES, M.O.M.; FACANALI, R.; RIBEIRO, J.E.L.S. Volatiles of foliar rachis, branches and resin elicited by insects from Protium hebetatum grows wild in Amazon. Journal of Essential Oil Bearing Plants, Abingdon, v.13, p.699-703, 2010.
- SANTOS, N.N.; MENEZES, L.R.; DOS SANTOS, J.A.; MEIRA, C.S.; GUIMARHES, E.T.; SOARES, M.B.; NEPEL, A.; BARISONE, A.; COSTA, E.V. A new source of (R)-limonene and rotundifolone from leaves of Lippia pedunculosa (Verbenaceae) and their trypanocidal properties. Natural Product Communications, Westerville, v.9, p.737-739, 2014.
- SARTORELLI, P.; SANTANA, J.S.; GUADAGNIN, R.C.; LAGO, J.H.G.; PINTO, E.G.; TEMPONE, A.G.; STEFANI, H.A.; SOARES, M.G.; SILVA, A.M. In vitro trypanocidal evaluation of pinane derivatives from essential oils of ripe fruits from Schinus terebinthifolius Raddi (Anacardiaceae). Química Nova, São Paulo, v.35, p.743-747, 2012.
- SIANI, A.C.; RAMOS, M.F.; MONTEIRO, S.S.; DOS SANTOS, R.R.; SOARES, R.O.A. Essential oils of the oleoresins from Protium heptaphyllum growing in the Brazilian southeastern and their cytotoxicity to neoplasic cell lines. Journal of Essential Oil Bearing Plants, Abingdon, v.14, p.373-378, 2011.
- SILVA, A.F.; RABELO, M.F.R.; ENOQUE, M.M. Diversidade de angiospermas e espécies medicinais de uma área de Cerrado. Revista Brasileira de Plantas Medicinais, Campinas, v.17, n.4, p.1016-1030, 2015.
- SOEIRO, M.N.C.; CASTRO, S.L. Screening of potential anti-Trypanosoma Cruzi candidates: in vitro and in vivo studies. Opening Medicinal Chemistry Journal, Sharjah, v.5, p.21-30, 2011.
- ZENG, Q.I.; JIN, H.Z.; QIN, J.J.; FU, J.J.; HU, X.J.; LIU, J.H.; YAN, L.; CHEN, M.; ZHANG, W.D. Chemical constituents of plants from the genus Dracocephalum. Chemistry &Biodiversity, Zurich, v.7, p.1911-1929, 2010.
- ZOGHBI, M.G.B.; ANDRADE, E.H.A.; LIMA, M.P.; SILVA, T.M.D.; DALY, D.C. The essential oils of five species of Protium growing in the North of Brazil. Journal of Essential Oil Bearing Plants, Abingdon, v.8, p.312-317, 2005.
Publication Dates
-
Publication in this collection
2018
History
-
Received
27 Oct 2016 -
Accepted
11 July 2017