Abstract
The aim of this work is the production and characterization of plasma polymerized acetaldehyde thin films. These films show highly polar species, are hydrophilic, organophilic and easily adsorb organic reactants with CO radicals but only allow permeation of reactants with OH radicals. The good step coverage of films deposited on aluminum trenches is useful for sensor development. Films deposited on hydrophobic substrates may result in a discontinued layer, which allows the use of preconcentration in sample pretreatment. Deposition on microchannels showed the possibility of chromatographic columns and/or retention system production to selectively detect or remove organic compounds from gas flows.
microcolumns for chromatography; plasma polymerization; adsorbent organic thin films
ARTIGO
Plasma polymerized acetaldehyde thin films for retention of volatile organic compounds
Leonardo Frois HernandezI; Roberto da Rocha LimaII; Alexsander Tressino de CarvalhoIII; Nicole Raymonde DemarquetteIII; Maria Lúcia Pereira da SilvaIII, * * e-mail: malu@lsi.usp.br
IFaculdade de Tecnologia de São Paulo, Pça. Cel. Fernando Prestes, 30, 01124-060 São PauloSP, Brasil
IIInstituto de Física, Universidade de São Paulo, Rua do Matão, 187, Trav. R, 05508-090 São PauloSP, Brasil
IIIEscola Politécnica, Universidade de São Paulo, Av. Prof. Luciano Gualberto, 158, Trav. 3, 05508-090 São PauloSP, Brasil
ABSTRACT
The aim of this work is the production and characterization of plasma polymerized acetaldehyde thin films. These films show highly polar species, are hydrophilic, organophilic and easily adsorb organic reactants with CO radicals but only allow permeation of reactants with OH radicals. The good step coverage of films deposited on aluminum trenches is useful for sensor development. Films deposited on hydrophobic substrates may result in a discontinued layer, which allows the use of preconcentration in sample pretreatment. Deposition on microchannels showed the possibility of chromatographic columns and/or retention system production to selectively detect or remove organic compounds from gas flows.
Keywords: microcolumns for chromatography; plasma polymerization; adsorbent organic thin films.
INTRODUCTION
Volatile organic compounds (VOC's) have taken a lot of attention due to their harmful environmental effect in the atmosphere and the hydrologic cycle as well. VOC's analyses are normally made by gas chromatography and eventually used preconcentrators due to the small amount of these contaminants in the environment.1
Moreover, miniaturized systems have been proposed for preconcentration and posterior analysis of VOC's present in the air.2-4 The commonest analysis system is a micro gas chromatograph capable of determining vapor mixtures at parts per billion levels in the environment and in less than 20 min. On this system, the preconcentrator uses 200 µm diameter graphite spheres, is quickly heated by microheaters5,6 and the preconcentration obtained is enough to analysis of indoor air quality. Recently, volatile organic compounds in groundwater, or even soil, were also detected using Chemiresistor and Surface Acoustic Wave (SAW) devices7,8 but the correct analysis required preconcentration in order to minimize chemical interferents and other phenomena that affect the results.9-12 Therefore, up to now the development of gas phase preconcentrators is principally related to the manufacturing of analytical equipment and miniaturization. Good examples are the use of portable gas chromatography13 allied with devices for preconcentration, such as the design proposed by Mitra.14
Preconcentration requires the production of high-surface area adsorbent materials. High-surface area can be obtained through some different ways, such as porous silicon layers15 or anodic etching of aluminum films16,17 to latter modification aiming VOC's adsorption. Another high superficial areas include nanofibers or nanofibers modified by the addition of particles inside,18 conducting polymers for nanowires manufacturing and the detection of H2O219 sorptive polymers,20 which provide a fast adsorption and desorption cycle, etc. Volatile sulfur compounds can also be detected using active carbon for preconcentration21 and semi-volatile organic compounds in aqueous matrix by the membrane extraction.22 Aside the high superficial area, preconcentration requires quick desorption and consequently thin films must be produced. Therefore, plasma deposited thin films are good choice for preconcentration or removal of organic compounds from gas or liquid phases.
In former works several adsorbent thin films were obtained by plasma deposition.23-26 It is also possible to produce a nanochanneled thin film27 useful as a chromatographic stationary phase. These films change surface characteristics and can be useful for VOC's adsorption. Furthermore, a structure similar to a capillary chromatography microcolumn was developed26 and some modifications were tested,28 such as the addition of microspheres to enhance flow dispersion. This microcolumn consists of three-dimensional microchannels obtained using conventional tools, which assures low manufacturing costs.
Most of the adsorbent films produced are oxygenated polar organic films but acetaldehyde was not tested. One of the most surprising characteristics is the possibility of discontinued deposition of 2-propanol plasma polymerized film on hydrophobic substrates. This discontinued layer enhances the surface area for adsorption.25 Moreover, the microcolumn tested was not packed. However, acetaldehyde polymerization is possible19,30 and packed columns present several advantages, such as higher retention time on sample pretreatment. Therefore, the aim of this work was to evaluate the plasma polymerization of acetaldehyde and to verify the possibility of using the packed three-dimensional microchannels for sample pretreatment.
EXPERIMENTAL
Films were deposited on five different substrates: silicon wafer, P type, <100>, 10- 20 Ω.cm, 3" diameter (Silicon Sence Inc., USA) for film characterization; piezoelectric quartz crystal (PQC), 4.096 MHz, 11 mm diameter and 0,8 mm thickness (Hosonic Industrial do Brasil LTDA, Brazil) for adsorption analysis using Quartz Crystal Measurements (QCM); square pieces 5 mm thick polymethylmetacrylate (acrylic - Plasttotal LTDA, Brazil) for evaluation of these films as surface protection agent; plasma polymerized hexamethyldisilazane (HMDS) film, for testing of deposition on highly hydrophobic substrates; aluminum trenches deposited on silicon wafer for evaluating the conformity of the deposited films. These trenches are composed by 10 lines with a depth of 3 µm and width varying from 1 to 70 µm. Films were produced using a homemade capacitive plasma reactor, with a 40 KHz power supply.31
The deposition time was always 5 min and the reactant flow roughly 1 g/min. Two deposition modes was used, the so-called open and closed chamber.26,27 In closed chamber mode, there is no flow of reactant to the chamber during the plasma discharge. Therefore, the deposition rate increases since the plasma's active species present a long resident time. Furthermore surface characteristics probably change because the active species may continue react after the plasma shut off.
The films were characterized using several techniques. The thickness and deposition rate was determined by profilemeter (Dektak. 3030, Veeco Instruments Inc., USA). Infrared Spectroscopy (FTS-40, Bio-Rad Laboratories, USA) and X-Ray Photoelectron Spectroscopy (PHI-5600 MultisystemPhysical Electronics, USA) were used to unravel the chemical structure and the superficial composition. Contact angle measurements using distilled water and aqueous solutions of organic reactants (2-propanol, acetone and n-hexane) were carried out at room temperature with a Goniometer (Rame Hart). Optical and Scanning Electron Microscopy (SEM) was used to evaluate conformal deposition on aluminum trenches, island or wrinkle formation on HMDS thin films and peeling on silicon substrates.
Adsorption characteristics of films deposited on piezoelectric quartz crystal were determined by Quartz Crystal Measurements (QCM). The procedure for these tests is the injection of small amount (mg) of reactant in a fluid (N2 or water). Reactants in a large range of polarity (water, 2-propanol, acetone, n-hexane) and the injection occurred in a short time (<<1 s) were used.
Tests of retention on microchannels were carried out machining a three-dimensional structure26 in acrylic and then depositing the thin film. In order to increase the retention, the microchannel was filled with glass microspheres. These spheres were also recovered with the thin film. Therefore, the fulfilled structure resembles a packed chromatography column. These structures were tested using QCM analysis in a similar procedure that was carried out for adsorption characterization. The structure was positioned as close as possible to the detector, a piezoelectric quartz crystal recovered by plasma polymerized HMDS thin film, i.e. an adsorbent film, acts like the detector. All QCM analysis was carried out in homemade equipment.32
All chemical reactants are p.a. grade (Casa Americana, Brazil), except for hexamethyldisilazane, which is industrial grade (Hoechst). Distilled water was used to prepare all solutions. Glass microspheres, 30 µm in diameter (Filite Co., Brazil), were used to pack microchannels of a three-dimensional structure.
RESULTS AND DISCUSSION
This section describes not only the film production and characterization but also its possible uses for surface protection, sensor development and sample pretreatment.
Production and characterization of thin films
Acetaldehyde plasma polymerized thin films were obtained in a large range of power: from 20 to 150 W in any of the deposition modes, i.e. open or closed chamber. However, pressure deposition on open chamber mode varies from 0.1 to 2.0 Torr but closed chamber mode requires a minimum pressure of 0.6 Torr. The higher pressure required on closed chamber mode indicates that the deposition mechanism changes. On closed chamber mode, probably gas phase reactions remove plasma's active species and prevent deposition. Moreover, high pressure means low surface bombardment which probably changes the surface composition of the films. There is no particulate formation for all pressure and power range used and the increase in pressure or power increase the deposition rate.
The optimal conditions to obtain thin films are 1.2 Torr/50 W and 0.8 Torr/100 W for closed and open chamber modes, respectively. On these conditions the films present a high amount of polar species and the highest deposition rate, approximately 400 Å/min. Infrared spectroscopy (FTIR) showed polar species, mainly CO and OH, besides the carbonic chain (CHn, CH2 and CH3). Adsorbed water can also be detected. CO and OH relative intensity do not show meaningful variations by FTIR analysis; however a higher relative intensity of adsorbed water can be obtained on closed chamber mode, probably due to the impossibility of byproducts removal during plasma deposition.
XPS analysis showed not only CO and OH but also COOH for all samples analyzed. In any deposition mode (open or closed chamber) the C:O ratio is 3:1, thus the oxygen content on the surface does not change significantly if the deposition mode is varied. However, the oxygen species present on the surface changes mainly due to ion bombardment once the deposition on low power favors reduction of the CO radical that produces OH but not COOH. Therefore, on the surface the oxidation of the carbonyl species occurs probably due to ion bombardment during deposition.
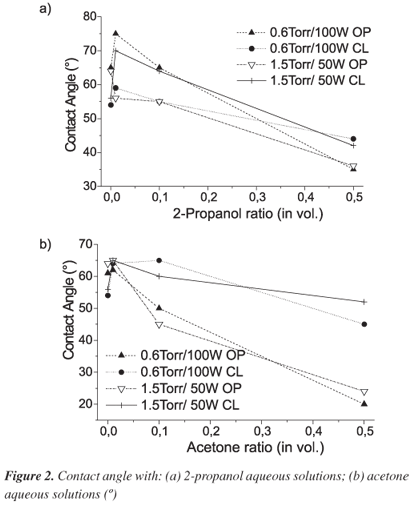
Table 1 shows the main FTIR and XPS results obtained and Figure 1 shows typical results. Just for comparison Table 1 also shows contact angle measurements, with water and n-hexane saturated aqueous solution, and deposition rate.
Contact angle measurements always indicated that all surfaces are hydrophylic and organophylic, with average values of 64º for water drops and wettability (0º) for pure organic compounds. Furthermore, the contact angle measurements are almost the same for water and n-hexane saturated aqueous solutions; therefore the film shows low affinity for the organic reactant probably due to the different polarities. The films showed meaningful variations on contact angle measurement only for acetone aqueous solution. Figure 2 shows typical results where it is noticeable the same behavior for all films deposited and the lower angles obtained with acetone aqueous solution compared to 2-propanol aqueous solutions. The similar values obtained for most of the water contact angle measurements and the selectivity for acetone solutions are in good agreement with the low variation on surface composition and are probably due to the presence of the CO radical on the film surface, as determined by XPS analysis.
Films with high amount of oxygenated species, determined by FTIR and XPS analysis, were characterized for adsorption of organic compound using QCM measurements. The analysis carried out using N2 showed a high selectivity on film adsorption once the most polar compounds, such as 2-propanol and acetone, are easily adsorbed, but not the non-polar n-hexane. Figure 3a shows a typical QCM spectrum obtained with a sequential injection of different organic compounds. As can be noticed in the figure, the injection of n-hexane does not change the descendent trend on the graphic due to low reactant affinity to the film surface. On the other hand, the changes on amount of acetone injected quickly changes the crystal frequency. Once the film shows good selectivity for polar organic compounds, tests using solutions of acetone and n-hexane or 2-propanol were carried out. Figure 3b shows a sequential injection of different solutions proportions and it is possible to observe only a small and quick change for acetone/n-hexane solutions. Thus, n-hexane seems to hinder acetone adsorption; however acetone/2-popanol solutions showed a big change on frequency values due to the adsorption of both compounds. Moreover, the baseline changes after 2-propanol or 2-propanol solutions injection, which seems to indicate that 2-propanol can percolate the film whereas acetone only adsorb on it. Table 2 summarizes the results obtained in Figure 3a and 3b, the minimum adsorbed amount of acetone detected is approximately 28 µg/mm2. A similar behavior was obtained for n-hexane and acetone using water as the fluid. However 2-propanol was not detected on this condition due to the competition between hydrogen bridge formation with water and adsorption on film surface. Furthermore water can also be adsorbed on these surfaces due to the hydrophilic character of these films.
The films showed good adhesion and without peeling to all substrates used. Moreover, deposition on plasma polymerized HMDS films shows some interesting characteristics. As previously observed in the introduction, double layers of HMDS and organic films may produce discontinuous deposition, named island formation. Therefore acetaldehyde thin films were deposited on 2000 Å HMDS thin films and the samples were evaluated using optical microscopy during at least one week. Table 3 shows the main results. The acetaldehyde deposition rate is higher than in silicon substrate for all conditions indicating that deposition mechanism changes probably due to the surface characteristics of HMDS films that favors adsorption of organic molecules on this surface. Island formation is achieved only on high ion bombardment condition, which is in good agreement with previous data obtained for acetaldehyde deposition (Table 1). The differences on wrinkle condition and island size also indicate that acetaldehyde films are not strongly bonded to the substrate.
Figure 4 shows images obtained by optical microscopy of island coalescence and wrinkle development on double layers. As can be notice in these figures, the island and wrinkle sizes change drastically in one week. Another important difference on island samples is the surface composition. XPS analysis showed an atomic percentage 4:1 for COH and CO radicals and no presence of COOH radical. Therefore, the deposition mechanism changes and probably relies on adsorption of acetaldehyde in the surface and polymerization occurs due to ion bombardment, which favors reduction of the molecule. This data is also in good agreement with previous measurements (Table 1).
Discontinuous deposition was obtained only using 2-propanol and acetaldehyde, which seems to indicate that the reactant polarity plays an important role. However, ion bombardment is also major parameter at low frequency plasmas. The polarity on these oxygenated molecules varies from ethers and esters, acetaldehyde and 2-propanol. Therefore, ethers and esters, less polar molecules that are polymerized only in a low bombardment condition, showed only wrinkle on their double layers.26 The 2-propanol molecule, a high polar compound, also requires ion bombardment for deposition. Thus, on double layer formation, the bombardment produces dangling bonds that favor adsorption and island formation. For this molecule the island size will be dependent on deposition conditions, such as power and time. Finally, acetaldehyde shows an intermediary position, with lower bombardment and weakly bonded surfaces, which provides conditions for island coalescence.
POSSIBLE USES
Acetaldehyde films showed promising characteristics. Once the films show selectivity properties with probable percolation of 2-propanol molecule and island formation, a possible use for them is on sensor development; therefore deposition on aluminum trenches were performed to evaluate step coverage. The films adsorb polar organic compounds, which can be used on chromatography columns. Furthermore, percolation can also be used for retention in sample pretreatment. Thus, a chromatography microcolumn that can also be used for retention purposes were developed.
DEPOSITION ON ALUMINUM TRENCHES
Deposition on aluminum trenches showed good step coverage for acetaldehyde and double layers thin films. Figure 5 shows SEM images for acetaldehyde (0.8Torr, 100W) and double layers thin films (acetaldehyde: 0.8 Torr, 100 W; HMDS: 0.5 Torr, 50 W). It is possible to observe that acetaldehyde film deposited on the aluminum trench (Figure 5a) and that in the double layer islands were formed not only on the bottom but also on the top of the structure (Figure 5b). However, the size of the island changes and they are bigger on the top of the structure than on the substrate, probably due to border effect33 that favored coalescence. Nonetheless, it was not observed island on the walls of the trenches, probably due to the low bombardment. Therefore, these films can be useful for sensor development.
DEPOSITION IN CHROMATOGRAPHY MICROCOLUMNS
Microchannels covered with plasma polymerized adsorbent film were already used for adsorption of volatile organic compounds.26 However the manufactured structure resembled a capillary column and it was tested only for gaseous samples. Therefore, some improvements were added to this previous design.
The new manufactured structure was designed with a long path to fluid in order to increase retention. A possible solution is the manufacturing of a packed microchromatography column. The only modification needed is the addition of microspheres to the microchannel. Silva28 has already tested this configuration but using non-adsorbent silica microspheres. Therefore, in this new approach the microcolumn was filled with microspheres, which were previously covered with acetaldehyde thin film. Once both microchannel and microspheres are covered with adsorbent film the retention can be enhanced due to the increase in adsorbent surface area. Figure 6 shows an illustrative design of the packed column.
The microcolumn was tested with sequential injections of vaporized organic reactants in N2 or liquid compounds in water. Figure 7 shows typical results for injection in N2 flow and Table 4 summarizes the sequential amount of injection and the correspondent frequency peak and elution time. The microchannels recovered with acetaldehyde thin films show higher elution times for polar organic compounds (Figure 7a). Moreover, this elution time is much bigger than the ones obtained with plasma polymerized HMDS film, a high adsorbent film for polar and non-polar organic compounds (Figure 7b). Therefore, the surface of the acetaldehyde film retains more strongly the organic compounds. On the other hand, the reproducibility of the elution time is lower, probably due to this strong interaction. This non-homogeneity is more evident for 2-propanol injection, which is in good agreement with QCM measurements that point out the possibility of percolation of the reactant through the film. Packed acetaldehyde microcolumns (Figure 7c) require many sequential injections before the detection of a small signal. Furthermore, this signal appears in a much higher elution time and the frequency peak is usually low (Figure 7c). A noticeable characteristic in Figure 7c is the relatively low amount even for n-hexane, which do not adsorb on acetaldehyde films, as determined by QCM analysis. A possible explanation is the dispersion of the fluid inside the channel due to the microspheres. This apparent lack of coherence on flow transport inside the structure may be minimized just decreasing the channel length. The minimum retained amount using nitrogen was: 10 µg/mm2 for all reactants, even n-hexane. These values are big if compared with the amount detected in QCM analysis. Therefore, others effects are acting in retention, such as capillary effects. Measurements with water as the fluid show similar results.
Once strong interaction occurs on acetaldehyde film deposited on microchannels and the packed microcolumn enhances significantly the adsorption area, this structure and film are highly indicate for retention of organic compounds, for instance on µTAS for sample pretreatment.
CONCLUSIONS
Acetaldehyde plasma polymerized thin films were obtained and presented interesting characteristics. The films were deposited with good adhesion on several substrates: silicon, quartz, acrylic, glass microspheres and aluminum. Films with high amount of oxygenated species show good adsorption and selectivity characteristics for polar organic compounds. Deposition of acetaldehyde thin films on HMDS plasma polymerized films leads to a discontinuous layer, which increases the adsorption area. This increase in surface area linked with the good step coverage that these films present indicate that these films have promising characteristics for sensor development. The strong interaction of polar organic reactants with film surface and the possible percolation of 2-propanol molecules throughout the film point out the use of such films as selective membranes, for instance in µTAS development. A simple structure that can be used as packed microcolumn was proposed and tested. The preliminary results showed that this device can be used on sample pretreatment for the retention of undesirable compounds, mainly if an adsorbent film, such as the proposed plasma polymerized acetaldehyde thin film, is deposited on the microchannel and microspheres that compose the structure. This device is not expensive and easily manufactured using conventional tools, such as a mechanical lathe.
ACKNOWLEDGEMENTS
The authors would like to thank Prof. E. Fachini, University of Puerto Rico, for XPS analysis and J. de O. Alves for English review. FAPESP, CNPq in Brazil and Advance it Program in University of Puerto Rico for financial support.
Recebido em 10/8/07; aceito em 31/1/08; publicado na web em 20/8/08
- 1. Winberry, W. T.; Murphy, N. T.; Riggan, R. M.; Center for Environmental Research Information Office of Research and Development U.S., Environmental Protection Agency: Cincinnati , 2nd ed., 1999.
- 2. Lu, C. J.; Zellers, E. T.; Analyst 2002, 127, 1061.
- 3. Lu, C. J.; Zellers, E. T.; Anal. Chem. 2001, 73, 3449.
- 4. Zellers, E. T.; Wise, K. D.; Najafi, K.; Aslam, D.; Brown, R. B.; Cai, Q. Y.; Driscoll, J.; Flynn, M.; Giachino, J.; Gordenker, R.; Hsieh, M. D.; Nguyen, C. T.-C.; Bergstrom, P.; Drelich, J.; Friedrich, C.; Gamble, E.; Kaviany, M.; Lu, C. J.; Matzger, A.; Oborny, M.; Pang, S.; Potkay, J.; Sacks, R.; Tian, W.-C.; Steinecker, W.; Whiting, J.; Zhong, Q.; Technical Report of University of Michigan, Michigan: USA, 2003, 1.
- 5. Tian, W. C.; Pang, S. W.; Lu, C. J.; Zellers, E. T.; J. Microelectromechanical Systems 2003, 12, 264.
- 6. Tian, W. C.; Pang, S. W.; J. Vac. Sci. Technol., B: Microelectron. Nanometer Struct. -- Process., Meas., Phenom. 2002, 20, 1008.
- 7. Ho, C. K.; Wright, J.; McGrath, L. K.; Lindgren, E. R.; Rawlinson, K. S.; Lohrstorfer, C. F.; Sandia Report 2003, SAND2003-0799.
- 8. Staples, E. J.; Prepr. Ext. Abstr. ACS Natl. Meet., Am. Chem. Soc., Div. Environ. Chem., 219, 236-ANYL Part 1, USA, 2000.
- 9. Ho, C. K.; Lohrstorfer, C. F.; Ground Water Monitoring and Remediation 2003, 23, 85.
- 10. Ho, C. K.; Lindgren, E. R.; Rawlinson, K. S.; McGrath, L. K.; Wright, J. L.; Sensors 3 2003, 7, 236.
- 11. Rivera, D.; Alam, M. K.; Davis, C. E.; Ho, C. K.; Sens. Actuators, B 2003, 92, 110.
- 12. Ho, C. K.; Hughes, R. C.; Sensors 2 2002, 1, 23.
- 13. Yashiin, Y. A.; Yashin, A. Y.; J. Anal. Chem. 2001, 56, 794.
- 14. Kim, M.; Mitra, S.; J. Chromatogr., A 2003, 1, 996.
- 15. Bell, T. E.; Gennissen, P. T. J.; DeMunter, D.; Kuhl, M.; J. Micromechanics Microengineering 1996, 6, 361.
- 16. Perez, G. P.; Yelton, W. G.; Cernosek, R. W.; Simonson, R. J.; Crooks, R. M.; Anal. Chem. 2003, 75, 3625.
- 17. Yelton, W. G.; Pfeifer, K. B.; Staton, A. W.; J. Electrochem. Soc 2002, 149, H1.
- 18. Pinto, N. J.; da Silva, A. N. R.; Fachini, E.; Carrión, P.; Furlan, R.; Ramos, I.; 226th Am. Chem. Soc. Meeting, New York , USA, 2003.
- 19. Liu, J.; Lin, Y. H.; Liang, L.; Voigt, J. A.; Huber, D. L.; Tian, Z. R.; Coker, E.; Mckenzie, B.; Mcdermott, M. J.; Chem. Eur. J. 2003, 9, 605.
- 20. Grate, J. W.; Nelson, D. A.; Proceedings IEEE 2003, 91, 881.
- 21. Nakamoto, J.; Ito, T.; Uematsu, H.; Sens. Actuators, B 2004, 99, 431.
- 22. Guo, X. M.; Mitra, S.; J. Chromatogr., A 2000, 904, 1189.
- 23. Carvalho, A. T.; Carvalho, R. A. M.; Silva, M. L. P.; Demarquette, N. R.; Mater. Res. 2006, 9, 9.
- 24. Silva, M. L. P.; Tan, I. H.; Nascimento Filho, A. P.; Galeazzo, E.; Jesus, D. P.; Sens. Actuators, B 2003, 91, 362.
- 25. Nascimento Filho, A. P.; Silva, M. L. P.; Galeazzo, E.; Demarquette, N. R.; Sens. Actuators, B 2003, 91, 370.
- 26. Lima, R. R.; Carvalho, R. A. M.; Nascimento Filho, A. P.; Silva, M. L. P.; Demarquette, N. R.; Sens. Actuators, B 2005, 108, 435.
- 27. Carvalho, R. A. M.; Lima, R. R.; Nascimento Filho, A. P.; Silva, M. L. P.; Demarquette, N. R.; Sens. Actuators, B 2005, 108, 955.
- 28. Silva, L. M.; Lima, R. R.; Carvalho, A. T.; Silva, M. L. P.; Madaleno, J. C.; Pereira, L.; Mater. Sci. Forum 2006, 514, 1250.
- 29. Gong, X.; Griesser, H. J.; Plasmas Polym. 1997, 2, 261.
- 30. Atkin, R.; Craig, V. S. J.; Hartley, P. G.; Wanless, E. J.; S.; Langmuir 2003,19, 4222.
- 31. Pereira, G. J.; Silva, M. L. P.; Tan, I. H.; Gouvea, D.; J. Mater. Chem. 2000,10, 259.
- 32. Santos, L. C.; Beraldo, F. P.; Hernandez, L. F.; Carvalho, R. A. M.; Silva, M. L. P.; J. Braz. Vacuum Soc. 2006, 25, 75.
- 33. Tarraf, A.; Daleiden, J.; Irmer, S.; Prasai, D.; Hillmer, H.; J. Micromechanics Microengineering 2004, 14, 317.
Publication Dates
-
Publication in this collection
10 Oct 2008 -
Date of issue
2008
History
-
Accepted
31 Jan 2008 -
Received
10 Aug 2007