Resumos
Este artigo descreve uma investigação da virulência/atenuação de recombinantes do herpesvírus bovino tipo 5 (BoHV-5) com deleções nos genes da glicoproteína E (BoHV-5gEΔ), timidina quinase (BoHV-5TKΔ), e ambos gE e TK (BoHV-5gEΔTKΔ). Bezerros soronegativos (80-90 dias de idade) inoculados com o vírus parental SV-507/99 (n=5) excretaram o vírus em secreções nasais por até 15 dias (média de 10,8 dias). Nos animais inoculados com os recombinantes, a duração da excreção viral foi de 11 dias (BoHV-5gEΔ), 9,6 dias (BoHV-5TKΔ) e 6,2 dias (BoHV-5gEΔTKΔ). Os maiores títulos foram observados entre os dias 1 e 6 pós-inoculação (pi), sendo de 10(6,8)TCID50/mL para o SV-507/99, 10(5,1)TCID50/mL (BoHV-5gEΔ), 10(5,9)TCID50/mL (BoHV-5TKΔ) e 10(4,7)TCIΔ50/mL (BoHV-5gEΔTKΔ). Os bezerros inoculados com o vírus parental apresentaram anorexia e apatia; três deles mostraram apatia profunda e perda da condição corporal. Dois bezerros foram eutanasiados in extremis nos dias 10 e 11 pi, respectivamente e o vírus foi isolado de várias regiões do encéfalo. Já os bezerros inoculados com os recombinantes permaneceram saudáveis; alguns apresentaram uma secreção nasal serosa transitória. Administração de dexametasona (Dx) no dia 42 pi resultou em excreção viral por todos os bezerros inoculados com o vírus parental (duração média de 3,7 dias), por 2 de 5 bezerros dos grupos BoHV-5TKΔ (dois dias) e BoHV-5gEΔ (um dia). Os bezerros inoculados com o duplo mutante BoHV-5gEΔTKΔ não excretaram o vírus após o tratamento com Dx. Pesquisa de DNA viral por PCR no dia 30 pós-Dx revelou uma ampla distribuição do DNA do vírus parental no encéfalo; poucas seções (3/30) foram positivas no encéfalo dos animais do grupo BoHV-5gEΔ, e não detectou-se DNA latente no encéfalo dos animais dos grupos BoHV-5TKΔ e BoHV-5gEΔTKΔ. Esses resultados demonstram que os mutantes simples (gE and tk-deletados) são atenuados para bezerros e estabelecem e/ou reativam infecção latente ineficientemente. Já o duplo mutante BoHV-5gEΔTKΔ é atenuado e parece não estabelecer e/ou não reativar eficientemente a infecção latente. Portanto, os vírus recombinantes, e em especial o duplo mutante BoHV-5gEΔTKΔ apresentam um fenótipo compatível com a sua inclusão em vacinas vivas modificadas.
BoHV-5; patogenia; mutantes; atenuação; virulência
This article describes an investigation on the virulence/attenuation of bovine herpesvirus type 5 (BoHV-5) recombinants deleted in the genes encoding glycoprotein E (BoHV-5gEΔ), thymidine kinase (BoHV-5TKΔ), and both gE and TK (BoHV-5gEΔTKΔ). Seronegative calves (80 to 90 days-old) inoculated with the parental strain (SV-507/99, n=5) shed virus in nasal secretions for up to 15 days (average 10.8 days). Duration of virus shedding was 11 days for BoHV-5gΔ, 9.6 days for BoHV-5TKΔ and 6.2 days for BoHV-5gEΔTKΔ groups. The highest titers were observed between days 1 and 6 post-infection (pi) for SV-507/99 (10(6.8)TCID50/mL), 10(5.1)TCID50/mL (BoHV-5gEΔ), 10(5.9)TCID50/mL (BoHV-5TKΔ) and 10(4.7)TCID50/mL (BoHV-5gEΔTKΔ). Calves inoculated with the parental virus presented anorexia, profound apathy and loss of body condition. Two calves were euthanized in extremis on days 10 and 11 pi; infectious virus was recovered from several areas of the brain. In contrast, calves inoculated with the recombinants remained healthy and a few presented a mild and transient nasal secretion. Dexamethasone (Dx) administration at day 42 pi resulted in virus shedding by all controls calves (mean duration 3.7 days), by 2/5 of BoHV-5TKΔ calves (two days) and 2/5 of BoHV-5gEΔ (one day). No virus shedding was detected in BoHV-5gEΔTKΔ calves upon Dx treatment. PCR examination of brain sections of calves euthanized at day 30 post Dx treatment revealed the presence of latent viral DNA widely distributed in the brain of SV-507/99 calves. Latent viral DNA was detected in a few sections (3/30) of the brains of BoHV-5gEΔ calves and was not detected in the brains of calves inoculated with BoHV-5TKΔ and BoHV-5gEΔTKΔ. These results show that the single BoHV-5 mutants (gE and tk-deleted) are attenuated for calves and establish and/or reactivate latent infection inefficiently. The double mutant BoHV-5gEΔTKΔ is fully attenuated and appears not to establish or not reactivate efficiently from latent infection. Thus, these recombinants, especially the double mutant BoHV-5gEΔTKΔ, display an adequate phenotype for use in modified-live vaccine formulations.
BoHV-5; pathogenesis; mutants; attenuation; virulence
LIVESTOCK DISEASES
Experimental infection of calves with recombinants of bovine herpesvirus 5 defective in glycoprotein E (gE), thymidine kinase (TK) and both, gE/TK
Infecção experimental de bezerros com recombinantes do herpesvírus bovino tipo 5 defectivos na glicoproteína E (gE), timidina quinase (TK) e ambos, gE/TK
Cyndia Mara B. dos SantosI; Deniz AnzilieroII; Fernando V. BauermannI; Mário Celso S. BrumII; Rudi WeiblenIII; Eduardo F. FloresIII,* * Corresponding author: eduardofurtadoflores@gmail.com
IPrograma de Pós-Graduação em Medicina Veterinária (PPGMV), Centro de Ciências Rurais (CCR), Universidade Federal de Santa Maria (UFSM), Santa Maria, RS 97105-900, Brazil
IIFaculdade de Veterinária, Universidade do Pampa (Unipampa), BR 472 Km 585, Uruguaiana, RS 97500-970, Brazil
IIISetor de Virologia, Departamento de Medicina Veterinária Preventiva (DMVP), CCR-UFSM, Santa Maria, RS
ABSTRACT
This article describes an investigation on the virulence/attenuation of bovine herpesvirus type 5 (BoHV-5) recombinants deleted in the genes encoding glycoprotein E (BoHV-5gEΔ), thymidine kinase (BoHV-5TKΔ), and both gE and TK (BoHV-5gEΔTKΔ). Seronegative calves (80 to 90 days-old) inoculated with the parental strain (SV-507/99, n=5) shed virus in nasal secretions for up to 15 days (average 10.8 days). Duration of virus shedding was 11 days for BoHV-5gΔ, 9.6 days for BoHV-5TKΔ and 6.2 days for BoHV-5gEΔTKΔ groups. The highest titers were observed between days 1 and 6 post-infection (pi) for SV-507/99 (106.8TCID50/mL), 105.1TCID50/mL (BoHV-5gEΔ), 105.9TCID50/mL (BoHV-5TKΔ) and 104.7TCID50/mL (BoHV-5gEΔTKΔ). Calves inoculated with the parental virus presented anorexia, profound apathy and loss of body condition. Two calves were euthanized in extremis on days 10 and 11 pi; infectious virus was recovered from several areas of the brain. In contrast, calves inoculated with the recombinants remained healthy and a few presented a mild and transient nasal secretion. Dexamethasone (Dx) administration at day 42 pi resulted in virus shedding by all controls calves (mean duration 3.7 days), by 2/5 of BoHV-5TKΔ calves (two days) and 2/5 of BoHV-5gEΔ (one day). No virus shedding was detected in BoHV-5gEΔTKΔ calves upon Dx treatment. PCR examination of brain sections of calves euthanized at day 30 post Dx treatment revealed the presence of latent viral DNA widely distributed in the brain of SV-507/99 calves. Latent viral DNA was detected in a few sections (3/30) of the brains of BoHV-5gEΔ calves and was not detected in the brains of calves inoculated with BoHV-5TKΔ and BoHV-5gEΔTKΔ. These results show that the single BoHV-5 mutants (gE and tk-deleted) are attenuated for calves and establish and/or reactivate latent infection inefficiently. The double mutant BoHV-5gEΔTKΔ is fully attenuated and appears not to establish or not reactivate efficiently from latent infection. Thus, these recombinants, especially the double mutant BoHV-5gEΔTKΔ, display an adequate phenotype for use in modified-live vaccine formulations.
Index terms: BoHV-5, pathogenesis, mutants, attenuation, virulence.
RESUMO
Este artigo descreve uma investigação da virulência/atenuação de recombinantes do herpesvírus bovino tipo 5 (BoHV-5) com deleções nos genes da glicoproteína E (BoHV-5gEΔ), timidina quinase (BoHV-5TKΔ), e ambos gE e TK (BoHV-5gEΔTKΔ). Bezerros soronegativos (80-90 dias de idade) inoculados com o vírus parental SV-507/99 (n=5) excretaram o vírus em secreções nasais por até 15 dias (média de 10,8 dias). Nos animais inoculados com os recombinantes, a duração da excreção viral foi de 11 dias (BoHV-5gEΔ), 9,6 dias (BoHV-5TKΔ) e 6,2 dias (BoHV-5gEΔTKΔ). Os maiores títulos foram observados entre os dias 1 e 6 pós-inoculação (pi), sendo de 106,8TCID50/mL para o SV-507/99, 105,1TCID50/mL (BoHV-5gEΔ), 105,9TCID50/mL (BoHV-5TKΔ) e 104,7TCIΔ50/mL (BoHV-5gEΔTKΔ). Os bezerros inoculados com o vírus parental apresentaram anorexia e apatia; três deles mostraram apatia profunda e perda da condição corporal. Dois bezerros foram eutanasiados in extremis nos dias 10 e 11 pi, respectivamente e o vírus foi isolado de várias regiões do encéfalo. Já os bezerros inoculados com os recombinantes permaneceram saudáveis; alguns apresentaram uma secreção nasal serosa transitória. Administração de dexametasona (Dx) no dia 42 pi resultou em excreção viral por todos os bezerros inoculados com o vírus parental (duração média de 3,7 dias), por 2 de 5 bezerros dos grupos BoHV-5TKΔ (dois dias) e BoHV-5gEΔ (um dia). Os bezerros inoculados com o duplo mutante BoHV-5gEΔTKΔ não excretaram o vírus após o tratamento com Dx. Pesquisa de DNA viral por PCR no dia 30 pós-Dx revelou uma ampla distribuição do DNA do vírus parental no encéfalo; poucas seções (3/30) foram positivas no encéfalo dos animais do grupo BoHV-5gEΔ, e não detectou-se DNA latente no encéfalo dos animais dos grupos BoHV-5TKΔ e BoHV-5gEΔTKΔ. Esses resultados demonstram que os mutantes simples (gE and tk-deletados) são atenuados para bezerros e estabelecem e/ou reativam infecção latente ineficientemente. Já o duplo mutante BoHV-5gEΔTKΔ é atenuado e parece não estabelecer e/ou não reativar eficientemente a infecção latente. Portanto, os vírus recombinantes, e em especial o duplo mutante BoHV-5gEΔTKΔ apresentam um fenótipo compatível com a sua inclusão em vacinas vivas modificadas.
Termos de indexação: BoHV-5, patogenia, mutantes, atenuação, virulência.
INTRODUCTION
Bovine herpesvirus type 5 (BoHV-5) is a neurovirulent alphaherpesvirus associated with severe meningoencephalitis in cattle (Kahrs 2001). Outbreaks of BoHV-5 neurological disease have been described in several South American countries, noticeably Brazil and Argentina (Carrillo et al. 1983, Salvador et al. 1998, Rissi et al. 2006). BoHV-5 is closely related genetically and antigenically to bovine herpesvirus 1 (BoHV-1), the agent of infectious bovine rhinotracheitis (IBR), infectious pustular vulvovaginits and balanopostitis (IPV/IBP) and abortions in cattle (Kahrs 2001). BoHV-1 infection is distributed worldwide, with the exception of some European countries that recently eradicated the infection (Ackermann & Engels 2006). Bovine herpesviruses 1 and -5 are large, enveloped DNA viruses belonging to the family Herpesviridae, subfamily Alphaherpesvirinae (Roizman et al. 1992) and, as such, are capable of establishing lifelong latent infections in their hosts (Rock 1994, Vogel et al. 2003).
A number of BoHV-1 vaccines, most of them based on conventional inactivated viruses and, at least one containing a temperature-sensitive, modified live virus (MLV), are currently in use in South America (Flores E.F., unpublished data). A few vaccines contain BoHV-5 antigens in their formulations, none of them based on live virus. Nevertheless, the antigenic similarity and serological cross-reactivity between BoHV-1 and -5 demonstrated in vitro and in vivo indicates that proper immunization with BoHV-1 vaccines would confer protection against BoHV-5 and vice versa (Bratanich et al. 1991, Vogel et al. 2002, Del Medico Zajac et al. 2006, Anziliero et al. 2010, Brum et al. 2010b). In any case, an important restriction for the use of conventional vaccines in control/eradication programs is the impossibility of serological differentiation between vaccinated and naturally, latently infected animals (Van Oirschot et al. 1996). Differential vaccines (DIVA, for differentiating infected from vaccinated animals), produced by gene deletion of the vaccine strains have been successfully used in control/eradication of BoHV-1 infections in several countries (Van Oirschot et al. 1996, Van Drunen-Littel van den Hurk 2006). A gE-deleted BoHV-1 recombinant strain has been constructed out a Brazilian BoHV-1.2 isolate (Franco et al. 2002a), but is not yet commercially available.
The non-essential viral envelope glycoprotein E (gE) has been largely used as the antigenic marker in BoHV-1 differential vaccines (Kaashoek et al. 1996, 1998, Van Oirschot et al. 1996). Glycoprotein E is required for efficient transport of viral particles through synaptically connected neurons such gE-deleted herpesviruses are poorly transported to the brain after nasal replication (Chowdhury et al. 2000). Thus, in addition to provide an antigenic marker, gE deletion from BoHV-1 and BoHV-5 genomes contributes for virus attenuation (Kaashoek et al. 1996, 1998, Franco et al. 2002a,b, Silva et al. 2010). Nevertheless, an additional deletion may be required to confer complete attenuation for BoHV-5 strains (Silva et al. 2010, Chowdhury S.I. unpublished observations). The gene encoding the enzyme thymidine kinase (tk) has been a suitable target for deletion towards attenuation of BoHV-1 and of other alphaherpesviruses as well, noticeably pseudorabies virus (Kit et al. 1985, Kaashoek et al. 1996). TK is an enzyme involved in the synthesis of deoxyribonucleotides for viral DNA synthesis and genome replication, an activity which is required for virus replication in non-dividing cells (Enquist et al. 1998, Ferrari et al. 2000).Our group described the construction of three recombinants, deleted in the genes coding for gE (BoHV-5gEΔ), TK (BoHV-5TKΔ) and both genes (BoHV-5gEΔTKΔ) out of a neurovirulent Brazilian BoHV-5 strain (Brum et al. 2010a). The recombinants BoHV-5TKΔ and BoHV-5gEΔTKΔ were shown to be fully attenuated and immunogenic in a rabbit model of infection (Silva et al. 2010). The present article describes an investigation on the virulence/attenuation of these recombinants for calves, the natural hosts.
MATERIALS AND METHODS
Experimental design. Twenty-one calves seronegative to BoHV-5 were randomly allocated in four groups. Animals from each group were inoculated intranasally (IN) with the parental virus (SV-507/99, n=5) or with each of the recombinants (BoHV-5gE [n=5], BoHV-5TKΔ [n=5] and BoHV-5gEΔTKΔ [n=6]). Following virus inoculation, animals were monitored in clinical, virological and serological aspects. At day 42 post-inoculation (pi), the inoculated animals were submitted to dexamethasone (Dx) treatment to reactivate latent infection and were monitored as described above. Thirty days later, calves were euthanized, the brain was collected and different brain sections were submitted to PCR to investigate the presence of latent viral DNA.
Cells and viruses. A Madin-Darby bovine kidney (MDBK)-derived cell line named CRIB (Flores & Donis 1995) was used for all procedures of virus amplification, quantitation, isolation from secretions and tissues and for virus-neutralizing (VN) assays. Cells were maintained on minimal essential medium (MEM), containing ampicillin (1.6mg/L), streptomycin (0.4mg/L), amphotericin (2mg/L), supplemented with 10% fetal bovine serum (Cultilab, Brazil). The viruses used for inoculation were the BoHV-5 parental strain SV-507/99 and the recombinants lacking TK (BoHV-5TKΔ), gE (BoHV-5gEΔ) and gE/TKD (BoHV-5 gEΔTKΔ) previously described by Brum et al. (2010a). Stocks of the parental virus and recombinants were produced in CRIB cells, aliquoted and stored at -80ºC. All stocks used for inoculation contained viruses at the same passage level (n=5).
Animals, virus inoculation and monitoring. Experimental calves were 80 to 90-days-old, males and tested negative for BoHV-1 and BoHV-5 neutralizing antibodies prior to the experiment. The experimental groups were maintained in separated barns, without contact among groups, and received water and food ad libitum. Calves were inoculated by IN instillation of 2mL of a virus suspension containing 107.5TCID50 of the respective virus SV507/99; BoHV-5TKΔ; BoHV-5gEΔ; BoHV-5gEΔTKΔ. Virus inoculation into the nostrils was followed by a light friction with cotton swabs to distribute the inoculums over the nasal mucosa. During 20 days after virus inoculation, the animals were monitored clinically: Body temperature, nasal, respiratory, systemic (appetite, alertness) and neurological signs. Clinical examinations were performed daily by two veterinarians who were not aware of the experimental groups.
Dexamethasone (Dx) administration and monitoring. At day 42 pi, inoculated calves were submitted to Dx administration (Decadronal, Achè, Brazil) in a regimen of five daily dosis of 0.1mg/kg/day by the intramuscularly (IM) route. After Dx treatment, the animals were monitored on a daily basis up to day 15 pDx as described during acute infection. Clinical examination was performed, nasal swabs were collected for virus isolation and quantitation; serum samples were collected for VN assays.
Sample processing. Nasal swabs collected after virus inoculation and Dx treatment were submitted to virus isolation. The swabs were vortexed vigorously, low-speed centrifuged and the supernatants were inoculated onto CRIB cells monolayers and submitted to three passages of five days each. The infectivity of the samples that were positive for virus were subsequently quantified by limiting dilution, the titers were calculated according to Reed & Muench (1938) and expressed as log10TCID50/mL. Blood samples for serology were collected at days 0, 32pi and 42 pi (day of Dx administration); and 30 days post-treatment (pDx) (72pi). Serum samples were submitted to a standard VN assay, testing two-fold dilutions of sera against a fixed dose (100-200TCID50) of the respective virus. The virus titers, expressed as the reciprocal of the highest dilution that prevented virus replication, were transformed in GMT (geometric mean titer [Thrusfield 1986]) for the calculation of the mean antibody titers of each group.
Tissue collection, DNA extraction and PCR. At day 30 pDx, calves were euthanized for collection of the brain. After removal, the brain was carefully sectioned in the following areas: olfactory bulb (BO), anterior cortex (AC), ventro-lateral cortex (VLC), thalamus (Th), pons (Po) and trigeminal ganglia (TG). Tissues were submitted to total DNA extraction using DNAzol reagent (Invitrogen, Carlsbad, CA, USA). The extracted DNA was solubilized in buffer Tris-EDTA (0.1-0.2mL) and stored at -20ºC until testing. DNA concentration was determined in a UV spectrophotometer at 260 nm. Total DNA was submitted to a nested PCR using two set of primers corresponding to positions 57.338 and 57.782 (primers 1 and 2) and 57.372 and 57.666 (primers 3 and 4) of the glycoprotein B gene coding region of the BoHV-5 strain SV-507/99. Nested-PCR reaction was performed essentially as described by Diel et al. (2007). Total DNA extracted from the brain of a control non-infected calf and from a calf with acute BoHV-5 infection was used as negative and positive controls, respectively. PCR products were analysed under UV light after electrophoresis in an 1.5% agarose gel stained with ethidium bromide. Tissues positive for viral DNA by PCR were subsequently submitted to virus isolation as described above.
Statistical analysis. The duration (days) and titers of virus shedding by each experimental group were compared statistically by submitting the individual values to analysis of variance followed by student t-test with a confidence interval of 0.05.
All procedures of animal handling and experimentation were conducted under veterinary supervision and according to recommendations by the Brazilian Committee of Animal Experimentation (COBEA, law #6.638 of May, 8th, 1979). The experiment was approved by an Institutional Animal Ethics Committee (UFSM, approval # 44/2008).
RESULTS
Acute infection
Calves inoculated with the recombinants developed only mild and transient, serous nasal and ocular secretion. No overt systemic signs were observed and the body temperature remained within normal limits (data not shown). In contrast, in addition to nasal discharge, animals inoculated with the parental virus developed anorexia, profound apathy, loss of body condition and severe weakness. Three animals developed profound apathy and neurological signs (# 348, 350, 381) begining at days 7 and 8 post-inoculation (pi). Calves # 350 and 348 were euthanized in extremis at day 10 and 11pi, respectively. Infectious virus was recovered from their brains upon inoculation of a pool tissue homogenates in CRIB cell cultures (not shown). Histological examination of brain sections of these animals revealed typical non-suppurative meningo-encephalitis. Calf # 381 recovered after a few days. The other three calves from this group also recovered after a period of 3-4 days of moderate apathy and reduction of food intake.
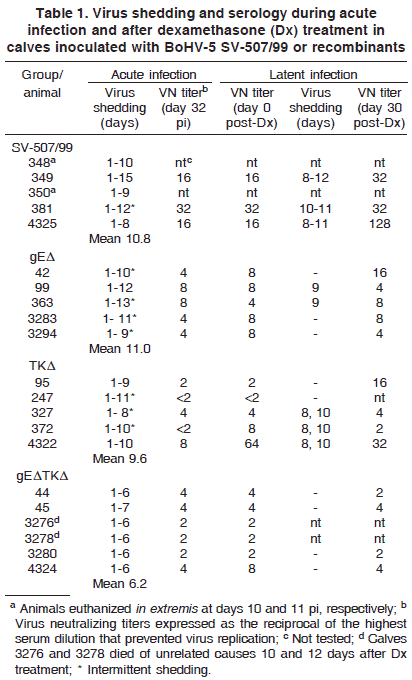
Virus shedding in nasal secretions was detected in all groups in the days following inoculation, yet in different titers and duration (Table 1, Fig.1). Duration of virus shedding was 10.6 days (SE ± 0.81) for SV-507/99 group; BoHV-5gEΔ (11 ± SE 0.71); BoHV-5TKΔ (9.6 ± SE 0.51) and BoHV-5gEΔTKΔ (6.1 ± SE 0.16). The duration of virus shedding was significantly reduced (p<0.01) in the double mutant group, comparing to the other three groups, which did not differ among them. In some animals, virus shedding was intermittent (Table 1). The highest titers were observed between days 1 e 6 pi for SV507/99 (106.8TCID50/mL), 105.1TCID50/mL for BoHV-5gEΔ, 105.9TCID50/mL for BoHV-5TKΔ and 104.7TCID50/mL for BoHV-5gEΔTKΔ group (Fig.1). The recombinant was shed in titers significantly lower than those of the parental from day 1 to 8 (p<0.05). Single mutants were shed in titers intermediary between the parental and the double mutant virus. The statistical analysis of the magnitude of virus shedding for each recombinant is summarized in the legend of Figure 1.
Seroconversion was observed in all four groups albeit at different levels (Table 1). In general, VN antibodies were more consistently detected and in higher titers in SV-507/99 group, followed by BoHV-5gEΔ calves. Animals inoculated with BoHV-5TKΔ and with the double mutant BoHV-5gEΔTKΔ seroconverted to lower titers and VN antibodies were not detected at 1:2 dilution of sera in all animals (Table 1).
Taken together, these results demonstrate that the three recombinants are attenuated for 80-90 days-old calves upon IN inoculation since replicated to lower titers and did not produce clinical disease. In contrast, the parental virus was virulent and produced severe disease in three out of five animals. Considering virus shedding and seroconversion as indicators of virus replication, the double mutant BoHV-5gEΔTKΔ was the most attenuated, followed by the recombinant lacking TK (BoHV-5TKΔ). The recombinant BoHV-5gEΔ was also attenuated yet to a lesser extent, since it replicated for a period similar to SV-507/99 strain and consistently induced VN antibodies (Table 1).
Latent infection
Nasal swabs collected at the day of Dx treatment were negative for virus, demonstrating the absence of virus replication. Following Dx administration, virus shedding was detected in swabs collected from all three calves of the SV-507/99 group (Table 1). Virus shedding was first detected at day 8 pDx and lasted from 2 to 5 days (average of 3.7 days). Excretion of infection virus was detected in 3/5 (40%) calves of group BoHV-5gE (one day) and in 3/5(60%) calves inoculated with BoHV-5TKΔ (two days). No infectivity was detected in nasal secretions collected from calves inoculated with the double mutant BoHV-5gEΔTKΔ group following Dx treatment. One calf from the group BoHV-5TKΔ presented an 8-fold increase in VN titer after Dx, suggesting that might have also reactivated the infection. Two calves of group SV-507/99 died of unrelated causes at days 13 and 15 post-Dx, respectively.
PCR examination of different brain sections collected at day 30 post-Dx revealed the presence of latent viral DNA in several areas of the brain of calves from SV-507/99 group including the TGs (Table 2). Latent viral DNA was also detected in a few areas of the brain of some calves of group BoHV-5gEΔ, but not in calves inoculated with BoHV-5TKΔ or BoHV-5gEΔTKΔ. No infectious virus was recovered from PCR positive sections upon inoculation of CRIB cells with tissue homogenates, confirming the status of latent infection.
These results show that the double mutant BoHV-5gEΔTKΔ is defficient in establishing and/or reactivating from latency since no virus shedding was detected after Dx treatment nor viral DNA was detected in brain sections examined by PCR. The single recombinants BoHV-5gEΔ and BoHV-5TKΔ showed a markedly reduced ability to establish and/or reactivate from latent infection. Virus shedding was detected in only a few animals and lasted one or two days. Latent viral DNA was detected in a few brain sections of some BoHV-5gEΔ-inoculated calves but not in calves inoculated with the recombinant lacking TK.
DISCUSSION
The results presented herein demonstrate that single deletions in the gE or tk genes sufficed to produce adequate attenuation of BoHV-5 SV-507/99 strain for calves. Consequently, the double mutant BoHV-5gEΔTKΔ was also fully attenuated. None of the recombinants induced important clinical signs in inoculated calves, in contrast with severe disease observed in 3 out of 5 calves inoculated with the parental virus. Considering virus shedding and VN titers as indicators of virus replication (and virulence thereof), the double mutant was the most attenuated, followed by BoHV-5TKΔ. The replication efficiency of BoHV-5gEΔ was not drastically reduced compared to the parental virus, yet its replication did not result in clinical signs. The double mutant BoHV-5gEΔTKΔ was not recovered from nasal secretions following Dx treatment, indicating that is defective in reaching the TG, establishing and/or reactivating from latency. The single mutants BoHV-5gE and BoHV-5TKΔ were inconsistently detected in nasal swabs after Dx administration, indicating they are also deffective - yet to a lesser extent - in the establishment and/or reactivation from latent infection.
Therefore, the three recombinants are attenuated for three-months-old calves and, would represent potential vaccine candidates. In particular, the double mutant BoHV-5gEΔTKΔ may be useful for inclusion in a modified live vaccine formulation. A parallel experiment demonstrated that this recombinant is completely attenuated for calves upon intramuscular administration and induced a protective immune response against BoHV-5 and BoHV-1 challenge (Anziliero et al. 2010). Moreover, the single mutants would be useful to study the function of the deleted gene products (gE and TK) in the biology and pathogenesis of BoHV-5 infection. The mechanisms underlying the inefficient establishment and/or reactivation from latency would be especially worthy of further investigation.
The length and magnitude of virus shedding during acute infection were significantly reduced (p<0.01) in the BoHV-5gEΔTKΔ group and, to a lesser extent, in the BoHV-5TKΔ-inoculated calves. Calves inoculated with the recombinants remained healthy, showing only a transient and mild nasal secretion. The degree of attenuation of these recombinants was somehow similar in rabbits where the frequency (number of animals excreting virus) and duration of virus shedding were significantly reduced (Silva et al. 2010). In contrast, the recombinant BoHV-5gEΔ, fully attenuated for calves, was partially neurovirulent for rabbits, producing neurological disease in 3 out of 8 animals (Silva et al. 2010). The envelope glycoprotein E plays an important role in the transport of alphaherpesviruses along circuits of synaptically connected neurons and, consequently, influences virus invasion of the brain (Chowdhury et al. 2000). Hence, BoHV-5 gE-defective strains showed reduced neuroinvasiveness and neurovirulence in rabbits (Chowdhury et al. 2000). The complete attenuation of BoHV-5gEΔ for calves, in spite of an efficient replication in the nasal mucosa, would probably be due to a reduced ability to invade and to disseminate within the brain. On the other hand, the differential expression of neurovirulence by recombinant BoHV-5gEΔ in rabbits (Silva et al. 2010) and calves (this experiment) would deserve further investigation. It is reasonable to speculate that the neural pathways and dynamics of invasion of the brain might somehow differ among these species, each putative route being differentially affected by gE deletion.
Thymidine kinase (TK) is an enzyme involved in the metabolism of deoxyribonucleotides (dNTPs) necessary for DNA synthesis and genome replication in neurons (Enquist et al. 1998, Ferrari et al. 2000). Thus, tk deletion of herpes simplex virus (HSV) and pseudorabies virus (PRV) has been associated with reduced neurovirulence (Coen et al. 1989, Moormann et al. 1990, Chen et al. 2004). Bovine herpesvirus-1 (BoHV-1) mutants lacking TK activity are also attenuated, albeit to a lesser extent (Kit et al. 1985, Kaashoek et al. 1996, 1998). Ours and previous data (Silva et al. 2010) indicated that the sole deletion of tk gene would suffice for attenuation of BoHV-5 for rabbits and calves. As BoHV-5 neuropathogenesis is associated which virus replication and dissemination within the brain (Chowdhury et al. 2000), we assume that functional TK would be necessary for the full expression of neurovirulence in vivo. Conceivably, the attenuation of TK-defective BoHV-5 (and of other alphaherpesviruses as well) may reflect a combination of deficient replication in the port of entry (nasal epithelium) plus a defective replication in neurons. In this sense, the recombinant BoHV-5TKΔ replicated much less efficiently than the parental virus in the nose of rabbits (Silva et al. 2010) and calves (present experiment).
Both single mutants BoHV-5gEΔ and BoHV-5TKΔ showed a reduced ability to reactivate from latent infection in comparison with the parental virus (Table 1). Poor reactivation by these recombinants may likely reflect inefficient invasion and/or replication in the trigeminal ganglia (TG) during acute infection and/or deficient replication and/or transport to the nose after Dx treatment. As latent viral DNA was barely detected in some brain areas of a few animals of BoHV-5gEΔ group (and not detected in BoHV-5TKΔ animals), the amount of latent DNA in TGs was probably very low, below the detection limit of our PCR. These findings contrast with a previous experiment in rabbits in which latent DNA of both single recombinants was consistently detected in the TG/OB of inoculated animals, in spite of an inability to reactivate the infection upon Dx treatment (Silva et al. 2010).
Reactivation of latent infection by TK-defective alphaherpesviruses is also significantly impaired or even abolished, especially for HSV in mice (Coen et al. 1989, Chen et al. 2004). Attempts to reactivate latent infection by TK-negative BoHV-1 and PRV strains have yielded conflicting results, probably reflecting differences in the viruses, animals and experimental procedures (Whetstone et al. 1992, Gilliam et al. 1993, Kaashoek et al. 1996). Despite these conflicting results, it is generally accepted that TK-defective alpha-herpesviruses replicate poorly (if so) in neurons and, therefore, are not expected to reactivate efficiently from latency. In rabbits, the recombinants BoHV-5gEΔ and BoHV-5TKΔ, but not the double mutant, established latent infection in TG/OB albeit they were not reactivated upon Dx treatment. The lack of excretion of BoHV-5gEΔ after Dx treatment may be related to defficient anterograde axonal transport as demonstrated for a gE-negative BoHV-1 strain in calves (Liu et al. 2008, Brum et al. 2009). In this sense, defficient anterograde transport of gE-negative BoHV-1 may adversely affect viral invasion of the brain during acute infection and transport to peripheral sites during viral reactivation (Chowdhury et al. 2000). Alternatively, this virus was not reactivated in the ganglia by corticosteroid treatment. Further studies are currently underway to determine the step(s) of the latency-reactivation cycle at which the single mutants are impaired.
By comparing the biology of these recombinants in rabbits and calves, some differences were noticed: (1) BoHV-5gEΔ was fully attenuated for calves, but was partially neurovirulent for rabbits (Silva et al. 2010); (2) Single mutants BoHV-5gEΔ and BoHV-5TKΔ consistently established latent infection in TG/OB of rabbits, but only BoHV-5gEΔ DNA was detected in a few brain sections of a few calves; (3) The single mutants were barely reactivated in calves and were not reactivated in rabbits. The reasons for these differences may be worthy of investigation and - if confirmed - may reflect differences in the biology of the virus in these hosts.
In summary, our results showed that single deletions in gE and tk genes sufficed to produce attenuation of BoHV-5 SV-507/99 for calves during acute infection. These mutants also showed a significantly reduced ability to establish and/or reactivate latent infection. The double mutant BoHV-5gEΔTKΔ was fully attenuated during acute infection and incapable of reactivating latency and, thus represents a potential vaccine candidate.
Received on October 11, 2010.
Accepted for publication on November 16, 2010.
- Ackermann M. & Engels M. 2006. Pro and contra IBR eradication. Vet. Microbiol. 113(3/4):293- 302.
- Anziliero D., Santos C.M.B., Bauermann F.B., Brum M.C.S., Weiblen R. & Flores E.F. 2011. Immunization of calves with a recombinant bovine herpesvirus 5 defective in thymidine kinase and glycoprotein E confers protection against BoHV-5 and BoHV-1 challenge. (Manuscript in preparation)
- Bratanich A.C., Sardi A.I., Smitsäart E.N. & Schudel A.A. 1991. Comparative studies of BHV-1 variants by in vivo and in vitro tests. J. Vet. Med. B. 38:41-48.
- Brum M.C., Coats C., Sangena R.B., Doster A., Jones C. & Chow-dhury S.I. 2009. Bovine herpesvirus type 1 (BoHV-1) anterograde neuronal transport from trigeminal ganglia to nose and eye requires glycoprotein E. J. Neurovirol. 15:196-201.
- Brum M.C., Weiblen R., Flores E.F. & Chowdhury S.I. 2010a. Construction and growth properties of bovine herpesvirus type 5 recombinants defective in the glycoprotein E or thymidine kinase gene or both. Braz. J. Med. Biol. Res. 43:217-224.
- Brum M.C.S., Caron L., Chowdhury S.I., Weiblen R. & Flores E.F. 2010b. Immunogenicity of an inactivated bovine herpesvirus type 5 strain defective in thymidine kinase and glycoprotein E. Pesq. Vet. Bras. 30:57-62.
- Carrillo B.J., Ambrogi A., Schudel A.A., Vazquez M., Dahme E. & Pospischil A. 1983. Meningoencephalitis caused by IBR virus in calves in Argentina. Zentralbl. Veterinärmed B 30:327-332.
- Chen S.H., Pearson A. & Coen D.M. 2004. Failure of thymidine kinase-negative herpes simplex virus to reactivate from latency following efficient establishment. J. Virol. 78:520-523.
- Coen D.M., Kosz-Vnenchak M., Jacobson J.G., Leib D.A., Bogard C.L., Schaffer P.A., Tyler K.L. & Knipe D.M. 1989. Thymidine kinase-negative herpes simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc. Natl Acad. Sci. U.S.A. 86:4736-4740.
- Chowdhury S.I., Lee B.J., Ozkul A. & Weiss M.L. 2000. Bovine herpesvirus 5 glycoprotein E is important for neuroinvasiveness and neurovirulence in the olfactory pathway of the rabbit. J. Virol. 74:2094-2106.
- Del Médico Z.M.P., Puntel M., Zamorano P.I., Sadir A.M. & Romera S.A. 2006. BHV-1 vaccine induces cross-protection against BHV-5 disease in cattle. Res. Vet. Sci. 81(3):327-334.
- Enquist L.W., Husak P.J., Banfield B.W. & Smith G.A. 1998. Infection and spread of alphaherpesviruses in the nervous system. Adv. Virus Res. 51:237-347.
- Ferrari M., Mettenleiter T.C., Romanelli M.G., Cabassi E., Corradi A., Dal Mas N. & Silini R. 2000. A comparative study of pseudorabies virus (PRV) strains with defects in thymidine kinase and glycoprotein genes. J. Comp. Pathol. 123:152-163.
- Flores E.F. & Donis R. 1995. Isolation of a mutant MDBK cell line resistant to bovine virus diarrhea virus (BVDV) due to a block in viral entry. Virology 208:565-575.
- Franco A.C., Rijsewijk F.A.M., Flores E.F., Weiblen R. & Roehe P.M. 2002a. Construction and characterization of a glycoprotein E deletion mutant of bovine herpesvirus type 1.2 strain isolated in Brazil. Braz. J. Microbiol. 33(3):274-278.
- Franco A.C., Spilki F.R., Esteves P.A., Lima M., Weiblen R., Flores E.F., Rijsewijk F.A.M. & Roehe P.M. 2002b. A Brazilian Glycoprotein E-negative bovine herpesvirus type 1.2a (BHV-1.2a) mutant is attenuated for cattle and induces protection against wild-type virus challenge. Pesq. Vet. Bras. 22(4):135-140.
- Gilliam S.E., Thackray A.M., Brown G.A. & Field H.J. 1993. The pathogenesis of wild type and drug resistant mutant strains of bovine herpesvirus-1 (BHV-1) in the natural host. Archs Virol. 128:43-54.
- Kaashoek M.J., Van Engelenburg F.A., Moerman A., Gielkens A.L., Rijsewijk F.A. & Van Oirschot J.T. 1996. Virulence and immuno-genicity in calves of thymidine kinase and glycoprotein E-nega-tive bovine herpesvirus 1 mutants. Vet. Microbiol. 48(1/2):143-53.
- Kaashoek M.J., Rijsewijk F.A., Ruuls R.C., Keil G.M., Thiry E., Pastoret P.P. & Van Oirschot J.T. 1998. Virulence, immunogenicity and reactivation of bovine herpesvirus 1 mutants with a deletion in the gC, gG, gI, gE, or in both the gI and gE gene. Vaccine 16(8):802-809.
- Kahrs R.F. 2001. Infectious bovine rhinotrachitis and infectious pustular vulvovaginitis, p.159-170. In: Ibid. (Ed.), Viral Disease of Cattle. Iowa State University Press, Ames.
- Kit S., Qavi H., Gaines J.D., Billingsley P., McConnell S. 1985. Thymidine kinase-negative bovine herpesvirus type 1 mutant is stable and highly attenuated in calves. Archs Virol. 86:63-83.
- Liu Z.F., Brum M.C., Doster A., Jones C. & Chowdhury S.I. 2008. A bovine herpesvirus type 1 mutant virus specifying a carboxyl-terminal truncation of glycoprotein E is defective in anterograde neuronal transport in rabbits and calves. J. Virol. 82:7432-7442.
- Moormann R.J., de Rover T., Briaire J., Peeters B.P., Gielkens A.L. & van Oirschot J.T. 1990. Inactivation of the thymidine kinase gene of a gI deletion mutant of pseudorabies virus generates a safe but still highly immunogenic vaccine strain. J. Gen. Virol. 71:1591-1595.
- Reed L.J. & Muench H. 1938. A simple method for estimating fifty per cent end points. Am. J. Hyg. 27:493-497.
- Rissi D.R., Rech R.R., Flores E.F., Kommers G.D. & Barros C.S.L. 2007. Meningoencephalitis by bovine herpesvirus-5. Pesq. Vet. Bras. 27(7):251-260.
- Rock D.L. 1994. Latent infection with bovine herpesvirus type-1. Semin. Virol. :233-240.
- Roizman B., Desrosiers R.C., Fleckenstein B., Lopez C., Minson A.C. & Studdert M.J. 1992. The family Herpesviridae: An update. The Herpesvirus Study Group of the International Committee on Taxonomy of Viruses. Arch. Virol. 123:425-449.
- Salvador S.W.C., Lemos R.A.A., Riet-Correa F., Roehe P.M. & Osorio A.L.A.R. 1998. Meningoencefalite em bovinos causada por herpesvírus bovino-5 no Mato Grosso do Sul e São Paulo. Pesq. Vet. Bras. 18:76-83.
- Silva S.C., Brum M.C., Weiblen R., Flores E.F. & Chowdhury S.I. 2010. A bovine herpesvirus 5 recombinant defective in the thymidine kinase (TK) gene and a double mutant lacking TK and the glycoprotein E gene are fully attenuated for rabbits. Braz. J. Med. Biol. Res. 43:150-159.
- Thrusfield M. 1986. Serological epidemiology, p.175-186. In: Ibid. (Ed.), Veterinary Epidemiology. Butterworths, London. 280p.
- Van Drunen Littel-van den Hurk S. 2006. Rationale and perspectives on the success of vaccination against bovine herpesvirus-1. Vet. Microbiol. 113:275-282.
- Van Oirschot J.T., Kaashoek M.J., Rijsewijk F.A. & Stegeman J.A. 1996. The use of marker vaccines in eradication of herpesviruses. J. Biotechnol. 44:75-81.
- Vogel F.S.F., Flores E.F., Weiblen R. & Kunrath C.F. 2002. Atividade neutralizante anti-herpesvírus bovino tipos 1 (BHV-1) e 5 (BHV-5) no soro de bovinos imunizados com vacinas contra o BHV-1. Ciência Rural 32:881-883.
- Vogel F.S.F., Caron L., Flores E.F., Weiblen R., Winkelmann E.R., Mayer S.V. & Bastos R.G. 2003. Distribution of bovine herpesvirus type 5 DNA in the central nervous systems of latently, experimentally infected calves. J. Clin. Microbiol. 41:4512-4520.
- Whetstone C.A., Miller J.M., Seal B.S., Bello L.J. & Lawrence W.C. 1992. Latency and reactivation of a thymidine kinase-negative bovine herpesvirus 1 deletion mutant. Archs Virol. 122:207-214.
Datas de Publicação
-
Publicação nesta coleção
25 Abr 2011 -
Data do Fascículo
Abr 2011
Histórico
-
Aceito
16 Nov 2010 -
Recebido
11 Out 2010