Abstracts
The authors address the value of endoscopic retrograde cholangiopancreatography, ultrasonography, computed tomography, magnetic resonance imaging and endoscopic ultrasound for the diagnosis of cystic and solid neoplasms of the pancreas, demonstrating that each of them is of great importance to undoubtedly increase the diagnostic accuracy of the biliopancreatic system diseases. The best method for each of several tumors is then determined.
Diagnostic imaging; Neoplasms; Neoplasms, cystic, mucinous, and serous; Pancreas
Os autores fazem uma revisão considerando o valor da colangiopancreatografia endoscópica retrógrada, da ultrassonografia, da tomografia computadorizada, da ressonância magnética e da ecoendoscopia para o diagnóstico das neoplasias císticas e sólidas do pâncreas, demonstrando que cada um deles tem grande importância para aumentar, de forma inconteste, a acurácia diagnóstica das doenças do sistema biliopancreático. determinando qual o melhor método para cada um dos diversos tumores.
Diagnóstico por imagem; Neoplasias; Neoplasias císticas mucinosas e serosas; Pâncreas
REVIEW
IProfessor, Department of Surgery and Anatomy, Faculty of Medicine of Ribeirão Preto - University of São Paulo, São Paulo - SP, Brazil
IIAffiliate Professor, Department of Diagnostic Imaging DDI, São Paulo Federal University - UNIFESP, São Paulo - SP, Brazil
IIIStaff, Second General Surgery Clinic, Rio de Janeiro State Servers Hospital - Rio de Janeiro - RJ, Brazil
Mailing address
ABSTRACT
The authors address the value of endoscopic retrograde cholangiopancreatography, ultrasonography, computed tomography, magnetic resonance imaging and endoscopic ultrasound for the diagnosis of cystic and solid neoplasms of the pancreas, demonstrating that each of them is of great importance to undoubtedly increase the diagnostic accuracy of the biliopancreatic system diseases. The best method for each of several tumors is then determined.
Key words: Diagnostic imaging. Neoplasms. Neoplasms, cystic, mucinous, and serous. Pancreas.
INTRODUCTION
In the 1960s the biliopancreatic system was considered a restricted area for an approach with diagnostic imaging methods. In the early 1970s the Endoscopic Retrograde Cholangiopancreatography (ERCP) was introduced and the accurate diagnosis, etiology and location began to be determined with a high level of accuracy1.
Abdominal ultrasound (US), introduced in the late 1970s, increased identification of diseases that affected the biliopancreatic tree in a less invasive way2. With the use of in real time, sectorial and high resolution systems for US, the gallbladder, the extrahepatic biliary tree and the pancreatic gland could be better studied3.
Also in the 1970s computed tomography (CT) was introduced in daily clinical practice and revolutionized medical diagnosis by images. The rapid acceptance of the method and its real diagnostic capacity gave the Nobel Prize for medicine to its creators, Godsfrey Hounsfield and Alan Cormack4.
The principle of magnetic resonance imaging (MRI) is known since the 1940s, but only in the 1970s the first medical images were obtained through MRI5.
As if all these were not enough, the endoscopic ultrasound experimentally begun almost at the same time. Also known as endosonography or ecoendoscopy (EE), it is a technique that allows the placement of a transducer ultrasound in all points accessible to classic endoscopy. Thus, organs and regions less accessible to other methods of image, such as the pancreas, the distal portion of the biliary tree and the papilla are studied with unparalleled accuracy6.
These methods have undergone modifications over time and their high problem-solving capacity has shown that each of them has a fundamental role to undoubtedly increase the diagnostic accuracy of the biliopancreatic system's diseases.
The purpose of this revision is to clearly and succinctly demonstrate the actual role of each of these exams for each of the various cystic and solid tumors of the pancreas, comparing them and determining which method is best for each of the several lesions.
This article will be presented in two parts: the first will address the methods of image in cystic Neoplasms; the second will address the study of methods of image in solid Neoplasms of the pancreas.
Cystic Neoplasms
Pancreatic cystic neoplasms are rare tumors, contributing with only 10% to 15% of all pancreatic cysts and 1% of pancreatic cancers. These tumors were classified as cystic neoplasms, composed by mucinous cystadenoma, cistadenocarcinoma, mucinous papillary intraductal neoplasm and, finally, the microcystic adenoma, also known as serous cystadenoma.
These clinical subtypes are very important, since the microcystic adenomas are benign and asymptomatic and therefore do not require any kind of treatment. While all the others are presently considered to be premalignant lesions of the pancreatic carcinoma.
Mucinous Cystic Neoplasm
All mucinous cystic neoplasms should be treated surgically, for it is believed that the mucinous cystadenomas coexist with mucinous cystadenocarcinomas or evolve into them.
Mucinous Cystadenoma
With characteristically macrocystic morphology, they are unilocular cystic lesions, with well differentiated walls from the rest of the pancreatic parenchyma and may be divided into multiple compartments by thin septa with or without a thick content that corresponds to mucin. The presence of localized thickening, irregularity of the wall or solid component suggests malignant degeneration to cystadenocarcinoma7. At US and EE these cystic neoplasms seem similar to pseudocysts. They contain cysts with diameters larger than 2 cm, anechoic, with posterior enhancement and internal septations. The septa are thin and, to the extent that gain is increased, the cystic areas get filled with echoes. In 10 to 18%, calcifications are present in the wall and are seen as echogenic areas with shadows. These cysts may not be defined as benign or malignant.
CT (Figures 1A and 1B) and MRI (Figure 2) have important roles in the diagnosis of this type of lesion, as well as its differentiation from pseudocysts. The differentiation is also based on clinical data, because the image aspects are sometimes superimposable. Many authors believe that if there is no history of acute pancreatitis with possibility of evolution to a pseudocyst, one must consider the a cystic lesion as potentially neoplastic and approach it surgically. Evaluation with these exams also includes staging of the lesion, searching for hepatic metastasis and peritoneal implants. Despite their current skills, the complete clinical characterization of such a lesion, when incidentally found, is around 25% 30%8-10.
The fine needle aspiration (FNA) guided by EE of this neoplasia is relatively easy and the cytological analysis of the aspirated liquid may show the presence of columnar epithelial cells (benign or malignant) and mucin. The most serious complication of a pancreatic cyst puncture is contamination and abscess formation, which may be avoided by completely emptying the cyst and with antibiotic prophylaxis (Figure 3)11.
Cistadenocarcinoma
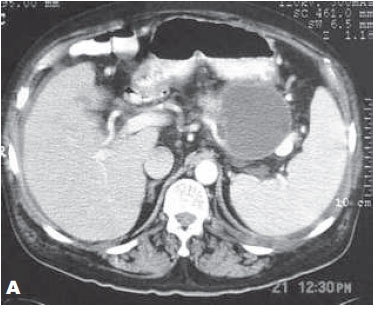
Some researchers identified four sonographic patterns associated with this type of cyst: 1) anechoic mass with posterior enhancement and irregular margins; 2) anechoic mass with inner homogeneous echoes that are laminated in supine position and moving with lateral decubitus; 3) anechoic mass, with internal regular vegetations that protrude into the lumen without showing movements; and 4) completely echogenic and heterogeneous mass12. The moderrn equipment of CT (Figure 4) and MRI with associated Magnetic Resonance Cholangiopancreatography (MRCP) (Figure 5) provide detailed information of pancreatic cysts, like: presence of septa, size, location and communication with the main pancreatic duct (MPD). In rare cases it may provide data on the presence of nodules or vegetation in the interior of the cysts. The critical analysis of these factors is important to differentiate a mucinous adenoma (MAC) cyst from a mucinous papillary intraductal neoplasia (MPIN)13,14.
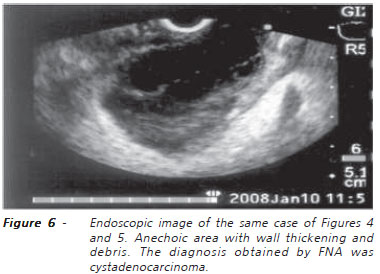
EE-FNA is relatively simple. With a single needle passage, the tip is placed in the center of the cyst and all content is aspirated. Once aspirated between 2-3 ml of clear liquid, the tip of the needle may be advanced through the wall or through a thin septum. This technique improves liquid outflow (Figure 6). Eventually, the viscosity of the liquid may impede the aspiration of its contents. The use of a needle of large caliber associated with prolonged aspiration usually provides enough material for analysis. Occasionally, bleeding may occur at the location of the puncture and this rarely happens inside the cyst. Should this happen, it tends to stop when the cyst fills with blood15.
Mucinous Papillary Intraductal Neoplasia
The MPIN consists not only of a distended MPD, but also peripheral cystic lesions, nodules or vegetations, with mass effect. It is difficult to differentiate the MPIN from chronic pancreatitis (PC) based on the dilatation of the MPD. If there are not parenchymal lesions that suggest chronic pancreatitis, the MPD should be aspirated. Sometimes a focal lesion adhered to the wall may be seen in the MPD of patients with MPIN. These nodules, when carefully studied and aspirated, may render diagnosis of focal malignancy. Cystic lesions associated to MPIN have a wide range of presentations and may simulate serous cystadenomas (SCA) or microcysts. The large unilocular cistadenomas are commonly found in advanced cases of MPIN and must be aspirated because of the possibility of an early stage malignant tumor. The lesions that have mass effect look similar to pancreatic adenocarcinoma and must be aspirated to the diagnosis of malignancy or of a focal chronic pancreatitis nodule.
At US these lesions are difficult to distinguish. Radiological methods demonstrate focal or diffuse ductal dilatation and sometimes filling failure images may be observed within the MPD, corresponding to the tumor or impacted mucin. As these tumors are small, the predominant radiological aspect may be similar to that observed in chronic pancreatitis with ductal dilatation and advanced atrophy. It is important to highlight that parenchymateous calcifications are usually not seen in cases of MPIN. They may occur only in extremely advanced cases (Figure 7).
Although MRCP may demonstrate ductal changes, endoscopic pancreatography is vital to the diagnosis, confirming the filling failure images, identifying the output of mucoid secretion through the duodenal papilla and enabling the access to such material and its cytological and histopathological exams (figures 8a and 8b). Cytology of material aspirated from the distended MPD or from a MPIN-associated cyst demonstrates an aspect similar to MAC, with malignant or benign columnar epithelial cells, generally associated with large amounts of mucin16,17.
Cystic Serous Neoplasia
Serous cystadenomas usually display a microcystic component, with a typical "honeycomb" endosonographic aspect, although they may also be macrocystic and unilocular, which may display area of fibrosis or a central scar. These injuries are potentially benign and their echotextural characteristics are very suggestive of this tumor type18.
In this disease the US very accurately identifies a nodular area with multiple microcysts. Abundant fibrous stroma and small dimensions of cysts may generate a radiological aspect of a solid neoplasm. However, the careful review of all phases of the exam generally allows the identification of small cysts. The hypervascularity of numerous fibrous septa causes an intense early enhancement after intravenous administration of contrast medium at CT and MRI (Figure 9). The ERCP has no diagnostic function in this type of cystic neoplasm13,14.
Pseudopapilary Solid-Cystic Epithelial Neoplasia (Frantz's tumor)
This is a pseudopapilary solid-cystic epithelial neoplasia located in the tail of the pancreas, which is often seen in young women (average age 24 years). It is an unusual, low-grade malignant tumor, curable by surgical removal. Usually the patient has no symptoms until the tumor is large. Often multiple areas of hemorrhage and cystic degeneration are found within the tumor, but mitotic figures are rarely seen. After surgical resection, prognosis is remarkably good, although duodenal and liver invasion and distant metastasis have been reported19,20. The lesion may appear similar to the cystadenoma or cystadenocarcinoma at EE and US, as there are septa in the cystic parts, possibly due to prominent papillae projecting into the space of cystic degeneration. At US, CT and MRI a solid-cystic mass may appear. Calcifications inside the mass are not common. Although the ecographic features are indistinguishable from those of cystadenoma and cystadenocarcinoma, the asymptomatic occurrence in young women, associated with a hemorragic component, must lead to suspicion of such a tumor19,20.
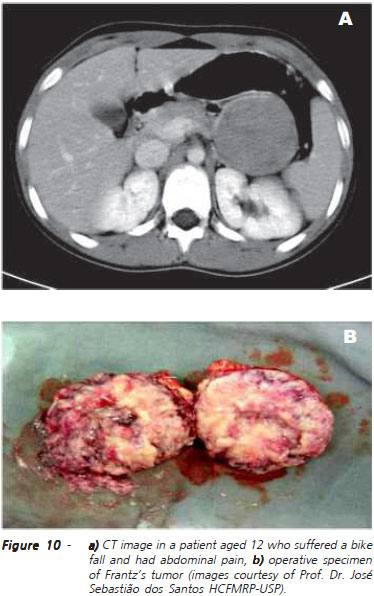
The radiological presentation of the solid-cystic epithelial neoplasia is dependent on the relationship between the solid and cystic components, with some cases being seen as predominantly solid, well demarcated masses and others with central areas of liquefaction (Figure 10). These, in turn, often present with hemorrhage, which may show increase of attenuation of the cystic content at CT or, more easily, the hiperintense signal in T1 and hypointense in at MRI; some of these tumors may present calcification in the periphery. EE-FNA of this type of injury has high accuracy and sensitivity21.
REFERENCES
- 1. McCune WS, Shorb PE, Moscovitz H. Endoscopic cannulation of the ampulla of vater: a preliminary report. Ann Surg 1968; 167(5):752-6.
- 2. Behan M, Kazam E. Sonography of the common bile duct: value of the right anterior oblique view. AJR Am J Roentgenol 1978; 130(4):701-9.
- 3. Kwa A, Bowie JD. Transducer selection for pancreatic ultrasound based on skin to pancreas distance in the supine and upright position. Radiology 1980; 134(2):541-2.
- 4. Hounsfield GN. Computed medical imaging. Nobel lecture, Decemberr 8, 1979. J Comput Assist Tomogr 1980; 4(5):665-74.
- 5. Hawkes RC, Holland GN, Moore WS, Worthington BS. Nuclear magnetic resonance imaging-an overview. Radiography 1980; 46(551):253-5.
- 6. Hawes RH. Normal endosonographic findings. Gastrointest Endosc 1996; 43(2 Pt 2):S6-10.
- 7. Moparty B, Logroño R, Nealon WH, Waxman I, Raju GS, Pasricha PJ, et al. The role of endoscopic ultrasound and endoscopic ultrasound-guided fine-needle aspiration in distinguishing pancreatic cystic lesions. Diagn Cytopathol 2007; 35(1):18-25.
- 8. Le Borgne J, de Calan L, Partensky C. Cystadenomas and cystadenocarcinomas of the pancreas: a multiinstitutional retrospective study of 398 cases. French Surgical Association. Ann Surg 1999; 230(2):152-61.
- 9. Planner AC, Anderson EM, Slater A, Phillips-Hughes J, Bungay HK, Betts M. An evidence-based review for the management of cystic pancreatic lesions. Clin Radiol 2007; 62(10):930-7.
- 10. Curry CA, Eng J, Horton KM, Urban B, Siegelman S, Kuszyk BS, et al. CT of primary cystic pancreatic neoplasms: can CT be used for patient triage and treatment ? AJR Am J Roentgenol 2000; 175(1):99-103.
- 11. Repák R, Rejchrt S, Bártová J, Malirová E, Tycová V, Bures J. Endoscopic ultrasonography (EUS) and EUS-guided fine-needle aspiration with cyst fluid analysis in pancreatic cystic neoplasms. Hepatogastroenterology 2009; 56(91-92):629-35.
- 12. Busilacchi P, Rizzatto G, Bazzocchi M, Boltro E, Candiani F, Ferrari F, et al. Pancreatic cystadenocarcinoma: diagnostic problems. Br J Radiol 1982; 55(656):558-61.
- 13. Edirimanne S, Connor SJ. Incidental pancreatic cystic lesions. World J Surg 2008; 32(9):2028-37.
- 14. Garcea G, Ong SL, Rajesh A, Neal CP, Pollard CA, Berry DP, et al. Cystic lesions of the pancreas. A diagnostic and management dilemma. Pancreatology 2008; 8(3):236-51.
- 15. Wu H, Cheng NS, Zhang YG, Luo HZ, Yan LN, Li J. Improved early diagnosis of cystadenocarcinoma of the pancreas. Hepatobiliary Pancreat Dis Int 2007; 6(1):87-91.
- 16. Ariyama J, Suyama M, Satoh K, Wakabayashi K. Endoscopic ultrasound and intraductal ultrasound in the diagnosis of small pancreatic tumors. Abdom Imaging 1998; 23(4):380-6.
- 17. Fukushima N, Mukai K, Kanai Y, Hasebe T, Shimada K, Ozaki H, et al. Intraductal papillary tumors and mucinous cystic tumors of the pancreas: clinicopathologic study of 38 cases. Hum Pathol 1997; 28(9):1010-7.
- 18. Sedlack R, Affi A, Vazquez-Sequeiros E, Norton ID, Clain JE, Wiersema MJ. Utility of EUS in the evaluation of cystic pancreatic lesions. Gastrointest Endosc 2002; 56(4):543-7.
- 19. Friedman AC, Lichtenstein JE, Fishman EK, Oertel JE, Dachman AH, Siegelman SS. Solid and papillary epithelial neoplasm of the pancreas. Radiology 1985; 154(2):333-7.
- 20. Lin JT, Wang TH, Wei TC, Sheu JC, Sung JL, How SW, et al. Sonographic features of solid and papillary neoplasm of the pancreas. J Clin Ultrasound 1985; 13(5):339-42.
- 21. Nadler EP, Novikov A, Landzberg BR, Pochapin MB, Centeno B, Fahey TJ, et al. The use of endoscopic ultrasound in the diagnosis of solid pseudopapillary tumors of the pancreas in children. J Pediatr Surg 2002; 37(9):1370-3.
Current role of imaging methods in the diagnosis of cystic solid pancreas neoplasms - part I
Publication Dates
-
Publication in this collection
16 June 2011 -
Date of issue
Apr 2011
History
-
Received
15 Jan 2010 -
Accepted
15 Feb 2010