Abstract:
A serological survey for antibodies against Leptospira interrogans, Brucella abortus, and Chlamydophila abortus was conducted in 21 clinically healthy, free-ranging giant ant- eaters (Myrmecophaga tridactyla) from Parque Nacional das Emas (Goiás State, Brazil; n=6), Parque Nacional da Serra da Canastra (Minas Gerais State, Brazil; n=9), and RPPN SESC Pantanal (Mato Grosso State, Brazil; n=6) between July 2001 and September 2006. Sera were screened for antibodies against 22 serovars of Leptospira interrogans with a microscopic agglutination test. Twelve tested positive for L. interrogans serovars sentot (n=5 in PN Emas, n=2 in PN Serra da Canastra), butembo (n=2 in PN Serra da Canastra), autumnalis, bataviae, and shermani/icterohaemorrhagiae (n=1 each in SESC Pantanal)One adult female tested positive for B. abortus with the buffered plate antigen test. All sera were negative for C. abortus using the complement fixation text. This is the first report of pathogens that may interfere with the reproduction and population dynamics of free-ranging giant anteaters.
Index Terms:
Anteaters; Myrmecophaga tridactyla; brucellosis; Brucella abortus; chlamydiosis; Chlamydophila abortus; leptospirosis; Leptospira interrogans; Pilosa; serological survey; Vermilingua; wildlife; Xenarthra.
Resumo:
Inquéritos sorológicos para detecção de anticorpos contra Leptospira interrogans, Brucella abortus, e Chlamydophila abortus foram realizados em 21 tamanduás-bandeira (Myrmecophaga tridactyla) de vida livre do Parque Nacional das Emas (Goiás, Brasil, n=6), o Parque Nacional da Serra da Canastra (Minas Gerais, Brasil, n=9) e RPPN SESC Pantanal (Mato Grosso, Brasil, n=6) entre julho de 2001 e setembro de 2006. Os sor os foram testados para anticorpos contra 22 sorotipos de Leptospira interrogans com um teste de aglutinação microscópica. Doze animais foram considerados positivos para L. interrogans sorovares sentot (n=5 em PN Emas, n=2 em PN Serra da Canastra), butembo (n=2 em PN Serra da Canastra), autumnalis, bataviae e shermani/icterohaemorrhagiae(n=1 para cada sorovar em SESC Pantanal). Uma fêmea adulta testou positivo para B. abortuscom o teste do antígeno tamponado. Todos os soros se mostraram negativos para C. abortusatravés do teste de fixação do complemento. Este é o primeiro relato de patógenos que podem interferir na dinâmica reprodutiva de populações de tamanduás em estado selvagem.
Termos de Indexação:
Tamanduás; Myrmecophaga tridactyla; brucelose; Brucella abortus; chlamydiose; Chlamydophila abortus; leptospirose; Leptospira interrogans; Pilosa; inquérito sorológico; Vermilingua; animais silvestres; Xenarthra.
Introduction
Diseases have been recognized as a growing problem in biodiversity conservation (McCallum & Dobson 1995McCallum H. & Dobson A.P. 1995. Detecting disease and parasite threats to endangered species and ecosystems. Trends Ecol. Evol. 10:190-194.). Due to the paucity of information on pathogens affecting wild giant anteaters (Myrmecophaga tridactyla), it is currently difficult to assess the role of diseases in the population declines that have led to the classification of M. tridactyla as Vulnerable by the IUCN Red List of Threatened Species (Miranda & Medri 2010Miranda F. & Medri I.M. 2010. Myrmecophaga tridactyla. Available at <Available at http://ww.iucnredlist.org > Accessed June 16, 2011.
http://ww.iucnredlist.org...
). Giant anteaters have small litter sizes, long gestations, and extended periods of parental care (Schauerte 2005Schauerte N. 2005. Untersuchungen zur Zyklus- und Graviditätsdiagnostik beim Grossen Ameisenbären (Myrmecophaga tridactyla). DoctoralThesis, Justus-Liebig-Universität, Giessen, Germany. 182p.). Pathogens that may interfere with their ability to produce viable offspring are therefore expected to have significant impacts on their population growth rate and bear the potential to drive this charismatic species to extinction (Boots & Sasaki 2001Boots M. & Sasaki A. 2001. Parasite-driven extinction in spatially explicit host-parasite systems. Am. Nat. 34:706-713.).
Leptospirosis, brucellosis, and chlamydiosis are important zoonoses with worldwide distribution that can lead to reduced fertility and abortion (Acha & Szyfres 2003Acha P.N. & Szyfres B. 2003. Zoonoses and communicable diseases common to man and animals. 3rd ed. Pan American Health Organization (PAHO), Washington, DC. 384p.). In Brazil, the causative agents of these diseases have been reported in domestic (Tomich et al. 2007Tomich R.G.P., Bomfim M.R.Q., Koury M.C., Pellegrin A.O., Pellegrin L.A., Ko A.I. & Barbosa-Stancioli E.F. 2007. Leptospirosis serosurvey in bovines from Brazilian Pantanal using IgG ELISA with recombinant protein LIPL32 and microscopic agglutination test. Braz. J. Microbiol. 38:674-680.) and wild animals (Ito et al. 1998Ito F.H., Vasconcellos S.A., Bernardini F., Nascimento A.A., Labruna M.B. & Arantes I.G. 1998. Evidencia sorológica de brucelose e leptospirose e parasitismo por ixodídeos em animais silvestres do Pantanal Sul-Matogrossense. Ars Vet. 14:302-310.) sharing their habitat with giant anteaters. Contact between giant anteaters and infected animals may lead to disease transmission, potentially affecting reproductive rates and leading to population declines in M. tridactyla.
The objective of this study was to investigate the presence of antibodies against Leptospira interrogans, Brucella abortus, and Chlamydophila abortus in wild giant anteaters inhabiting three protected areas of central Brazil.
Materials and Methods
Study area. Study animals were wild-caught in three different localities of Brazil: Parque Nacional das Emas (PNE; 18˚16'S, 52˚53'W), located in the Brazilian Central Plateau of western Brazil, which protects 131,800 ha of the Cerrado biome in southwestern Goiás State (Scardua et al. 2004Scardua F.P., Carvalho D.A. & Beserra M.M.L. 2004. Parque Nacional das Emas: plano de manejo. MMA/IBAMA, Brasília.893p.); Parque Nacional da Serra da Canastra (PNSC; 20˚00'-20˚30'S, 46˚15'-47˚00'W), which covers approximately 200,000 ha of the Cerrado biome in southwestern Minas Gerais State (IBAMA 2005Instituto Brasileiro do Meio Ambiente (IBAMA). 2005. Plano de Manejo do Parque Nacional Serra da Canastra (PNSC). MMA/IBAMA, Brasília. 94p.); and Reserva Particular do Patrimônio Natural SESC Pantanal (SESC; 16-17˚S, 56-57˚W), a privately owned conservation unit of approximately 88,000 ha located within the Pantanal Biosphere Reserve, in Mato Grosso State (Brandão et al. 2011Brandão L.G., Antas P.T.Z., Oliveira L.F.B., Pádua M.T.J., Pereira N.C. & Valutky W.W. 2011. Plano de manejo da Reserva Particular de Patrimônio Natural do SESC Pantanal. 2ª ed. SESC, Departamento Nacional, Rio de Janeiro. 148p.).
Sample collection. Line transects were performed in July 2001 and January to April 2003 (PNE); August 2003 (PNSC); and from July to September 2005 and June to September 2006 (SESC). Anteaters were darted with a blowpipe using 10mg/kg ketamine chloride (Ketalar, Laboratorios Pfizer, São Paulo, Brazil) with 1mg/kg midazolam (Dormonid, Roche, São Paulo, Brazil) or 9.56mg/kg ketamine chloride with 1.6mg/kg xylazine (Virbacyl, Virbac, São Paulo, Brazil). Sex and geographic location were recorded, and age was determined based on body mass and size. Morphometric measurements were taken and a clinical examination performed. Blood was collected into sterile test tubes by puncturing the external jugular vein or, less frequently, the cephalic or inner femoral vein. Serum was separated with a portable centrifuge, then aliquoted in Eppendorf tubes and stored in liquid nitrogen.
Permits to capture and sample wild giant anteaters were granted by the Brazilian Institute of Environment and Renewable Natural Resources (IBAMA).
Sample analysis. All analyses were performed at the Departamento de Medicina Preventiva, Universidade Estadual de Londrina, Brazil. A microscopic agglutination test (MAT) with live pathogen was used to detect antibodies against Leptospira interrogans (Cole et al. 1973Cole J.R.J., Sulzer C.R. & Pursell A.R. 1973. Improved microtechnique for the leptospiral Microscopic Agglutination Test. Appl. Microbiol. 25:976-980.). The following reference serovars were used: australis, bratislava, autumnalis, butembo, bataviae, canicola, castellonis, copenhageni, cynopteri, fortbragg, grippotyphosa, hardjo, hebdomadis, icterohaemorrhagiae, panama, pomona, pyrogenes, sentot, shermani, tarassovi, whitcomb, and wolffi. Sera were considered positive if agglutination was present at dilutions ≥1:10. Positive serum samples were serially diluted to determine the end point (Myers 1985Myers D.M. 1985. Manual de métodos para el diagnóstico de laboratorio de la leptospirosis. Centro Panamericano de Zoonosis, Buenos Aires. 46p.). If a sample showed cross-reactivity between two or more serovars of the same group, the one showing higher titers was considered positive.
The Buffered Plate Antigen test (BPA, Angus & Barton 1984Angus R.D. & Barton C.E. 1984. The production and evaluation of a buffered plate antigen for use in a presumptive test for brucellosis. Dev. Biol. Stand. 56:349-356.) was used to screen sera for antibodies against B. abortus. A 0.03 ml suspension of deactivated B. abortus cells was homogenized with the same volume of serum. Samples showing macroscopic agglutination after four minutes were considered reactive.
Sera were tested for antibodies against C. abortus using the complement fixation test (OIE 2008OIE 2008. Enzootic abortion of ewes (ovine chlamydiosis), p.1013-1020. In: OIE. (Ed.), Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. World Organization for Animal Health (OIE), Paris.). Reference strain C. abortus S26/3 (provided by Dr Carlo Turilli; Instituto Zooprofilattico Sperimentale delle Venezie, Padova, Italy) was used as antigen. Titers ≥1:32 were considered positive (OIE 2008OIE 2008. Enzootic abortion of ewes (ovine chlamydiosis), p.1013-1020. In: OIE. (Ed.), Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. World Organization for Animal Health (OIE), Paris.).
Results
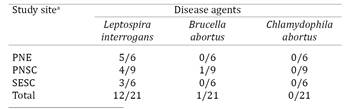
A total of 21 giant anteaters were captured: six at PNE (2 males, 4 females), nine at PNSC (4 males, 5 females), and six at SESC (3 males, 3 females). All animals were considered clinically healthy. Twelve sera (57.14%) were positive for Leptospira interrogans, one (4.76%) tested positive for B. abortus, and all were negative for antibodies against Chlamydophila abortus (Table 1).
The large majority of anteaters with antibodies against L. interrogans had low agglutinating titers, but two animals from PNE had titers >1:100 for L. interrogans serovar sentot(Fig.1). Determination of the more probable serovar was not possible in one serum sample from SESC because co-agglutination occurred and both serovars (shermani and icterohaemmorhagiae) were positive at the same maximum dilution.
Percentages of antibody against serovars of Leptospira interrogans in free-ranging giant anteaters (Myrmecophaga tridactyla) from three protected areas of Brazil
Serovar sentot was identified in seven giant anteaters from two protected areas. Antibodies against serovar butembo were restricted to anteaters from PNSC, and serovars autumnalis, bataviae, shermani, and icterohaemmorhagiae were only found in animals from SESC (Fig.1).
Discussion
This is the first report of pathogens that may interfere with the reproduction and population dynamics of free-ranging giant anteaters.
Twelve of 21 tested giant anteaters were seroreactive to Leptospira interrogans, over 50% of which were positive for serovar sentot. Epidemiological data on the occurrence of this serovar in Brazil are scarce, as it is not always included as a reference strain (e.g., Tomich et al. 2007Tomich R.G.P., Bomfim M.R.Q., Koury M.C., Pellegrin A.O., Pellegrin L.A., Ko A.I. & Barbosa-Stancioli E.F. 2007. Leptospirosis serosurvey in bovines from Brazilian Pantanal using IgG ELISA with recombinant protein LIPL32 and microscopic agglutination test. Braz. J. Microbiol. 38:674-680.; Freitas et al. 2010Freitas T.P.T., Keuroghlian A., Eaton D.P., Freitas E.B., Figueiredo A., Nakazato L., Oliveira J.M., Miranda F., Paes R.C.S., Monteiro L.A.R.C., Lima J.V.B., Neto A.A.C., Dutra V. & Freitas J.C. 2010. Prevalence of Leptospira interrogansantibodies in free-ranging Tayassu pecari of the Southern Pantanal, Brazil, an ecosystem where wildlife and cattle interact. Trop. Anim. Health 42:1695-1703.). It is possible that the wide variety of serovars observed in anteaters from SESC (Fig.1), as well as in domestic animals and other wildlife from this area (Freitas et al. 2010Freitas T.P.T., Keuroghlian A., Eaton D.P., Freitas E.B., Figueiredo A., Nakazato L., Oliveira J.M., Miranda F., Paes R.C.S., Monteiro L.A.R.C., Lima J.V.B., Neto A.A.C., Dutra V. & Freitas J.C. 2010. Prevalence of Leptospira interrogansantibodies in free-ranging Tayassu pecari of the Southern Pantanal, Brazil, an ecosystem where wildlife and cattle interact. Trop. Anim. Health 42:1695-1703.; Jorge et al. 2011Jorge R.S.P., Ferreira F., Ferreira Neto J.S., Vasconcellos S.A., Lima E.S., Morais Z.M. & Souza G.O. 2011. Exposure of free-ranging wild carnivores, horses and domestic dogs to Leptospira spp in the northern Pantanal, Brazil. Mem. Inst. Oswaldo Cruz 106:441-444.), is related to the area having favorable conditions for the survival of Leptospira in the environment, such as seasonal flooding and high temperature and humidity levels (Acha & Szyfres 2003Acha P.N. & Szyfres B. 2003. Zoonoses and communicable diseases common to man and animals. 3rd ed. Pan American Health Organization (PAHO), Washington, DC. 384p.). Emmons et al. (2004)Emmons L.H., Peña Flores R., Alpirre S.A. & Swarner M.J. 2004. Bathing behavior of giant anteaters (Myrmecophaga tridactyla). Edentata 6:41-43. repeatedly observed giant anteaters wallowing in mud and bathing in shallow ponds that are used by other species as watering place. This behavior may further increase the risk of anteaters becoming infected with L. interrogans.
No clinical signs of leptospirosis were observed in any of the examined anteaters. This was not unexpected, as wild animals rarely show symptoms or lesions attributable to Leptospira (Acha & Szyfres 2003Acha P.N. & Szyfres B. 2003. Zoonoses and communicable diseases common to man and animals. 3rd ed. Pan American Health Organization (PAHO), Washington, DC. 384p.) A captive giant anteater at Rio de Janeiro Zoo, however, showed clinical signs due to L. interrogans serovar icterohaemmorhagiae infection (Monteiro et al. 2003Monteiro R.V., Fedullo L.P.L., Albuquerque C.E. & Lilenbaum W. 2003. Leptospirosis in giant anteater (Myrmecophaga tridactyla, Linnaeus, 1758) at Rio de Janeiro Zoological Foundation, Brazil. Revta Bras. Ciênc. Vet. 10:126-127.).
A single giant anteater from PNSC tested positive for Brucella abortus. The adult female was clinically healthy, but it is not known whether its fertility was affected by the pathogen. PNSC lies within one of Brazil's most important areas of cattle ranching, where roughly 6% of herds are infected with bovine brucellosis (Gonçalves et al. 2009Gonçalves V.S.P., Delphino M.K.V.C., Dias R.A., Ferreira F., Amaku M., Ferreira Neto J.S., Porto T.B., Alves C.M., Figueiredo V.C.F. & Lôbo J.R. 2009. Situação epidemiológica da brucelose bovina no Estado de Minas Gerais. Arq. Bras. Med. Vet. Zootec. 61(Suppl.1):35-45.). Extensive cattle ranching occurs both in the vicinity of PNSC and on private properties within the national park (IBAMA 2005Instituto Brasileiro do Meio Ambiente (IBAMA). 2005. Plano de Manejo do Parque Nacional Serra da Canastra (PNSC). MMA/IBAMA, Brasília. 94p.). This situation bears the risk of close contact between domestic and wild animals, and cattle may have been the source of infection for this anteater. It is, however, also possible that the seropositive female acquired the pathogen from a wild reservoir host. Gonçalves et al. (2009)Gonçalves V.S.P., Delphino M.K.V.C., Dias R.A., Ferreira F., Amaku M., Ferreira Neto J.S., Porto T.B., Alves C.M., Figueiredo V.C.F. & Lôbo J.R. 2009. Situação epidemiológica da brucelose bovina no Estado de Minas Gerais. Arq. Bras. Med. Vet. Zootec. 61(Suppl.1):35-45. noted that the presence of wild cervids significantly increased the risk of cattle herds being infected with B. abortus. Nevertheless, the role of cervids as reservoirs requires confirmation because data on the prevalence of Brucella infection in cervids from the PNSC area are lacking.
None of the individuals from SESC and PNE was seropositive for B. abortus. It should, however, be noted that only a few anteaters were tested in these areas. Prevalence of bovine brucellosis is very high in the vicinity of PNE (21% seropositive cattle herds, Rocha et al. 2009Rocha W.V., Gonçalves V.S.P., Coelho C.G.N.F.L., Brito W.M.E.D., Dias R.A., Delphino M.K.V.C., Ferreira F., Amaku M., Ferreira Neto J.S., Figueiredo V.C.F., Lôbo J.R. & Brito L.A.B. 2009. Situaçäo epidemiológica da brucelose bovina no Estado de Goiás. Arq. Bras. Med. Vet. Zootec. 61 (Suppl.1): 27-34.) and of SESC (37% seropositive herds, Negreiros et al. 2009Negreiros R.L., Dias R.A., Ferreira F., Ferreira Neto J.S., Gonçalves V.S.P., Silva M.C.P., Figueiredo V.C.F., Lôbo J.R., Freitas J. & Amaku M. 2009. Situação epidemiológica da brucelose bovina no Estado de Mato Grosso. Arq. Bras. Med. Vet. Zootec. 61(Suppl.1):56-65.), and cattle ranching occurs in private farms near both protected areas. Antibodies against B. abortus have also been found in peccaries (Tayassu pecari) in the vicinity of PNE (Kashivakura et al. 2004Kashivakura C.K., Furtado M.M., Jácomo A.T.A., Marvulo M.F., Silva J.C.R., Suero D., Ferro C., Astete S.H., Tôrres N.M. & Silveira L. 2004. Brucelose em queixadas Tayassu pecari, de vida livre da região do Parque Nacional das Emas. XXV Congresso Brasileiro de Zoologia, Londrina, p.217-218. (Abstract)) and in pampas deer (Ozotoceros bezoarticus) from the southern Pantanal (Elisei et al. 2010Elisei C., Pellegrin A.O., Tomas W.M., Soares C.O., Araújo F.R., Funes-Huacca M.E. & Rosinha G.M.S. 2010. Evidência molecular de Brucella sp. em Ozotoceros bezoarticus (veado campeiro) do Pantanal Sul-Mato-Grossense. Pesq. Vet. Bras. 30:503-509.). Wild giant anteaters may therefore also be exposed to this pathogen in PNE and SESC. It remains to be studied whether infection with B. abortus leads to clinical symptoms in giant anteaters. Considering that only one out of 21 assessed anteaters was seropositive in spite of the elevated risk of exposure related to high prevalences in cattle and other wildlife, it is also possible that, similar to moose (Alces alces, Forbes et al. 1996Forbes L.B., Tessaro S.V. & Lees W. 1996. Experimental studies on Brucella abortus in moose (Alces alces). J. Wildl. Dis. 32:94-104.), Myrmecophaga tridactyla is highly susceptible to the disease and dies soon after infection.
None of the assessed giant anteaters was positive for Chlamydophila abortus. This pathogen of worldwide distribution is one of the most frequent causes of abortion in sheep and goats (Longbottom & Coulter 2003Longbottom D. & Coulter L.J. 2003. Animal chlamydioses and zoonotic implications. J. Comp. Pathol. 128:217-244.). Data on its occurrence in Brazilian wildlife are virtually non-existent, with the notable exception of a giant anteater kept in a zoo in São Paulo State that recently tested positive for Chlamydophila spp. (Miranda, unpublished data). According to its case history, the nulliparous female suffered spontaneous abortion in the final third of its first pregnancy, a symptom typical for infection with C. abortus in small ruminants (Longbottom & Coulter 2003Longbottom D. & Coulter L.J. 2003. Animal chlamydioses and zoonotic implications. J. Comp. Pathol. 128:217-244.). This case implies that exposure to this pathogen can interfere with reproduction in giant anteaters.
To our knowledge, no epidemiological studies on C. abortus have been performed in domestic or wild animals within or near our study sites, which complicates the assessment of the exposure risk of the giant anteaters studied here. Only few laboratories in Brazil are qualified to identify C. abortus, which may explain the limited data on its prevalence in this country and, especially, at our study sites (Silva et al. 2006Silva F.G., Freitas J.C. & Müller E.E. 2006. Chlamydophila abortus em animais de produção. Ciência Rural 36:342-348.). Additional screenings of giant anteaters inhabiting areas where presence of C. abortus has been confirmed in domestic or other wild animals will be needed to assess the importance of this pathogen in their reproduction and population dynamics.
Acknowledgments
We are thankful to all the persons who helped us with fieldwork and laboratory analyses . Special thanks are extended to Roberto Aguilar for his guidance. Financial support was provided by the IUCN/SSC Anteater, Sloth and Armadillo Specialist Group and Wildlife Conservation Society.
References
- Acha P.N. & Szyfres B. 2003. Zoonoses and communicable diseases common to man and animals. 3rd ed. Pan American Health Organization (PAHO), Washington, DC. 384p.
- Angus R.D. & Barton C.E. 1984. The production and evaluation of a buffered plate antigen for use in a presumptive test for brucellosis. Dev. Biol. Stand. 56:349-356.
- Boots M. & Sasaki A. 2001. Parasite-driven extinction in spatially explicit host-parasite systems. Am. Nat. 34:706-713.
- Brandão L.G., Antas P.T.Z., Oliveira L.F.B., Pádua M.T.J., Pereira N.C. & Valutky W.W. 2011. Plano de manejo da Reserva Particular de Patrimônio Natural do SESC Pantanal. 2ª ed. SESC, Departamento Nacional, Rio de Janeiro. 148p.
- Cole J.R.J., Sulzer C.R. & Pursell A.R. 1973. Improved microtechnique for the leptospiral Microscopic Agglutination Test. Appl. Microbiol. 25:976-980.
- Elisei C., Pellegrin A.O., Tomas W.M., Soares C.O., Araújo F.R., Funes-Huacca M.E. & Rosinha G.M.S. 2010. Evidência molecular de Brucella sp. em Ozotoceros bezoarticus (veado campeiro) do Pantanal Sul-Mato-Grossense. Pesq. Vet. Bras. 30:503-509.
- Emmons L.H., Peña Flores R., Alpirre S.A. & Swarner M.J. 2004. Bathing behavior of giant anteaters (Myrmecophaga tridactyla). Edentata 6:41-43.
- Forbes L.B., Tessaro S.V. & Lees W. 1996. Experimental studies on Brucella abortus in moose (Alces alces). J. Wildl. Dis. 32:94-104.
- Freitas T.P.T., Keuroghlian A., Eaton D.P., Freitas E.B., Figueiredo A., Nakazato L., Oliveira J.M., Miranda F., Paes R.C.S., Monteiro L.A.R.C., Lima J.V.B., Neto A.A.C., Dutra V. & Freitas J.C. 2010. Prevalence of Leptospira interrogansantibodies in free-ranging Tayassu pecari of the Southern Pantanal, Brazil, an ecosystem where wildlife and cattle interact. Trop. Anim. Health 42:1695-1703.
- Gonçalves V.S.P., Delphino M.K.V.C., Dias R.A., Ferreira F., Amaku M., Ferreira Neto J.S., Porto T.B., Alves C.M., Figueiredo V.C.F. & Lôbo J.R. 2009. Situação epidemiológica da brucelose bovina no Estado de Minas Gerais. Arq. Bras. Med. Vet. Zootec. 61(Suppl.1):35-45.
- Instituto Brasileiro do Meio Ambiente (IBAMA). 2005. Plano de Manejo do Parque Nacional Serra da Canastra (PNSC). MMA/IBAMA, Brasília. 94p.
- Ito F.H., Vasconcellos S.A., Bernardini F., Nascimento A.A., Labruna M.B. & Arantes I.G. 1998. Evidencia sorológica de brucelose e leptospirose e parasitismo por ixodídeos em animais silvestres do Pantanal Sul-Matogrossense. Ars Vet. 14:302-310.
- Jorge R.S.P., Ferreira F., Ferreira Neto J.S., Vasconcellos S.A., Lima E.S., Morais Z.M. & Souza G.O. 2011. Exposure of free-ranging wild carnivores, horses and domestic dogs to Leptospira spp in the northern Pantanal, Brazil. Mem. Inst. Oswaldo Cruz 106:441-444.
- Kashivakura C.K., Furtado M.M., Jácomo A.T.A., Marvulo M.F., Silva J.C.R., Suero D., Ferro C., Astete S.H., Tôrres N.M. & Silveira L. 2004. Brucelose em queixadas Tayassu pecari, de vida livre da região do Parque Nacional das Emas. XXV Congresso Brasileiro de Zoologia, Londrina, p.217-218. (Abstract)
- Longbottom D. & Coulter L.J. 2003. Animal chlamydioses and zoonotic implications. J. Comp. Pathol. 128:217-244.
- McCallum H. & Dobson A.P. 1995. Detecting disease and parasite threats to endangered species and ecosystems. Trends Ecol. Evol. 10:190-194.
- Miranda F. & Medri I.M. 2010. Myrmecophaga tridactyla. Available at <Available at http://ww.iucnredlist.org > Accessed June 16, 2011.
» http://ww.iucnredlist.org - Monteiro R.V., Fedullo L.P.L., Albuquerque C.E. & Lilenbaum W. 2003. Leptospirosis in giant anteater (Myrmecophaga tridactyla, Linnaeus, 1758) at Rio de Janeiro Zoological Foundation, Brazil. Revta Bras. Ciênc. Vet. 10:126-127.
- Myers D.M. 1985. Manual de métodos para el diagnóstico de laboratorio de la leptospirosis. Centro Panamericano de Zoonosis, Buenos Aires. 46p.
- Negreiros R.L., Dias R.A., Ferreira F., Ferreira Neto J.S., Gonçalves V.S.P., Silva M.C.P., Figueiredo V.C.F., Lôbo J.R., Freitas J. & Amaku M. 2009. Situação epidemiológica da brucelose bovina no Estado de Mato Grosso. Arq. Bras. Med. Vet. Zootec. 61(Suppl.1):56-65.
- OIE 2008. Enzootic abortion of ewes (ovine chlamydiosis), p.1013-1020. In: OIE. (Ed.), Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. World Organization for Animal Health (OIE), Paris.
- Rocha W.V., Gonçalves V.S.P., Coelho C.G.N.F.L., Brito W.M.E.D., Dias R.A., Delphino M.K.V.C., Ferreira F., Amaku M., Ferreira Neto J.S., Figueiredo V.C.F., Lôbo J.R. & Brito L.A.B. 2009. Situaçäo epidemiológica da brucelose bovina no Estado de Goiás. Arq. Bras. Med. Vet. Zootec. 61 (Suppl.1): 27-34.
- Scardua F.P., Carvalho D.A. & Beserra M.M.L. 2004. Parque Nacional das Emas: plano de manejo. MMA/IBAMA, Brasília.893p.
- Schauerte N. 2005. Untersuchungen zur Zyklus- und Graviditätsdiagnostik beim Grossen Ameisenbären (Myrmecophaga tridactyla). DoctoralThesis, Justus-Liebig-Universität, Giessen, Germany. 182p.
- Silva F.G., Freitas J.C. & Müller E.E. 2006. Chlamydophila abortus em animais de produção. Ciência Rural 36:342-348.
- Tomich R.G.P., Bomfim M.R.Q., Koury M.C., Pellegrin A.O., Pellegrin L.A., Ko A.I. & Barbosa-Stancioli E.F. 2007. Leptospirosis serosurvey in bovines from Brazilian Pantanal using IgG ELISA with recombinant protein LIPL32 and microscopic agglutination test. Braz. J. Microbiol. 38:674-680.
Publication Dates
-
Publication in this collection
May 2015
History
-
Received
27 June 2013 -
Accepted
17 Dec 2014