Abstracts
Blackleg is caused by Clostridium chauvoei, whereas malignant oedema is caused by C. chauvoei, C. septicum, C. sordellii, C. perfringens type A, and/or C. novyi type A. Anti-C. chauvoei, anti-C. septicum, anti-C. sordellii and anti-C. novyi type A polyclonal antibodies were produced in rabbits and purified in a column of DEAE-cellulose. Aliquots of the antisera were conjugated with fluorescein isothiocyanate and the remaining was used for the streptavidin biotin peroxidase technique (SBPT). SBPT was standardized to detect C. chauvoei, C. septicum, C. sordellii and C. novyi type A in formalin-fixed, paraffin-embedded tissues of guinea pigs. SBPT was compared to a fluorescent antibody technique (FAT). Sections and smears of muscle from inoculation area (MIA), heart, liver, spleen and kidney, were obtained for both SBPT and FAT. Cross-reactions between the different Clostridial species were not observed. C. chauvoei and C. septicum were detected in all specimens from the animals inoculated with these microorganisms, while only sections of muscle obtained from all the animals inoculated with C. sordellii and C. novyi type A were positive. The same results observed by the SBPT, were obtained on tissue smears of these microorganisms stained by the FAT. The results indicate that SBPT is suitable for detection of C. chauvoei, C. septicum, C. sordellii and C. novyi type A in formalin-fixed, paraffin-embedded tissues of guinea pigs.
Blackleg; malignant oedema; Clostridia; guinea pigs; fluorescent antibody technique; streptavidin biotin peroxidase technique
O carbúnculo sintomático é causado pelo Clostridium chauvoei, enquanto o edema maligno é causado pelo C. chauvoei, C. septicum, C. sordellii, C. perfringens tipo A, e/ou C. novyi tipo A. Corpos policlonais anti-C. chauvoei, anti-C. septicum, anti-C. sordellii and anti-C.novyi tipo A foram produzidos em coelhos e purificados em uma coluna de DEAE-celulose. Alíquotas das imunoglobulinas foram conjugadas com isotiocianato de fluoresceína e o restante foi usado na técnica de streptavidina biotina peroxidase (SBP). SBP foi padronizada para detectar C. chauvoei, C. septicum, C. sordellii e C. novyi tipo A em tecidos de cobaias fixados em formol e incluídos em parafina. A mesma foi comparada com a técnica de imunofluorescência direta (IFD). Secções e impressões do músculo da área de inoculação, coração, fígado, baço e rim, foram obtidas para ambas as técnicas. Nenhuma reação cruzada foi observada quando tecidos inoculados com outras espécies de Clostridia foram tratadas com esses quatro anticorpos primários. C. chauvoei e C. septicum foram detectados em todos os espécimes provenientes dos animais inoculados com esses microrganismos, enquanto somente secções de músculo obtidas de todos os animais inoculados com C. sordellii e C. novyi tipo A foram positivas. Os mesmos resultados observados pela técnica de SBP, foram obtidos em impressões de tecidos desses microrganismos corados pela IFD. Os resultados indicam que a técnica de SBP permite a detecção de C. chauvoei, C. septicum, C. sordellii e C. novyi tipo A em tecidos de cobaias fixados em formol e incluídos em parafina.
Carbúnculo sintomático; edema maligno; Clostridia; cobaias; imunofluorescência direta; streptavidina biotina peroxidase
Immunohistochemical detection of Clostridia species in paraffin-embedded tissues of experimentally inoculated guinea pigs
Detecção imunohistoquímica de várias espécies de Clostridia em tecidos parafinizados de cobaias inoculadas experimentalmente
Ronnie A. AssisI, * * Author for correspondence. E-mail: assisra@rwnet.com.br ; Francisco C.F. LobatoI; Rogéria SerakidesII; Renato L. SantosII; Guilherme R.C. DiasI; Ricardo. A.P. NascimentoIII; Vera. L.V. AbreuI; Patrícia M. ParreirasIV; Francisco A. UzalV
IDepartamento de Medicina Veterinária Preventiva, Escola de Veterinária da Universidade Federal de Minas Gerais (UFMG), Av. Presidente Antônio Carlos 6627, Cx. Postal 567, Belo Horizonte, MG 30123-970, Brazil
IIDepartamento de Clínica e Cirurgias Veterinárias, Escola de Veterinária da UFMG
IIILaboratório Regional de Apoio Animal do Ministério da Agricultura, Pecuária e Abastecimento (LARA), Av. Rômulo Joviano s/n, Pedro Leopoldo, MG 33600-000, Brazil
IVInstituto Oswaldo Cruz, Fiocruz, Av. Brasil 4365, Rio de Janeiro, RJ 21045-900, Brazil
VCalifornia Animal Health and Food Safety Laboratory System, San Bernardino Branch, School of Veterinary Medicine, University of California Davis, 105 W Central Ave, San Bernardino, CA 92408, USA. E-mail: fuzal@cahfs.ucdavis.edu
ABSTRACT
Blackleg is caused by Clostridium chauvoei, whereas malignant oedema is caused by C. chauvoei, C. septicum, C. sordellii, C. perfringens type A, and/or C. novyi type A. Anti-C. chauvoei, anti-C. septicum, anti-C. sordellii and anti-C. novyi type A polyclonal antibodies were produced in rabbits and purified in a column of DEAE-cellulose. Aliquots of the antisera were conjugated with fluorescein isothiocyanate and the remaining was used for the streptavidin biotin peroxidase technique (SBPT). SBPT was standardized to detect C. chauvoei, C. septicum, C. sordellii and C. novyi type A in formalin-fixed, paraffin-embedded tissues of guinea pigs. SBPT was compared to a fluorescent antibody technique (FAT). Sections and smears of muscle from inoculation area (MIA), heart, liver, spleen and kidney, were obtained for both SBPT and FAT. Cross-reactions between the different Clostridial species were not observed. C. chauvoei and C. septicum were detected in all specimens from the animals inoculated with these microorganisms, while only sections of muscle obtained from all the animals inoculated with C. sordellii and C. novyi type A were positive. The same results observed by the SBPT, were obtained on tissue smears of these microorganisms stained by the FAT. The results indicate that SBPT is suitable for detection of C. chauvoei, C. septicum, C. sordellii and C. novyi type A in formalin-fixed, paraffin-embedded tissues of guinea pigs.
Index terms: Blackleg, malignant oedema, Clostridia, guinea pigs, fluorescent antibody technique, streptavidin biotin peroxidase technique.
RESUMO
O carbúnculo sintomático é causado pelo Clostridium chauvoei, enquanto o edema maligno é causado pelo C. chauvoei, C. septicum, C. sordellii, C. perfringens tipo A, e/ou C. novyi tipo A. Corpos policlonais anti-C. chauvoei, anti-C. septicum, anti-C. sordellii and anti-C.novyi tipo A foram produzidos em coelhos e purificados em uma coluna de DEAE-celulose. Alíquotas das imunoglobulinas foram conjugadas com isotiocianato de fluoresceína e o restante foi usado na técnica de streptavidina biotina peroxidase (SBP). SBP foi padronizada para detectar C. chauvoei, C. septicum, C. sordellii e C. novyi tipo A em tecidos de cobaias fixados em formol e incluídos em parafina. A mesma foi comparada com a técnica de imunofluorescência direta (IFD). Secções e impressões do músculo da área de inoculação, coração, fígado, baço e rim, foram obtidas para ambas as técnicas. Nenhuma reação cruzada foi observada quando tecidos inoculados com outras espécies de Clostridia foram tratadas com esses quatro anticorpos primários. C. chauvoei e C. septicum foram detectados em todos os espécimes provenientes dos animais inoculados com esses microrganismos, enquanto somente secções de músculo obtidas de todos os animais inoculados com C. sordellii e C. novyi tipo A foram positivas. Os mesmos resultados observados pela técnica de SBP, foram obtidos em impressões de tecidos desses microrganismos corados pela IFD. Os resultados indicam que a técnica de SBP permite a detecção de C. chauvoei, C. septicum, C. sordellii e C. novyi tipo A em tecidos de cobaias fixados em formol e incluídos em parafina.
Termos de indexação: Carbúnculo sintomático, edema maligno, Clostridia, cobaias, imunofluorescência direta, streptavidina biotina peroxidase.
INTRODUCTION
Blackleg is an "endogenous" infection produced by Clostridium chauvoei (Gyles 1993). In contrast, malignant oedema is considered to be an "exogenous" infection produced by one or more of the following microorganisms: Clostridium septicum, C. chauvoei, C. novyi type A, C. sordellii and C. perfringens type A (Sterne & Batty 1975).
Although a presumptive diagnosis of blackleg and malignant oedema can be made based on clinical and pathological findings, a final diagnosis of these diseases needs identification of the causative microorganisms (Buxton & Donachie 1991).
Traditionally, confirmation of the diagnosis of blackleg and malignant oedema is based on culture and isolation of the microorganisms involved. However, these procedures are not always successful due to difficulties in obtaining, submitting and processing the samples in the laboratory. Also, some of the Clostridia causing blackleg and malignant oedema are extremely sensitive to oxygen, and they tend to be easily overgrown by other microorganisms present in the samples (Assis et al. 2001). Furthermore, culture and identification of Clostridia takes from 4 days to one week to complete, and differentiation between C. chauvoei and C. septicum is difficult due to morphological and biochemical similarities between these organisms (Sterne & Batty 1975).
The fluorescence antibody test (FAT) is frequently used for identification of these microorganisms on tissue or culture smears (Batty & Walker 1963, Pinto & Abreu 1992). This technique requires a fluorescent microscope and labeled antibodies are difficult to obtain commercially, the sections can be stored for only a few hours (Lozano & Ulrich 1968, Larsson 1988), and the tissue morphology is poor. In contrast, immunohistochemistry (IHC) is free of most of these hindrances, and it has been used to identify several microorganisms, including Clostridia, in smears and in tissue sections (Conesa et al. 1995, Vanelli & Uzal 1996a).
Of the many IHC techniques currently available, the streptavidin biotin peroxidase technique (SBPT) is amongst the most sensitive (Gimeno 1995). In this study, a SBPT was optimized to detect C. chauvoei, C. septicum, C. sordellii and C. novyi type A in tissues of guinea pigs experimentally infected with these microorganisms and the results of this technique were compared with those of FAT.
MATERIALS AND METHODS
Tissues
Tissues from fifty guinea pigs that had been previously inoculated intramuscularly with different species of Clostridia were available for this study. The animals had been divided in 5 groups of 10 animals each: animals in groups 1 to 4 had been inoculated with Clostridium chauvoei (strain ATCC 10092), C. septicum (strain ATCC 12464), C. sordellii (strain ATCC 9714), and C. novyi type A (strain ATCC 19402), respectively. All the inocula had been diluted in a sterile calcium chloride solution. Animals from group 5 (control) had been inoculated by the same route with a mixture of calcium chloride and sterile Tioglycollate broth (Barcelona, Dignolab, Spain). Infected animals had been killed when they presented clinical signs. In average, the animals inoculated with C. chauvoei, C. septicum, C. sordellii, and C. novyi type A, were killed at 24, 34, 26, and 36 hours after inoculation, respectively. The control animals were killed at similar intervals as the guinea pigs inoculated with bacterial cultures.
Production of primary antibodies and conjugates
For the FAT and SBPT, the conjugates and the primary antibodies were prepared as previously described by Assis et al. (2001). Briefly, immunoglobulins against C. chauvoei, C. septicum, C. sordellii and C. novyi type A were produced in rabbits and precipitated with a saturated solution (80%) of ammonium sulphate, before being dialyzed against PBS 0.01 M, pH 7.2. The IgG fractions were purified by immunoafinity using a Diethylaminoethyl (DEAE) cellulose column balanced and eluted with Tris/HCL buffer, pH 8.8. The protein concentration was determined using the Biuret method (Gornall et al. 1949) in a spectrophotometer by measuring the absorbance at 560 nm and comparing the results with a 10 mg/ml bovine serum albumin solution (BSA-Sigma, St Louis, USA) standard curve (Harlow & Lane 1988). Part of the purified IgG was used as primary antibody in the SBPT, and the remaining was conjugated. The conjugation of the immunoglobulins was done with fluorescein isothyocyanate (FITC- Sigma, St. Louis, USA). Unconjugated fluorescein was removed by passing the conjugates through of a Sephadex G-50 column balanced and eluted with PBS 0.01 M, pH 7.2. The fractions of conjugates obtained were mixed with glycerol in the proportion of 1:1 and stored at - 20ºC. Sera obtained prior to the immunization of the rabbits were used as normal rabbit sera (NRS) for control slides in the SBPT.
Streptavidin Biotin Peroxidase Technique (SBPT)
Samples of muscle from the inoculation area (MIA), heart, spleen, liver, and kidney were fixed by immersion in 10% buffered formalin, pH 7.2, for 24 hours, embedded in paraffin wax, cut at 5mm and mounted onto chrome alum-gelatin coated glass slides (Merck, Darmstadt, Germany). Prior to SBPT staining, the slides were incubated at 56ºC during 15 minutes. The SBPT was performed using a commercial Kit (Dako LSAB-peroxidase K675, Dako Corporation, Carpinteria, CA 93013, USA) according to the manufacture's recommendations. DAB (3,3-diaminobenzidine tetrahydrochloride) was used as chromogen, Kit (SK-4100, Vector laboratories, Burlingame, CA 94010), following the manufacturer's recommendations. The sections were then counterstained with Harris's haematoxylin, coversliped and examined under a light microscope. In order to establish the appropriate dilutions, temperature and incubation period for the primary antibodies, sections of MIA from animals in groups 1 to 4 in which Gram-positive bacilli were observed in sections stained with Maccallum-Goodpasture (Luna 1968), were tested by SBPT under different conditions. Primary antibodies and NRS were incubated for periods ranging from 15 min to 2 hours at room temperature, and for 12 hours at 4ºC. Dilutions of primary antibodies and NRS of 1:100 to 1:16,000 in phosphate buffer saline, pH 7.2 (PBS), were tested. After establishing the optimal working dilution, temperature and duration of incubation, sections from all tissue samples were tested using the corresponding primary antibody or NRS.
In order to test the laboratory specificity of the technique, each primary antibody was tested using SBPT on sections of MIA from guinea pigs inoculated with the Clostridium species other than the one against which the antibody was produced, and on sections of MIA from control animals.
Fluorescent Antibody Technique (FAT)
Smears of MIA, heart, spleen, liver, and kidney were prepared, air dried and fixed in anhydrous acetone during 30 minutes at-20ºC and processed for a FAT according to Pinto & Abreu (1992). FITC conjugates were used diluted 1:512, 1:256, 1:1024 and 1:1024, for C. septicum, C. chauvoei, C. sordellii and C. novyi type A, respectively (Assis et al. 2001). Briefly, the conjugates were diluted in a 2% Tween 80 solution in PBS pH 7.2. The tissue smears were incubated in a moist chamber at 37ºC by 30 minutes. The slides were speedily washed with PBS pH 7.2, immersed in PBS during 15 minutes and washed with distilled water. After this they were counterstained with a 0.005% Evan's Blue (Sigma, St. Louis, USA) solution in PBS for 10 minutes, washed for three times in PBS, mounted with glycerol pH 7.2 and examined under fluorescent microscope (Carl Zeiss, Oberkochen, Germany). These same evaluation criteria described for SBPT were used to determine the laboratory specificity of the FAT.
Diagnostic sensitivity and specificity
For calculation of diagnostic sensitivity and specificity of both techniques, the FAT was considered the gold standard. Diagnostic sensitivity was defined as the proportion of true positive samples (FAT positive) that were correctly identified as such by the SBPT, while diagnostic specificity was defined as the proportion of true negatives (FAT negative) that were correctly identified by the same test (Altman 1991).
RESULTS
At necropsy, severe muscular and subcutaneous oedema, associated with haemorrhage, and gas bubbles were observed involving the entire right thigh of all guinea pigs inoculated with clostridial cultures. Histologically, muscular lesions were similar in all infected groups and were characterized by a neutrophilic inflammatory response associated with degenerative changes including hypercontraction and hyalinization of muscle fibers and loss of striations. These changes were associated with variable degrees of interstitial oedema and haemorrhage. Basophilic rods were consistently observed only in the muscle tissue of all infected groups with frequency ranging from 60 to 90% of animals within each group. These rods were located in the interstitium. All uninfected controls had muscular lesions similar to those observed in infected animals although no bacteria were seen. Mild hyperaemia and focal haemorrhages were observed in sections of the liver, spleen, kidney, and myocardium in all groups. Clusters of basophilic rods were observed in sections of the liver, spleen, heart, and kidney from animals infected with Clostridium septicum.
C. chauvoei and C. septicum were detected by FAT in all tissue smears from guinea pigs of G1 and G2, respectively. C. sordellii and C. novyi type A were detected only in MIA smears from all the animals of G3 and G4, respectively. Each conjugate stained only the tissue smears from animals inoculated with the corresponding microorganisms. No fluorescence was observed when the conjugates were applied on smears of MIA from the control animals.
For the SBPT, optimal dilutions of the primary antibodies against C. chauvoei, C. septicum, C. novyi type A and C. sordellii , were 1:2000, 1:4000, 1:5000 and 1:8000, respectively. Preferred incubation times were 15 min, 40 min, 15 min and 1 hour, respectively for these microorganisms. Best staining was obtained when all incubations were performed at room temperature. Under these conditions a very low background staining was observed in all the sections from of G1 to G4 examined. Each primary antibody stained only the tissue samples from animals inoculated with the corresponding microorganism and staining was not observed in any tissue when the primary antibodies were replaced by NRS or in MIA tissues. Cross reactions between the different Clostridial species were not observed.
C. chauvoei and C. septicum were detected by the SBPT in all the sections from of G1 and G2, respectively, whereas C. novyi type A and C. sordellii were detected only in sections of the MIA from all animals of G3 and G4, respectively.
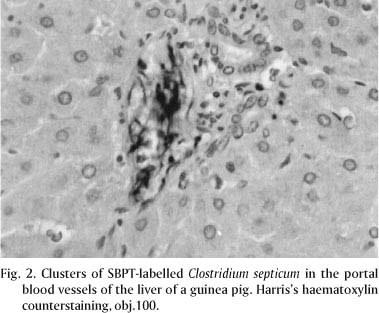
The analysis of the sections from animals inoculated with C. chauvoei and C. septicum stained by the SBPT, revealed that both agents presented the same pattern of distribution and the same intensity of staining. However, as a general rule, larger amounts of rods were observed in sections of animals inoculated with C. septicum than with C. chauvoei. C. chauvoei and C. septicum were found in high amounts only in sections of MIA. In the other organs, they were found in small quantities. SBPT revealed diffusely distributed, isolated or clustered dark brown bacilli in the interstitium of the MIA of groups 1 to 4 (Fig. 1). Rods were detected inside cardiac blood vessels and between myocardial muscular fibers of guinea pigs inoculated with C. chauvoei and C. septicum. In the liver of the animals inoculated with these microorganisms, positively stained rods were observed in the portal blood vessels (Fig. 2) and periacinar interstitium. Clusters of rods were also observed in the interstitium of the splenic red pulp, and in the interstitium and blood vessels of the renal cortex and medulla of guinea pigs inoculated with C. chauvoei and C. septicum (Fig. 3).
Diagnostic sensitivity of SBPT for all Clostridia species in MIA was 100%. For C. chauvoei and C. septicum, sensitivity was also 100% in the other organs. However, for C. novyi type A and C. sordellii, it was not possible to determine the sensitivity in the other organs, because rods were not detected in these tissues by FAT. Diagnostic specificity of SBPT for all these microorganisms was 100%.
DISCUSSION
The finding of Clostridium chauvoei and C. septicum, but not C. sordellii and C. novyi type A in organs other than MIA suggests that the former are more invasive. However, because the guinea pigs were killed as soon as they developed clinical signs (data not shown) we can not be certain about the state of the infection when the animals were killed, and we can not, therefore withdraw final conclusions about the comparative invasiveness of these microorganisms. The larger amounts of C. septicum observed in comparison with C. chauvoei might be explained by the higher replication potential of this agent (Batty & Walker 1965, Sterne 1981, Smith 1984).
The material most frequently submitted to the laboratory for diagnosis of blackleg and malignant oedema is skeletal muscle. However, the presence of C. chauvoei and C. septicum in other organs in this study, suggests that these organs are suitable for the diagnosis of these diseases by the FAT and SBPT, when either of these microorganisms are involved. Nevertheless, our study was performed in guinea pigs and we do not know if the same principle applies to sheep, cattle or other animal species.
The use of purified antibodies in this study allowed us to use high dilutions of these reagents, as well as reduced incubation periods, in comparison with previous reports (Conesa et al. 1995, Vanelli & Uzal 1996a,b), which was possibly responsible for the very low background observed. Our results show that the primary antibodies produced for this study were specific, since they stained only MIA sections from animals inoculated with the Clostridial species against which the primary antibody was produced and no cross reactions with other Clostridia or staining of control tissues were observed.
In this work, the FAT was considered the gold standard since Sterne & Batty (1975, ) stated that positivity to this technique is one of the criteria for diagnosis of blackleg and malignant oedema. Our results confirmed the validity of the mentioned criterion because the FAT detected the corresponding micro-organisms in 100% of the animals inoculated with them.
This study demonstrated that SBPT is a simple and quick method to specifically identify C. chauvoei, C. septicum, C. sordellii and C. novyi type A in formalin-fixed, paraffin-embedded tissues of guinea pigs, and it may complement the bacteriological procedures for the diagnosis of blackleg and malignant oedema, especially where laboratory facilities for anaerobic culture are not readily available, or when specimens for diagnostic are to be sent from areas far from diagnostic centers. This technique also allows retrospective studies using paraffin-embedded tissues.
Finally, SBPT may significantly improve the success rate for the diagnosis of blackleg and malignant oedema. This, in turn, will help establishing the prevalence of these diseases in countries such as Brazil where laboratory facilities are not always readily available. In Brazil epidemiological information about blackleg and malignant oedema is scanty (Correa et al. 1980, Baldassi et al. 1985), and originates mainly from clinical and necropsy findings (Assis et al. 2002).
ACKNOWLEDGEMENTS
Our thanks to the Ministério da Agricultura, Pecuária e Abastecimento (LARA, Pedro Leopoldo, Minas Gerais, Brazil), for providing the experimental animals, to E. N. Vidál (Argentina), Marilene de Almeida Campos and Mardelene Geisa Gomes (Brazil), for their invaluable technical assistance, and to Dr Maurício B. Carvalho Filho (LARA-MG, Brazil), for the critical reading of this manuscript.
Received on April 20, 2004.
Accepted for publication on August 27, 2004.
- Altman D.G. 1991. Pratical Statistics for Medical Research.1st ed. Chapman & Hall/CRC Press, Boca Raton, Florida, p.410.
- Assis R.A., Lobato F.C.F., Dias L.D., Uzal F.A, Martins N.E. & Silva N. 2001. Producción y evaluación de conjugados fluorescentes para o diagnóstico de mancha y gangrena gaseosa. Revta Med. Vet., Buenos Aires, 82:68-70.
- Assis R.A., Lobato F.C.F., Martins N.E., Nascimento R.A.P., Abreu V.L.V. & Uzal F.A. 2002. An outbreak of malignant edema in cattle. Revta Port. Ciênc. Vet., Lisboa, 97: 143-145.
- Baldassi L., Hipólito M., Calil E.M.B., Chiba S. & Moulin A.A.P. 1985. Observações sobre a incidência de gangrena gasosa e carbúnculo sintomático durante 10 anos, 1970-79, no estado de São Paulo. Biológico, São Paulo, 51:161-165.
- Batty I. & Walker P.D. 1963. Differentiation of Clostridium septicum and Clostridium chauvoei by use of fluorescent labelled antibodies. J. Pathol. Bacteriol. 85:517-520.
- Batty I & Walker P.D. 1965. Colonial morphology and fluorescent labeled antibody staining in the identification of species of the Genus Clostridium J. Appl. Bacteriol. 28:112-118.
- Buxton D & Donachie W. 1991. Clostridial diseases. p.104-114. In: Martin W.B. & Aitken I.D. (ed.) Diseases of the Sheep. 2nd ed. Blackwell Scientific Publications, Oxford.
- Conesa L.C.G., Vanneli S.A. & Uzal F.A. 1995. Detection of Clostridium chauvoei in formalin-fixed, paraffin-embedded tissues of sheep by the peroxidase-antiperoxidase (PAP) technique. Vet. Res. Commun. 19:451-456.
- Correa W.M., Correa C.N.N., Lopes C.A.M., Langoni H. & Modolo J.F. 1980. Enfermidades por clostrídios 1969-1978 (clostridial diseases 1969-1978). Arq. Bras. Med. Vet. Zootec. 32:369-374.
- Gimeno E.J. 1995. Fundamentos da inmunohistoquímica aplicada a Patología Veterinária, p.17-51. In: Anais do 7ş Encontro Nacional de Patologia Veterinária (Enapave), Belo Horizonte.
- Gornall A.G., Bardawill C.J. & David M.M. 1949. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 177:751-766.
- Gyles C.L. 1993. Histotoxic Clostridia, p.106-113. In: Gyles C.L. & Thoen C.O. (ed.) Pathogenesis of Bacterial Infections in Animals. Iowa State University Press, Ames.
- Harlow E.D. & Lane D. 1988. Antibodies. A Laboratory Manual. Cold Spring Harbor Laboratory, Spring Harbor, USA. 726p.
- Larson L.I. 1988. Immunocytochemistry: Theory and Practice. CRC Press, Boca Raton, Florida. 368p.
- Lozano E.A. & Ulrich J.T. 1968. Temperature studies on chicken inoculations to aid differentiation between Clostridium chauvoei and Clostridium septicum Cornell. Vet. 58: 333-343.
- Luna L.G. 1968. Manual of the Histologic Staining Methods of the Armed Forces Institute of Pathology. 3rd ed, McGraw Hill, New York.
- Pinto M.P. & Abreu, V.L.V. 1992. Comparação de técnicas para preparo de conjugados anti-Clostridium septicum e anti-Clostridium chauvoei. Arq. Bras. Med. Vet. Zootec. 44:513-520.
- Smith L.D.S. 1984. The Pathogenic Anaerobic Bacteria. 3rd ed. Springfield, Illinois. 550p.
- Sterne M. & Batty I. 1975. Pathogenic Clostridia. Butterworths, London.144p.
- Sterne M. 1981. Clostridial infections. Brit. Vet. J.137:443-454.
- Vannelli S.A. & Uzal F.A. 1996a. Clostridium septicum detection by the peroxidase-antiperoxidase (PAP) technique, in formalin-fixed, paraffin-embedded tissues of sheep. Arch. Med. Vet. 28:125-127.
- Vannelli S.A & Uzal F.A. 1996b. Identificación de Clostridium sordellii por una técnica de peroxidasa-antiperoxidasa (PAP) en improntas y en tejidos ovinos fijados en formol e incluídos en parafina. Revta Med. Vet., Buenos Aires, 77:306-312.
Publication Dates
-
Publication in this collection
13 June 2005 -
Date of issue
Mar 2005
History
-
Received
20 Apr 2004 -
Accepted
27 Aug 2004