Abstracts
Many attempts have been made to establish the control of foodborne pathogens through Lactobacillus isolates and their metabolism products with success being obtained in several situations. The aim of this study was to investigate the antagonistic effect of eight Lactobacillus isolates, including L. casei subsp. pseudoplantarum, L. plantarum, L. reuteri and L. delbrueckii subsp. delbrueckii, on the pathogenic Escherichia colistrain O157:H7. The inhibitory effect of pure cultures and two pooled cultures supernatants of Lactobacillus on the growth of pathogenic bacteria was evaluated by the spot agar method and by monitoring turbidity. Antimicrobial activity was confirmed for L. reuteri and L. delbrueckii subsp. delbrueckii and for a pool of lactic acid bacteria. The neutralized supernatant of the pool exerted a higher antimicrobial activity than that of the individual strains. Furthermore, D-lactic acid and acetic acid were produced during growth of the Lactobacillus isolates studied.
Lactobacillus; antagonism; Escherichiacoli O157:H7; organic acid
Muitas tentativas têm sido feitas para se estabelecer o controle de patógenos de origem alimentar através do uso de estirpes de Lactobacillus e dos seus produtos de metabolismo, com sucesso sendo sucedido em várias situações. O objetivo deste trabalho foi investigar o efeito antagônico do sobrenadante de culturas de oito isolados de Lactobacillus, incluindo L. casei subsp. pseudoplantarum, L. plantarum L. reuteri e L. delbrueckii subsp. delbrueckii, sobre Escherichia coli amostra O157:H7. Os efeitos inibidores de culturas puras e de dois "pools" de cultura de Lactobacillus sobre o crescimento da bactéria foram avaliados através do método de inibição em ágar e através do monitoramento da turbidez da cultura bacteriana. A atividade antimicrobiana foi confirmada para Lactobacillus reuteri e Lactobacillus delbrueckii subsp. delbrueckii e para o "pool" de bactérias acido-láctica. O sobrenadante neutralizado do "pool" de Lactobacillus exerceu uma atividade antimicrobiana mais elevada do que aquela das estirpes individuais. Além disso, ácido D-láctico e ácido acético foram produzidos durante o crescimento dos Lactobacillusestudados
Lactobacillus; antagonismo; Escherichiacoli O157:H7; ácido orgânico
Introduction
Escherichia coli O157:H7 represents one of the most important enteropathogenic bacteria; it is responsible for numerous reports of diarrhea is transmitted through food, water and the environment, affecting mainly infants and immunosuppressed adults, all over the world (Monteiro-Neto et al. 1997Monteiro-Neto V., Campos L.C., Ferreira A.J.P., Gomes T.A.T. & Trabulsi L.R. 1997. Virulence properties of Escherichia coli 0111:H12 strains. FEMS Microbiol. Lett. 146:123-128., Fábrega et al. 2002Fábrega V.L.A., Ferreira A.J.P., Patrício F.R.S., Brinkley C. & Scaletsky I.C.A. 2002. Cell-detaching Escherichia coli (CDEC) strains from children with diarrhea: identification of a protein with toxigenic activity. FEMS Microbiol. Lett. 217:191-197.). The pathogenicity of E. coli O157:H7 is attributed to the production of potent enterotoxins, the Shiga-like toxins (Stx1 and Stx2, also called verocytotoxins), that directly affect the activity of cells in the intestinal wall, resulting in hemorrhage and thousands of deaths annually (Kudva et al. 1996Kudva T.I., Hatfield P.G. & Hovde C.J. 1996. Escherichia coli O157:H7 in microbiota flora of sheep. J. Clin. Microbiol. 34:431-433., Cantarelli et al. 2000Cantarelli V., Nagayama K., Takahashi A., Honda T., Cauduro P., Dias G.A.G., Mezzari A. & Brodt T. 2000. Isolation of Shiga toxin-producing Escherichia coli (STEC) serotype O91:H21 from a child with diarrhea in Porto Alegre city, RS, Brazil. Braz. J. Microbiol. 31:266-270., Garcia et al. 2010Garcia A., Fox J.G. & Besser T.E. 2010. Zoonotic enterohemorrhagic Escherichia coli: a one health perspective. ILAR J. 51:221-232.). Many different types of food were identified as potential sources of Shiga-toxin-producing Escherichia coli (STEC), including raw and undercooked foodstuffs (Garcia et al. 2010Garcia A., Fox J.G. & Besser T.E. 2010. Zoonotic enterohemorrhagic Escherichia coli: a one health perspective. ILAR J. 51:221-232.). The natural hosts of Escherichia coli O157:H7 are wildlife and farm ruminants, mainly cattle and swine. In the state of Rio de Janeiro (Brazil), studies conducted by Cerqueira et al (1999Cerqueira A.M.F., Guth B.E.C., Joaquim R.M. & Andrade J.R.C. 1999. High occurrence of Shiga toxin-producing Escherichia coli (STEC) in healthy cattle in Rio de Janeiro State, Brazil. Vet. Microbiol. 70:111-121.) reported the presence of STEC in 71 % of the fecal samples of healthy cattle from dairy farms, beef farms and slaughterhouses. This was the first report concerning the isolation of STEC from the intestines of dairy and beef cattle in Brazil, although several studies had already reported the presence of other enteropathogenic strains in the food industry (Cerqueira et al. 1999Cerqueira A.M.F., Guth B.E.C., Joaquim R.M. & Andrade J.R.C. 1999. High occurrence of Shiga toxin-producing Escherichia coli (STEC) in healthy cattle in Rio de Janeiro State, Brazil. Vet. Microbiol. 70:111-121.).
One approach that led to the reduction and, in a number of cases, the elimination of intestinal pathogenic bacteria in humans and animals includes the ingestion of probiotics in the diary diet (Guarner & Schaafsma 1998Guarner F. & Schaafsma G.J. 1998. Probiotics. Int. J. Food Microbiol. 39: 237-238., Gopal et al. 2001Gopal P.K., Prasad J., Smart J. & Gill H.S. 2001. In vitro adherence properties of Lactobacillus rhamnosus DR20 and Bifidobacterium lactis DR10 strains and their antagonistic activity against enterotoxigenic Escherichia coli. Int. J. Food Microbiol. 67:207-216.). Probiotics are live microorganisms that, when administered in adequate amounts, confer beneficial effects on the host by altering indigenous microbiota and preventing infections (FAO/WHO 2001FAO/WHO2001. Joint FAO/WHOExpert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Available at <http://www.who.int/foodsafety/publications/fs_management/en/probiotics.pdf> Accessed Febr. 12, 2014.
http://www.who.int/foodsafety/publicatio...
). Lactic acid bacteria (LAB) with probiotic properties, such as Bifidobacterium spp. and Lactobacillus spp. were used to prevent some intestinal pathogenic infections and to stimulate the host's immune system in both humans and animals (Perdigón et al. 1999Perdigón G., Vintiñi E., Alvarez S., Medina M. & Medici M. 1999. Study of the possible mechanisms involved in the mucosal immune system activation by lactic acid bacteria. J. Dairy Sci. 82:1108-1114., Fang et al. 2000Fang H., Elina T., Heikki A. & Seppo S. 2000. Modulation of humoral immune response through probiotic intake. FEMS Immunol. Med.Microbiol. 29:47-52., Nakazato et al. 2011Nakazato G., Paganelli F.L., Lago J.C., Aoki F.H., Mobilon C., Brocchi M., Stehling E.G. & Silveira W.D. 2011. Lactobacillus acidophilus decreases Salmonella Typhimurium invasion in vivo. J. Food Saf. 31:284-289.). It is well documented that Lactobacillus spp. with probiotic properties prevent the growth and toxin production of bacteria such as Campylobacter jejuni, Listeria monocytogenes, Helicobacter pylori, Salmonella, Shigella and Escherichia coli (Jin et al. 1996Jin L.Z., Ho Y.W., Ali M.A., Abdullah N. & Jalaludin S. 1996. Effect of adherent Lactobacillus spp. on in vitro adherence of salmonellae to the intestinal epithelial cells of chicken. J. Appl. Bacteriol. 81:201-206., Kalantzopoulos 1997Kalantzopoulos G. 1997. Fermented products with probiotic qualities. Anaerobe 3:15-19., Servin & Coconnier 2003Servin A.L. & Coconier M.H. 2003. Adhesion of probiotic strains to the intestinal mucosa and interaction with pathogens. Best Pract. Res. 17:741-754., Poppi et al. 2008Poppi L.B., Mancilha I.M., Ferreira A.J.P.& Leal D.D.M. 2008. Evaluation of the antagonistic effect of Lactobacillus on Listeria monocytogenes in vitro. Braz. J. Food Technol. 11:113-119., Scapin et al. 2013Scapin D., Grando W.F., Rossi E.M., Perez K.J., Malheiros P.S. & Tondo E.C. 2013. Antagonistic activity of Lactobacillus acidophilus LA10 against Salmonella enterica serovar Enteritidis SE86 in mice. Braz. J. Microbiol. 44:57-61.).
The antagonist activity of probiotics on pathogenic bacteria could be associated with the competition for nutrients and sites of adhesion in the mucosa of the small intestine and the production of carbon dioxide, hydrogen peroxide and diacetyl (Gopal et al. 2001Gopal P.K., Prasad J., Smart J. & Gill H.S. 2001. In vitro adherence properties of Lactobacillus rhamnosus DR20 and Bifidobacterium lactis DR10 strains and their antagonistic activity against enterotoxigenic Escherichia coli. Int. J. Food Microbiol. 67:207-216.). Furthermore, the inhibitory effect on the growth of several enteropathogenic bacteria is likely associated with the antimicrobial compounds produced by lactic acid bacteria, such as bacteriocin and lactic, acetic and other short-chain organic acids, which are responsible for a reduction in the intestinal pH (Servin & Coconnier 2003Servin A.L. & Coconier M.H. 2003. Adhesion of probiotic strains to the intestinal mucosa and interaction with pathogens. Best Pract. Res. 17:741-754., Cheng et al. 2003Cheng H.Y., Yu R.C. & Chou C.C. 2003. Increased acid tolerance of Escherichia coli O157:H7 as affected by acid adaptation time and conditions of acid challenge. Food Res. Int. 36:49-56., Varalakshmi et al. 2013Varalakshmi S., Balasubramanyam B.V., Surendranath B., Bagath M. & Rajendran D. 2013. Use of novel lactic acid bacterial strains with antagonistic activity for the preparation of safe indigenous fermented dairy foods (Dahi and Raita). J. Food Safty 34:26-33.). Lactic acid represents the main antimicrobial compound present in cultures of lactic acid bacteria (Earnshaw 1992Earnshaw R.G. 1992. The antimicrobial action of lactic acid bacteria: natural food preservation systems, p.211-232. In: Wood B.J.B. (Ed.), The Lactic Acid Bacteria: the lactic acid bacteria in health and disease. Chapman and Hall, London, UK., Navarro et al. 2000Navarro L., Zarazaga M., Sáenz J., Ruiz-Larrea F. & Torres C. 2000. Bacteriocin production of by lactic acid bacteria isolated from Rioja red wines. J. Appl. Microbiol. 88:44-51., Todorov & Dicks 2005Todorov S.D. & Dicks L.M.T. 2005. Lactobacillus plantarum isolated from molasses produces bacteriocins active against Gram-negative bacteria. Enzyme Microb. Technol. 36:318-326., Rossland et al. 2005Rossland E., Langsrud T., Granum P.E. & Sorhaug T. 2005. Production of antimicrobial metabolites by strains of Lactobacillus or Lactococcus co-cultured with Bacillus cereus in milk. Int. J. Food Microbiol. 98:193-200., Moraes et al. 2013Moraes P.M., Perin L.M., Silva Jr A. & Nero L.A. 2013. Comparison of phenotypic and molecular tests to identify lactic acid bacteria. Braz. J. Microbiol. 44:109-112.). Weak acids possess higher antimicrobial activity than strong acids, which ionize completely in an aqueous solution (Axe & Bailey 1995Axe D.D. & Bailey J.E. 1995. Transport of lactate and acetate through the cytoplasmic membrane of Escherichia coli. Biotechnol. Bioeng. 47:8-19.). The non-dissociated forms of organic acids are able to function as protonophores, inducing the acidification of the cytoplasm and the accumulation of toxic anions. The decrease in the cell's internal pH affects the influx of protons through the cell membrane, which dissipates the proton-motive force, reducing cellular energy (ATP) and affecting substrate uptake in the cell (Axe & Bailey 1995Axe D.D. & Bailey J.E. 1995. Transport of lactate and acetate through the cytoplasmic membrane of Escherichia coli. Biotechnol. Bioeng. 47:8-19., Diez-Gonzalez & Russell 1997Diez-Gonzalez F. & Russell J.B. 1997. The ability of Escherichia coli O157:H7 to decrease its intracellular pH and resist the toxicity of acetic acid. Microbiol. 143:1175-1180.).
Several in vitro and in vivo studies demonstrated the antagonism of numerous strains of Lactobacillus, including L. delbrueckii var delbrueckii, L. plantarum, L. acidophilus, L. reuteri and L. casei, against a variety of pathogens (Poppi et al. 2008Poppi L.B., Mancilha I.M., Ferreira A.J.P.& Leal D.D.M. 2008. Evaluation of the antagonistic effect of Lactobacillus on Listeria monocytogenes in vitro. Braz. J. Food Technol. 11:113-119., Servin & Coconnier 2003Servin A.L. & Coconier M.H. 2003. Adhesion of probiotic strains to the intestinal mucosa and interaction with pathogens. Best Pract. Res. 17:741-754., Kalantzopoulos 1997Kalantzopoulos G. 1997. Fermented products with probiotic qualities. Anaerobe 3:15-19., Jin et al. 1996Jin L.Z., Ho Y.W., Ali M.A., Abdullah N. & Jalaludin S. 1996. Effect of adherent Lactobacillus spp. on in vitro adherence of salmonellae to the intestinal epithelial cells of chicken. J. Appl. Bacteriol. 81:201-206.). In spite of many detailed studies concerning the antagonistic effects of these bacteria on pathogens, there is still a need for new bacterial strains with antimicrobial properties for clinical and commercial benefits (Chaucheyras-Durand & Durand 2010Chaucheyras-Durand F. & Durand H. 2010. Probiotics in animal nutrition and health. Beneficial Microbes 1:3-9.). In vitro screening methods such as agar spotting and the monitoring of turbidity represent a fast and effective tool for this purpose. Therefore, it is desirable to use these methods to select promising strains of Lactobacillus for the development of new probiotic preparations at the industrial scale.
The main objective of this work was to evaluate the in vitroperformance of eight strains of Lactobacillus isolated from poultry litter with respect to their inhibitory effect on the growth of Escherichia coli O157:H7.
Materials and Methods
Lactobacillus isolates and Escherichia colistrain O157:H7
Eight isolates of Lactobacillus, previously isolated from poultry litter (Paço et al. 2003Paço R.S., Leme I.L., Bottino J.A. & Ferreira A.J.P. 2003. Identification of Lactobacillus spp. from broiler litter in Brazil. Braz. J. Microbiol. 34:236-237.), were selected to study their antagonistic effects on an Escherichia coli O157:H7 strain that was kindly provided by Dr. Isabel Scaletsky (Department of Microbiology, Immunology and Parasitology, Federal University of São Paulo, São Paulo, Brazil). The bacterial isolates L. casei subsp. pseudoplantarum (isolates 30b and 30c), L. plantarum (isolates 11fb, 22c and 41b), L. reuteri (isolates 18fa and 19fa) and L. delbrueckii subsp. delbrueckii (isolate 17fb) were obtained from the Laboratory of Avian Diseases at the School of Veterinary Medicine and Animal Science of the University of São Paulo, Brazil. The Lactobacillus isolates were maintained at 4°C in MRS (Manosa-Rogosa and Sharpe) agar slant. The pathogenic strain of Escherichia coli 0157:H7 was grown in BHI broth (Difco, Sparks, MD, USA) under aerobic conditions at 37°C for 18h.
The physiological characteristics and purity of the Lactobacillusisolates were analyzed by observation of their morphological characteristics using a light microscope, Gram staining, a catalase test, a motility test and their ability to ferment various substrates. Sugar and sugar- alcohol assimilation tests were performed in test tubes by inoculation of the isolates into sterilized media, as described. The following substrates were used: peptone (10 g.L-1), yeast extract (5 g.L-1) , potassium phosphate (5 g.L-1), ammonium citrate (2 g.L-1) sodium acetate (5 g.L-1), manganese sulfate (0.5 g.L-1), magnesium sulfate (0.01 g.L-1), Tween 80 (0.05 g.L-1), phenol red (0.5 g.L-1). All products have been purchased from Merck, Darmstadt, Germany. The carbohydrate sources used: arabinose, cellobiose, galactose, glucose, lactose, maltose, mannitol, mannose, melezitose, melibiose, raffinose, rhamnose, salicin, sucrose, trehalose and xylose (Sigma, St. Louis, MO, USA) at concentration 10g.L-1 for each sugar type. The stock solutions were prepared from a 20% (w/v) solution previously filtered through a 0.45 μm membrane filter (Millipore Corp., Billerica, MA, USA). The tubes were inoculated and incubated at 37oC for 7 days to observe red phenol reduction by the sugar test (Kandler & Weiss 1986Kandler O. & Weiss N. 1986. Regular, nonsporing Gram-positive rods, p.1209-1234. In: Sneath P.H.A., Mairm N.S. & Sharpe M.E. (Eds), Bergey's Manual of Systematic Bacteriology. Williams and Wilkins, Baltimore, USA.).
Growth conditions
The strains of Lactobacillus were activated by transferring a full loop from the stock culture to a 125mL Erlenmeyer flask containing 45mL of MRS broth, followed by incubation at 37°C for 18 h. After three successive propagations in the same conditions, strains were grown independently in test tubes containing 5.0mL of MRS broth at 37°C for 16h.
Two different pools of Lactobacillus were prepared, which were named pool A (PA) and pool B (PB). PA was prepared by mixing 4.0-mL of each pre-activated culture (32-mL total) in a 500-mL flask containing 268-mL sterile MRS broth and incubating the mixture at 37°C for 18 h. PB consisted of the same mixture as PA, but without the subsequent incubation. Cells of individual and pooled cultures were collected by centrifugation (10,000xg at 4°C for 10 min), washed twice with 0.1 M PBS (phosphate saline buffer, pH 7.4), suspended in 20 % sterile sucrose solution and frozen at -80°C for later use. The supernatants from these cultures were used to study their inhibitory capability against Escherichia coliO157:H7. All of the experiments were carried out at least in duplicate. Replicates differ by less than 10%, and typically by less than 5%. The statistical significance was evaluated by the Student's t test.
Antibacterial activity against Escherichia coli O157:H7 - agar spot test
The antimicrobial activity of Lactobacillus strains against Escherichia coli O157:H7 was analyzed by a spot agar test as described by Schillinger & Lüke (1989)Schillinger U. & Lücke F.K. 1989. Antibacterial activity of Lactobacillus sake isolated from meat. Appl. Environ. Microbiol. 55:1901-1906.. Aliquots of 2μL of concentrated cell suspensions of pure cultures and pool A of Lactobacillus were spotted on the surface of MRS agar plates and MRS agar plates buffered with 35 mM sodium bicarbonate. The plates were dried out at room temperature for 30 min and incubated microaerobically at 37°C for 24 h. Afterward, the plates were covered with 10mL of soft BHI (Brain Heart Infusion) agar containing 108 CFU/mL of an overnight culture of Escherichia coliO157:H7 suspension. Then, the plates were incubated under anaerobic condition at 37°C for an additional 24h. The formation of clear zones of growth inhibition around Lactobacillus colonies and their diameters were recorded. Inhibition was considered positive if the diameter of the clear zone that formed around colonies was 5 mm or larger.
Inhibitory effect of Lactobacillus supernatants on Escherichia coli O157:H7
The supernatants from each culture and pool of Lactobacillus were separated into two groups, a neutralized fraction and an acidic fraction. One fraction was adjusted to pH 7.0 with sodium bicarbonate, while the pH of the other fraction remained unaltered. Both fractions were filtered using a 0.45 μm membrane filter (Millipore Corp., Billerica, MA, USA) and stored at -80°C for further use.
The direct antagonism of compounds contained in Lactobacillus cell-free supernatants against E. coli O157:H7 was monitored by turbidimetry. Aliquots (300 μL) of the neutralized fraction and the acidic fraction of supernatants from each pure culture and PA and PB were transferred to sterile tubes containing 300μL of an E. coli O157:H7 cell suspension (108 CFU/mL) and incubated at 37°C for 7h. The blank used for standardization consisted of a mixture of 300μL each of sterile MRS and BHI broth. As a control run, 300μL of sterile MRS broth was added to 300 μL of the pathogenic cell suspension, followed by incubation as indicated above. Samples were taken at intervals of 1 hour, and their OD was determined using a spectrophotometer Biomate 3 (Thermo Fisher Scientific, Waltham, MA, USA) at 600 nm. After each absorbance measurement, cell viability was confirmed using a BHI agar plate incubated in the same conditions.
Effect of pH on Escherichia coli O157:H7
To investigate the survival of Escherichia coli O157:H7 in the culture media at different pH values. Afterwards, pH was adjusted to 3.6 and 4.2, respectively, with 1.0 N HCl. The bacterial cultures were inoculated with an aliquot of Escherichia coli O157:H7 cell suspension (108 CFU/mL) and incubated at 37°C for 7h. The cell growth was monitored following the procedures described above.
Lactic acid and acetic acid assays
The D-lactic acid and acetic acid concentrations were determined by HPLC Waters 786 (Spectralab Scientific, Ontario, Canada) with a refractive index (IR) detector and Bio-Rad HPX-87-H (300 x 7.8mm) column at 45°C using 5 mM sulfuric acid as the eluent, a flow rate of 0.6ml.min-1 and a sample volume of 20 μl. All samples were conveniently diluted and filtered using a Sep Pak C18 column (Millipore Corp., Billerica, MA, USA).
Results and Discussion
The physiological characteristics of Lactobacillus strains previously isolated from poultry litter (Paço et al. 2003Paço R.S., Leme I.L., Bottino J.A. & Ferreira A.J.P. 2003. Identification of Lactobacillus spp. from broiler litter in Brazil. Braz. J. Microbiol. 34:236-237.) were analyzed with regard to their morphology, physiology and biochemical characteristics. All strains were confirmed as Lactobacillus using characteristics and properties expected to be on this bacterium, such as a rod shape, positive Gram staining, lack of motility, catalase-negativity, absence of endospores, fermentation of most of the sugars and sugar alcohols tested and resistance to the phenol compound. The strains exhibited a carbohydrate fermentation profile similar to L. plantarum, L. reuteri, L. delbruecki subsp. delbruecki and L. casei subsp. pseudoplantarum (Kandler & Weiss 1986Kandler O. & Weiss N. 1986. Regular, nonsporing Gram-positive rods, p.1209-1234. In: Sneath P.H.A., Mairm N.S. & Sharpe M.E. (Eds), Bergey's Manual of Systematic Bacteriology. Williams and Wilkins, Baltimore, USA.).
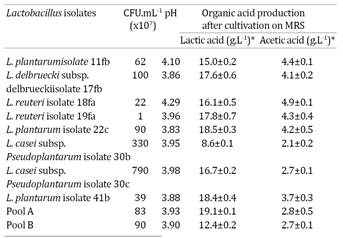
Table 1 shows the total viable cells, final pH, lactic and acetic acid concentrations of cell-free supernatants obtained from pure and pooled cultures of Lactobacillus. The total number of colony formation units (CFU) of the strains were generally high, ranging from 1.107 to 1.109 CFU mL-1. These results were similar to those obtained by Avonts & De Vuyst (2001)Avonts L. & De Vuyst L. 2001. Antimicrobial potential of probiotic lactic acid bacteria. Meded Rijksuniv. Gent., Fak. Landbouwkd. Toegep. Biol. Wet. 66:543-550. using seven commercial strains of lactobacilli grown in MRS broth under the same conditions, also as shown in Table 2.
All strains, except L. casei subsp. pseudoplantaumisolate 30b, were able to produce lactic acid at concentrations higher than 12.4g. L-1. The highest concentration was attained with pool A (19.1g.L-1), followed by L. plantarum isolate 41b (18.4g.L-1) and L. plantarum isolate 22c (18.5g.L-1). In addition, all lactobacilli produced acetic acid at concentrations higher than 2.0g.L-1. These short-chain organic acids are known to exhibit high antimicrobial activity against microorganisms due to the easy diffusion of the non-dissociated form through the cell membranes of pathogens (Axe & Bailey 1995Axe D.D. & Bailey J.E. 1995. Transport of lactate and acetate through the cytoplasmic membrane of Escherichia coli. Biotechnol. Bioeng. 47:8-19.). Cheng et al. (2003)Cheng H.Y., Yu R.C. & Chou C.C. 2003. Increased acid tolerance of Escherichia coli O157:H7 as affected by acid adaptation time and conditions of acid challenge. Food Res. Int. 36:49-56. studied the influence of organic acids, including acetic acid, propionic acid and lactic acid on the growth and survival of E. coli O157:H7. The authors reported that lactic acid exerts greater inhibitory effects on the growth of the pathogenic bacteria than acetic acid and propionic acid; however, the combination of the organic acids exhibited an important synergic effect in the inhibition of this pathogen (Cheng et al. 2003Cheng H.Y., Yu R.C. & Chou C.C. 2003. Increased acid tolerance of Escherichia coli O157:H7 as affected by acid adaptation time and conditions of acid challenge. Food Res. Int. 36:49-56., Varalakshmi et al. 2013Varalakshmi S., Balasubramanyam B.V., Surendranath B., Bagath M. & Rajendran D. 2013. Use of novel lactic acid bacterial strains with antagonistic activity for the preparation of safe indigenous fermented dairy foods (Dahi and Raita). J. Food Safty 34:26-33.). According to Diez-Gonzalez & Russell (1997)Diez-Gonzalez F. & Russell J.B. 1997. The ability of Escherichia coli O157:H7 to decrease its intracellular pH and resist the toxicity of acetic acid. Microbiol. 143:1175-1180. small amounts of sodium acetate (1.6g. L-1) can inhibit the growth of E. coli O157:H7. This level of acetate is similar to the levels observed in the present work.
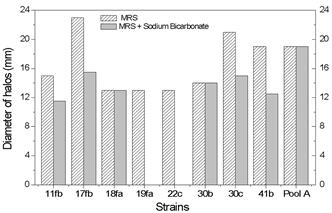
The antagonistic effects of pure and pooled cultures of Lactobacillusstrains on E. coli O157:H7 were studied using the agar spot method. Based on the data in Figure 1, in non-neutralized media, all of the strains and the pool of Lactobacillus strains were antagonistic towards E.coli O157:H7 (Table 2). The strains L. delbrueckii isolate 17fb and L. casei subsp. pseudoplantarumisolate 30c displayed significantly greater (p<0.05) inhibitory effects on the pathogen than the other studied strains, with zones of inhibition larger than 20mm (Fig.1). Pool A and L. plantarum isolate 41b exhibited a similar pattern (19mm of inhibition halo). Among the strains studied, differences between 10 and 77% of the diameter of clear zone formation were observed. The total colony count in 2 μL of cell suspension obtained by parallel culture on an MRS plate ranged from 1.105 to 1.108 CFUmL-1, while the inoculum concentration of E. coli O157:H7 was 108CFU in 10 mL of liquefied BHI agar. The lower level of inhibition by L. reuteri isolate 18fa, L. reuteri isolate 19fa and L. plantarum isolate 22c in MRS media was likely related to their slower growth capacity (Table 2).
Inhibitory effects of Lactobacillus isolates supernatant on Escherichia coli O157:H7 by the spot agar method using MRS agar and MRS after treatment with sodium bicarbonate.
Furthermore, when the same strains and pools of Lactobacillus were tested on neutralized MRS medium (with sodium bicarbonate, 3g.L-1), the mixed strains (Pool A) exhibited higher inhibitory activity (19mm halo diameter) than the pure cultures (0 to 15mm). Differences in the halo diameter of between 30 and 100% were observed for cultures in MRS and MRS-neutralized agar medium (Fig.1). Similar results have been described in a study that examined the inhibitory effects of different strains of Bifidobacterium against E. coli; halo diameters in the range of 13 to 30mm were reported for the un-neutralized agar media associated with a considerable reduction in halo size during growth in media containing sodium bicarbonate (Gagnon et al. 2004Gagnon M., Kheadr E.E., Blay G.L. & Fliss I. 2004. In vitro inhibition of Escherichia coli O157:H7 by bifidobacterial strains of human origin. Int. J.Food Microbiol. 92:69-78.).
In our study, the E. coli O157:H7 inhibition by the Lactobacillus isolates in the presence of sodium bicarbonate may be associated with non-acidic substances, such as hydrogen peroxide and bacteriocins. Furthermore, it has been confirmed that the failure of L. plantarum isolate 22c and L. reuteri isolate 19fa to grow in the media containing sodium bicarbonate was due to an unfavorable pH for the growth of these microorganism.
The combination of strains in pool A displayed greater inhibitory activity toward E. coli O157:H7 than the individual cultures in almost all cases, regardless of the presence or absence of sodium bicarbonate (Fig.1). These results demonstrate variation in the inhibitory strength of pure cultures of Lactobacillus, as well as in the synergistic effect of co-cultures against E. coli O157:H7, emphasizing the importance of the precise selection of the strains for probiotic applications.
Figure 2A shows the optical density of E. coli O157:H7 grown in media containing the supernatants from different cultures of Lactobacillus. The absorbance values demonstrate that E. coli was able to grow in media containing the neutralized fraction of supernatants from pure and pooled cultures of Lactobacillus. However, the pathogenic strain exhibited slower growth rate and reached lower final OD values in neutralized media compared to the control (Fig.2A).
Growth of Escherichia coli O157:H7 in cultural media containing the neutralized fraction of supernatants of Lactobacillus cultures (A) and the acidic fraction of supernatants (B) of L. plantarum-11fb (
 ), L. delbruecki subsp. delbruecki-17fb (
), L. delbruecki subsp. delbruecki-17fb ( ), L. reuteri -18fa (
), L. reuteri -18fa ( ), L. reuteri-19fa (
), L. reuteri-19fa ( ), L. plantarum -22c (
), L. plantarum -22c ( ), L. casei subsp. pseudoplantarum -30b (
), L. casei subsp. pseudoplantarum -30b ( ), L. casei subsp. pseudoplantarum-30c (
), L. casei subsp. pseudoplantarum-30c ( ), L. plantarum -41b( ), pool A ( ), pool B ( ) and control(
), L. plantarum -41b( ), pool A ( ), pool B ( ) and control( ).
).
The results suggest that pH adjustment results in the loss of the antibacterial properties of the supernatants. However, inhibitory activity was observed in all of the media containing the acidic fractions of the supernatants, as confirmed by optical density values that were near zero. Indeed, it is well known that most of the antagonistic effects of Lactobacillus on E. coligrowth could be due to the presence of organic acids that are produced during cell growth (Axe & Bailey 1995Axe D.D. & Bailey J.E. 1995. Transport of lactate and acetate through the cytoplasmic membrane of Escherichia coli. Biotechnol. Bioeng. 47:8-19., Diez-Gonzalez & Russell 1997Diez-Gonzalez F. & Russell J.B. 1997. The ability of Escherichia coli O157:H7 to decrease its intracellular pH and resist the toxicity of acetic acid. Microbiol. 143:1175-1180.).
According to the results shown in Figure 2B, substances present in the supernatants exerted bacteriostatic or bactericidal effects on the pathogenic Escherichia coli. The supernatants from L. delbruecki subsp. Delbrueckii isolate 17fb, L. reuteri isolate 19fa and pool B cultures exhibited bactericidal activity on E. coli; after 7 h of exposure to these supernatants, no colony formation was observed on specific agar media (data not shown). Some strains, such as L. reuteri, can produce reuterin, a non-proteic substance that is soluble in water and possesses antibacterial, antifungal and antiprotozoan properties (Sung et al. 2003Sung H.W., Chen C.N., Liang H.F. & Hong M.H. 2003. A natural compound (reuterin) produced by Lactobacillus reuteri for biological-tissue fixation. Biomaterials 24:1335-1347.).
Lash et al. (2005)Lash B.W., Mysliwiec T.H., Gourama H. & Mysliwiec T.H. 2005. Detection and partial characterization of a broad-range bacteriocin produced by Lactobacillus plantarum (ATCC 8014). Food Microbiol. 22:199-204. studied the inhibitory effects and stability of L. plantarum supernatants at different temperatures, pHs and after treatment with proteolytic enzymes. The results revealed that the supernatant from L. plantarum lost its antimicrobial activity when the pH was adjusted to values higher than 5.0 and lower that 4.0, suggesting that the compound responsible for the inhibition was active only in this pH range. In the present work, a similar effect was detected with the supernatants from Lactobacillus isolates 17fb and 19fa, and pool B, which exhibited antimicrobial activity toward E. coli O157:H7 except at pH 7.0, where the pathogenic bacteria grew normally.
Ogawa et al. (2001)Ogawa M., Shimizu K., Nomoto K., Tanaka R., Hamabata T., Yamasaki S., Takeda T. & Takeda Y. 2001. Inhibition of in vitro growth of Shiga toxin-producing Escherichia coli O157:H7 by probiotic bacteria Lactobacillus strains due to production of latic acid. Int. J. Food Microbiol. 68:135-140. reported that L. casei strain Shirota and L. acidophilus strain YIT 0070 exerted inhibitory effects on E. coli growth. Their antimicrobial activity was assayed in co-culture, suggesting that the antimicrobial effect of Lactobacillus depends on the pH, due to accumulation of lactic acid in the culture medium. The authors also tested the antagonistic effect of L. brevis; however, this organism was unable to affect E. coligrowth. The lack of antagonistic effects indicates that, as observed in the present work, the antimicrobial activity is dependent on the Lactobacillusstrain. On the other hand, Aricia et al. (2004)Aricia M., Bilgin B., Sadgic O. & Ozdemir C. 2004. Some characteristic of Lactobacillus isolates from infant faeces. Food Microbiol. 21:19-24.observed a decrease in the antibacterial activity of supernatants of Lactobacillus that were adjusted to pH 6.5 and contained catalase, suggesting that hydrogen peroxide and organic acids promoted the inhibition of the growth of pathogens, including E. coli.
The pH exerts an important negative effect on bacterial metabolism and growth. In this study, the E. coli O157:H7 was subjected to a moderately acidic environment at 37°C for 7 h. Table 2 shows the optical density (OD 600 nm) of the pathogenic E. coli inoculated in BHI and MRS broth which had been adjusted to different pH values. The results demonstrate that E. coli O157:H7 was unable to grow in both media at pH 3.6 and 4.2; the effect on pathogen growth was bacteriostatic. However, the acidified media did not exert a bactericidal effect on E. coli; after 7h of exposure, the pathogenic strains were able to grow on BHI agar plates (data not shown). Previous studies showed that E. coli and other pathogenic bacteria are sensitive to pH 3.5 and that exposure to low pH can result in adaptive resistance to acidic media (Koutsoumanis & Sofos 2004Koutsoumanis K.P. & Sofos J.N. 2004. Comparative acid stress response of Listeria monocytogenes, Escherichia coli O157:H7 and Salmonella Typhimurium after habituation at different pH conditions. Lett. Appl. Microbiol. 38:321-326.; Cheng et al. 2003Cheng H.Y., Yu R.C. & Chou C.C. 2003. Increased acid tolerance of Escherichia coli O157:H7 as affected by acid adaptation time and conditions of acid challenge. Food Res. Int. 36:49-56.). E.coli and others pathogens bacteria can survive under conditions of extreme acids stress thanks to an acidic-resistance (AR) systems, which is a group of amino acid decarboxylases and antiporters amino-acid dependent. Cells that have grown in amino-acid deficient medium (minimal medium) can succumb at pH 2.5 given the lack of glutamate or arginine (Foster 2004Foster J.W. 2004. Escherichia coli acid resistance: tales of an amateur acidophile. Nature Rev. Microbiol. 2:898-907.).
Cheng et al. (2003)Cheng H.Y., Yu R.C. & Chou C.C. 2003. Increased acid tolerance of Escherichia coli O157:H7 as affected by acid adaptation time and conditions of acid challenge. Food Res. Int. 36:49-56. reported that the percentage of E. coli O157:H7 cells that survived in MRS broth at pH 3.0 were 4.2%. However, the authors mentioned that the bactericidal effect was not observed after 120 min of exposure to these conditions and that by increasing the pH of the culture broth to 4.0, the percentage of survival grew to 70% of the control growth (pH 7.0). Previous researches showed that many Lactobacillus isolates may prevent the binding of pathogens bacteria in the human and animal intestinal cells. After the banning of growth promoters antibiotics (GPA) in worldwide, many alternatives were launched, but still the Lactobacillus strains are the best choice for many situations, like the use as probiotics for chicken, cattle, swine and others production animals, such as meat-type or egg-type. Nevertheless, we must consider the metabolism product of these bacteria for use in animal production feeding. The Lactobacillus studied could prove useful as dietary supplements as well as antimicrobial agents in food and packaging applications.
Conclusions
Almost all strains of Lactobacillus investigated in this study exhibited bactericidal effects on Escherichia coli O157:H7.
The highest inhibitory activity corresponded to Lactobacillus delbrueckii subsp. delbrueckii 17fb and L. casei subsp. pseudoplantarum 30c and, suggesting that antimicrobial compounds production, such as hydrogen peroxide or bacteriocins may be responsible by bacterial inhibition.
All strains tested produced lactic and acetic acids.
The amount of lactic acid produced has no demonstrable influence on the inhibition; in pool A, the supernatants with the highest lactic acid concentrations were unable to exert a more pronounced bactericidal effect on the pathogenic bacteria than the other strains.
Thus, the strains of Lactobacillus studied could be used as antimicrobial agents in packaging or as dietary supplements to control pathogenic microorganisms such as Escherichia coli O157:H7.
Acknowledgements
The authors gratefully acknowledge the financial support of CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo), and CNPq (Conselho Nacional de Pesquisa e Desenvolvimento Tecnológico). A.J. Piantino Ferreira is recipient of CNPq fellowship.
- Aricia M., Bilgin B., Sadgic O. & Ozdemir C. 2004. Some characteristic of Lactobacillus isolates from infant faeces. Food Microbiol. 21:19-24.
- Avonts L. & De Vuyst L. 2001. Antimicrobial potential of probiotic lactic acid bacteria. Meded Rijksuniv. Gent., Fak. Landbouwkd. Toegep. Biol. Wet. 66:543-550.
- Axe D.D. & Bailey J.E. 1995. Transport of lactate and acetate through the cytoplasmic membrane of Escherichia coli. Biotechnol. Bioeng. 47:8-19.
- Cantarelli V., Nagayama K., Takahashi A., Honda T., Cauduro P., Dias G.A.G., Mezzari A. & Brodt T. 2000. Isolation of Shiga toxin-producing Escherichia coli (STEC) serotype O91:H21 from a child with diarrhea in Porto Alegre city, RS, Brazil. Braz. J. Microbiol. 31:266-270.
- Cerqueira A.M.F., Guth B.E.C., Joaquim R.M. & Andrade J.R.C. 1999. High occurrence of Shiga toxin-producing Escherichia coli (STEC) in healthy cattle in Rio de Janeiro State, Brazil. Vet. Microbiol. 70:111-121.
- Chaucheyras-Durand F. & Durand H. 2010. Probiotics in animal nutrition and health. Beneficial Microbes 1:3-9.
- Cheng H.Y., Yu R.C. & Chou C.C. 2003. Increased acid tolerance of Escherichia coli O157:H7 as affected by acid adaptation time and conditions of acid challenge. Food Res. Int. 36:49-56.
- Diez-Gonzalez F. & Russell J.B. 1997. The ability of Escherichia coli O157:H7 to decrease its intracellular pH and resist the toxicity of acetic acid. Microbiol. 143:1175-1180.
- Earnshaw R.G. 1992. The antimicrobial action of lactic acid bacteria: natural food preservation systems, p.211-232. In: Wood B.J.B. (Ed.), The Lactic Acid Bacteria: the lactic acid bacteria in health and disease. Chapman and Hall, London, UK.
- Fábrega V.L.A., Ferreira A.J.P., Patrício F.R.S., Brinkley C. & Scaletsky I.C.A. 2002. Cell-detaching Escherichia coli (CDEC) strains from children with diarrhea: identification of a protein with toxigenic activity. FEMS Microbiol. Lett. 217:191-197.
- Fang H., Elina T., Heikki A. & Seppo S. 2000. Modulation of humoral immune response through probiotic intake. FEMS Immunol. Med.Microbiol. 29:47-52.
- FAO/WHO2001. Joint FAO/WHOExpert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Available at <http://www.who.int/foodsafety/publications/fs_management/en/probiotics.pdf> Accessed Febr. 12, 2014.
» http://www.who.int/foodsafety/publications/fs_management/en/probiotics.pdf - Foster J.W. 2004. Escherichia coli acid resistance: tales of an amateur acidophile. Nature Rev. Microbiol. 2:898-907.
- Gagnon M., Kheadr E.E., Blay G.L. & Fliss I. 2004. In vitro inhibition of Escherichia coli O157:H7 by bifidobacterial strains of human origin. Int. J.Food Microbiol. 92:69-78.
- Garcia A., Fox J.G. & Besser T.E. 2010. Zoonotic enterohemorrhagic Escherichia coli: a one health perspective. ILAR J. 51:221-232.
- Gopal P.K., Prasad J., Smart J. & Gill H.S. 2001. In vitro adherence properties of Lactobacillus rhamnosus DR20 and Bifidobacterium lactis DR10 strains and their antagonistic activity against enterotoxigenic Escherichia coli. Int. J. Food Microbiol. 67:207-216.
- Guarner F. & Schaafsma G.J. 1998. Probiotics. Int. J. Food Microbiol. 39: 237-238.
- Jin L.Z., Ho Y.W., Ali M.A., Abdullah N. & Jalaludin S. 1996. Effect of adherent Lactobacillus spp. on in vitro adherence of salmonellae to the intestinal epithelial cells of chicken. J. Appl. Bacteriol. 81:201-206.
- Kalantzopoulos G. 1997. Fermented products with probiotic qualities. Anaerobe 3:15-19.
- Kandler O. & Weiss N. 1986. Regular, nonsporing Gram-positive rods, p.1209-1234. In: Sneath P.H.A., Mairm N.S. & Sharpe M.E. (Eds), Bergey's Manual of Systematic Bacteriology. Williams and Wilkins, Baltimore, USA.
- Koutsoumanis K.P. & Sofos J.N. 2004. Comparative acid stress response of Listeria monocytogenes, Escherichia coli O157:H7 and Salmonella Typhimurium after habituation at different pH conditions. Lett. Appl. Microbiol. 38:321-326.
- Kudva T.I., Hatfield P.G. & Hovde C.J. 1996. Escherichia coli O157:H7 in microbiota flora of sheep. J. Clin. Microbiol. 34:431-433.
- Lash B.W., Mysliwiec T.H., Gourama H. & Mysliwiec T.H. 2005. Detection and partial characterization of a broad-range bacteriocin produced by Lactobacillus plantarum (ATCC 8014). Food Microbiol. 22:199-204.
- Monteiro-Neto V., Campos L.C., Ferreira A.J.P., Gomes T.A.T. & Trabulsi L.R. 1997. Virulence properties of Escherichia coli 0111:H12 strains. FEMS Microbiol. Lett. 146:123-128.
- Moraes P.M., Perin L.M., Silva Jr A. & Nero L.A. 2013. Comparison of phenotypic and molecular tests to identify lactic acid bacteria. Braz. J. Microbiol. 44:109-112.
- Nakazato G., Paganelli F.L., Lago J.C., Aoki F.H., Mobilon C., Brocchi M., Stehling E.G. & Silveira W.D. 2011. Lactobacillus acidophilus decreases Salmonella Typhimurium invasion in vivo. J. Food Saf. 31:284-289.
- Navarro L., Zarazaga M., Sáenz J., Ruiz-Larrea F. & Torres C. 2000. Bacteriocin production of by lactic acid bacteria isolated from Rioja red wines. J. Appl. Microbiol. 88:44-51.
- Ogawa M., Shimizu K., Nomoto K., Tanaka R., Hamabata T., Yamasaki S., Takeda T. & Takeda Y. 2001. Inhibition of in vitro growth of Shiga toxin-producing Escherichia coli O157:H7 by probiotic bacteria Lactobacillus strains due to production of latic acid. Int. J. Food Microbiol. 68:135-140.
- Paço R.S., Leme I.L., Bottino J.A. & Ferreira A.J.P. 2003. Identification of Lactobacillus spp. from broiler litter in Brazil. Braz. J. Microbiol. 34:236-237.
- Perdigón G., Vintiñi E., Alvarez S., Medina M. & Medici M. 1999. Study of the possible mechanisms involved in the mucosal immune system activation by lactic acid bacteria. J. Dairy Sci. 82:1108-1114.
- Poppi L.B., Mancilha I.M., Ferreira A.J.P.& Leal D.D.M. 2008. Evaluation of the antagonistic effect of Lactobacillus on Listeria monocytogenes in vitro. Braz. J. Food Technol. 11:113-119.
- Rossland E., Langsrud T., Granum P.E. & Sorhaug T. 2005. Production of antimicrobial metabolites by strains of Lactobacillus or Lactococcus co-cultured with Bacillus cereus in milk. Int. J. Food Microbiol. 98:193-200.
- Scapin D., Grando W.F., Rossi E.M., Perez K.J., Malheiros P.S. & Tondo E.C. 2013. Antagonistic activity of Lactobacillus acidophilus LA10 against Salmonella enterica serovar Enteritidis SE86 in mice. Braz. J. Microbiol. 44:57-61.
- Servin A.L. & Coconier M.H. 2003. Adhesion of probiotic strains to the intestinal mucosa and interaction with pathogens. Best Pract. Res. 17:741-754.
- Schillinger U. & Lücke F.K. 1989. Antibacterial activity of Lactobacillus sake isolated from meat. Appl. Environ. Microbiol. 55:1901-1906.
- Sung H.W., Chen C.N., Liang H.F. & Hong M.H. 2003. A natural compound (reuterin) produced by Lactobacillus reuteri for biological-tissue fixation. Biomaterials 24:1335-1347.
- Todorov S.D. & Dicks L.M.T. 2005. Lactobacillus plantarum isolated from molasses produces bacteriocins active against Gram-negative bacteria. Enzyme Microb. Technol. 36:318-326.
- Varalakshmi S., Balasubramanyam B.V., Surendranath B., Bagath M. & Rajendran D. 2013. Use of novel lactic acid bacterial strains with antagonistic activity for the preparation of safe indigenous fermented dairy foods (Dahi and Raita). J. Food Safty 34:26-33.
Publication Dates
-
Publication in this collection
Apr 2015
History
-
Received
13 Jan 2015 -
Accepted
21 Apr 2015