Abstracts
The objectives of the study were to evaluate the presence/production of beta-lactamases by both phenotypic and genotypic methods, verify whether results are dependent of bacteria type (Staphylococcus aureus versus coagulase-negative Staphylococcus - CNS) and verify the agreement between tests. A total of 200 bacteria samples from 21 different herds were enrolled, being 100 CNS and 100 S. aureus. Beta-lactamase presence/detection was performed by different tests (PCR, clover leaf test - CLT, Nitrocefin disk, and in vitro resistance to penicillin). Results of all tests were not dependent of bacteria type (CNS or S. aureus). Several S. aureus beta-lactamase producing isolates were from the same herd. Phenotypic tests excluding in vitro resistance to penicillin showed a strong association measured by the kappa coefficient for both bacteria species. Nitrocefin and CLT are more reliable tests for detecting beta-lactamase production in staphylococci.
Beta-lactamase; blaZ; coagulase-negative Staphylococcus; Staphylococcus aureus
Os objetivos do presente estudo foram avaliar a presença/produção de beta-lactamases por ambos os métodos fenotípicos e genotípicos, verificar se os resultados são dependentes do tipo de bactéria (Staphylococcus aureus contra Staphylococcus coagulase negativa - CNS) e verificar a concordância entre os testes. Um total de 200 amostras bactérianas oriundas de 21 rebanhos distintos foram incluídos, sendo 100 CNS e 100 S. aureus. A presença/detecção de beta-lactamase foi realizada por diferentes testes (PCR, teste trevo (clover leaf test) - CLT, disco Nitrocefin e resistência in vitro à penicilina). Os resultados de todos os testes não foram dependentes do tipo de bactérias (CNS ou S. aureus). Vários isolados de S. aureus produtores de beta-lactamase eram de um mesmo rebanho. Testes fenotípicos excluindo resistência in vitro à penicilina mostraram uma forte associação medida pelo coeficiente kappa para ambas as espécies de bactérias. Nitrocefina e CLT são testes mais confiáveis para detectar a produção de beta-lactamase em estafilococos.
Beta-lactamase; blaZ; Staphylococcus coagulase negativa; Staphylococcus aureus
LIVESTOCK DISEASES
Beta-lactamase detection in Staphylococcus aureus and coagulase-negative Staphylococcus isolated from bovine mastitis
Detecção de beta-lactamase em Staphylococcus aureus e Staphylococcus coagulase negativa isolados de mastite bovina
Bruno F. Robles; Diego B. Nóbrega; Felipe F. Guimarães; Guido G. Wanderley; H. Langoni* * Corresponding author: hlangoni@fmvz.unesp.br
Departamento de Higiene Veterinária e Saúde Pública, Universidade Estadual Paulista (Unesp), Distrito de Rubião Júnior s/n, Botucatu, SP 18618-900, Brazil
ABSTRACT
The objectives of the study were to evaluate the presence/production of beta-lactamases by both phenotypic and genotypic methods, verify whether results are dependent of bacteria type (Staphylococcus aureus versus coagulase-negative Staphylococcus - CNS) and verify the agreement between tests. A total of 200 bacteria samples from 21 different herds were enrolled, being 100 CNS and 100 S. aureus. Beta-lactamase presence/detection was performed by different tests (PCR, clover leaf test - CLT, Nitrocefin disk, and in vitro resistance to penicillin). Results of all tests were not dependent of bacteria type (CNS or S. aureus). Several S. aureus beta-lactamase producing isolates were from the same herd. Phenotypic tests excluding in vitro resistance to penicillin showed a strong association measured by the kappa coefficient for both bacteria species. Nitrocefin and CLT are more reliable tests for detecting beta-lactamase production in staphylococci.
Index terms: Beta-lactamase, blaZ, coagulase-negative Staphylococcus, Staphylococcus aureus.
RESUMO
Os objetivos do presente estudo foram avaliar a presença/produção de beta-lactamases por ambos os métodos fenotípicos e genotípicos, verificar se os resultados são dependentes do tipo de bactéria (Staphylococcus aureus contra Staphylococcus coagulase negativa - CNS) e verificar a concordância entre os testes. Um total de 200 amostras bactérianas oriundas de 21 rebanhos distintos foram incluídos, sendo 100 CNS e 100 S. aureus. A presença/detecção de beta-lactamase foi realizada por diferentes testes (PCR, teste trevo (clover leaf test) - CLT, disco Nitrocefin e resistência in vitro à penicilina). Os resultados de todos os testes não foram dependentes do tipo de bactérias (CNS ou S. aureus). Vários isolados de S. aureus produtores de beta-lactamase eram de um mesmo rebanho. Testes fenotípicos excluindo resistência in vitro à penicilina mostraram uma forte associação medida pelo coeficiente kappa para ambas as espécies de bactérias. Nitrocefina e CLT são testes mais confiáveis para detectar a produção de beta-lactamase em estafilococos.
Termos de indexação: Beta-lactamase, blaZ, Staphylococcus coagulase negativa, Staphylococcus aureus.
INTRODUCTION
Staphylococcus aureus (S. aureus) is one of the most common causes of contagious bovine mastitis (Melchior et al. 2006). Coagulase-negative Staphylococcus (CNS) are commonly isolated from mastitis cases in several countries (Pitkala et al. 2004, de Freitas Guimarães et al. 2013), with limited knowledge and published papers regarding antimicrobial resistance mechanisms. In recent years, treatment of CNS-caused infections has become an important topic. As S. aureus, CNS can harbor resistance genes to several antimicrobials (Hammad et al. 2012, Silva et al. 2013).
Beta-lactam compounds such as penicillin continues to be one of the most frequently used drugs in veterinary medicine (Pitkala et al. 2007). Two primary resistance mechanisms to beta-lactams are noteworthy in Staphylococcus spp.: the expression of beta-lactamase enzymes encoded by the blaZ gene, and production of the penicillin-binding protein 2a resulting in a higher-level of resistance encoded by the mecA gene (Fuda et al. 2005). Prevalence of penicillin resistance in staphylococci causing animal diseases is most commonly due to the blaZ gene (Malik et al. 2007, Pitkala et al. 2007).
Different tests can be performed to evaluate beta-lactamase production in staphylococci. A qualitative procedure for detecting production of beta-lactamase is the usage of Nitrocefin disks. The reaction is based on the production of a colored compound when the substrate (nitrocefin) is exposed to a beta-lactamase-producing bacteria. The clover leaf test (CLT) is an alternative with high sensitivity and specificity for investigating beta-lactamase production in staphylococci (Bergan et al. 1997).
Studies regarding beta-lactamase production in Staphylococcus isolates and comparison of tests are scarce. Comparison of beta-lactamase activity in coagulase positive and negative isolates would prove to be valuable, adding more results to available literature.
The objectives of the present study were to evaluate the presence/production of beta-lactamases by both phenotypic and genotypic methods, verify whether results are dependent of bacteria type (coagulase positive versus coagulase negative Staphylococcus) and verify the agreement between tests. Our hypotheses were that more coagulase-positive Staphylococcus isolates would present beta-lactamase enzymes and tests would have a high agreement coefficient.
MATERIALS AND METHODS
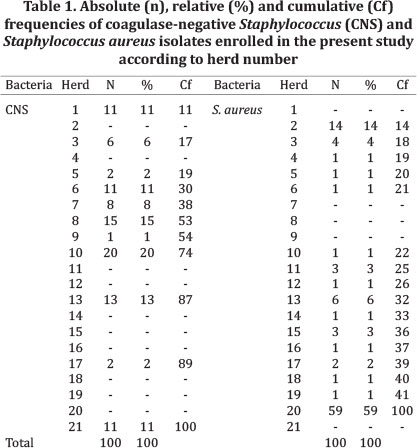
Samples. A total of 200 bacteria samples from 21 different herds located in São Paulo State were enrolled, being 100 CNS and 100 Staphylococcus aureus (Table 1). Isolates were taken from cows with diagnosed intramammary infection (IMI) from 2009 to 2012 and stored at -80°C.
Microbiological procedures. In vitro antimicrobial susceptibility testing was conducted by disc diffusion method using the two following antimicrobial agents: oxacillin and penicillin (Oxoid Ltd., Basingstoke, Hampshire, England), and interpreted according to the Clinical and Laboratory Standards Institute (CLSI 2008). Beta-lactamases detection was performed by three methods.
Nitrocefin®Disks. Nitrocefin® commercial disks (Becton Dickinson Microbiology Systems, Cockeysville, United States) were acquired and stocked at -10o C until usage. Disks were initially embedded in saline solution, and with a sterile loop, colonies were streaked onto its surface. Disks were observed within 60 minutes at ambient temperature. A positive reaction was considered as a change of colour from yellow to pink. S. aureus ATCC 29213 and S. aureus 25923 were used as positive and negative control respectively.
Clover Leaf Test (Hodge Test). The clover leaf test was performed according to Bergan et al. (1997). Presence of an irregular inhibition zone was considered as a positive result. S. aureus ATCC 29213 and S. aureus 25923 were used as positive and negative control respectively.
PCR detection of blaZ gene. PCR reactions aiming the blaZ gene were performed with primers blaZf (5 'AAGAGATTTGCCTATGCTTC 3) and blaZr (5' GCTTGACCACTTTTATCAGC 3') (Haveri et al. 2005). Extraction and purification were performed by physical methods (boiling and centrifugation) (Malik et al. 2007). S. aureus ATCC 29213 and S. aureus 25923 were used as positive and negative control respectively.
Statistical analysis. Descriptive analyses were performed using the PROC FREQ procedure of SAS software (SAS 2008). Agreement between tests was achieved by Kappa coefficient and McNemar test. Differences in results frequencies regarding bacteria type were determined by hierarchical models. Farm and animal effect were first assessed using the chi-square test. Variables individually associated with result test at a P-value < 0.25 were selected to build a final generalized mixed model. Farm was included as an explanatory variable whereas animal did not enter the final models. To verify whether farm should be included as a fixed or random effect, individual generalized mixed models were constructed for each result test including farm as a random effect. A compound symmetry covariance structure was used to account for clustering of isolates from same farm. In cases of estimated V correlation values ≥0.20, models were building considering farm as a random effect. This applies to all beta-lactamases tests results (PCR, clover leaf test and Nitrocefin disk), whereas to penicillin susceptibility results, the model hierarchical structure assumed farm as a fixed effect (estimated V correlation value = 0.16). Each final model provided the best fit for the data, assessed using Akaike and Bayesian information criteria. Statistical significance was defined as P < 0.05. All statistical analyses were performed with the SAS 9.2 software.
RESULTS
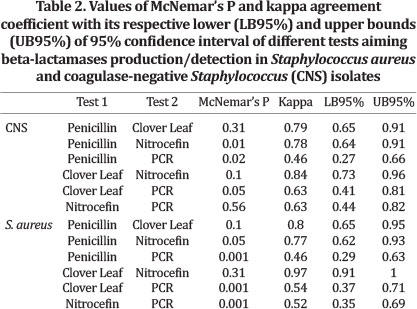
PCR detection of blaZ gene was not performed in four isolates of CNS and two isolates of Staphylococcus aureus. Hence, these bacteria were excluded from pairwise analyses. Resistance to penicillin was detected in more S. aureus isolates (83/100) than CNS ones (32/100). Clover Leaf Test, Nitrocefin disks and PCR showed similar results, with 79.0%, 78.0% and 62.2% of positivity for S. aureus, and 29.0% 25.0% and 20.8% for CNS for the three tests respectively. Several isolates from S. aureus and CNS displayed the beta-lactamase production phenotype detected by all three methods, whereas PCR results were negative (Table 3). Hence, agreement coefficients involving PCR test were lower when compared to other coefficients, particularly in S. aureus isolates (Table 2). For CNS, CLT/Nitrocefin and penicillin resistance/CLT had the two highest Kappa coefficients (0.84 and 0.79 respectively). The same was observed for S. aureus (CLT/Nitrocefin = 0.97; penicillin resistance/CLT = 0.80). Despite the high coefficient observed between penicillin resistance and Nitrocefin test for both species, McNemar's Test was significant for two tests, and in all three tests involving the PCR results for S. aureus.
According to hierarchical models applied, results of penicillin resistance (P=0.63), Nitrocefin (P=0.10), PCR (P=0.61) and CLT (P=0.18) were not dependent of bacteria type (CNS or S. aureus). Several S. aureus beta-lactamase producing isolates were from the same herd (herd 20, Table 1).
DISCUSSION
This study addressed an important topic: beta-lactamase production in Staphylococcus aureus and CNS, and comparison of diagnostic methods. Unsuccessful treatments are usually observed for mastitis caused by beta-lactamase producing isolates, particularly S. aureus (Sol et al. 2000). S. aureus is accepted as a major mastitis pathogen with laborious treatment, whereas CNS remains as a minor pathogen (Sampimon et al. 2009). In many countries, CNS have become the most common mastitis-causing agents (Pyorala & Taponen 2009), and is proving to be as pathogenic as S. aureus, at least concerning the presence of antimicrobial resistance genes. Studies regarding detection of resistance genes including blaZ, showed that none of the S. aureus possessed more than three genes, whereas 25% of CNS isolates harbored, at least, four genes encoding resistance to antibiotics (Podkowik et al. 2012).
In the present study, several S. aureus and CNS isolates showed beta-lactamase phenotype, in contrast to other studies (Johler et al. 2011). For all tests, S. aureus isolates showed beta-lactamase activity in a higher percentage than CNS. However, no significant difference was observed in all tests. Our hypothesis that more S. aureus isolates would produce beta-lactamases was not proved, despite the results observed in Table 3. We expected that due to a relative high rate of unsuccessful treatments (Leitner et al. 2003), more S. aureus isolates would present beta-lactamases enzymes. Our study showed that no difference was present between S. aureus and CNS isolates, and probably, beta-lactamase production is herd-dependent, although this was not studied. Recent studies showed that there is an association between antimicrobial use and antimicrobial resistance in dairy farms (Saini et al. 2012), even if there is very little evidence supporting an increase in antimicrobial resistance due to mastitis treatments (Erskine et al. 2002). Mastitis therapy differs between herds, which may contribute to different resistance profiles of similar bacteria. Herd 20 presented nearly 40% of all animals infected with S. aureus (data not shown), all harboring the blaZ gene. Unfortunately, we did not have access to information regarding mastitis therapy and prophylaxis from this herd, which could contribute to the understanding of S. aureus mastitis epidemiology.
In general, phenotypic tests showed a strong association measured by the kappa coefficient for both CNS and S. aureus (Table 2). The blaZ gene is widely spread among both S. aureus and CNS (Duran et al. 2012). In our study, we observed that several S. aureus and CNS isolates did not harbor the blaZ gene, and phenotypic tests showed beta-lactamase activity. This resulted in decreased kappa values, and in some cases, a significant statistical P value for McNemar's test (< 0.05; i.e., PCR x Penicillin resistance for CNS, PCR x CLT and PCR x Nitrocefin for S. aureus) showing that tests had a low agreement detecting beta-lactamase activity. Beta-lactamase phenotype could be result of expression of more than one gene, and there is more than one mechanism that grants staphylococci beta-lactam resistance other than the expression of blaZ gene (Malik et al. 2007). Moreover, the detection of a gene does not necessarily means it is expressed. Detection of RNAm could lead to more conclusions.
Two tests for both CNS and S. aureus involving resistance in vitro to penicillin had significant P-values, indicating that tests did not perform similarly detecting beta-lactamases. Several mechanisms could be involved in resistance to penicillin, and should be interpreted with caution to avoid misleading conclusions. In general, both Nitrocefin and CLT are specific to detect beta-lactamases production, and are more reliable tests for this purpose.
We choose to not assume PCR detection of the blaZ gene as a gold standard method for the same reasons we listed before, not calculating underestimated values of sensitivity and specificity for phenotypic tests, even with studies showing high sensitivity and specificity for Clover Leaf Test and Nitrocefin disks for detecting beta-lactamase production in staphylococci using the blaZ detection as the gold standard (Pitkala et al. 2007).
CONCLUSIONS
Staphylococcus aureus and CNS did not differ regarding production of beta-lactamases and detection of the blaZ gene.
All phenotypic tests performed similarly for both species, excluding in vitro resistance to penicillin.
Result of PCR detection of the blaZ gene had a low association with all phenotypic tests for both bacteria species.
There is a strong possibility that beta-lactamase production is herd-dependent.
Acknowledgements.- This study was funded by FAPESP (The São Paulo State Official Foundation to Support Research) B.F. Robles (Grant 2011/08533-2) for the grant and the financial received for this research.
Received on January 4, 2014.
Accepted for publication on March 1, 2014.
- Bergan T., Bruun J.N., Digranes A., Lingaas E., Melby K.K. & Sander J. 1997. Susceptibility testing of bacteria and fungi. Report from "the Norwegian Working Group on Antibiotics". Scand. J. Infect. Dis. (Suppl.)103:1-36.
- CLSI 2008. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; Approved Standard. 3rd ed. Clinical and Laboratory Standards Institute, Wayne, PA.
- de Freitas Guimarães F., Nóbrega D.B., Richini-Pereira V.B., Marson P.M., Figueiredo Pantoja J.C. & Langoni H. 2013. Enterotoxin genes in coagulase-negative and coagulase-positive staphylococci isolated from bovine milk. J. Dairy Sci. 96:2866-2872.
- Duran N., Ozer B., Duran G.G., Onlen Y. & Demir C. 2012. Antibiotic resistance genes and susceptibility patterns in staphylococci. Indian J. Med. Res. 135:389-396.
- Erskine R.J., Walker R.D., Bolin C.A., Bartlett P.C. & White D.G. 2002. Trends in antibacterial susceptibility of mastitis pathogens during a seven-year period. J. Dairy Sci. 85:1111-1118.
- Fuda C.C., Fisher J.F. & Mobashery S. 2005. Beta-lactam resistance in Staphylococcus aureus: the adaptive resistance of a plastic genome. Cell. Mol. Life Sci. 62:2617-2633.
- Hammad A.M., Watanabe W., Fujii T. & Shimamoto T. 2012. Occurrence and characteristics of methicillin-resistant and -susceptible Staphylococcus aureus and methicillin-resistant coagulase-negative staphylococci from Japanese retail ready-to-eat raw fish. Int. J. Food Microbiol. 156:286-289.
- Haveri M., Suominen S., Rantala L., Honkanen-Buzalski T. & Pyorala S. 2005. Comparison of phenotypic and genotypic detection of penicillin G resistance of Staphylococcus aureus isolated from bovine intramammary infection. Vet. Microbiol. 106:97-102.
- Johler S., Layer F. & Stephan R. 2011. Comparison of virulence and antibiotic resistance genes of food poisoning outbreak isolates of Staphylococcus aureus with isolates obtained from bovine mastitis milk and pig carcasses. J. Food Protect. 74:1852-1859.
- Leitner G., Lubashevsky E. & Trainin Z. 2003. Staphylococcus aureus vaccine against mastitis in dairy cows, composition and evaluation of its immunogenicity in a mouse model. Vet. Immunol. Immunop. 93:159-167.
- Malik S., Christensen H., Peng H. & Barton M.D. 2007. Presence and diversity of the beta-lactamase gene in cat and dog staphylococci. Vet. Microbiol. 123:162-168.
- Melchior M.B., Fink-Gremmels J. & Gaastra W. 2006. Comparative assessment of the antimicrobial susceptibility of Staphylococcus aureus isolates from bovine mastitis in biofilm versus planktonic culture. J. Vet. Med. B, Infect. Dis. Vet. Publ. Health 53:326-332.
- Pitkala A., Haveri M., Pyorala S., Myllys V. & Honkanen-Buzalski T. 2004. Bovine mastitis in Finland 2001-prevalence, distribution of bacteria, and antimicrobial resistance. J. Dairy Sci. 87:2433-2441.
- Pitkala A., Salmikivi L., Bredbacka P., Myllyniemi A.L. & Koskinen M.T. 2007. Comparison of tests for detection of beta-lactamase-producing staphylococci. J. Clin. Microbiol. 45:2031-2033.
- Podkowik M., Bystron J. & Bania J. 2012. Prevalence of antibiotic resistance genes in staphylococci isolated from ready-to-eat meat products. Pol. J. Vet. Sci. 15:233-237.
- Pyorala S. & Taponen S. 2009. Coagulase-negative staphylococci-emerging mastitis pathogens. Vet. Microbiol. 134:3-8.
- Saini V., McClure J.T., Scholl D.T., DeVries T.J. & Barkema H.W. 2012. Herd-level association between antimicrobial use and antimicrobial resistance in bovine mastitis Staphylococcus aureus isolates on Canadian dairy farms. J. Dairy Sci. 95:1921-1929.
- Sampimon O.C., Barkema H.W., Berends I.M., Sol J. & Lam T.J. 2009. Prevalence and herd-level risk factors for intramammary infection with coagulase-negative staphylococci in Dutch dairy herds. Vet. Microbiol. 134:37-44.
- SAS Institute 2008. SAS/STAT User's Guide. Version 9.2. Sas Incorporation, Cary, NC. 7886p.
- Silva N.C.C., Guimarães F.F., Manzi M.P., Budri P.E., Gómez-Sanz E., Benito D., Langoni H., Rall V.L.M. & Torres C. 2013. Molecular characterization and clonal diversity of methicillin-susceptible Staphylococcus aureus in milk of cows with mastitis in Brazil. J. Dairy Sci. 96:6856-6862.
- Sol J., Sampimon O.C., Barkema H.W. & Schukken Y.H. 2000. Factors associated with cure after therapy of clinical mastitis caused by Staphylococcus aureus J. Dairy Sci. 83:278-284.
Publication Dates
-
Publication in this collection
23 June 2014 -
Date of issue
Apr 2014
History
-
Accepted
01 Mar 2014 -
Received
04 Jan 2014