Abstracts
PURPOSE: To evaluate parameters related with arterial pressure and metabolic profile in women with polycystic ovary syndrome (POS). METHODS: This monocentric study at the University Hospital Endocrinology Section included 60 women aged 18-45 years, 42 being diagnosed with POS and acting as 18 controls. All women were subjected to transvaginal ultrasound and monitored for arterial pressure for 24 h in the ambulatory (MAP). Venous blood samples were taken between 07.00 and 09.00, after 12 h fasting. Basal (BG) and fasting glucose concentrations, total cholesterol and its fractions, triglycerides and insulin (to calculate the homeostatic assay insulin-resistance, HOMA-IR) were measured. Collected data were the mean arterial blood pressure (24-h awake/sleep cycle), arterial pressure nocturnal descensus, glycemia and fasting glucose for HOMA-IR, and lipid profile. The Student's t test was used to compare homogeneous variables; the Mann-Whitney test was used to compare non-homogeneous variables; the Pearson's correlation coefficient was used to search for correlation between the variables. The c² test was used for comparison of the absence of nocturnal descensus. Significance was taken as p<0.05. RESULTS: The mean age of the patients with POS was 27.4±5.5 (18-45 years, n=42) and the body mass index (BMI) was 30.2±6.5 kg/m² (18.3-54.9). In the Control Group, the mean age was 31.4±6.1 (18-45 years) and the BMI was 27.1±6.2 kg/m² (18.3-54.9, n=18). No difference in the metabolic parameters and insulin resistance was observed between the two groups. Comparison between these parameters and MAP showed that the only parameter with a correlation was the BMI, independent of the POS diagnosis. This was not seen in nocturnal descensus, which was uncorrelated with POS and any of the other studied parameters. CONCLUSION: POS women do not show higher arterial blood pressure, glycemia, HDL-col, TG, HOMA-IR and BMI compared to non-POS women. However, POS patients showed correlation between arterial pressure and BMI, suggesting that obesity is a primary factor involved in arterial pressure changes in these patients.
Cardiovascular diseases; Cardiovascular diseases; Metabolic diseases; Metabolic diseases; Polycystic ovary syndrome; Polycystic ovary syndrome; Polycystic ovary syndrome
OBJETIVO: Avaliar os parâmetros relacionados com a pressão arterial e o perfil metabólico em portadoras de SOP. MÉTODOS: Estudo monocêntrico aberto no qual foram avaliadas 60 mulheres em idade fértil, entre 18 e 45 anos, sendo que 42 mulheres preenchiam os critérios diagnósticos para SOP, e 18 que não preenchiam critérios formaram o Grupo Controle. Todas as mulheres foram submetidas a ultrassonografia transvaginal e a monitorização ambulatorial da pressão arterial por 24 horas (MAPA). Amostras de sangue venoso foram coletadas entre 7h00min e 9h00min, após jejum prévio de 12 horas, sendo medidos glicose de jejum ou basal (GB), colesterol total e frações, triglicerídeos e insulina (para cálculo do HOMA-IR). Dados coletados: valores médios de pressão arterial-PA (no período de vigília, sono de 24hs), descenso noturno de PA; glicemia e insulina de jejum para cálculo do HOMA-IR; perfil lipídico. Para comparar as variáveis com distribuição homogênea foi utilizado o teste t de Student e para as variáveis não homogêneas foi utilizado o teste não-paramétrico de Mann-Whitney; e para correlacionar as variáveis foi avaliado o coeficiente de correlação de Pearson. Para comparação das proporções da ausência de descenso noturno foi realizado o teste do c². Em todas as análises, foi considerado o nível de significância de 5% (p<0,05). RESULTADOS: A média de idade das 42 pacientes com diagnóstico de SOP foi de 27,4±5,5 (18-45 anos) e do IMC de 30,2±6,5 kg/m² (18,3-54,9), e a média de idade das 18 mulheres controle foi de 31,4±6,1 (18-45 anos) e do IMC de 27,1±6,2 kg/m² (18,3-54,9). Não foi observada diferença significativa nos parâmetros metabólicos e de resistência a insulina e pressão arterial entre os grupos. Comparando esses parâmetros com as médias das pressões arteriais, registradas pela MAPA, foi observado que o único fator que teve correlação foi o índice de massa corporal, independente do diagnóstico de SOP. O mesmo não foi observado com o DN, o qual não teve relação significante com o diagnóstico de SOP e com os parâmetros estudados. CONCLUSÃO: Mulheres com SOP não apresentam maiores níveis de pressão arterial, glicemia, HDL-col, TG, HOMA-IR e IMC do que mulheres sem SOP. Todavia, entre as pacientes com SOP, o único parâmetro que apresentou correlação com os valores médios de pressão arterial foi o IMC, sugerindo que a obesidade é o fator primordial para alteração do comportamento de pressão arterial nessas pacientes.
Doenças cardiovasculares; Doenças cardiovasculares; Doenças metabólicas; Doenças metabólicas; Síndrome do ovário policístico; Síndrome do ovário policístico; Obesidade
ARTIGOS ORIGINAIS
Arterial hypertension and metabolic profile in patients with polycystic ovary syndrome
Hipertensão arterial e perfil metabólico em pacientes com síndrome dos ovários policísticos
Renata do Sacramento Monte de OliveiraI; Renato Galvão RedoratI; Gisele Hart ZieheI; Vera Aleta MansurII; Flávia Lúcia ConceiçãoIII
IEndocrinology Section Faculdade de Medicina e Hospital Universitário Clementino Fraga Filho, Universidade Federal do Rio de Janeiro UFRJ Rio de Janeiro (RJ), Brazil
IIHospital dos Servidores do Estado Rio de Janeiro (RJ), Brazil
IIIFaculdade de Medicina e Hospital Universitário Clementino Fraga Filho, Universidade Federal do Rio de Janeiro UFRJ Rio de Janeiro (RJ), Brazil
Correspondence Correspondence Hospital Universitario Clementino Fraga Filho Rua Rodolfo Paulo Rocco, 255 Department of Endocrinology, 9º andar Ilha do Fundão CEP: 95010-003 Rio de Janeiro (RJ), Brazil
ABSTRACT
PURPOSE: To evaluate parameters related with arterial pressure and metabolic profile in women with polycystic ovary syndrome (POS).
METHODS: This monocentric study at the University Hospital Endocrinology Section included 60 women aged 18-45 years, 42 being diagnosed with POS and acting as 18 controls. All women were subjected to transvaginal ultrasound and monitored for arterial pressure for 24 h in the ambulatory (MAP). Venous blood samples were taken between 07.00 and 09.00, after 12 h fasting. Basal (BG) and fasting glucose concentrations, total cholesterol and its fractions, triglycerides and insulin (to calculate the homeostatic assay insulin-resistance, HOMA-IR) were measured. Collected data were the mean arterial blood pressure (24-h awake/sleep cycle), arterial pressure nocturnal descensus, glycemia and fasting glucose for HOMA-IR, and lipid profile. The Student's t test was used to compare homogeneous variables; the Mann-Whitney test was used to compare non-homogeneous variables; the Pearson's correlation coefficient was used to search for correlation between the variables. The c2 test was used for comparison of the absence of nocturnal descensus. Significance was taken as p<0.05.
RESULTS: The mean age of the patients with POS was 27.4±5.5 (1845 years, n=42) and the body mass index (BMI) was 30.2±6.5 kg/m2 (18.354.9). In the Control Group, the mean age was 31.4±6.1 (1845 years) and the BMI was 27.1±6.2 kg/m2 (18.354.9, n=18). No difference in the metabolic parameters and insulin resistance was observed between the two groups. Comparison between these parameters and MAP showed that the only parameter with a correlation was the BMI, independent of the POS diagnosis. This was not seen in nocturnal descensus, which was uncorrelated with POS and any of the other studied parameters.
CONCLUSION: POS women do not show higher arterial blood pressure, glycemia, HDL-col, TG, HOMA-IR and BMI compared to non-POS women. However, POS patients showed correlation between arterial pressure and BMI, suggesting that obesity is a primary factor involved in arterial pressure changes in these patients.
Keywords: Cardiovascular diseases/etiology; Cardiovascular diseases/metabolism; Metabolic diseases/complications ; Metabolic diseases/metabolism; Polycystic ovary syndrome/complications; Polycystic ovary syndrome/metabolism; Obesity/complications
RESUMO
OBJETIVO: Avaliar os parâmetros relacionados com a pressão arterial e o perfil metabólico em portadoras de SOP.
MÉTODOS: Estudo monocêntrico aberto no qual foram avaliadas 60 mulheres em idade fértil, entre 18 e 45 anos, sendo que 42 mulheres preenchiam os critérios diagnósticos para SOP, e 18 que não preenchiam critérios formaram o Grupo Controle. Todas as mulheres foram submetidas a ultrassonografia transvaginal e a monitorização ambulatorial da pressão arterial por 24 horas (MAPA). Amostras de sangue venoso foram coletadas entre 7h00min e 9h00min, após jejum prévio de 12 horas, sendo medidos glicose de jejum ou basal (GB), colesterol total e frações, triglicerídeos e insulina (para cálculo do HOMA-IR). Dados coletados: valores médios de pressão arterial-PA (no período de vigília, sono de 24hs), descenso noturno de PA; glicemia e insulina de jejum para cálculo do HOMA-IR; perfil lipídico. Para comparar as variáveis com distribuição homogênea foi utilizado o teste t de Student e para as variáveis não homogêneas foi utilizado o teste não-paramétrico de Mann-Whitney; e para correlacionar as variáveis foi avaliado o coeficiente de correlação de Pearson. Para comparação das proporções da ausência de descenso noturno foi realizado o teste do c2. Em todas as análises, foi considerado o nível de significância de 5% (p<0,05).
RESULTADOS: A média de idade das 42 pacientes com diagnóstico de SOP foi de 27,4±5,5 (1845 anos) e do IMC de 30,2±6,5 kg/m2 (18,354,9), e a média de idade das 18 mulheres controle foi de 31,4±6,1 (1845 anos) e do IMC de 27,1±6,2 kg/m2 (18,354,9). Não foi observada diferença significativa nos parâmetros metabólicos e de resistência a insulina e pressão arterial entre os grupos. Comparando esses parâmetros com as médias das pressões arteriais, registradas pela MAPA, foi observado que o único fator que teve correlação foi o índice de massa corporal, independente do diagnóstico de SOP. O mesmo não foi observado com o DN, o qual não teve relação significante com o diagnóstico de SOP e com os parâmetros estudados.
CONCLUSÃO: Mulheres com SOP não apresentam maiores níveis de pressão arterial, glicemia, HDL-col, TG, HOMA-IR e IMC do que mulheres sem SOP. Todavia, entre as pacientes com SOP, o único parâmetro que apresentou correlação com os valores médios de pressão arterial foi o IMC, sugerindo que a obesidade é o fator primordial para alteração do comportamento de pressão arterial nessas pacientes.
Palavras-chave: Doenças cardiovasculares/etiologia; Doenças cardiovasculares/metabolismo; Doenças metabólicas/complicações; Doenças metabólicas/metabolismo; Síndrome do ovário policístico/complicações; Síndrome do ovário policístico/metabolismo; Obesidade/complicações
Introduction
Polycystic ovary syndrome (POS) is a heterogeneous clinical condition affecting 1 in 15 women in the world1, and is the most common endocrine disorder at a reproductive age2,3, varying from 610%, according to diagnosing criteria4,5. In spite of its physiopathology being not fully understood, it is of multifactorial genesis, with genetical, metabolic and neuroendocrine influences6,7, and a fundamental insulin-resistance background8.
In addition to classic chronic anovulation and hyperandrogenism, POS leads to higher risk of cardiovascular problems related to insulin resistance9, such as systemic arterial hypertension (SAH), glucose intolerance and type 2 diabetes mellitus, dyslipidemia, obesity and metabolic syndrome10-13.
One of the risk factors for cardiovascular diseases is SAH, diagnosed in one third of the population worldwide14,15. Given its high prevalence16 and high morbid-mortality rates, it is of socio-economic importance.
An association between SAH and POS is not completely established9. Insulin resistance and hyperinsulinemia are predisposing factors for SAH in POS women17, but no study has shown a direct correlation between the arterial pressure and the insulin resistance parameters in these patients. In spite of the comorbidity related to insulin resistance in POS patients influencing arterial pressure18, it is not known if the POS patients are characterized by differential arterial pressure (AP) compared to other women, and whether its parameters would be correlated with other cardiovascular risk factors. Mean arterial pressure (MAP) studies to evaluate arterial pressure in POS patients are scarce.
Thus we decided to study the changes in arterial pressure and metabolic profile in POS patients.
Methods
Sixty women who attended the Hospital Clementino Fraga Filho (HUCFF) Endocrinology Section voluntarily between 2010 and 2012 were enrolled in this study. The study was approved by the Hospital's Ethics and Research Committee under the number 085/09. All volunteers agreeing to participate in the study signed an informed consent form, according to the National Health Council (Resolution 196/96).
Women aged 18 to 45 included in the study presented symptoms related to hyperandrogenism (hirsutism, acne, alopecia), anovulation (amenorrhea, oligomenorrhea, infertility) and insulin resistance (obesity, acantose nigricans). The POS diagnosing criteria were those established by the Rotterdam consensus19, and included two of the three following criteria: oligomenorrhea or anovulation; clinical or biochemical signs of hyperandrogenism, polycystic ovaries confirmed by ultrasonography (presence of 12 or more 29 mm diameter follicles in each ovary
and/or ovarian volume >10 mm3). Women included in the Control Group were in the same age range, had similar complaints as the ones from the POS group, but did not fulfill the diagnostic criteria.
Women showing other correlated diseases resulting in hyperandrogenism and/or anovulation, such as thyroid disfunction, hyperprolactinemia, classical and non-classical congenital adrenal hyperplasia, Cushing syndrome and androgen-producing tumors of the adrenal gland and ovaries, were not included in the study. Women administered with medicine contributing to false-positive results (corticoids, anticoagulants, oral contraceptives, anti-lipidemic or hypoglycemic drugs etc.) were also excluded. Some patients interrupted contraceptive treatment to take part in the study.
Evaluated parameters were age, symptoms associated with hyperandrogenism and/or anovulation, comorbidities, medications in use, physiological and familiar risk factors. Patients were given a clinical examination to determine body mass (kg), height (m), waist circumference (WC), and arterial pressure. WC was measured at the mean point between the costal margin and the anterosuperior iliac crest. BMI was determined by the Quetelet's20 rate between body mass (kg) divided by height in m2. A diagnosis of overweight/obesity was determined according to the WHO criteria20: normal (18.524.9 kg/m2), overweight (2529.9 kg/m2), and obese (>30 kg/m2). In addition, insulin resistance signs, such as acantose nigricans in skin folding region and signs of hyperandrogenism, such as hirsutism, acne and alopecia. Hirsutism was diagnosed according to the modified semiquantitative Ferriman-Gallwey score, using a cut off of 821.
All women were submitted to transvaginal ultrasound examination to determine the ovarian topography to determine ultrasound criteria established by the Rotterdam consensus.
MAP was measured for 24 h, using a Dynamapa monitor that involved the oscilometric method. MAP along the awake and sleep cycle and nocturnal descensus were determined. The results were interpreted according to the V Brazilian Directions for Ambulatory Monitoring of Arterial Pressure22.
Criteria established by the American Diabetes Association23 were to determine glucose value and those from NCEP, ATP III24, for lipid profiles. Venous blood samples were collected between 07.00 and 09.00 after 12 h fasting. Fasting and basal (BG) glucose levels, total cholesterol and fractions, triglycerides and insulin (to calculate HOMA-IR) levels were measured. Serum glucose was measured enzymatically, with a level lower than
99 mg/dL as reference. Total cholesterol was determined by a colorimetric enzymatic method, with a reference level <200 mg/dL; LDL-col was measured by the Friedwald formula; triglycerides were determined by a colorimetric enzymatic method, using a reference level lower than 150 mg/dL; insulin was determined by a electrochemoluminescence method, using reference levels between 2 and 13 mcU/mL (for BMI up to 25), 2 and 19 mcU/mL (for BMIs of 2530), and 2 and 23 mcU/mL (for BMI>30).
Roche modular equipment P was used for glucose (sensibility 2 mg/dL, intra essay variation coefficient (IaVC) 1% and inter essay variation (IeVC) 1.7%), cholesterol (sensibility 3 mg/dL, IaVC 0.8 % and IeVC 1.7%), and triglycerides (sensibility 4 mg/dL, IaVC 1.5% and IeVC 1.8%). Roche modular equipment E was used to quantify insulin
(sensibility 2 mcU/mL, IaVC 0.71.5% and IeVC 2.44.9%).
Student's t-test was used to compare the mean of the variables in arterial pressure, glucose, HDL-col, triglycerides and HOMA-IR between the obese and non-obese POS patients. To compare the distribution of the means of variables arterial pressure, glucose, HDL-col, triglycerides, and HOMA-IR between the control
and POS groups, and within the POS group those with and without nocturnal descensus, the non-parametric Mann Whitney test. To establish any correlation between the metabolic paramenters and insulin resistance with the mean arterial pressure within the POS group, the Pearson correlation coefficient was used (p>0=positive correlation; p<0=negative correlation; p=0: no correlation; p: 03.0=weak correlation; p: 3.07.0=moderate correlation; p>7,0=strong correlation). For those parameters showing some correlation, a simple linear regression helped to quantify the influence of these parameters in MAP. To compare the proportion of the absence of nocturnal descensus between the control and POS groups, and between the obese and non-obese POS, the c2 test was employed, the values being expressed as percentages.
The results have been expressed as the mean ± standard deviation, and p<0.05 was taken as statistically significant. Statistical Analysis System (SAS) software for Windows, version 9.1, was used throughout this work.
Results
Clinical and biochemical characteristics of the groups are presented in Table 1. The mean age of the 42 POS patients was 27.4±5.5 (1845 years) and the BMI was 30.2±6.5 kg/m2 (18.354.9). Fifty-two percent (22/42) were obese (IMC>30) and forty-eight percent (20/42) were non-obese (IMC<29.9). The mean age of the 18 women in the Control Groups was 31.4±6.1 (1845 years), and the BMI was 27.1±6.2 kg/m2 (18.354.9). Thirty-nine percent of them (7/18) were obese (IMC>30) and sixty-one percent (11/18) were non-obese (IMC<29.9).
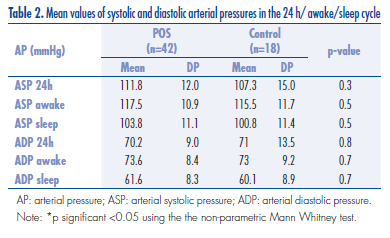
The mean values of systolic and diastolic arterial pressures in the 24 h awake/sleep cycle are given in Table 2. Sixteen POS patients (36.8%) and six controls (33.3%) did not show nocturnal descensus, with no difference between the two groups (p=0.95). There was no significant difference between POS and control with respect to the metabolic parameters, insulin resistence and MAP parameters (mean values for systolic and diastolic arterial pressure, and nocturnal descensus). This was also observed when the obese women in the two groups were compared.
By comparing the metabolic parameters and the HOMA-IR with mean arterial pressure, the only variable with a positive correlation was with BMI. In POS patients, a positive correlation between BMI and MAP was apparent. A moderate correlation existed between the BMI and the systolic arterial pressure, and a weak correlation existed between BMI and diastolic arterial pressure (Table 3).
Within the POS group, there were significant differences between the non-obese (BMI<29.9) and obese women (BMI>30) with respect to glycemia, triglycerides, HOMA-IR, and the mean arterial pressure (Table 4).
There was no difference with respect to the metabolic profile, HOMA-IR, and mean arterial pressure when the POS patients with and without nocturnal descensus were compared to each other (Table 5).
Finally, the presence or absence of nocturnal descensus did not show statistically significant differences with respect to POS (p=0.9). The same was observed for the BMI, meaning that the nocturnal descensus was not correlated with obesity (p=0.2).
Discussion
There was no difference between the metabolic parameters (RI, glucose, HDL and triglycerides) between the women diagnosed with POS and Control Group. However, the prevalence of this metabolic alteration was significantly higher in obese and non-obese POS women, revealing the relevant role of obesity in the metabolic profile of POS patients.
In spite of the prevalence of metabolic disorders in POS patients25,26, no difference was seen in the glucose, HDL, triglycerides, HOMA-IR and BMI levels between the POS and control women. This might be correlated with the fact that both groups showed metabolic syndrome symptoms, hyperandrogenism and anovulation. This similarity between the clinical presentations in the two groups could perhaps result from equivalent metabolic characteristics. However, when obese and non-obese POS patients were compared, significantly different levels of glucose, triglycerides and HOMA-IR were found. Studies on the BMI as a risk factor for POS are conflicting. Some authors stress that BMI influences the prevalence of metabolic disorders in POS patients, while other claim that a poorer metabolic profile of POS patients is independent of BMI27,28.
SAH is a risk factor for different diseases and, as such, it is of high socio-economic concerns. The association between POS and SAH is not well established. The actual mechanism responsible for SAH in POS patients is a matter of great controversy. In addition to a crucial role in SAH in POS patients, other mechanisms may be involved, such as the characteristic pro-inflammatory state in the syndrome, hyperandrogenism, and the influence of both the angiotensin-aldosterone and sympathetic system10,29,30.
MAP is an appropriate way to inspect cardiovascular status in these patients22. By keeping indirect and intermittent registers of arterial pressure for 24 h, MAP could detect circadian cycle variations in arterial pressure, which had considerable prognostic implications. Among the parameters studied, the most indicative were the mean arterial pressure and the absence of nocturnal descensus. The mean arterial systolic pressure (ASP) and the mean arterial diastolic pressure (ADP) obtained in a 24 h period, including the awake/sleep cycle, show consistent correlations with lesions in the target organs and cardiovascular morbidity and mortality31,32. When comparing the mean arterial pressure detected by MAP in POS and control patients, no difference was found. These results are difficult to consider, since most of the results in the literature are controversial and use casual ambulatorial arterial pressure measurements, and MAP studies are scarce.
The only cardiovascular risk factor analysed in our investigation that showed a correlation with the arterial systolic and diastolic pressures was BMI. Two interesting results were a moderate correlation between the mean arterial systolic pressure, and a weak correlation between the mean arterial diastolic pressure, and POS. The same influence of BMI occurred between the mean arterial pressure and obesity in POS patients. A 5% significance was found in the comparison between these parameters, confirming previous reports indicating an association between obesity and arterial pressure33,34. Nonetheless, the differences between the levels of glycemia, triglycerides and HOMA-IR in the obese and non-obese groups was significant, confirming an interaction between the adipose tissue and the comorbidities related to the insulin resistance.
The adipose tissue is recognized as a complex endocrine organ, capable of influencing the sympathetic system and producing chemical signal that, together with insulin resistance, influence arterial pressure in POS women. Overstimulation of sympathetic system in obesity promotes an expansion of the extracellular volume and regional flux, causing increase cardiac debit35. Obese patients show higher plasma rennin activity, increasing the plasma levels of angiotensinogen, increased activity of tissue converting enzyme and higher plasma levels of aldosterone, implicating the renin-angiotensin-aldosterone system in ovary physiology and pathology36. Another important aspect of adipose tissue physiology is hyperinsulinemia that compensates in the insulin resistance, which exercises a trophic effect on blood vessel smooth muscle, causing increased vascular resistance and arterial pressure37. POS might share a common genetic background with arterial hypertension and obesity; finding this trait could elucidate the mechanisms involved in these diseases and other comorbidities due to insulin resistance38. However, it is not possible to conclude that POS patients are at greater risk of cardiovascular diseases, and the validity of this association needs testing.
The correlation between nocturnal descensus and risk factors for cardiovascular disease remains debatable since the results are ontroversial22,31,39. In the present study, the risk of presenting nocturnal descensus was similar between the POS, the controls and their BMIs. No correlation existed between nocturnal descensus and the mean values of glycemia, HDL-col, triglycerides and HOMA-IR. Similarly, no correlation was found between MAP.These findings, in general, contradict those in the literature. This might be attributed to the fact that sleep disorders, such as obstructive dyspnea, were not excluded and could influence the prevalence of nocturnal descensus and present a relevant bias in our study40.
One advantage of our study was the uniform profile of the patients seeking treatment in our institution by presenting suspected POS, all of whom had the same diagnosis. In both groups POS and Control the complaints were related to hyperandrogenism, anovulation and obesity. POS diagnosis was careful and followed the Rotterdam consensus; other causes of hyperandrogenism were excluded. These criteria were fundamental to the reliability of the study. However, the small number of patients in the Control Group might have introduced some bias to the analyses.
Acknowledgements
To Clínica Prev Total Laboratório de Imagem LTDA for the MAP measurements and Laboratório Sérgio Franco for the laboratorial tests.
Received 08/22/2012
Accepted with modifications 11/26/2012
Conflict of interest: none.
Study carried out at Department of Endocrinology at the Hospital Universitário Clementino Fraga Filho, Universidade Federal do Rio de Janeiro UFRJ Rio de Janeiro (RJ), Brazil.
- 1. Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370(9588):685-97.
- 2. Elghblawi E. Polycystic ovary syndrome and female reproduction. Br J Nurs. 2007;16(18):1118-21.
- 3. March WA, Moore VM, Wilson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25(2):544-51.
- 4. Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352(12):1223-36.
- 5. Marcondes JAM, Barcellos CRG, Rocha MP. Dificuldades e armadilhas no diagnóstico da síndrome dos ovários policísticos. Arq Bras Endocrinol Metab. 2011;55(1):6-15.
- 6. Costa LOBF, Viana AOR, Oliveira M. Prevalence of the metabolic syndrome in women with polycystic ovary syndrome. Rev Bras Ginecol Obstet. 2007;29(1):10-7.
- 7. Burt Solorzano CM, Beller JP, Abshire MY, Collins JS, McCartney CR, Marshall JC. Neuroendocrine dysfunction in polycystic ovary syndrome. Steroids. 2012;77(4):332-7.
- 8. de Paula Martins W, Santana LF, Nastri CO, Ferriani FA, de Sa MF, Dos Reis RM. Agreement among insulin sensitivity indexes on the diagnosis of insulin resistance in polycystic ovary syndrome and ovulatory women. Eur J Obstet Gynecol Reprod Biol. 2007;133(2):203-7.
- 9. Iftikhar S, Collazo-Clavell ML, Roger VL, St Sauver J, Brown RD Jr, Cha S, et al. Risk of cardiovascular events in patients with polycystic ovary syndrome. Neth J Med. 2012;70(2):74-80.
- 10. Azevedo MF, Costa EC, Oliveira AIN, Silva IBO, Marinho JCDB, Rodrigues JAM, et al. Elevated blood pressure in women with polycystic ovary syndrome: prevalence and associated risk factors. Rev Bras Ginecol Obstet. 2011;33(1):31-6.
- 11. El-Mazny A, Abou-Salem N, El-Sherbiny W, El-Mazny A. Insulin resistance, dyslipidemia, and metabolic syndrome in women with polycystic ovary syndrome. Int J Gynaecol Obstet. 2010;109(3):239-41.
- 12. Kandaraki E, Christakou C, Diamanti-Kandarakis E. Metabolic syndrome and polycystic ovary syndrome... and vice versa. Arq Bras Endocrinol Metab. 2009;53(2):227-37.
- 13. Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose intolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2010;16(4):347-63.
- 14. Baracat EC, Soares-Junior JM. Polycystic ovaries: insulin resistance and metabolic syndrome. Rev Bras Ginecol Obstet. 2007;29(3):117-9.
- 15. Sathyapalan T, Atkin SL. Recent advances in cardiovascular aspects of polycystic ovary syndrome. Eur J Endocrinol. 2012;166(4):575-83.
- 16. Magalhães MEC, Brandão AA, Pozzan R, Campana EMG, Fonseca FL, Pizzi OL, et al. Prevention of arterial hypertension: when to start and with whom? Rev Bras Hipertens. 2010;17(2):93-7.
- 17. Williams B. The year in hipertension. J Am Coll Cardiol. 2009;55(1): 65-73.
- 18. Kargili A, Karakurt F, Kasapoglu B, Derbent A, Koca C, Selcoki Y. Association of polycystic ovary syndrome and a non-dipping blood pressure pattern in young women. Clinics (Sao Paulo). 2010;65(5):475-9.
- 19. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19-25.
- 20. World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO Consultation on Obesity. Geneva: WHO; 1997.
- 21. Hatch R, Rosenfield RL, Kim MH, Tredway D. Hirsutism: implications, etiology, and management. Am J Obstet Gynecol. 1981;140(7):815-30.
- 22. Sociedades Brasileira de Cardiologia, de Hipertensão e de Nefrologia. V Diretrizes Brasileiras de Monitorização Ambulatorial de Hipertensão Arterial (MAPA). Rev Bras Hipertens. 2011;18(1):7-17.
- 23. Expert Commitee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Commitee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20(7):1183-97.
- 24. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486-97.
- 25. Romano LGM, Bedoschi G, Melo AS, Albuquerque FO, Rosa e Silva ACJ, Ferriani RA, et al. Metabolic abnormalities in polycystic ovary syndrome women: obese and non obese. Rev Bras Ginecol Obstet. 2011;33(6):310-6.
- 26. Melo AS, Macedo CSV, Romano LGM, Ferriani RA, Navarro PAAS. Women with polycystic ovary syndrome have a higher frequency of metabolic syndrome regardless of body mass index. Rev Bras Ginecol Obstet. 2012;34(1):4-10.
- 27. Costa EC, Soares EMM, Lemos TMA, Maranhão TMO, Azevedo GD. Índices de obesidade central e fatores de risco cardiovascular na síndrome dos ovários policísticos. Arq Bras Cardiol. 2010;94(5):633-8.
- 28. de Groot PC, Dekkers M, Romijn JA, Dieben SW, Helmerhorst FM. PCOS, coronary heart disease, stroke and the influence of obesity: a systematic review and meta-analysis. Hum Reprod Update. 2011;17(4):495-500.
- 29. Lansdown A, Rees DA. The Sympathetic nervous system in polycystic ovary syndrome: a novel therapeutic target? Clin Endocrinol (Oxf). 2012;77(6):791-801.
- 30. Reckelhoff JF. Polycystic ovary syndrome: androgens and hypertension. Hypertension. 2007;49(6):1220-1.
- 31. Vaz-de-Melo RO, Toledo JCY, Loureiro AAC, Cipullo JP, Moreno Júnior H, Martin JFV. Ausência de descenso noturno se associa a acidente vascular cerebral e infarto do miocárdio. Arq Bras Cardiol. 2010;94(1):79-85.
- 32. Conen D, Bamberg F. Noinvasive 24-h ambulatory blood pressure and cardiovascular disease: a systematic review and meta-analysis. J Hypertens. 2008;26(7):1290-9.
- 33. Luque-Ramírez M, Alvarez-Blasco F, Mendieta-Azcona C, Botella-Carretero JI, Escobar-Morreale HF. Obesity is the major determinant of the abnormalities in blood pressure found in young women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92(6):2141-8.
- 34. Kotchen T. Obesity-related hypertension: epidemiology, pathophysiology, and clinical management. Am J Hypertens. 2010;23(11):1170-8.
- 35. Landsberg L. Obesity, metabolism, and hypertension. Yale J Biol Med. 1989;62(5):511-9.
- 36. Raposo-Costa AP, Reis AM. O sistema renina-angiotensina em ovário. Arq Bras Endocrinol Metab. 2000;44(4):306-13.
- 37. Martins WP, Soares GM, Vieira CS, dos Reis RM, Silva de Sá MF, Ferriani RA. Cardiovascular risk markers in polycystic ovary syndrome in women with and without insulin resistance. Rev Bras Ginecol Obstet. 2009;31(3):111-6.
- 38. Leibel NI, Baumann EE, Kocherginsky M, Rosenfield RL. Relationship of adolescent polycystic ovary syndrome to parental metabolic syndrome. J Clin Endocrinol Metab. 2006;91(4):1275-83.
- 39. Ben-Dov IZ, Kark JD, Ben-Ishay D, Mekler J, Ben-Arie L, Bursztyn M. Predictors of all-cause mortality in clinical ambulatory monitoring: unique aspects of blood pressure during sleep. Hypertension. 2007;49(6):1235-41.
- 40. Attal P, Chanson P. Endocrine aspects of obstructive sleep apnea. J Clin Endocrinol Metab. 2010;95(2):483-95.
Correspondence
Publication Dates
-
Publication in this collection
11 Jan 2013 -
Date of issue
Jan 2013
History
-
Received
22 Aug 2012 -
Accepted
26 Nov 2012