Abstracts
OBJECTIVE: To evaluate the bone mineral density variability in patients with systemic lupus erythematosus, before the beginning and at 30 and 60 days from the beginning of glucocorticoid therapy. MATERIALS AND METHODS: Fifteen premenopausal women with systemic lupus erythematosus were referred for bone densitometry before being submitted to glucocorticoid therapy. RESULTS: It was demonstrated that the bone mineral density in g/cm² in the lumbar spine presented a significant decrease in the span between the first analysis previously to the beginning of the glucocorticoid therapy and the third analysis after 60 days under therapy. On the other hand no significant difference was found in the femoral neck. CONCLUSION: The authors conclude that, at short term, rather than only at long term as previously reported by other authors, corticotherapy in patients with systemic lupus erythematosus results in a decrease in the bone mineral density in the lumbar spine.
Bone densitometry; Premenopause; Systemic lupus erythematosus
OBJETIVO: Avaliar a variabilidade da densidade mineral óssea em pacientes com lúpus eritematoso sistêmico, antes do início e após 30 e 60 dias do início de glicocorticoterapia. MATERIAIS E MÉTODOS: Estudo efetuado em 15 mulheres em pré-menopausa com lúpus eritematoso sistêmico encaminhadas para realização de densitometria óssea e que fariam uso de corticosteróides logo após o exame. RESULTADOS: Demonstrou-se que a densidade mineral óssea, em g/cm², na coluna lombar reduziu-se significativamente entre a análise prévia ao uso e a análise em 60 dias de uso da medicação, mas no colo femoral não houve diferença significativa. CONCLUSÃO: Concluiu-se que o uso de corticoterapia em pacientes com lúpus eritematoso sistêmico, em curto prazo, reduz significativamente a densidade mineral óssea na coluna lombar, não somente em longo prazo como descrito anteriormente por outros autores.
Densitometria óssea; Pré-menopausa; Lúpus eritematoso sistêmico
ORIGINAL ARTICLE
Study of the bone mineral density in patients with systemic lupus erythematosus before and after glucocorticoid treatment*
Fernanda do AmaranteI; Simara Santos de SouzaII; Volnei BorgesIII; Carlos Jader FeldmanIV
IMaster, Specialist in Clinical Densitometry, Professor at Faculdade de Tecnologia em Radiologia Fatipuc, Canoas, RS, Brazil
IIProfessor for the Technical Course of Medical Radiology IPUC, Canoas, RS, Brazil
IIIPhD, Professor at Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, RS, Brazil
IVPhD, MD, Radiologist at SIDI Medicina por Imagem, Porto Alegre, RS, Brazil
Mailing address
ABSTRACT
OBJECTIVE: To evaluate the bone mineral density variability in patients with systemic lupus erythematosus, before the beginning and at 30 and 60 days from the beginning of glucocorticoid therapy.
MATERIALS AND METHODS: Fifteen premenopausal women with systemic lupus erythematosus were referred for bone densitometry before being submitted to glucocorticoid therapy.
RESULTS: It was demonstrated that the bone mineral density in g/cm² in the lumbar spine presented a significant decrease in the span between the first analysis previously to the beginning of the glucocorticoid therapy and the third analysis after 60 days under therapy. On the other hand no significant difference was found in the femoral neck.
CONCLUSION: The authors conclude that, at short term, rather than only at long term as previously reported by other authors, corticotherapy in patients with systemic lupus erythematosus results in a decrease in the bone mineral density in the lumbar spine.
Keywords: Bone densitometry; Premenopause; Systemic lupus erythematosus.
INTRODUCTION
Osteoporosis is a systemic metabolic disease characterized by decreased bone mass and deterioration of bony microarchitecture with the consequential increase in risk for fractures(1). The disease is caused by an imbalance between osteoblastic bone formation and osteoclastic bone resorption(2,3). Most frequently, osteoporosis affects postmenopausal women with relevant morbidity and mortality rates, but may also develop very early in patients with hypercortisolism like those affected by systemic lupus erythematosus.
Corticotherapy has been widely utilized because of anti-inflammatory and immunosuppressive effects of the method in the management of several diseases such as systemic lupus erythematosus (SLE)(4). Hypogonadism in cases of hypercortisolism has been associated with the suppressive effect of gonadotropin-releasing hormone (GnRH) release or yet with the suppression of the hypophysis sensitivity to this hormone(5). Additionally to these mechanisms, it is already known that synthetic glucocorticoid can suppress adrenocorticotrophic hormone (ACTH) secretion, leading to an adrenal atrophy and consequential androgens decrease(6). The main mechanisms of glucocorticoid osteoporosis are: decrease in the bone matrix deposition by osteoblasts, increase in the bone resorption by osteoclasts, decrease in the intestinal resorption and increase in the urinary excretion of calcium(7,8).
The incidence of osteoporosis in patients with systemic lupus erythematosus still remains unknown, but the wide utilization of corticotherapy may be responsible for the development of osteoporosis not only in patients with SLE but also in other diseases(4).
Current evidences indicate that, most of times, the catabolic effect of glucocorticoids is due to the direct action of these steroids over the bone cells activity, besides the increase in the bone resorption(9). Osteoporosis is the most frequently found iatrogenic complication associated with the chronic treatment with pharmacological doses of these steroids. The bone loss severity is related to factors such as dose and therapy duration. It is known that this bone loss is greater in the first six months of therapy and may persist in patients receiving higher doses of glucocorticoids(10).
The present study was aimed at evaluating the variability of bone mineral density in patients with SLE with medical indication for glucocorticoid therapy, before the beginning and at 30 and 60 days from the beginning of the therapy.
MATERIALS AND METHODS
The present case-control study has been developed in the Unit of Bone Densitometry at SIDI Medicina por Imagem, Porto Alegre, RS, with the participation of Faculdade de Tecnologia em Radiologia Fatipuc, Canoas, RS, Brazil.
Fifteen women under the age of 48, who were not undergoing treatment with hormones or other drugs, like calcium, that could influence the study results, and with previous diagnosis of SLE (at least three criteria for SLE classification, according to the American College of Radiology [ACR]) and who had agreed to participate in the study were evaluated. Only those women who would be submitted to corticoid therapy right after the bone densitometry, were invited to participate.
The patients answered a questionnaire about clinical data such as body weight, height, date of disease onset, previous fractures, life style (physical activities, alcoholism, tobacco smoking) and use of medications.
Bone mineral density was measured by a specialized technologist in the lumbar spine and femoral head by means of dual energy x-ray absorptiometry in a DPX model densitometer (Lunar Radiation Corporation; Wisconsin, USA). Coefficients of variation of the densitometric technique in the period of data collection were measured on the lumbar spine and proximal femur, respectively with the following results: 1.5% and 0.9%(11).
According to the diagnostic criteria proposed by Kanis(12) and Sampaio Netto et al.(13), the results can be divided into four groups:
Normal: higher bone mineral density values than a lower-than-expected standard deviation for a young woman. Bone density in the lumbar spine > 1,05 g/cm², and > 0,83 g/cm² in the femoral neck.
Decreased bone mass: bone mineral density values ranging between 1.0 and 2.5 lower-than-expected standard deviation for a young woman. Bone density in the lumbar spine ranging between 1.05 and 0.88 g/cm², and between 0.83 and 0.71 g/cm² in the femoral neck.
Osteoporosis: bone mineral density values < 2.5 lower-than-expected standard deviation for a young woman. Bone density in the lumbar spine < 0.88 g/cm², and < 0.71g/cm² in the femoral neck
Established osteoporosis: bone mineral density values < 2.5 lower-than-expected standard deviation (SD) for a young woman, in the presence of one or more fractures(14).
The first bone densitometry study was performed before the beginning of the glucocorticoid therapy, and the second and third evaluations, after respectively 30 and 60 days under therapy.
The ANOVA test for repeated measurements was utilized for statistical analysis, with Bonferroni correction for multiple comparisons between mean values. The SPSS-10 statistical software was utilized and statistical significance level corresponded to p < 0.05.
RESULTS
Fifteen premenopausal women previously diagnosed with SLE and referred for bone densitometry were evaluated in the period between February-June 2007.
In the present study, median for age was 43 years (range, 3248 years) and all the women were white.
Previous or current history of tobacco smoking was reported by 13.3% of the patients. Nine patients (60%) were sedentary, five (33.3%) presented a familial history of osteoporosis, only three (20%) had a previous history of fracture, and four (26.7%) utilized oral calcium supplements.
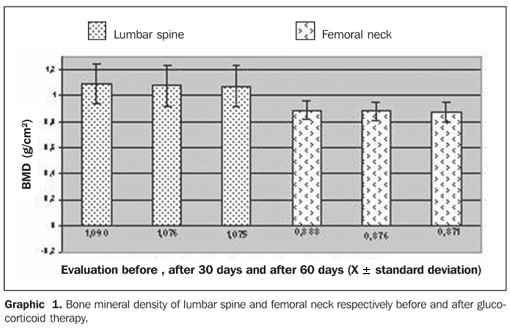
Graphic 1 demonstrates the bone mineral density in g/cm² in both segments evaluated the lumbar spine and femoral neck at three different moments: before the beginning of the glucocorticoid therapy, and after 30 and 60 days under therapy. The patients received doses considered as low (< 7.5 mg/day) during the evaluation. The mean value for the lumbar spine at the first evaluation was 1.090 ± 0.159 g/cm², at the second evaluation, after 30 days, 1.076 ± 0.164 g/cm², and at the third evaluation, after 60 days, 1.075 ± 0.161 g/cm². No statistically significant difference was found in the comparison between mean values for bone mineral densities at the first versus second evaluation, and second versus third evaluation. However, in the comparison between mean values in the first and third evaluations, there was a statistically significant difference (p = 0.023).
Also, mean values for bone mineral density of femoral neck are demonstrated on Graphic 1: at the first evaluation, 0.888 ± 0.075 g/cm², second, 0.876 ± 0.068 g/cm², and third, 0.871 ± 0,070 g/cm². No statistically significant difference was found in the comparison between evaluations.
Bone mineral densities are translated as t-scores according to the diagnostic guidelines proposed by the World Health Organization (WHO). Graphic 2 demonstrates the t-scores corresponding to the evaluations for the lumbar spine and femoral neck before the beginning of the glucocorticoid therapy, after 30 days and after 60 days under therapy. The first evaluation of the lumbar spine resulted in a t-score corresponding to 0.680 ± 1.1, the second evaluation, 0.820 ± 1.3, and the third evaluation, 1.030 ± 1.4, with no statistically significant difference. However, according to the WHO criteria, the first and second evaluations demonstrated normal levels of bone mineral density, and the third evaluation demonstrated osteopenia (decreased bone mass). Mean t-scores for femoral neck are also demonstrated on Graphic 2: at the first evaluation, 0.760 ± 0.7, at the second, 0.860 ± 0.6, and at the third, 0.900 ± 0.6. No statistically significant difference was found in the comparison between these evaluations.
DISCUSSION
Osteoporosis is a disease that may develop in the presence of several conditions, and its pathogenesis may be associated with factors such as the presence of osteodegenerative disorders like rheumatoid arthritis and SLE whose treatment involves drugs which affect the bone mass.
Some of these medications are the low-dose glucocorticoids. The harmful effect of these drugs on the bone mass seems to be observed early in the course of the therapy, although this is still not well determined(15). The glucocorticoids effects on the bone and pathogenesis in the induction of osteoporosis have been exhaustively reviewed and seem to be both direct and indirect, inhibiting bone formation and increasing bone resorption(16).
In 2006, Natsui et al. demonstrated a significant loss of bone mineral density, particularly in the lumbar spine, after a two-month high-dose glucocorticoid therapy, showing that the intensive use of this drug induces to a fast loss of trabecular bone mineral density (17).
Recently, a meta-analysis demonstrated an increased risk for fracture within three-six months after the beginning of high-dose corticosteroid therapy(18).
In the present study, patients undergoing low-dose glucocorticoid therapy presented decreased bone mineral density in the lumbar spine (L1L4) two months after the beginning of the therapy, with a statistically significant difference (p = 0.023) as compared with the previous bone mineral density in the same patients. Previously, studies also had demonstrated that glucocorticoid therapy for at least three months would induce bone mineral density decrease in men and postmenopausal women(19).
Also in cases of some respiratory disorders, such as asthma, corticosteroid therapy is frequently utilized and, probably, presents the same effect on bone tissues which should be further researched.
Glucocorticoid-induced osteoporosis represents a relevant problem caused not only by high-dose therapies, and new studies on their mechanisms of action have been developed. Although effective preventive and management therapies for glucocorticoid-induced osteoporosis are well described, they have not been routinely adopted in the clinical practice(20).
Finally, the present study demonstrated the significant decrease in the bone mineral density in the lumbar spine of women in the age range between 32 and 48 years, with SLE under low-dose glucocorticoid therapy for 60 days, indicating that there is a significant variability as a result of the utilization of these drugs at short term.
REFERENCES
- 1. Keen RW, Kelly PJ. Genetic factors in osteoporosis. What are the implications for prevention and treatment? Drugs Aging. 1997;11:3337.
- 2. Zaidi M, Alam AS, Shankar VS, et al. Cellular biology of bone resorption. Biol Rev Camb Philos Soc. 1993;68:197264.
- 3. Manolagas SC, Jilka RL. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med. 1995;332:30511.
- 4. Godinho F, Santos MJP, Silva JC. Osteoporose induzida pelos glicocorticóides: conhecimento e estratégias preventivas nos doentes com lúpus. Acta Reumatol Port. 2004;29:10510.
- 5. Veldhuis JD, Lizarralde G, Iranmanesh A. Divergent effects of short term glucocorticoid excess on the gonadotropic and somatotropic axes in norml men. J Clin Endocrinol Metab. 1992;74: 96102.
- 6. Doerr P, Pirke KM. Cortisol-induced suppression of plasma testosterone in normal adult males. J Clin Endocrinol Metab. 1976;43:6229.
- 7. Weinstein RS, Jilka RL, Parfitt AM, et al. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest. 1988;102:27482.
- 8. Sambrook P, Lane NE. Corticosteroid osteoporosis. Best Pract Res Clin Rheumatol. 2001;15: 40113.
- 9. Lanna CMM, Montenegro Jr RM, Paula FJA. Fisiopatologia da osteoporose induzida por glicocorticóide. Arq Bras Endocrinol Metab. 2003;47: 918.
- 10. Adachi JD. Corticosteroid-induced osteoporosis. Am J Med Sci. 1997;313:419.
- 11. Defavori CG, Sarriés GA. A correlação de métodos DEXA e CDEXA em absortimetria mineral óssea. Radiol Bras. 2007;40:1837.
- 12. Kanis JA. Bone density measurements and osteoporosis. J Intern Med. 1997;241:1735.
- 13. Sampaio Netto O, Coutinho LOL, Souza DC. Análise da nova classificação de laudos de densitometria óssea. Radiol Bras. 2007;40:235.
- 14. Szejnfeld VL, Atra E, Baracat EC, et al. Bone density in white Brazilian women: rapid loss as the time around the menopause. Calcif Tissue Int. 1995;56:18691.
- 15. LoCascio V, Bonucci E, Imbimbo B, et al. Bone loss in response to long-term glucocorticoid therapy. Bone Miner. 1990;8:3951.
- 16. Baylink DJ. Glucocorticoid-induced osteoporosis. N Engl J Med. 1983;309:3068.
- 17. Natsui K, Tanaka K, Suda M, et al. High-dose glucocorticoid treatment induces rapid loss of trabecular bone mineral density and lean body mass. Osteoporos Int. 2006;17:1058.
- 18. van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteporos Int. 2002;13:77787.
- 19. McIlwain HH. Glucocorticoid-induced osteoporosis: pathogenesis, diagnosis, and management. Prev Med. 2003;36:2439.
- 20. Woolf AD. An update on glucocorticoid-induced osteoporosis. Curr Opin Rheumatol. 2007;19: 3705.
Publication Dates
-
Publication in this collection
29 Aug 2008 -
Date of issue
Aug 2008
History
-
Received
06 July 2007 -
Accepted
16 Oct 2007