Abstracts
The American bullfrog (Rana catesbeiana), recently named Lithobates catesbeianus is currently farmed for commercial purposes throughout various regions of Brazil. Stressful situations such as problems of management, inadequate facilities and environmental changes with consequent reduction of immunity are common in intensive production. The assessments of these situations of stress allow us detect these problems decreasing the injuries causes by confinement. The main objective of this study was to use the biological markers of plasma cortisol and glucose level and hematological parameters to evaluate the response of bullfrog tadpoles submitted to stressed mechanisms of capture and hypoxia. The animals were subjected to three treatments: stress due to individual capture with a hand net; stress due to batch capture with a hand net; and stress due to capture by emptying. The results obtained demonstrated that there were no statistically significant differences in the parameters tested when comparing the treatments with and without exposure to air (normoxia and hypoxia). Based on these results we can conclude that the stressful stimuli tested were not adequate to alter the biomarker tested. For the cortisol, probably this should have occurred due to the synergistic action between this hormone and thyroxin, which induces metamorphosis in these animals.
Frogculture; stress; cortisol; Lithobates catesbeianus; frog; amphibian
A rã-touro americana (Rana catesbeiana) recentemente denominada Lithobates catesbeianus é cria da com propósito comercial em várias regiões do Brasil. Situações estressantes tais como problemas de manejo, criação inadequada e alterações ambientais com consequente redução da imunidade são comuns em produções intensivas. A avaliação destas situações de estresse permite-nos detectar estes probemas e diminuir as injurias causadas pelo confinamento. O principal objetivo deste estudo foi utilizar os marcadores biológicos de cortisol, glicemia e dados hematológicos para avaliar a resposta de girinos de rã-touro submetidos aos mecanismos estressores de captura e hipóxia. Os animais foram distribuídosem três tratamentos: estresse por captura individual com puçá; estresse por captura em massa com puçá e estresse por captura por escoamento. Os resultados obtidos demostraram não haver diferenças estatisticamente significativas entre os parametros avaliados quando comparou-se os grupos com e sem exposição ao ar (normoxia e hipoxia). Com base nestes resultados pode-se concluir que os estímulos estressores avaliados não foram adequados para alterar os valores plasmáticos dos marcadores biológicos testados. Para o cortisol, isto ocorreu provavelmente em virtude da ação sinérgica deste hormônio e a tiroxina, que induz a metamorfose nestes animais.
Ranicultura; estresse; cortisol; Lithobates catesbeianus; rã; anfíbios
ANIMAL MORPHOPHYSIOLOGY
Physiological response of American bullfrog tadpoles to stressor conditions of capture and hypoxia
Resposta fisiológica de girinos de rã-touro americana submetidos aos mecanismos estressores de captura e hipóxia
Guilherme C. RochaI; Cláudia M. FerreiraI; Patrícia C. TeixeiraII; Danielle C. DiasII; Fernanda M. FrançaI; Antonio M. AntonucciI; Adriana S. MarcantonioIII; Marcelo LaurettoIV
ILaboratório de Patologia de Organismos Aquáticos, Instituto de Pesca, Av. Francisco Matarazzo 455, São Paulo, SP 05001-900, Brazil. E-mail: claudia@pesca.sp.gov.br
IICentro de Aqüicultura (CA), Universidade Estadual Paulista (Unesp), Campus de Jaboticabal, Via de acesso Prof. Paulo Donato Castellane s/n, Jaboticabal, SP 14884-900, Brazil
IIIPólo Regional de Desenvolvimento Tecnológico dos Agronegócios, Agência Paulista de Tecnologia dos Agronegócios (APTA), Av. Sagrados Corações s/n, Pindamonhangaba, SP 12420-000, Brazil
IVEscola de Artes e Ciências e Humanidades (EACH), Universidade de São Paulo (USP), Av. Prof. Dr. Orlando Marques de Paiva 87, São Paulo, SP 05508-270.
ABSTRACT
The American bullfrog (Rana catesbeiana), recently named Lithobates catesbeianus is currently farmed for commercial purposes throughout various regions of Brazil. Stressful situations such as problems of management, inadequate facilities and environmental changes with consequent reduction of immunity are common in intensive production. The assessments of these situations of stress allow us detect these problems decreasing the injuries causes by confinement. The main objective of this study was to use the biological markers of plasma cortisol and glucose level and hematological parameters to evaluate the response of bullfrog tadpoles submitted to stressed mechanisms of capture and hypoxia. The animals were subjected to three treatments: stress due to individual capture with a hand net; stress due to batch capture with a hand net; and stress due to capture by emptying. The results obtained demonstrated that there were no statistically significant differences in the parameters tested when comparing the treatments with and without exposure to air (normoxia and hypoxia). Based on these results we can conclude that the stressful stimuli tested were not adequate to alter the biomarker tested. For the cortisol, probably this should have occurred due to the synergistic action between this hormone and thyroxin, which induces metamorphosis in these animals.
INDEX TERMS: Frogculture, stress, cortisol, Lithobates catesbeianus, frog, amphibian.
RESUMO
A rã-touro americana (Rana catesbeiana) recentemente denominada Lithobates catesbeianus é cria
da com propósito comercial em várias regiões do Brasil. Situações estressantes tais como problemas de manejo, criação inadequada e alterações ambientais com consequente redução da imunidade são comuns em produções intensivas. A avaliação destas situações de estresse permite-nos detectar estes probemas e diminuir as injurias causadas pelo confinamento. O principal objetivo deste estudo foi utilizar os marcadores biológicos de cortisol, glicemia e dados hematológicos para avaliar a resposta de girinos de rã-touro submetidos aos mecanismos estressores de captura e hipóxia. Os animais foram distribuídosem três tratamentos: estresse por captura individual com puçá; estresse por captura em massa com puçá e estresse por captura por escoamento. Os resultados obtidos demostraram não haver diferenças estatisticamente significativas entre os parametros avaliados quando comparou-se os grupos com e sem exposição ao ar (normoxia e hipoxia). Com base nestes resultados pode-se concluir que os estímulos estressores avaliados não foram adequados para alterar os valores plasmáticos dos marcadores biológicos testados. Para o cortisol, isto ocorreu provavelmente em virtude da ação sinérgica deste hormônio e a tiroxina, que induz a metamorfose nestes animais.
TERMOS DE INDEXAÇÃO: Ranicultura, estresse, cortisol, Lithobates catesbeianus, rã, anfíbios.
Introduction
The American bullfrog (Rana catesbeiana), recently named Lithobates catesbeianus (Frost et al. 2006), was introduced to Brazil in 1935 and became a farmed commodity to this day. When compared to native Brazilian anurans, the American bullfrog has several zootechnical advantages including ease of handling, rapid growth, high fecundity and the fact that it is rarely affected by epidemic-type pathological causes of death (Ferreira et al. 2002). However, various factors can lead to undesirable physiologic responses, such as temperature, water quality, age, physiological conditions, social circumstances, different species and strains, abrupt or extreme changes in physical environment, social interactions and human interference (including physical, prophylactic and sanitary handling, and inadequate feeding) (Wendelaar Bonga 1997, Ferreira et al. 2001). These factors, individually or in unison, can cause stress to the organisms with regard to reproductive dysfunction, growth and health, for example. Stress can be defined as a condition in which the dynamic equilibrium of the animal (homeostasis) is disturbed, as a result of an intrinsic and/or extrinsic stimulus, called a stressor). Animals submitted to stressful conditions can undergo adaptations to a perturbed homeostasis by compensatory physiological changes (Wendelaar Bonga 1997). We can be divided the response to stress into three steps. In the first step, there is the activation of brain system center, which culminates in an intense release of catecholamines and corticoids (mainly cortisol). In the second step, the immediate actions and effects of these hormones occur which are released into the blood stream and tissues. In the third step, the states of exhaustion are characterized which cause a fall in performance and decrease in resistance to diseases (Barcelos et al. 2000). The latter can lead to infection of the animal by opportunistic agents, including bacteria and fungi that could potentially lead to death (Barton & Iwama 1991). Some indicators of stress can be detected in the blood. Plasma cortisol and glucose, for example, are the markers most utilized today and are thought to be accurate indicators of stress. Cortisol was chosen as a biological marker in this study because it is a hormone used in routine diagnostic tests. It is therefore easily accessible and cheaper than other available markers at this time (e.g. corticosterone).
Cortisol or hydrocortisone is a glucocorticoid that is related to metabolism, inflammation and stress (Voet et al. 2000). It exerts an effect on various functions of the body, among them are effects on the metabolism of carbohydrates, proteins and fats. When in excess, it causes a decrease in muscle protein, immune function, performance of the animal, and at the same time disrupts hydromineral balance and increases levels of hepatic glycogen and fatty acids. Glucose is a metabolite whose main biological function is to serve as an energy substrate for cells. It is stored in the form of glycogen in the liver. In situations where blood cortisol levels are high, gluconeogenesis occurs, that is, hepatic cells convert proteins and glycerol into glucose (Feder & Burggren 1992).
There are not many scientific reports on stress in captive amphibians such as L. catesbeianus, but these physiological processes probably also occur in this type of animal. Based on experimental studies on the behavior of frogs Duellman & Trueb (1994) reported that these animals are easily stressed. According to Ferreira et al. (2001), stress can be the cause of one of the main problems in frog farming diseases. In various phases of development, characteristics as a consequence of exposure to stress factors can be observed. Symptoms most often noted in tadpoles include a lack of appetite, lethargy and disorientation, among others. In the pre-growing and growing phases, the animals pile on each other in the corners of the pens and jump in a disoriented manner. Additionally, excess skin is seen in moist areas and sometimes mucus can be observed in the form of froth. In the reproductive period, females, when stressed, abort and produce characteristic cries, while males quit croaking (Lima & Agostinho 1992, Ferreira et al. 2001).
The aim of the present study was to evaluate the physiological response of bullfrog tadpoles (Lithobates catesbeianus) to stress caused by different means of capture and by hypoxia. Plasma cortisol and glucose and hematological parameters were determined.
Material and methods
Bullfrog tadpoles (Lithobates catesbeianus) in the pro-metamorphosis stage were obtained from the Frog Farming of the Aquacultural Center of São Paulo State University, Jaboticabal, SP (CAUnesp). The tadpoles utilized in the collections had a mean weight of 9.05±1.69g and mean length of 11.96 ±1.31cm. For estimate values form control group the blood was drawn from each individual tadpole upon immediate removal from their original tanks. Following transportation to the lab, animals were acclimated for 7 days in polyethylene tanks (500 liters), at a density of 1 tadpole/liter at the Experimental Frog Farming of Aquacultural Center (PRDTAVP), Agricultural Department of São Paulo State, Pindamonhangaba, Brazil. These tanks were conditioned using an agricultural oven, and the surface was covered with polyethylene plastic and the sides with nylon screen. The treatments performed were: stress due to individual capture with a hand net (Treatment 1); stress due to batch capture with a hand net (Treatment 2); and stress due to capture by emptying (Treatment 3), which is the handling traditionally used in Brazil frog farming. Each treatment was carried out in simultaneous triplicate in a random order, and 12 individuals were used (6 normoxia - blood collected immediately, and 6 hypoxia - blood collected after 15 min exposure to air). Time to hypoxia was determined through preliminary tests.
There were two sets of collections with an interval of 5 days between them (totaling 72 tadpoles for the whole study). Following exposure to each of the stress inducing conditions, prior to the collection of blood, animals were placed in water for 30 min to facilitate the release of cortisol. Results of the blood samples obtained at the end of the study were compared those samples collected before the onset of the study.
The animals that eventually died during the study period were removed from the tanks in the morning so as not to degrade the quality of the water. Also at this time, the animals were fed rations containing 45% crude protein, 6% crude fiber 9% ether extract, once a day, in an amount of 1% of live weight. The water of each tank was changed completely at various intervals throughout the day (continuous flow system). The ambient temperature, relative humidity of the air and physical-chemical parameters of the water (temperature, electrical conductivity, pH, oxygen) was monitored daily. Total ammonia and nitrite were measured weekly.
For physiological analyses, an aliquot of blood was obtained from the rupture of the caudal vessel with the aid of disposable needles and heparinized tips, after the application of Lidocaine for local anesthesia of tails of the tadpoles. In accordance with the circadian rhythm of the animals, blood collections took place in the morning hours. The animals were subsequently anesthesiated with benzocaine (1:10) and sacrificed. The cortisol and glucose levels were determined in the plasma obtained by centrifugation. Cortisol was assayed by an ELISA method (Active-Cortisol EIA DSL10, Diagnostic System Labs USA) with limit of detection of 0.1ìg/dL and coefficient of variation for intra- and inter-assay precision of 5.9 and 8.7%, respectively. Glucose was determined by means of the LABTEST® kit (GLUCOSE PAP Liquiform).
The hematological parameters determined and the methods used were those recognized internationally: total number of erythrocytes (RBC) counted in a Neubauer chamber using Hayem's solution as diluent; hematocrit (Ht), hemoglobin (Hb) total and differential leukocyte counts in blood smears stained with May-Grünwald and Giemsa.
Physiological and hematological variables were statistically described. After normality (D'Agostino-Pearson) and homocedasticity (Bartlett's test) verifications, a two-way analysis of variance was conducted (both factors fixed), followed by a Tukey test (Zar 1999). Statistical tests were performed in the BioEstat 4.0 and Minitab® 15, considering p=0.05.
Results and discussion
The results of the monitoring the physical and chemical parameters of the water did not show a significant difference among the tanks used for the different treatments. The temperature of the water was 23.91±0.30oC, electrical conductivity 54.58±3.27mS/cm, pH 6.87±0.11 and dissolved oxygen 3.20±0.55mg/L. Mean ammonia was 0.05mg/L of NH4, which corresponds to 0.19mg/L of NH3. Nitrite was not detected in any of the tanks. In relation to ambient temperature, the minimal temperature was 19.50±2.00oC and maximal 32.70±2.54oC, with an air relative humidity of 75%. During the entire study, mortality was minimal at 1.57% of the total animals.
The data for the plasma cortisol followed a normal distribution (K2=2.89; p=0.2355) and the variances were
homogeneous (B=3.27; p=0.351). Even with a small number of data (n=82) the ANOVA was sufficient hard to be applied, considering the fact of normality and homocedasticity have been attended. The results of ANOVA showed no significative effect of the factors tested (p>0.05). No statistically significant differences were found between the varying capture methods or the time between collections (individual capture with a hand net, batch capture with a hand net or capture by emptying), providing no justification for the application of a posteriori test. In other words, all these methods could be utilized without causing substantial changes in circulating levels of cortisol and impairing the health of Lithobates catesbeianus tadpoles. Since the capture method by emptying the tank is the most rapid and efficient, it would therefore be the most indicated in commercial frog farming. Barton & Iwama (1991) noted that the pattern of response for cortisol, after acute disturbances, consists of a rapid elevation in levels (minutes) followed by a slow decline (hours or days), in the majority of fish species. Besides the different types of capture tested in this study, another stressor condition was 15min of hypoxia followed by 30min rest in water, before drawing blood samples, with the intention of following the standard model for the release of cortisol in vertebrates, which is 30 min after the stressor event (Barcellos et al. 2000, Sloman et al. 2001). However, this time delay before blood sampling may not have been sufficient to demonstrate an elevation in plasma levels of this hormone, suggesting that additional studies are needed with serial measurements of plasma or tissue cortisol in this species. In the literature, cortisol values were found to vary greatly, which could be attributed to events that affect the release of cortisol in the same organism, such as stage of development and sexual maturation, time of year, temperature, circadian rhythm and the method utilized for drawing blood (Iwama 1993).
The mean plasma cortisol levels for bullfrog tadpoles obtained during this study varied 2.14 to 5.0ng/mL, higher than those found by Wright et al. (2003), working with tadpoles of the same species and stage of development, which varied 0.8 to 1.08ng/mL. Our results were probably influenced by the low levels of dissolved oxygen in the tanks, while those reported by the above authors were from experiments carried out in the laboratory with a more controlled photoperiod and physical and chemical conditions of the water, besides using a radioimmunoassay to determine plasma cortisol. The levels detected by Krug et al. (1983) varied between 12 and 22.3ng/mL, at pro-metamorphosis and climax, respectively. However, these latter investigators reported qualitative uncertainties regarding the hormone measured, calling these substances quantified as "cortisol-like material."
A study performed with rainbow trout, Oncorhynchus mykiss, submitted to stress by exposure to air for 30 sec and with blood collection 30 min after this procedure, showed evidence of significantly higher cortisol levels in fish that were exposed to air compared to those not exposed (Sloman et al. 2001). Barcellos et al. (2000) observed that specimens of catfishes, Rhamdia quelen, subjected to procedures of capture and transfer to tanks, showed a peak cortisol level one hour after the procedure.
Another parameter examined to indicate was plasma glucose level. The same procedure's prior stage to plasma cortisol realizing the descriptive statistic and normality's test was followed. As with the results for cortisol, the plasma glucose concentrations detected, also did not show significant differences when comparing the different treatments (methods of capture) and with and without exposure to air (hypoxia or normoxia). However, our analyses indicate that there was a significant difference in plasma glucose levels between the 1st and 2nd collections, indicating that collection time had a substantial impact on plasma glucose level (p=0.015). Elevated glycemia in fish, as a response to stress, is due to the action of catecholamines which stimulate glycogenolysis, while increased levels of plasma glucose are maintained by cortisol, which acts after adrenalin (Wijayan et al. 1994). Hyperglycemia is generally observed in animals exposed to some type of adverse stimulus (Pottinger et al. 1999). However, in the same manner as with cortisol, it is not known with certainty at what time glucose levels peak after a stress stimulus, for the species in question.
The mean values found for this parameter in the literature vary considerably. Byrne & White (1975) reported for blood glucose in bullfrog values of 40 and 50mg% in natural populations of this species during the reproductive period.
Wang & Chang (1994) found glucose levels varying between 20 and 64mg/dL, and Stefani (1996) values from 48.97 to 55.86mg%. Hutchison & Turkey (1975) determined mean blood glucose levels of 24.5±3.0mg% for Rana pipiens, while Smith (1954) observed in Rana temporaria mean values of 38±1.4mg%. Baseline levels of blood glucose found in the present study varied between 49.33 and 104.33mg/dL (76.07±18.50mg/dL).
According to Herman (1977), the differences in blood glucose concentrations among different species can be attributed to differences in handling, procedure for blood drawing and/or analytical method used. The same reasoning can be applied to the glucose levels found in the species studied here, L. catesbeianus. Moreover, it should be taken into consideration as well the question of stage of development (tadpole to adult frog) and the presence or not of a stressor stimulus.
The physiological tools of cortisol and glucose levels for identifying stress in L. catesbeianus tadpoles were complemented with the study of some hematological indicators. The responses of the hematological parameters to the stressor conditions imposed are shown in Table 1.
These results did not indicate significant differences in the hematological parameters in animals subjected to stress by hypoxia and capture, independent of the comparison between treatments and between the two collection periods. In studies on stress, Pickering et al. (1982) utilizing trout (Salmo trutta) and Ellsaesser & Clem (1986) utilizing catfish
Physiological response of American bullfrog tadpoles to stressor conditions of capture and hypoxia
(Ictalurus punctalus), also showed no significant differences in the number of erythrocytes and other hematological parameters. However, Kebus et al. (1992) and Iwama (1993) stated that immunodeficiency resulting from the response to stress was due to a negative correlation of the number of circulating lymphocytes with plasma cortisol level. Pickering et al. (1982) observed that the simple stress of handling in two specimens of Salmo trutta caused lymphopenia after 8 h, which required 72 h for recovery of lymphocytes to baseline levels.
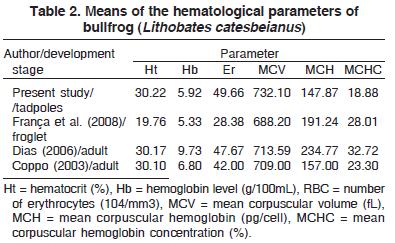
Ferreira et al. (2003) reported that the most abundant leukocytes in the blood of tadpoles of L. catesbeianus are the lymphocytes, which was also found in the differential leukocyte count of the present study: lymphocytes (Lp) 91.85±1.40%. The other leukocytes determined were neutrophils (Nt) at 1.36±0.94 %, basophils (Bs) at 5.18±1.178% and eosinophils (Es) at 1.65±0.50%, with no detection of monocytes (Mn). The mean results obtained in the differential leukocyte count are similar to those of Ferreira et al. (2003): Lp: 88.0±1.4%; Nt: 3.8±0.9%; Bs: 5.8±0.7%; Es: 2.0±0.3% and Mn: 0.4±0.1% and also similar to those of França et al. (2008) who worked with froglets recently metamorphosed: Lp: 82.64±2.86%; Nt: 8.61±1.98%; Bs: 6.38±1.45%; Es: 1.82±0.41% and Mn: 0.56±0.24%. For comparison, the results of França et al. (2008), Dias (2006) and Coppo (2003) for mean values found in individuals of L. catesbianus at different stages of development are presented in Table 2.
Acknowledgements
To FAPESP for the financial support (Project 05/53070-0), and Dr. Lisa M. Schloegel (Consortium for Conservation Medicine, New York, USA) to have revised this manuscript.
Conclusion
The results of our study indicate that neither capture methods nor hypoxia appear to significantly impact plasma cortisol and glucose levels or hematological parameters in tadpoles of the species Lithobates catesbeianus, changes that are typically observed in other aquatic organisms in response to stress. It is possible that the intensity of the stressor stimuli were not sufficient to significantly alter the plasma values measured in this study. Probably this should have occurred due to the synergistic action of the hormone thyroxin with cortisol, which induces metamorphosis in these animals Additional tests should also be conducted to determine if additional markers are better indicators of stress in tadpoles (e.g. corticosterone).
Received on July 14, 2010.
Accepted for publication on August 23, 2010.
- Barcellos L.J.G., Souza S.M.G. & Woehl V.M. 2000. Estresse em peixes: fisiologia da resposta ao estresse, causas e conseqüências (Revisão). Bolm Inst. Pesca 26(1):99-111.
- Barton B.A. & Iwama G.K. 1991. Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Ann. Rev. Fish Dis. 10:3-26.
- Byrne J.J. & White R.J. 1975. Cyclic changes in liver and muscle glycogen tissue lipid and blood glucose in a naturally occurring population of Rana catesbeiana Comp. Bioch. Physiol. 50A:709-715.
- Coppo J.A. 2003. El médio interno de la rana toro Rana catesbeiana, Shaw 1802. Revta Veterinária 14(1):25-41.
- Dias D.C. 2006. Influência de probióticos no desempenho produtivo e fisiológico de rã-touro (Rana catesbeiana Shaw, 1802). Dissertação de Mestrado, Faculdade de Ciências Agrárias, Centro de Aqüicultura, Universidade Estadual Paulista, Jaboticabal, São Paulo. 103p.
- Duellman W.E. & Trueb L. 1994. Biology of Amphibians. Johns Hopkins University Press Baltimore, USA. 670p.
- Ellsaesser C.F. & Clem L.W. 1986. Haematological and immunological changes in channel catfish stressed by handling and transport. J. Fish Biol. 28:511-521.
- Feder M.E. & Burggren W.W. 1992. Environmental physiology of the amphibians. University of Chicago Press, Chicago, USA. 472p.
- Ferreira C.M., Dias D.C., França F.M. & Barbosa C.J.S. 2001. Estresse e sistemas de criação. 11º Encontro Nacional de Ranicultura, Academia Brasileira de Estudos Técnicos em Ranicultura, Bragança Paulista, p.37-40.
- Ferreira C.M., Pimenta A.G.C. & Paiva-Neto J.S. 2002. Introdução à Ranicultura. Bolm Tec. Inst. Pesca, São Paulo, 33:1-15.
- Ferreira C.M., Bueno-Guimarães H.M., Ranzani-Paiva M.J.T., Soares S.R.C., Rivero D.H.R.F. & Saldiva P.H.N. 2003. Marcadores hematológicos de toxicidade do cobre em girinos de Rana catesbeiana (Bullfrog). Revta Bras. Toxicol. 16(2):83-88.
- França F.M., Dias D.C., Teixeira P.C., Marcantônio A.S., De Stéfani M.V., Antonucci A., Rocha G., Ranzani-Paiva M.J.T. & Ferreira C.M. 2008. Efeito do probiótico Bacillus subtilis no crescimento, sobrevivência e fisiologia de rãs-touro (Rana catesbeiana), Bolm Inst. Pesca, São Paulo, 34(3):403-412.
- Frost D.R., Grant T., Faivovich J., Bain R.H., Haas A., Haddad C.F.B., De Sa R., Channing A., Wilkinson M., Donnellan S.C., Raxworthy C.J., Campbell J.A., Blotto B.L., Moler P., Drewes R.C., Nussbaum R.A., Lynch J.D., Green D.M. & Wheeler W.C. 2006. The amphibian tree of life. Bull. Am. Museum Natural History 297:1-370.
- Herman C.A. 1977. Comparative effects of epinephrine and norepinephrine on plasma glucose and hematocrit levels in the American bullfrog (Rana catesbeiana). Gen. Comp. Endocrinol. 32:321-329.
- Hutchison V.H. & Turkey L.D. 1975. Glucose and lactate concen-trations during activity in the leopard frog, Rana pipiens J. Comp. Physiol. B, Biochemical, Syst. Environ. Physiol. 99:287-295.
- Iwama G.K. 1993. Intensive Fish Production: Course manual UBC access guided independent study. University of British Columbia, Vancouver. 130p.
- Kebus M.J., Collins M.T., Brownfield M.S., Amundson C.H., Kayes T.B. & Malison J.A. 1992. Effects of rearing density on stress response and growth of rainbow trout. J. Med. Biol. Res. 22:1019-1022.
- Krug E.C., Honn K.V., Battista J. & Nicoll C.S. 1983. Corticosteroids in serum of Rana catesbeiana during development and metamorphosis. Gen. Comp. Endocrinol. 5:232-241.
- Lima S.L. & Agostinho C.A. 1992. A tecnologia da criação de rãs. Imprensa Universitária, Viçosa, MG. 76p.
- Pickering A.D., Pottinger T.G. & Christie P. 1982. Recovery of the trout, Salmo trutta L., from acute handling stress: A time-course study. J. Fish Biol.24:731-740.
- Pottinger T.G., Yeomasn W.E. & Carrick T.R. 1999. Plasma cortisol and 17-estradiol levels in roach exposed to acute and chronic stress. J. Fish Biol.54:525-532.
- Smith C.L. 1954. The relation between seasonal hyperglycaemia and thyroid activity in the frog (Rana temporaria). J. Endocrinol. 10:184-191.
- Sloman K.A., Metcalfe N.B., Taylor A.C. & Gilmour K.M. 2001. Plasma cortisol concentrations before and after social stress in rainbow trout and brown trout. Physiol. Biochem. Zool. 74:383-389.
- Stéfani M.V. 1996. Metabolismo e crescimento da rã-touro (Rana catesbeiana Shaw, 1802) alimentada com níveis crescentes de carboidratos. Tese de Doutorado, Faculdade de Ciências Agrárias, Centro de Aqüicultura, Universidade Estadual Paulista, Jaboticabal, SP.
- Voet D.I., Voet J.G.I. & Pratt C.W. 2000. Fundamentos de Bioquimica. Artmed, São Paulo. 1264p.
- Wang J.H. & Chang M.H. 1994. Studies on hematology of captive bullfrog Rana catesbeiana Coa Fish 46:69-87.
- Wendelaar Bonga S.E. 1997. the stress response in fish. Physiol. Rev. 77:591-625.
- Wijayan M.M., Reddy P.K., Leatherland J.F. & Moon T.W. 1994. The effects of cortisol on hepatocyte metabolism in rainbow trout: A study using the steroid analogue RU 486. Gen. Comp. Endocrinol. 96:75-84.
- Wright M.L., Guertin C.J., Duffy J.L., Szatkowski M.C., Visconti R.F. & Alves C.D. 2003. Developmental and diel profiles of plasma corticosteroids in the bullfrog, Rana catesbeiana Comp. Biochem. Physiol. 135A:585-595.
- Zar J.H. 1999. Biostatical Analysis. Pretice Hall, New Jersey, USA. 663p.
Publication Dates
-
Publication in this collection
06 Dec 2010 -
Date of issue
Oct 2010
History
-
Received
14 July 2010 -
Accepted
23 Aug 2010