Abstract
Determination of free urinary cortisol is a test of choice in the diagnosis of Cushing's syndrome. In this study, cortisol was quantified using reversed-phase high-performance liquid chromatography (RP-HPLC) in urine samples previously extracted with ether and using triamcinolone acetonide as internal standard (IS). A BDS-Hypersil-C18® column, water-acetonitrile (72:28; v/v), with a flow rate of 1.0 mL/min and detection at 243 nm were used. This method showed to be both effective and efficient, with sensitivity and linearity ranging from 2.50 to 150 μg/L, and can be used in substitution to the radioimmunoassay technique within this concentration range.
HPLC; cortisol; urine
NOTA TÉCNICA
Determination of free urinary cortisol in cushing's syndrome using reversed-phase high performance liquid chromatography
Eduardo Kinio Sugawara* * e-mail: du-kinio@hotmail.com ; Luciane Maria Ribeiro Neto; Ieda Terezinha do Nascimento Verreschi
Departamento de Medicina, Universidade Federal de São Paulo, Rua Pedro de Toledo, 781, 04039-032 São Paulo - SP, Brasil
ABSTRACT
Determination of free urinary cortisol is a test of choice in the diagnosis of Cushing's syndrome. In this study, cortisol was quantified using reversed-phase high-performance liquid chromatography (RP-HPLC) in urine samples previously extracted with ether and using triamcinolone acetonide as internal standard (IS). A BDS-Hypersil-C18® column, water-acetonitrile (72:28; v/v), with a flow rate of 1.0 mL/min and detection at 243 nm were used. This method showed to be both effective and efficient, with sensitivity and linearity ranging from 2.50 to 150 μg/L, and can be used in substitution to the radioimmunoassay technique within this concentration range.
Keywords: HPLC; cortisol; urine.
INTRODUCTION
Cortisol (F) determination in the different organic fluids has been used as a diagnostic aid in several pathologies as well as in clinical research studies.
Measurements of free F in urine and of F and corticotropin (ACTH) in plasma are tests of choice in the diagnosis of Cushing's syndrome.
Cushing's syndrome is a hormonal disorder caused by prolonged exposure of the body tissues to high levels of the hormone cortisol. Sometimes called hypercortisolism, Cushing's syndrome is relatively rare and most commonly affects adults aged 20 to 50 years. People who are obese and have type 2 diabetes, along with poorly controlled blood glucose and high blood pressure, have an increased risk of developing the disorder.1
Historically, several methods using the radioimmunoassay technique (RIA) were developed to determine F. This technique is still susceptible to cortisone interference and/or other endogenous steroidal metabolites and synthetic glucocorticoids.2 Another limitation of the RIA techniques is the impossibility of using an internal standard to monitor the recovery of F in the extraction process. Recent reviews comparing RIA with chromatographic methods in the determination of F clearly indicate that more accurate results are obtained with the latter.3-7 Limitations such as those observed using RIA in F determination led to the development of more specific methods based on liquid chromatography with ultraviolet detection (HPLC-UV),7-11 liquid chromatography combined with mass spectrometry (LC-MS)5,7,12 and gas chromatography combined with mass spectrometry (GC-MS).13-15 LC-MS/MS methods are among the most successful approaches to improve specificity problems inherent in many immunoassays. The latest generation of tandem mass spectrometers has superior limits of quantification, permitting omission of previously employed derivatization steps.16,17 Chromatographic methods have enabled interferent reduction not only in the quantification of cortisol, but also of cortisone and endogenous F metabolites.
In this study, a method was developed to quantify free F in urine using reversed-phase high-performance liquid chromatography (RP-HPLC) after liquid-liquid extraction18,19 and using triamcinolone acetonide (T) as internal standard.
EXPERIMENTAL
Reagents
Cortisol (98%) was provided by Sigma-Aldrich (St. Louis, MO, USA) and Triamcinolone acetonide (100%) by USP (Rockville, MD, USA). Methanol and acetonitrile were HPLC grade, and ether was analytical reagent grade (Merck, Darmstadt, Germany). Water was obtained with a Milli-Q purification system (Millipore, Bedford, MA USA). Both acetic acid (Merck, Darmstadt, Gemany) and sodium hydroxide (Vetec, Rio de Janeiro, Brazil) were used in 10% solutions.
Standards
Standard solutions (0.01, 0.10, 10.0, and 100 x 101μg/mL) of F and T were prepared in methanol and stored at -20 ºC. Triamcinolone acetonide was used as internal standard in the chromatographic analyses.
Sample preparation
Urine samples (5.0 mL) added with T (40.0 ng/mL) were extracted with ether (3.0 mL) by shaking in Vortex® and then centrifuged (2.1130 g, 15 min, 4 ºC). The organic phase was evaporated under a nitrogen stream. The residue was resuspended in the mobile phase (200 μL), filtered through a membrane (Durapore®, 0.45 μm, 13 mm; Millipore, São Paulo, BR) and injected (50.0 μL) onto the chromatographic column.
Optimal pH for extraction was determined through sample recovery tests with a known concentration (50.0 μg/L) of F in acid (5.0), neutral (7.0), or alkaline (9.0) pH, adjusted with acetic acid or sodium hydroxide.
RP-HPLC
A HP 1100 high-performance liquid chromatograph (Hewlett-Packard, Palo Alto, CA, USA) consisting of an automatic sampler, a quaternary pump and variable-wavelength (λ=243 nm) UV detector was used. Chromatographic separations were accomplished with a BDS-Hypersil-C18 analytic column® (250 x 4 mm, 5 μm), with equivalent guard-column, obtained from Agilent (USA), and maintained at 40 ºC during analysis. Water-acetonitrile (72:28; v/v) was used as mobile phase with a flow rate of 1.0 mL/min for 9.0 min, and 1.5 mL/min for 6.0 min. At 15.0 min, the mobile phase was changed to acetonitrile (100%), which was maintained for 2.0 min. At 17 min, the initial condition was reestablished and maintained for another 3 min, for a new chromatographic run.
Calibration curve
A calibration curve for F quantification was determined in aqueous media by adding methanolic standard solutions of nine different concentrations of F (2.50, 5.00, 10.0, 15.0, 25.0, 50.0, 70.0, 100, and 150 μg/L) and T (40.0 ng/mL). The curve was obtained using the experimental data from the graph representing the ratio between the peak areas of F and T versus different concentrations.
Analytical validation
Linearity was evaluated by repeated (n=5) analysis using different concentrations in the range from 2.50 to 150 μg/L. The relative standard deviation (RSD) was determined for each concentration, and the limit of quantification was defined as being the lowest concentration with RSD < 10%. Intra-assay precision was determined using samples added with different concentrations (2.50 to 150 μg/L) and analyzed in replicate (n=5). Inter-assay precision was evaluated in different concentrations for two and three consecutive days. The method accuracy was assessed by analyzing samples (n=5) with known concentrations prepared in the laboratory, using calibrated equipment and glassware. The results were expressed as percentages.
The F and T standard solution samples used to determine these parameters were prepared with water. Matrix interference was verified using urine from healthy individuals added with known concentrations of F (5.00, 10.0, 70.0, and 100 μg/L).
Sampling
The applicability of the method was evaluated by analyzing samples (n=30) from the laboratory routines of the Steroids Laboratory of the Endocrinology Division (Medicine Department of the Federal University of São Paulo) and the Hormones and Molecular Genetics Laboratory (LIM/42) of the Endocrinology Division (Medical School of the University of São Paulo). Urine samples were stored at -20 ºC until analysis.
RESULTS AND DISCUSSION
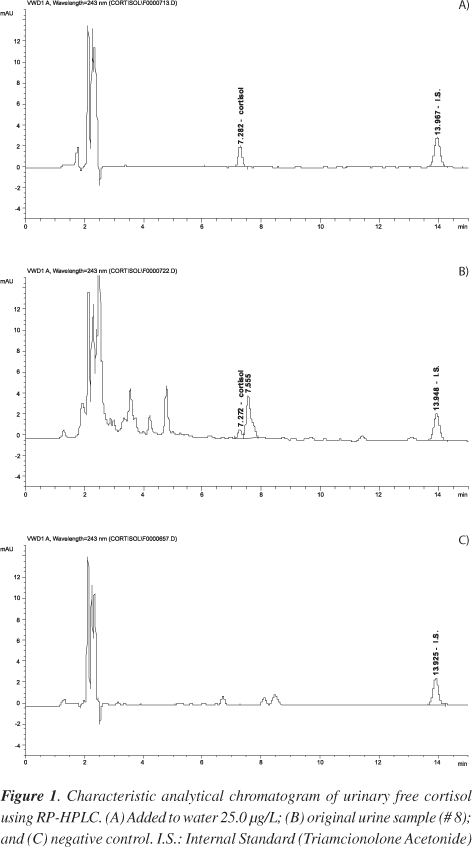
The method used here, RP-HPLC, was shown to be fast and efficient for determining free urinary F. The retention times of F and T were 7.3 and 14.1 min, respectively. The total time for chromatographic analysis was 20 min. The retention time was consistent with or inferior to the times reported in the literature, all superior to 20 min. 6,8,10 A typical chromatogram is depicted in Figure 1 (B). Ether extraction associates simplicity and low cost for RP-HPLC analysis. Furthermore, its use in extraction of steroids is frequently mentioned in the literature.18-21 No pH interference was observed in extraction recovery, once the relationship between F and T was maintained with the different pH values (0.91 ± 0.03). Replacing RIA by RP-HPLC allows a significant reduction in the total time of analysis, because the usually employed RIA methodology requires a minimum of 6 h for the incubation step. Moreover, this method confirms the methodology used for determination of serum cortisol22 in the routines of the Steroids Laboratory, contributing to laboratory practice and to the replacement RIA.
Linearity was observed in the concentration range from 2.50 to 150 μg/L with a determination coefficient (r2) of 0.9944, which meets the expected work range for a Cushing's syndrome diagnosis. Table 1 shows the precision and accuracy values obtained in the analytical validation study. The average values for the intra- and inter-assay RSD were 3.2% (from 2.50 to 150 μg/L) and 4.6% (from 5.00 to 100 μg/L), respectively. These findings were compatible with those reported in the literature for the same concentration range, reported to vary around 7.0%.5,6,23 The accuracy of the method is between 88.9 and 114%, which is also compatible with the reports of the literature showing values between 88 and 97%.5,24 The limit of quantification found was 2.50 μg/L. The sensitivity of this method was similar or even higher than that of other published methods, for which the limit of quantification was 5.0 μg/L.5,10,25,26
Tables 2 and 3 show the results obtained for samples submitted to the RP-HPLC method. In 30 determinations made with samples from 19 patients, the values ranged between 3.30 and 863 μg/24 h. Regarding the reference value (55.0 μg/24 h), 36.6% of the obtained results were suggestive of a Cushing's syndrome diagnosis. As these samples were obtained from patients under clinical evaluation, we were able to compare the results with the corresponding diagnoses, confirmed by the pathological, imaging, and petrosal sinus catheterism examinations.
The results of the samples numbered from 1 to 12 (Table 2) were evaluated for cortisol in serum and/or saliva and correspond to data supplied by the routine laboratory. Cortisol measurement by RIA was performed using an adapted laboratory routine.21 The concentration in μg/dL was calculated using the RIACALC software (WallacOy) and the results were corrected for the initial volume and the dilution of the samples. The serum cortisol levels within the clinical application interval lied between 2.00 and 25.0 μg/dL27,28, while, according to the literature, the morning levels of salivary cortisol in adults are higher than 486 ng/dL, and the nocturnal concentrations vary from 36.4 to 302 ng/dL.29 The samples numbered from 13 to 30 (Table 3) were evaluated for cortisol in total urine and, whenever possible, in serum. Discrepancy was observed in five (27.8%) evaluations of this group.
The results obtained were evaluated for efficiency in screening for Cushing syndrome by establishing a specific cutoff score, as proposed in the literature.30 Thus, values of 100.0 and 69.0% were obtained for specificity and sensitivity, respectively; these parameters represent the ability of the method to recognize a truly negative or positive condition, respectively. Based on a conclusive diagnosis of Cushing's syndrome and using the statistical tool described above, we suggested a reference value which is appropriate for screening this syndrome by measuring free urinary cortisol. The proposed reference value is 50.0 μg/24 h which provides an increase of sensitivity to 75% without harming the analytical specificity. Similarly, a borderline value of 41.0 μg/24 h was found for free urinary cortisol. Therefore, results in the range from 41.0 to 50.0 μg/24 h are suggestive of Cushing's syndrome, however inconclusive, due to the possibility of false-positive (7%) and -negative (19%) results.
CONCLUSION
A method using RP-HPLC is proposed that was shown to be sensitive and specific for the determination of free urinary cortisol, with advantages over radioimmunoassay because it does not produce radioactive waste and is less time-consuming. The results obtained indicate that the proposed methodology can be useful as a diagnostic tool in patients suspected to have Cushing's syndrome. Further studies should be conducted for a better evaluation of the possibility of establishing a new reference value.
ACKNOWLEDGEMENTS
This study received financial support from Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq (478243/2004-9).
Recebido em 29/10/08; aceito em 21/7/09; publicado na web em 12/1/10
-
1http://www.endocrine.niddk.nih.gov/pubs/cushings/cushings.htm, acessada em Fevereiro 2009.
» link - 2. Vieira, J. G. H.; Tachibana, T. T.; Noguti, K. O.; Ferrer, C. M.; Maciel, R. M. B.; J. Bras. Patol 1999, 35, 71.
- 3. Ching, S. Y.; Lim, E. M.; Beilby, J.; Bhagat, C.; Rossi, E.; Walsh, J. P.; Pullan, P.; Ann. Clin. Biochem 2006, 43, 402.
- 4. Lin, C.; Wu, T.; Machacek, D. A.; Jiang, N.; Kao, P. C.; J. Clin. Endocrinol. Metab. 1997, 82, 151.
- 5. Taylor, R. L.; Machacek, D.; Singh, R. J.; Clin. Chem. 2002, 48, 1511.
- 6. Turpeinen, U.; Markkanen, H.; Välimäki, M.; Stenman, U.; Clin. Chem. 1997, 43, 1386.
- 7. Turpeinen, U.; Stenman, U. H.; Scand. J. Clin. Lab. Invest. 2003, 63, 143.
- 8. Gatti, R.; Cappellin, E.; Zecchin, B.; Antonelli, G.; Spinella, P.; Mantero, E.; De Palo, E. F.; J. Chromatogr.. B 2005, 824, 51.
- 9. Rouits, E.; Celle, M. B.; Morel, A.; Gamelim, E.; J. Chromatogr., B 2003, 793, 357.
- 10. Furuta, T.; Mori, C.; Suzuki, A.; Shibasaki, H.; Yokokawa, A.; Kasuya, Y.; J. Chromatogr., B. 2004, 801,165.
- 11. Al Sharef, O.; Feely, J.; Kavanagh, P. V.; Scott, K. R. Sharma, S. C.; Biomed. Chromatogr. 2007, 21, 1201.
- 12. Vieira, J. G. H.; Nakamura, O. H.; Cravalho, V. M.; Arq. Bras. Endocrinol. Metab 2005, 49, 291.
- 13. Shibasaki, H.; Furuta, T.; Kasuya, Y.; J. Chromatogr., B 1997, 692, 7.
- 14. Furuta, T.; Matsuzawa, H.; Kasuya, Y.; J. Chromatogr., B 2000, 738, 367.
- 15. Palermo, M.; Shackleton, C. H.; Mantero, F.; Stewart, P. M.; Clin. Endocrinol. 1996, 45, 605.
- 16. Wood, L.; Ducroq, D. H.; Fraser, H. L; Gillingwater, S.; Evans, C.; Pickett, A. J.; Rees, D. W.; John, R.; Turkes, A.; Ann. Clin. Biochem 2008, 45, 380.
- 17. Soldin, S. J.; Soldin, O. P.; Clin. Chem 2009, 55, 1061.
- 18. Fernandes, V. T.; Ribeiro-Neto, L. M.; Lima, S. B.; Vieira, J. G. H.; Verreschi, I. T. N.; Kater, C. E.; J. Chromatogr. Sci. 2003, 41, 251.
- 19. Sugawara, E. K.; Ribeiro-Neto, L. M.; Fernandes, V. F. T.; Kater, C. E.; Verreschi, I. T. N.; Rev. Bras. Cien. Farm. 2004, 40, 327.
- 20. Vieira, J. G. H.; Russo, E. M. K.; Maciel, R. M. B.; Germek A. O.; Antunes, L. A. N.; Rev. Bras. Patol. Clin 1979, 15, 125.
- 21. Wei, J. Q.; Wei, J. L.; Zhou, X. T.; Cheng, J. P.; Biomed. Chromatogr 1990, 4, 161.
- 22. Sugawara, E. K.; Ribeiro Neto, L. M.; Oliveira, K. C.; Verreschi, I. T. N.; J. Bras. Patol. Med. Lab 2008, 44, 337.
- 23. Aburuz, S.; Millership, J.; Heaney, L.; Mcelnay, J.; J. Chromatotogr., B 2003, 798, 193.
- 24. Hu, Z.; Gong, Q.; Hu, X.; Wang, L.; Cao, Y.; Cao, W.; Yu, Q.; Cheng, Z.; J. Chromatogr., B 2005, 826, 238.
- 25. Zhang, Y.; Wu, H. L.; Ding, Y. J.; Xia, A. L.; Cui, H.; Yu, R. Q.; J. Chromatogr., B 2006, 840, 116.
- 26. Nassar, A. E.; Varshney, N.; Getek, T.; Cheng, L.; J. Chromatogr. Sci 2001, 39, 59.
-
27http://www.fleury.com.br/Sist/manual_exames/Pages/Coleta.aspx?usuario=medico, acessada em Novembro 2007.
» link -
28http://www.mayomedicallaboratories.com/test-catolog/Clinical+and+Interpretive/8545, acessada em Junho 2009.
» link - 29. Kiess, W.; Ptaffle, R.; J. Pediatr. 2007, 83, 97.
- 30. Portney, L. G.; Watkins, M. P.; Foundations of Clinical Research Applications to Practice, 1st ed., Appleton & Lange: Connecticut, 1993, cap. 6.
Publication Dates
-
Publication in this collection
12 Mar 2010 -
Date of issue
2010
History
-
Received
29 Oct 2008 -
Accepted
21 July 2009