Abstract
Two alkaloids, erysodine (1) and erysothrine (2) were isolated from the flowers of a Pakistani medicinal plant, Erythrina suberosa. These compounds were investigated for anxiolytic properties, and the results showed significant effect, in an acute oral treatment with 1-2, which were suspended in saline (NaCl 0.9%) plus DMSO 1%, and evaluated in 122 Swiss male mice exposed to two tests of anxiety - the elevated plus-maze (EPM) and the light/dark transition model (LDTM).
anxyolitic activity; E. suberosa; erythrinian alkaloids
ARTIGO
Anxiolytic-like effects of erythrinian alkaloids from erythrina suberosa
Maria Amélia Rodrigues SerranoI; Andrea Nastri de Luca BatistaI; Vanderlan da Silva BolzaniI; Luciana de Ávila SantosI, * * e-mail: deavilasantos@yahoo.com.br ; Paulo José de Campos NogueiraII; Ricardo Luiz Nunes-de-SouzaII; Abdul LatifIII; Mohammad ArfanIII
IInstituto de Química, Universidade Estadual Paulista, CP 355, 14801-970 Araraquara - SP, Brasil
IIFaculdade de Ciências Farmacêuticas, Universidade Estadual Paulista, 14801-902 Araraquara - SP, Brasil
IIIInstitute of Chemical Sciences, University of Peshawar, Peshawar, Pakistan
ABSTRACT
Two alkaloids, erysodine (1) and erysothrine (2) were isolated from the flowers of a Pakistani medicinal plant, Erythrina suberosa. These compounds were investigated for anxiolytic properties, and the results showed significant effect, in an acute oral treatment with 1-2, which were suspended in saline (NaCl 0.9%) plus DMSO 1%, and evaluated in 122 Swiss male mice exposed to two tests of anxiety - the elevated plus-maze (EPM) and the light/dark transition model (LDTM).
Keywords: anxyolitic activity; E. suberosa; erythrinian alkaloids.
INTRODUCTION
The genus Erythrina is comprised of about 100 species in the tropics, half of which have remained under investigation.1 The species of this genus are famous for different classes of compounds like alkaloids, flavonoids and terpenes.1,2 The alkaloids isolated from different species of Erythrina show activities like hypotensive, anticonvulsant, hypnotic, and analgesic.3Erythrina species have been a part of traditional folk medicines because of their anxiolytic properties.4Erythrina suberosa Roxb. Belonging to the family Fabaceae, is an ornamental tall tree found in Pakistan from Islamabad up north to Peshawar. This tree is planted on road sides, residential areas and public parks due to its bright red colored flowers in the form of bunches that bloom from May to June. It is used as a calming drink, and is prepared from the combined aqueous extract of the flowers of this tree and that of Hibiscus rosa sinensis that has refreshing, soothing and relaxing effect during hot summer season.
The ethanolic extract of the flowers of E. suberosa was fractionated to give an alkaloidal fraction which yielded two known alkaloids erysodine (1) and erysothrine (2) by silica gel column chromatographic separation. The structures of the two alkaloids were confirmed from spectroscopic analysis and by comparison with the previously available data.5-7 Herein, we report the anxiolytic activity of the two isolated alkaloids.
EXPERIMENTAL
Plant material
The flowers of Erythrina suberosa were collected in April 2007 from the campus of University of Peshawar, Peshawar, Pakistan where this plant is cultivated as an ornamental plant. A voucher specimen of the areal parts along with flowers has been identified by Mr. Samin Jan, Lecturer, Islamia College University, Peshawar, Pakistan and submitted to the herbarium at Islamia College University, Peshawar, Pakistan with a voucher number ES-123.
Preparation of extracts
Dried flowers (4.5 kg) were extracted with ethanol using cold percolation method. The solvent was evaporated under reduced pressure to afford a crude extract (715 g). This crude extract was dissolved in 800 mL of methanol/water (8:2 v/v) and acidified to pH 2 with 1N HCl. The acidified solution was extracted with ethyl acetate (3 × 800 mL) and, the resultant hydrochloric acid solution was then made alkaline (pH 10) using concentrated NH4OH. After that, it was re-extracted with ethyl acetate (3 × 730 mL) to afford a mixture of crude alkaloids (35 g).
Isolation and identification of active compounds
The alkaloidal fraction (35 g) was subjected to column chromatography over silica gel (200 µm, 25 × 7 cm) eluted with a gradient of dichloromethane-methanol (100:0 → 99.5:0.5 → 99:1 → 98.5:1.5 → 98:2 → 97.5:2.5 → 97:3 → 96.5:3.5 → 96:4 → 95.5:4.5 → 95:5 → 94:6 → 93:7) to yield 13 fractions (A to M, 15mL each). Fractions D-K were pooled together (9.6 g) and then subjected to another column chromatography over silica gel (200 µm, 23 × 5 cm) with a gradient elution using chloroform-methanol (100:0 → 99:1 → 98:2 → 97:3 → 96:4 → 95:5 → 94:6 → 93:7) yielding 8 fractions (A to H, 15 mL each). The fractions D and E afforded compound 1 (44 mg) and 2 (180 mg), respectively.
The compounds were identified as erysodine and erysothrine. Their structures were confirmed by using modern spectroscopic techniques of 1H, 13C, and 2D NMR (COSY, NOESY, HMBC, HMQC, and NOE techniques) using Varian Inova 500 FTNMR spectrometer and by comparison with the literature data.5,7
Subjects
Twelve groups (n=8-12/group) of Swiss male mice from São Paulo State University/UNESP, SP, Brazil, weighing 25-35 g, were housed in groups of 10 per cage (41 x 34 x 16 cm) and maintained under a normal 12-h light cycle (lights on 07:00 h) in a temperature/humidity controlled environment (23 ± 1 °C/55 ± 5%) with free access to food and water. All animals were experimentally naive. Experimental procedures were in compliance with the US National Institutes of Health Guide for Care and Use of Laboratory Animals and were approved by the Local Ethical Committee (UNESP-Araraquara/SP, nr 35/2004).
Compound administration
Erysodine and erysothrine were suspended in saline (NaCl 0.9%) + 1% Dimethyl sulfoxide (DMSO). Animals were orally treated by gavage with these compounds (3 and 10 mg/kg). These doses were based in previous studies from our laboratory.8,9 A positive control group receiving intraperitoneal injection of diazepam (DZP, 2 mg/kg) was also included in the study. All compounds were administered 30 min before testing.
Apparatus
Elevated plus-maze (EPM)
The EPM comprised two open arms (30 x 5 cm) and two closed arms (30 x 5 x 15 cm) that extended from a common central platform (5 x 5 cm). The apparatus was constructed from glass (clear walls) and wood (floor) and elevated 38.5 cm above floor level.
Light-dark transition model (LDTM)
The LDTM was a Plexiglas box (44.5 x 37 x 25 cm) divided by a barrier with a door way (7 x 7.5 cm) through which mice could cross between two chambers, one made by white walls (27 x 37 x 25 cm) and illuminated by a white light (500 lux; illuminated compartment) and one made by black walls (17 x 37 x 25 cm) and illuminated by a red light (25 lux, dark compartment).
Procedure
The mice were treated with erysodine or erysothrine (0, 3 or 10 mg/kg; p.o.) and 30 min later, they were individually exposed in the EPM or LDTM. EPM: Each animal was placed in the central square facing an open arm, and allowed to freely explore the EPM. The following spatio-temporal measures were recorded during a 5 min test: number of open and closed arm entries (arm entry: all four paws into an arm), % open entries [(open/total) x 100], and % open time [(time open/300) x 100].
Percentage open arm entries and percentage open arm time were used as anxiety indices, and frequency of closed arms entries was used as locomotor activity. LDTM: Each animal was placed at the center of the illuminated compartment and, after the first passage to the dark compartment, the behavior of the animal was video recorded for 5 min to further analysis of the number of transition between compartments and the time spent in the illuminated compartment. Entry into a compartment was recorded when the animal crossed with all four paws the line that separated the two compartments.
Statistics
All results were submitted to one-way analysis of variance (ANOVA), followed by Duncan's multiple comparison test when significant. In all cases a p value < 0.05 was considered significant.
RESULTS AND DISCUSSION
Isolated compounds
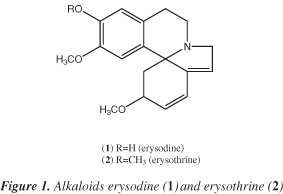
Fractionation of crude ethanolic extract led to the isolation of alkaloids erysodine (1) and erysothrine (2), Figure 1. They were identified by comparison of obtained spectral data (Table 1) with those reported in the literature for these compounds.5,7
Elevated plus-maze
Figure 2 shows the effects of erysodine (0, 3 or 10 mg/kg, p.o.), erysothrine (0, 3 or 10 mg/kg, p.o.) and DZP (2 mg/kg, i.p.) on the behavior of mice in the EPM. One-way ANOVA revealed significant differences of treatment on % open arms time [F(5,55)=12,21; p<0.01] and % open arms entries [F(5,55)=17,59; p<0.01]. Post hoc comparisons revealed that the significant differences were produced by DZP treatment in both parameters (p<0.01) and by erysothrine (10 mg/kg) in the % open arms entries (p<0.01). One-way ANOVA followed by Duncan's test also revealed that DZP treatment significantly decreased frequency of closed arms entries [F(5,55)= 2.78; p=0.03].
Light-dark transition model
Figure 3 shows the effects of erysodine (0, 3 or 10 mg/kg, p.o.), erysothrine (0, 3 or 10 mg/kg, p.o.) and DZP (2 mg/kg, i.p.) on the behavior of mice in the LDTM. One-way ANOVA revealed significant differences of treatment on time spent in the illuminated compartment [F(5,55)= 2,68; p=0.03] and transitions between both compartments [F(5,55)= 3,19; p=0.01]. Post hoc comparisons revealed that DZP and Erysodine (10 mg/kg) treated animals spent more time in the illuminated compartment (p<0.05). Duncan's test also revealed significant differences of treatment on the number of transitions between both compartments of the LDTM for erysodine (10 mg/kg, p=0.02) and erysothrine (3 mg/kg, p<0.01).
DISCUSSION
The results of the present study demonstrated that acute p.o. treatment with the E. suberosa alkaloids erysodine and erysothrine produced anxiolytic-like effects in mice exposed to a two widely used anxiety tests - the elevated plus-maze and the light-dark transition model.
In the EPM, only erysodine (10 mg/kg) increased percent of open arm entries and tended to increase percent of open arm time, the two conventional measures of anxiety in the EPM. Importantly, the anxiolytic-like effects of erysodine were selective for anxiety measures, since this alkaloid did not change closed arms entries, the measure widely used as an index of general activity in the EPM.10
In the LDTM, both erysodine and erysothrine produced anxiolytic-like effects. However, while erysodine (10 mg/kg) increased both time spent in the illuminated compartment and the number of transitions between compartments, erysothrine (3 mg/kg) increased the number of transitions only. Nevertheless, it must be emphasized that the lower dose of erysodine (3 mg/kg) and the two doses of erysothrine (3 and 10 mg) also tended (p < 0.08) to increase the time spent in the aversive place (i.e., the illuminated compartment). In addition, although both alkaloids have increased the number of transitions between compartments, which in turn could also be interpreted as an effect on locomotor behavior, neither erysodine nor erysotrine changed locomotion (i.e., the number of closed arm entries) in the EPM.
Evidence suggesting that plants from genus Erythrina have anti-anxiety effects have been demonstrated elsewhere.4,7-9 For instance, recently Flausino et al.8 have reported that a new erythrinian alkaloid, (+)-11-α-hydroxyerytravine (OH-Ery), and the two known erythrinian alkaloids, erytravine (Ery) and (+)-α-hydroxyerysothrine (Eryso), isolated from Erythrina mulungu impaired the inhibitory avoidance acquisition of the open arms in the elevated T-maze, an animal model of anxiety validated for rats11 and mice.12 Flausino et al.9 also observed that acute p.o. treatment with the alkaloids erythravine and (+)-11-α-hydroxyerythravine produced anxiolytic effects in the LDTM.
It is noteworthy that the benzodiazepine diazepam, a classic anxiolytic drug that was used as positive control group in the present study, also attenuated anxiety measures in both tests. Taken together these results strongly suggest that the alkaloids erysodine and erysothrine are markedly involved in the anti-anxiety activities of the E. suberosa.
ACKNOWLEDGMENT
The financial support from the Higher Education Commission, Pakistan under its Indigenous Ph.D (5000) scheme to one of the co-author (Abdul Latif) is gratefully acknowledged.
REFERENCES
1. Garcia-Mateos, R.; Soto-Hernandez, M.; Kelly, D.; Biochem. Syst. Ecol. 1998, 26, 545.
2. Sarragiotto, M. H.; Filho, H. L.; Marsaioli, A. J.; Can. J. Chem. 1981, 59, 2771; Mckee, T. C.; Bokesch, H. R.; McCormick, J. L.; Rashid, M. A.; Spielvogel, D.; Gustafson, K. R.; Alavanja, M. M.; Cardellina, J. H.; Boyd, M. R.; J. Nat. Prod. 1997, 60, 431.
3. Hargreaves, R.; Jonson, D.; Millington, D.; Mondal, M.; Beavers, W.; Becker, L.; Young, C.; Rinehari, K. L.; Lloydia 1974, 37, 569.
4. Garín-Aguilar, M. E.; Luna, J. E. R.; Soto-Hernández, M.; del Toro, G. V.; Vázquez, M. M.; J. Ethnopharmacol. 2000, 69, 189.
5. Amer, M. E.; Shamma, M.; Freyer, A. J.; J. Nat. Prod. 1991, 54, 329.
6. Amer, M. E.; El-Masry, S.; Shamma, M.; Freyer, A. J.; J. Nat. Prod. 1991, 54, 161.
7. Wandji, J.; Awanchiri, S. S.; Fomum, Z. T.; Tillequin, F.; Libot, F.; Phytochemistry 1995, 39, 677.
8. Flausino Jr., O. A.; Santos, L. A.; Verli, H.; Pereira, A. M.; Bolzani, V. S.; Nunes-de-Souza, R. L.; J. Nat. Prod. 2007, 70, 48.
9. Flausino Jr., O. A.; Pereira, A. M.; Bolzani, V. S.; Nunes-de-Souza, R. L.; Biol. Pharm. Bull. 2007, 30, 375.
10. Cruz, A. P. M.; Frei, F.; Graeff, F. G.; Pharmacol., Biochem. Behav. 1994, 49, 171.
11. Viana, M. B.; Tomaz, C.; Graeff, F. G.; Pharmacol., Biochem. Behav. 1994, 49, 549; Zangrossi Jr., H.; Graeff, F. G.; Brain Res. Bull. 1997, 44, 1.
12. Jardim, M. C.; Nogueira, R. L.; Graeff, F. G.; Nunes-de-Souza, R. L.; Brain Res. Bull. 1999, 48, 407; Carvalho-Netto, E. F.; Nunes-de-Souza, R. L.; Behav. Brain Res. 2004, 148, 119.
Recebido em 14/7/10; aceito em 25/11/10; publicado na web em 25/2/11
- 1. Garcia-Mateos, R.; Soto-Hernandez, M.; Kelly, D.; Biochem. Syst. Ecol 1998, 26, 545.
- 2. Sarragiotto, M. H.; Filho, H. L.; Marsaioli, A. J.; Can. J. Chem. 1981, 59, 2771;
- Mckee, T. C.; Bokesch, H. R.; McCormick, J. L.; Rashid, M. A.; Spielvogel, D.; Gustafson, K. R.; Alavanja, M. M.; Cardellina, J. H.; Boyd, M. R.; J. Nat. Prod. 1997, 60, 431.
- 3. Hargreaves, R.; Jonson, D.; Millington, D.; Mondal, M.; Beavers, W.; Becker, L.; Young, C.; Rinehari, K. L.; Lloydia 1974, 37, 569.
- 4. Garín-Aguilar, M. E.; Luna, J. E. R.; Soto-Hernández, M.; del Toro, G. V.; Vázquez, M. M.; J. Ethnopharmacol. 2000, 69, 189.
- 5. Amer, M. E.; Shamma, M.; Freyer, A. J.; J. Nat. Prod. 1991, 54, 329.
- 6. Amer, M. E.; El-Masry, S.; Shamma, M.; Freyer, A. J.; J. Nat. Prod 1991, 54, 161.
- 7. Wandji, J.; Awanchiri, S. S.; Fomum, Z. T.; Tillequin, F.; Libot, F.; Phytochemistry 1995, 39, 677.
- 8. Flausino Jr., O. A.; Santos, L. A.; Verli, H.; Pereira, A. M.; Bolzani, V. S.; Nunes-de-Souza, R. L.; J. Nat. Prod. 2007, 70, 48.
- 9. Flausino Jr., O. A.; Pereira, A. M.; Bolzani, V. S.; Nunes-de-Souza, R. L.; Biol. Pharm. Bull. 2007, 30, 375.
- 10. Cruz, A. P. M.; Frei, F.; Graeff, F. G.; Pharmacol., Biochem. Behav. 1994, 49, 171.
- 11. Viana, M. B.; Tomaz, C.; Graeff, F. G.; Pharmacol., Biochem. Behav 1994, 49, 549;
- Zangrossi Jr., H.; Graeff, F. G.; Brain Res. Bull. 1997, 44, 1.
- 12. Jardim, M. C.; Nogueira, R. L.; Graeff, F. G.; Nunes-de-Souza, R. L.; Brain Res. Bull. 1999, 48, 407;
- Carvalho-Netto, E. F.; Nunes-de-Souza, R. L.; Behav. Brain Res. 2004, 148, 119.
Publication Dates
-
Publication in this collection
18 July 2011 -
Date of issue
2011
History
-
Received
14 July 2010 -
Accepted
25 Nov 2010