ABSTRACT
Peritoneal carcinomatosis is the natural course of gastrointestinal, gynecologic, and primary peritoneal neoplasms. In recent years, our understanding of carcinomatosis has changed; it is no longer considered a disseminated condition, but rather a disease confined to the peritoneum. Thus, the combination of cytoreductive surgery and intraperitoneal chemotherapy has become the cornerstone of control of peritoneal metastases. Traditionally, intraperitoneal chemotherapy is delivered in the form of liquid solutions. However, a new mode of chemotherapy delivery to the abdominal cavity has arisen as an alternative to the conventional method. In Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC), the liquid solution is aerosolized into a spray, potentiating the distribution and penetration of the chemotherapeutic agent intraperitoneally. The present study aims to describe a novel form of this innovative surgical technique performed for the first time in Brazil, in a modification of the technique originally described for PIPAC: delivery through a single-port device.
Keywords:
Peritoneal Neoplasms; Cytoreduction Surgical Procedures; Antineoplastic Agents; Neoplasm Metastasis /prevention & control; Peritoneum
RESUMO
A carcinomatose peritoneal é a evolução natural das neoplasias gastrointestinais, ginecológicas e primárias do peritônio. Nos últimos anos, a carcinomatose passou a ser considerada uma doença confinada ao peritônio, e não mais uma doença disseminada. Desta forma, a associação de citorredução cirúrgica associada à quimioterapia intraperitoneal se tornou o ponto chave no controle das metástases peritoneais. Tradicionalmente, a quimioterapia intraperitoneal é aplicada utilizando soluções líquidas. Uma nova modalidade de infusão da quimioterapia na cavidade abdominal surge como uma alternativa ao método tradicional. A chamada PIPAC (Pressurized Intraperitoneal Aerosol Chemotherapy) transforma a solução terapêutica líquida em um spray aerossolizado, potencializando a distribuição e penetração da quimioterapia intraperitoneal. Este relato tem por objetivo descrever essa nova técnica cirúrgica inovadora, realizada pela primeira vez por um monoportal no Brasil, alterando a forma descrita originalmente para a aplicação da PIPAC.
Descritores:
Neoplasias Peritoneais; Procedimentos Cirúrgicos de Citorredução; Antineoplásicos; Metástase Neoplásica/prevenção & controle; Peritônio
INTRODUCTION
Peritoneal carcinomatosis is the natural course of gastrointestinal, gynecologic, and primary peritoneal neoplasms. The use of intraperitoneal chemotherapy as an alternative for the control of peritoneal metastases was first described in 1958, in the setting of carcinomatosis secondary to neoplasms of the colon11 Economou SG, Mrazek R, McDonald G, Slaughter D, Cole WH. The intraperitoneal use of nitrogen mustard at the time of operation for cancer. Ann N Y Acad Sci. 1958;68(3):1097-102.. Since then, intraperitoneal chemotherapy has been used to control peritoneal spread of cancer in several different settings. In 1996, Alberts et al.22 Alberts DS, Liu PY, Hannigan EV, O'Toole R, Williams SD, Young JA, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med. 1996;335(26):1950-5. reported improved survival with the combination of intraperitoneal chemotherapy and surgery for consolidation treatment of carcinomatosis secondary to ovarian malignancies. However, with the contemporary introduction of paclitaxel, which provided equivalent improvements in survival, this approach was abandoned and was never implemented as a routine treatment of ovarian neoplasms33 McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334(1):1-6..
Systemic chemotherapy for peritoneal metastases is palliative; its main focus is on improving quality of life and prolonging survival. The systemic chemotherapy approach to peritoneal carcinomatosis did not yield the same survival benefit observed for hematologic and lymphatic spread; patients with peritoneal disease continued to die a few months after diagnosis44 Chan CH, Cusack JC, Ryan DP. A critical look at local-regional management of peritoneal metastasis. Hematol Oncol Clin North Am. 2015;29(1):153-8.,55 Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88(2):358-63.. In the last 20 years, there has been a better understanding of carcinomatosis as part of the neoplastic spread process; it is now viewed as a disease state limited to a single "organ", the peritoneum, which has changed the outlook for treatment of this condition66 Sugarbaker PH. Observations concerning cancer spread within the peritoneal cavity and concepts supporting an ordered pathophysiology. Cancer Treat Res. 1996;82:79-100.. The combination of cytoreductive surgery and hiperthermic intraperitoneal chemotherapy (HIPEC) has since become the cornerstone of all new approaches and attempts to control peritoneal carcinomatosis77 Batista TP, Sarmento BJQ, Loureiro JF, Petruzziello A, Lopes A, Santos CC, Quadros CA, Akaishi EH, Cordeiro EZ, Coimbra FJF, Laporte GA, Castro LS, Batista RMSS, Aguiar S Júnior, Costa WL Júnior, Ferreira FO; BSSO/SBCO Committee on Peritoneal Surface Malignancies and HIPEC. A proposal of Brazilian Society of Surgical Oncology (BSSO/SBCO) for standardizing cytoreductive surgery (CRS) plus hyperthermic intraperitoneal chemotherapy (HIPEC) procedures in Brazil: pseudomixoma peritonei, appendiceal tumors and malignant peritoneal mesothelioma. Rev Col Bras Cir. 2017;44(5):530-44. Erratum in: Rev Col Bras Cir. 2017;44(6):665.. Direct delivery of chemotherapeutic agents to the intraperitoneal space has proved superior to systemic chemotherapy when evaluating characteristics such as drug concentration reached in the peritoneal space, penetration into peritoneal metastases, and chemotherapy-related toxicity88 Markman M. Intraperitoneal antineoplastic drug delivery: rationale and results. Lancet Oncol. 2003;4(5):277-83.. The evidence which led to the use of intraperitoneal chemotherapy is based on the idea of directly exposing peritoneal metastatic nodules to drug concentrations far higher than those that can be achieved with traditional systemic chemotherapy. Direct contact between tumor cells and chemotherapeutic agents in the peritoneal cavity is associated with a tumoral biological activity that is both superior and different from the performance of systemic chemotherapy, with particular advantages of intraperitoneal chemotherapy in the treatment of carcinomatosis99 Alberts DS, Young L, Mason N, Salmon SE. In vitro evaluation of anticancer drugs against ovarian cancer at concentrations achievable by intraperitoneal administration. Semin Oncol. 1985;12(3 Suppl 4):38-42..
Traditionally, intraperitoneal chemotherapy (IPC) is delivered by peritoneal lavage with a liquid solution carrying the chemotherapeutic agent. This form of delivery has several limitations regarding the homogeneity of distribution within the peritoneal cavity and poor tissue penetration. In recent years, a new modality for delivery has arisen as an alternative to conventional intraabdominal chemotherapy: Pressurized Intraperitoneal Aerosol Chemotherapy, or PIPAC. Aerosolization of intraperitoneal chemotherapy under positive pressure was first described in 2000, and has several advantages when compared with the use of liquid solutions for delivery of chemotherapy to the intraperitoneal space1010 Reymond MA, Hu B, Garcia A, Reck T, Köckerling F, Hess J, et al. Feasibility of therapeutic pneumoperitoneum in a large animal model using a microvaporisator. Surg Endosc. 2000;14(1):51-5.. This new mode of delivery is superior to traditional methods including HIPEC, early postoperative intraperitoneal chemotherapy (EPIC), and neoadjuvant intraperitoneal-systemic chemotherapy (NIPS). Administration of PIPAC involves several technical aspects related to the operative procedure itself, but also entails physical changes in operating rooms and institutional routines.
This descriptive paper aims to report on the process of implementation of this novel therapy at a dedicated peritoneal carcinomatosis unit and to describe the unique aspects of an innovative modification made to this technique, first performed in Brazil on December 12, 2017, at the Santa Rita Hospital - Porto Alegre Santa Casa de Misericórdia Complex: PIPAC delivery through a single-port device. The experimental procedure described herein was approved by the relevant Institutional Review Board (decision nº 2.172.647).
TECHNICAL NOTE
Physical aspects: the operating room
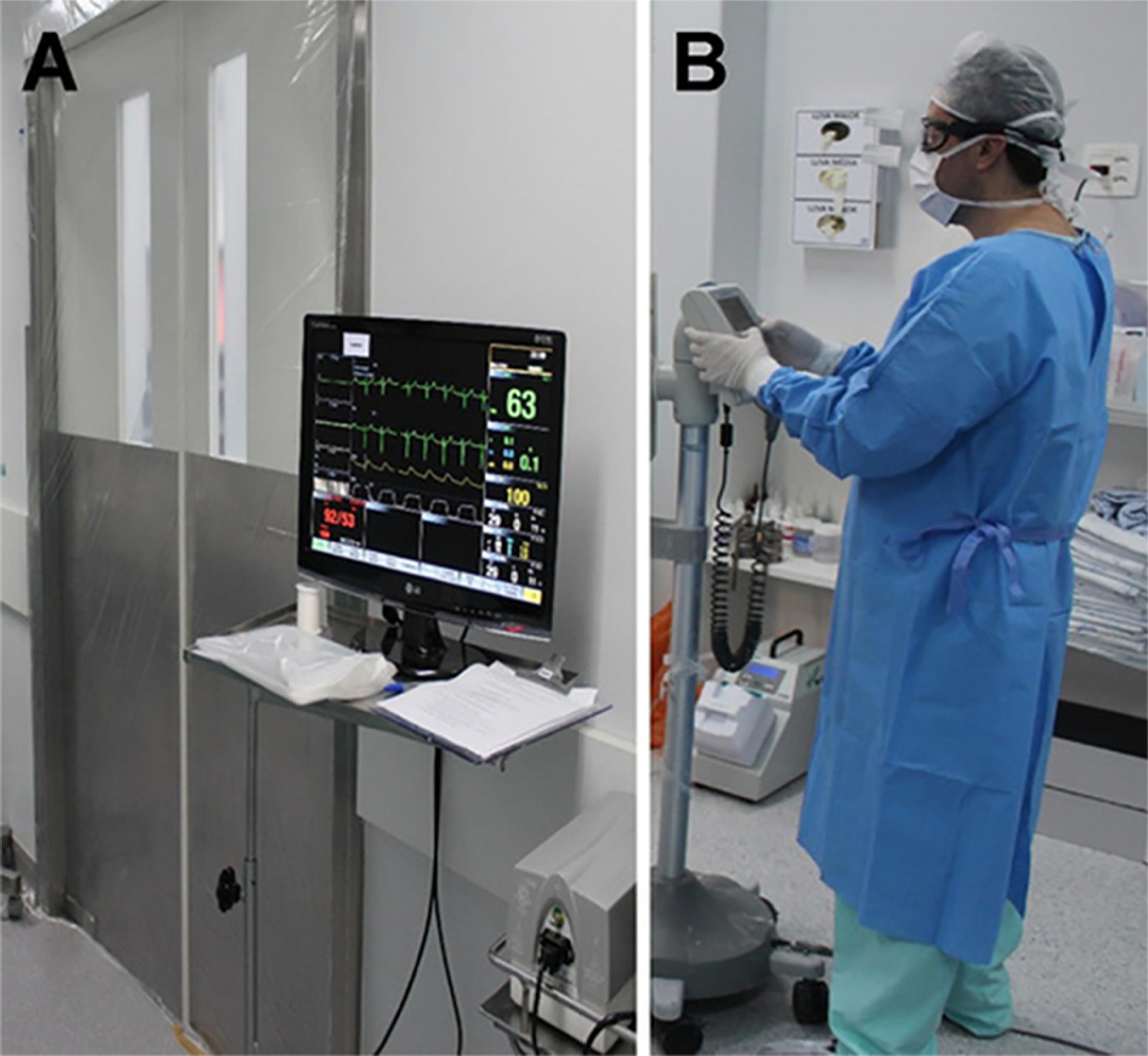
Before PIPAC is attempted, the operating room requires three basic features: negative-pressure, unidirectional (laminar) airflow ventilation and hermetically sealing doors. If these are not already available, the room must be prepared accordingly by the hospital engineering department. Unidirectional, or laminar, airflow should be achieved with exhaustion pumps that establish a negative flow of air between the corridor and the operating room. Hermetically sealing doors can be achieved by installing appropriate sliding doors or simply by fitting plastic sheetings around the doorframe, all the way to the floor (Figure 1A).
Protective equipment. A) Sealed operating room doors and remote monitoring. B) Surgeon wearing personal protective equipment while handling the chemotherapy infusion pump.
Patient monitoring must be visible outside the operating room by using a remote monitor or extension of the anesthetic monitoring screen. The control panel of the contrast injector should be available outside the operating room for activation and control. The video system should be placed to the right, at shoulder height (as for a classic laparoscopic cholecystectomy), but must be able to rotate to foot level to allow appropriate visualization of the pelvis. The infusion pump and microparticle aspirator unit should be positioned to the left of the patient. A long venous catheter with three-way stopcocks should be positioned outside the room and remain continuously accessible to the anesthesiologist throughout the procedure.
Technical aspects: personal safety and disposal of material
Handling of cytotoxic drugs in the operating room must follow strict checking and administration protocols. All supplies that are in direct contact with the surgical field should be discarded as potentially contaminated by cytotoxic agents. On should follow the routine institutional disposal procedure for such materials. This includes surgical drapes and gowns, which, for this reason, should preferably be disposable. During handling of the chemotherapeutic agent for preparation, before administration, and upon returning to the operating room after the aerosol treatment, all individuals participating in the process must wear disposable waterproof gowns, closed-vent goggles, and respirators with a microparticulate filter (Figure 1B).
Description of the procedure
Patient positioning, personal protective equipment, and special materials
Patients should be placed in the supine position and prepared as for a standard laparoscopy. The operating table should be positioned as to ensure adequate visualization of the patient (especially the head) from outside the operating room. The necessary supplies for the procedure are as follows: 1) disposable drapes; 2) BhioQap Centryport trocar; 3) BhioQap safety sheath; 4) Unidirectional BhioQap device; 5) Three-way BhioQap Centryport seal; 6) BhioQap attachment arm; 7) Operating table cover sheet; 8) Suction unit with a microparticulate filter and two sets of tubings; 9) 5-mm laparoscopic aspirator tip; 10) 5-mm laparoscopic scissors; 11) 0°, 10-mm scope; 12) chemotherapy injection set and tubing; 13) Dust and mist respirator equipped with a filter for nuisance levels of organic vapors; 14) Goggles; 15) Plastic operating-room door cover (if a hermetically sealing sliding door is not available); and 16) Sterile colostomy wafer/barrier.
Equipment
-
BhioQap: registered with the Brazilian National Health Surveillance Agency (ANVISA) with certificate of marketing authorization, no 80381210067, cleared for use since October 2017;
-
Microparticle aspirator (Buffalo Pumps);
-
IV contrast injection system with remote actuation (Bracco);
-
Visible anesthesia monitors, with remote terminal outside the operating room.
Operative technique and routine procedures
This procedure was first performed, in Brazil, at the Santa Casa de Misericórdia Complex of Porto Alegre on December 12, 2017, after systematic meetings involving surgeons, the anesthesia team, the engineering and occupational health and safety departments, the chemotherapy pharmacy, the hospitality department, and the operating room management staff. This involvement is essential for the development of any routine procedure that will include sectors outside the operating room, and must be tailored to the procedure. For the first application, a longitudinal midline incision should be made approximately 4-5cm above the umbilicus. In subsequent procedures, the incision should be performed by completely resecting the previous scar, but maintaining all other characteristics as for the previous procedure. The peritoneal cavity must be accessed using Hasson's open technique. The BhioQap Centryport should be introduced into the peritoneal cavity with the aid of a specific trocar. Pneumoperitoneum is established with a laparoscopic insufflator until the intra-abdominal pressure reaches 15mmHg, as for routine laparoscopy. The procedure can be systematically divided into two stages: 1) diagnostic; and 2) therapeutic. The first stage consists of routine ascitic fluid aspiration, quantification of carcinomatosis, and any necessary biopsies. At the Porto Alegre Santa Casa Peritoneal Diseases Treatment Program, we use the Peritoneal Cancer Index (PCI), developed by Dr. Paul Sugarbaker, for assessment and follow-up of carcinomatosis1111 Jacquet P, Sugarbaker PH. Current methodologies for clinical assessment of patients with peritoneal carcinomatosis. J Exp Clin Cancer Res. 1996;15(1):49-58.. The ascitic fluid should be measured and sent for cytology examination. Biopsies are obtained from the parietal peritoneum at sites with the highest concentration of metastatic nodules or in the same region biopsied in the previous procedure. The second stage involves the use of the BhioQap unidirectional device for aerosolization of intraperitoneal chemotherapy (Figure 2), and consists of the following sequence of procedures.
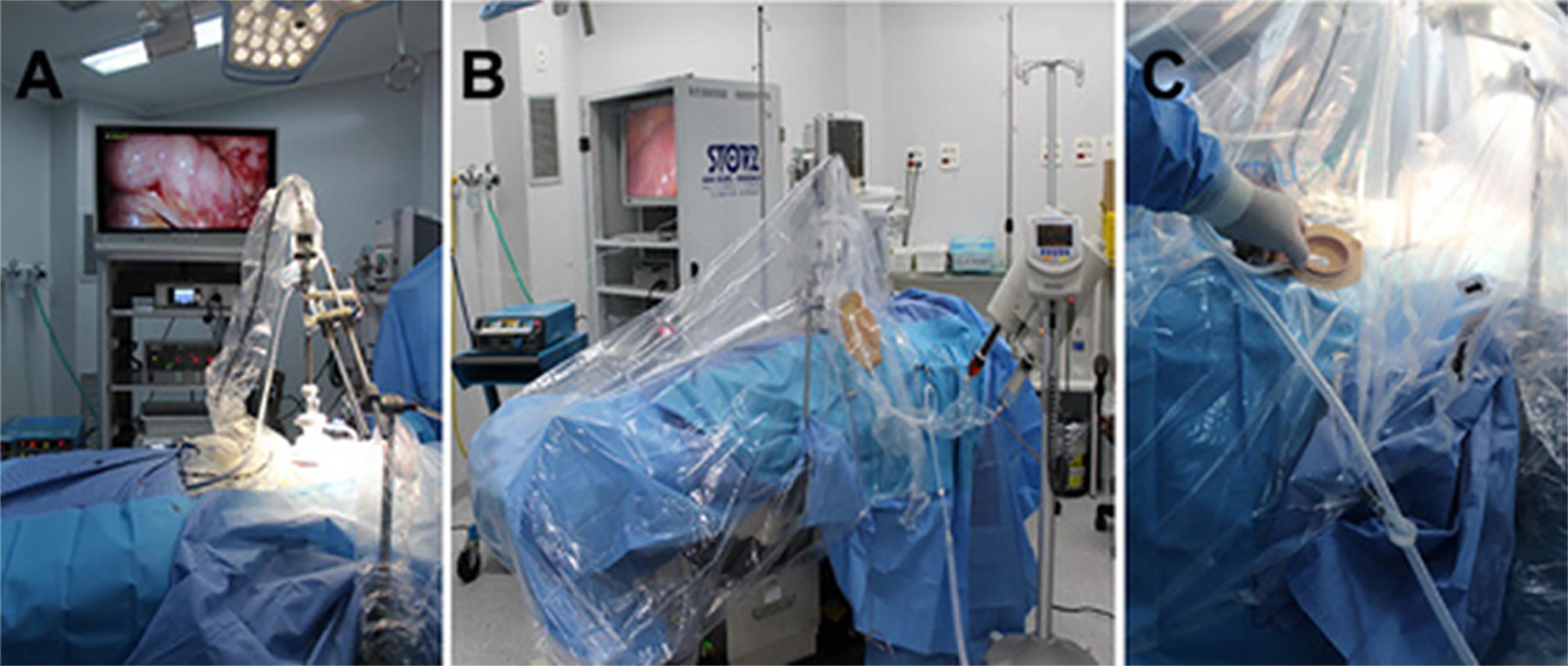
The insufflator is closed off and the peritoneal cavity is emptied. The silicone sheath of the Centryport device is opened. The BhioQap is attached to the Centryport. The insufflator tubing is attached to one Luer-Lock connector (kept open), and the microparticle aspirator tubing, to the other (kept closed). Pneumoperitoneum is established up to 12mmHg and stability is tested for approximately five minutes, to rule out any visible variation in the insufflator flow. This ensures that the peritoneal cavity is properly sealed. The 10-mm optic is positioned; once adequate visualization of the peritoneal cavity has been achieved, it is attached securely to the mechanical arm (Figure 3A). The chemotherapy injection tubing is attached to the Luer-Lock connector of the active portion of the BhioQap device. The entire operating table, mechanical arm, and optics are covered with plastic sheeting, from the patient's feet to shoulder height, and sealed with self-adhesive tape (Figure 3B). The colostomy barrier is placed onto the dome formed by the plastic sheeting and the suction tubing of the microparticle aspirator unit it passees through the barrier (Figure 3C). The chemotherapeutic agent is checked and loaded into the power injector, which is then prepared for delivery.
Procedure. A) Mechanical arm holding optics scope in place within the abdominal wall. B) BhioQap device and mechanical arm covered by a "sealed chamber" made from protective plastic sheeting. C) Suction tip passing through the colostomy baseplate into the sealed chamber.
All staff then leave the operating room, which should be closed by a hermetically sealing sliding door or sealed with plastic sheeting. The injector must be set to administer the volume calculated and compounded by the pharmacy. The infusion rate should be set to 3ml/s, and must not exceed a pressure of 300psi. Once aerosolization is completed, an intraperitoneal pressure of 12mmHg must be maintained for a period of 30 minutes (therapeutic pneumoperitoneum). After the therapeutic pneumoperitoneum period is complete, all staff return to the operating room, wearing personal protective equipment (disposable waterproof apron, dust and mist respirator equipped with a nuisance-level organic vapor filter, and eye protection). The insufflator is shut off. The aerosolized chemotherapy is suctioned through a filter into the aspirator unit. The 10-mm scope is removed. The plastic cover is opened and the BhioQap system is removed. The cavity is inspected and the abdominal wall is closed in conventional fashion, with interrupted Prolene and Monocryl 4-0 sutures.
DISCUSSION
In the setting of peritoneal carcinomatosis, PIPAC has emerged as a promising approach to a condition that still carries a guarded prognosis. It should not be understood as an alternative to the already established combination approach of cytoreductive surgery with hyperthermic chemotherapy. PIPAC seems to be more appropriate for patients who do not benefit from this aggressive surgical approach to the treatment of carcinomatosis. Aerosolization appears to be particularly useful in patients who fail to achieve complete surgical cytoreduction with zero visible disease (CC-0), have a high PCI, or have not been selected for neoadjuvant chemotherapy. In a systematic review of published articles, the complication rate was 12%, and the mean postoperative length of stay was three days1212 Grass F, Vuagniaux A, Teixeira-Farinha H, Lehmann K, Demartines N, Hübner M. Systematic review of pressurized intraperitoneal aerosol chemotherapy for the treatment of advanced peritoneal carcinomatosis. Br J Surg. 2017;104(6):669-78.. Low morbidity1313 Blanco A, Giger-Pabst U, Solass W, Zieren J, Reymond MA. Renal and hepatic toxicities after pressurized intraperitoneal aerosol chemotherapy (PIPAC). Ann Surg Oncol. 2013;20(7):2311-6., rapid recovery, and the possibility of repeated procedures makes this technique interesting in cases where intraperitoneal chemotherapy is proposed as a therapeutic plan for patients with peritoneal carcinomatosis. These features, which have made minimally invasive surgery of peritoneal disease possible, may eventually lead to a combination of resection with the PIPAC technique in patients with a high risk of carcinomatosis. This idea still requires scientific validation. However, initial studies have suggested an increase in the complication rate with such approaches, especially when the PIPAC technique is combined with cytoreductive surgery1414 Tempfer CB, Celik I, Solass W, Buerkle B, Pabst UG, Zieren J, et al. Activity of Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) with cisplatin and doxorubicin in women with recurrent, platinum-resistant ovarian cancer: preliminary clinical experience. Gynecol Oncol. 2014;132(2):307-11..
The single-port procedure described herein explores the concept of reducing the possibility of abdominal wall implants, and was first described by Robella et al.1515 Robella M, Vaira M, De Simone M. Safety and feasibility of pressurized intraperitoneal aerosol chemotherapy (PIPAC) associated with systemic chemotherapy: an innovative approach to treat peritoneal carcinomatosis. World J Surg Oncol. 2016;14:128.. The understanding of carcinomatosis as a chronic process of inflammation and implantation of neoplastic cells in the peritoneal cavity gave rise to the concept of "tumor cell entrapment". The single-port procedure, even though it is probably associated with greater inflammation than conventional laparoscopy, as suggested in previous studies1616 de Carvalho GL, Cavazzola LT. Can mathematic formulas help us with our patients? Surg Endosc. 2011;25(1):336-7., provides the benefit of a single, planned scar on the abdominal wall. This feature allows future resection of this scar during definitive treatment of carcinomatosis, helping control the disease in the abdominal wall. The rate of failure or impossibility of this procedure has ranged from 0-17%, even when using the Hasson's open technique1212 Grass F, Vuagniaux A, Teixeira-Farinha H, Lehmann K, Demartines N, Hübner M. Systematic review of pressurized intraperitoneal aerosol chemotherapy for the treatment of advanced peritoneal carcinomatosis. Br J Surg. 2017;104(6):669-78..
The key distinctive feature of PIPAC is the process of converting a liquid solution of chemotherapy into an aerosolized spray, thereby potentiating its effects, when aerosolized liquids take on the general physical characteristics, behavior, and distribution of a gas. Two of these properties are essential for the application of intraperitoneal chemotherapy. The first is a homogeneous and rapid distribution in the physical space within which the gas is contained. This property has been challenged in studies demonstrating that the drug concentrations achieved are close to those obtained with the conventional delivery model1717 Khosrawipour V, Khosrawipour T, Falkenstein TA, Diaz-Carballo D, Förster E, Osma A, et al. Evaluating the effect of Micropump© position, internal pressure and Doxorubicin dosage on efficacy of Pressurized Intra-peritoneal Aerosol Chemotherapy (PIPAC) in an ex vivo model. Anticancer Res. 2016;36(9):4595-600.. In a previous validation study of the BhioQap single-port device, we explored a device with a unique feature designed to minimize this effect, namely, multidirectional delivery. However, the distribution pattern identified in the animal models was erratic, and did not improve distribution homogeneity in different compartments of the abdomen. Recognition of the peritoneal fluid circulation process and exposure of the peritoneal cavity to the aerosolized substance for more than 30 minutes seem to minimize the dependence of the technique on multidirectionality. The second desired property concerns the depth of tissue penetration of the chemotherapeutic agent. This property is modified substantially during PIPAC, as delivery is influenced both by the physical characteristics of the chemotherapeutic agent in a gaseous state and by the pressure of the pneumoperitoneum1818 Nadiradze G, Giger-Pabst U, Zieren J, Strumberg D, Solass W, Reymond MA. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) with low-dose Cisplatin and Doxorubicin in gastric peritoneal metastasis. J Gastrointest Surg. 2016;20(2):367-73.. Intraperitoneal pressure modifies the so-called peritoneal permeability by altering hydrostatic forces in the tissue, doubling the concentration of intraperitoneal substances in the extracellular space and increasing in fivefold the hydraulic conductivity of the fluid, driving it into the core of peritoneal metastases1919 Zakaria el-R, Lofthouse J, Flessner MF. In vivo hydraulic conductivity of muscle: effects of hydrostatic pressure. Am J Physiol. 1997;273(6 Pt 2):H2774-82.,2020 Zakaria ER, Lofthouse J, Flessner MF. In vivo effects of hydrostatic pressure on interstitium of abdominal wall muscle. Am J Physiol. 1999;276(2 Pt 2):H517-29.. This consolidates the concept of therapeutic pneumoperitoneum as a mechanism superior to all other modalities for delivery of intraperitoneal chemotherapy used to date. Hence, the use of therapeutic pneumoperitoneum may explain the encouraging initial results obtained with PIPAC in the treatment of peritoneal carcinomatosis secondary to gastric cancer1818 Nadiradze G, Giger-Pabst U, Zieren J, Strumberg D, Solass W, Reymond MA. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) with low-dose Cisplatin and Doxorubicin in gastric peritoneal metastasis. J Gastrointest Surg. 2016;20(2):367-73., colon cancer2121 Demtröder C, Solass W, Zieren J, Strumberg D, Giger-Pabst U, Reymond MA. Pressurized intraperitoneal aerosol chemotherapy with oxaliplatin in colorectal peritoneal metastasis. Colorectal Dis. 2016;18(4):364-71., and ovarian cancer1414 Tempfer CB, Celik I, Solass W, Buerkle B, Pabst UG, Zieren J, et al. Activity of Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) with cisplatin and doxorubicin in women with recurrent, platinum-resistant ovarian cancer: preliminary clinical experience. Gynecol Oncol. 2014;132(2):307-11.. Neoadjuvant use of PIPAC may be a promising approach for these patients, and may allow debulking of high-PCI peritoneal carcinomatosis to a more localized disease state, which may then be amenable to cytoreductive surgery with hyperthermic chemotherapy2222 Girshally R, Demtröder C, Albayrak N, Zieren J, Tempfer C, Reymond MA. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) as a neoadjuvant therapy before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J Surg Oncol. 2016;14(1):253..
In summary, encouraging initial data suggest that PIPAC can improve quality of life in a significant number of patients with peritoneal carcinomatosis, and is a potential tool for palliative treatment of patients with peritoneal metastases2323 Odendahl K, Solass W, Demtröder C, Giger-Pabst U, Zieren J, Tempfer C, et al. Quality of life of patients with end-stage peritoneal metastasis treated with Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC). Eur J Surg Oncol. 2015;41(10):1379-85.. The response rates reported thus far are still based on small, retrospective cohort or case-control studies. Nevertheless, the unique features of PIPAC described above lead us to believe that this technique holds promise for the treatment of peritoneal carcinomatosis, and that the available evidence for its utility should not be disregarded.
-
Source of funding: none.
REFERÊNCIAS
-
1Economou SG, Mrazek R, McDonald G, Slaughter D, Cole WH. The intraperitoneal use of nitrogen mustard at the time of operation for cancer. Ann N Y Acad Sci. 1958;68(3):1097-102.
-
2Alberts DS, Liu PY, Hannigan EV, O'Toole R, Williams SD, Young JA, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med. 1996;335(26):1950-5.
-
3McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334(1):1-6.
-
4Chan CH, Cusack JC, Ryan DP. A critical look at local-regional management of peritoneal metastasis. Hematol Oncol Clin North Am. 2015;29(1):153-8.
-
5Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88(2):358-63.
-
6Sugarbaker PH. Observations concerning cancer spread within the peritoneal cavity and concepts supporting an ordered pathophysiology. Cancer Treat Res. 1996;82:79-100.
-
7Batista TP, Sarmento BJQ, Loureiro JF, Petruzziello A, Lopes A, Santos CC, Quadros CA, Akaishi EH, Cordeiro EZ, Coimbra FJF, Laporte GA, Castro LS, Batista RMSS, Aguiar S Júnior, Costa WL Júnior, Ferreira FO; BSSO/SBCO Committee on Peritoneal Surface Malignancies and HIPEC. A proposal of Brazilian Society of Surgical Oncology (BSSO/SBCO) for standardizing cytoreductive surgery (CRS) plus hyperthermic intraperitoneal chemotherapy (HIPEC) procedures in Brazil: pseudomixoma peritonei, appendiceal tumors and malignant peritoneal mesothelioma. Rev Col Bras Cir. 2017;44(5):530-44. Erratum in: Rev Col Bras Cir. 2017;44(6):665.
-
8Markman M. Intraperitoneal antineoplastic drug delivery: rationale and results. Lancet Oncol. 2003;4(5):277-83.
-
9Alberts DS, Young L, Mason N, Salmon SE. In vitro evaluation of anticancer drugs against ovarian cancer at concentrations achievable by intraperitoneal administration. Semin Oncol. 1985;12(3 Suppl 4):38-42.
-
10Reymond MA, Hu B, Garcia A, Reck T, Köckerling F, Hess J, et al. Feasibility of therapeutic pneumoperitoneum in a large animal model using a microvaporisator. Surg Endosc. 2000;14(1):51-5.
-
11Jacquet P, Sugarbaker PH. Current methodologies for clinical assessment of patients with peritoneal carcinomatosis. J Exp Clin Cancer Res. 1996;15(1):49-58.
-
12Grass F, Vuagniaux A, Teixeira-Farinha H, Lehmann K, Demartines N, Hübner M. Systematic review of pressurized intraperitoneal aerosol chemotherapy for the treatment of advanced peritoneal carcinomatosis. Br J Surg. 2017;104(6):669-78.
-
13Blanco A, Giger-Pabst U, Solass W, Zieren J, Reymond MA. Renal and hepatic toxicities after pressurized intraperitoneal aerosol chemotherapy (PIPAC). Ann Surg Oncol. 2013;20(7):2311-6.
-
14Tempfer CB, Celik I, Solass W, Buerkle B, Pabst UG, Zieren J, et al. Activity of Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) with cisplatin and doxorubicin in women with recurrent, platinum-resistant ovarian cancer: preliminary clinical experience. Gynecol Oncol. 2014;132(2):307-11.
-
15Robella M, Vaira M, De Simone M. Safety and feasibility of pressurized intraperitoneal aerosol chemotherapy (PIPAC) associated with systemic chemotherapy: an innovative approach to treat peritoneal carcinomatosis. World J Surg Oncol. 2016;14:128.
-
16de Carvalho GL, Cavazzola LT. Can mathematic formulas help us with our patients? Surg Endosc. 2011;25(1):336-7.
-
17Khosrawipour V, Khosrawipour T, Falkenstein TA, Diaz-Carballo D, Förster E, Osma A, et al. Evaluating the effect of Micropump© position, internal pressure and Doxorubicin dosage on efficacy of Pressurized Intra-peritoneal Aerosol Chemotherapy (PIPAC) in an ex vivo model. Anticancer Res. 2016;36(9):4595-600.
-
18Nadiradze G, Giger-Pabst U, Zieren J, Strumberg D, Solass W, Reymond MA. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) with low-dose Cisplatin and Doxorubicin in gastric peritoneal metastasis. J Gastrointest Surg. 2016;20(2):367-73.
-
19Zakaria el-R, Lofthouse J, Flessner MF. In vivo hydraulic conductivity of muscle: effects of hydrostatic pressure. Am J Physiol. 1997;273(6 Pt 2):H2774-82.
-
20Zakaria ER, Lofthouse J, Flessner MF. In vivo effects of hydrostatic pressure on interstitium of abdominal wall muscle. Am J Physiol. 1999;276(2 Pt 2):H517-29.
-
21Demtröder C, Solass W, Zieren J, Strumberg D, Giger-Pabst U, Reymond MA. Pressurized intraperitoneal aerosol chemotherapy with oxaliplatin in colorectal peritoneal metastasis. Colorectal Dis. 2016;18(4):364-71.
-
22Girshally R, Demtröder C, Albayrak N, Zieren J, Tempfer C, Reymond MA. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) as a neoadjuvant therapy before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J Surg Oncol. 2016;14(1):253.
-
23Odendahl K, Solass W, Demtröder C, Giger-Pabst U, Zieren J, Tempfer C, et al. Quality of life of patients with end-stage peritoneal metastasis treated with Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC). Eur J Surg Oncol. 2015;41(10):1379-85.
Publication Dates
-
Publication in this collection
20 Aug 2018 -
Date of issue
2018
History
-
Received
08 May 2018 -
Accepted
29 May 2018