Abstract:
This study describes the development of the central nervous system in guinea pigs from 12th day post conception (dpc) until birth. Totally, 41 embryos and fetuses were analyzed macroscopically and by means of light and electron microscopy. The neural tube closure was observed at day 14 and the development of the spinal cord and differentiation of the primitive central nervous system vesicles was on 20th dpc. Histologically, undifferentiated brain tissue was observed as a mass of mesenchymal tissue between 18th and 20th dpc, and at 25th dpc the tissue within the medullary canal had higher density. On day 30 the brain tissue was differentiated on day 30 and the spinal cord filling throughout the spinal canal, period from which it was possible to observe cerebral and cerebellar stratums. At day 45 intumescences were visualized and cerebral hemispheres were divided, with a clear division between white and gray matter in brain and cerebellum. Median sulcus of the dorsal spinal cord and the cauda equina were only evident on day 50. There were no significant structural differences in fetuses of 50 and 60 dpc, and animals at term were all lissencephalic. In conclusion, morphological studies of the nervous system in guinea pig can provide important information for clinical studies in humans, due to its high degree of neurological maturity in relation to its short gestation period, what can provide a good tool for neurological studies.
Index Terms:
Nervous system; embryology; animal models; histology.
Resumo:
Este estudo descreve o desenvolvimento do sistema nervoso central em guinea pig do 12º dia pós-concepção (dpc) até ao nascimento. No total, 41 embriões e fetos foram analisados macroscopicamente e por microscopia de luz e eletrônica. O fechamento do tubo neural foi observado no dia 14 e o desenvolvimento da medula espinhal e diferenciação das vesículas primitivas do sistema nervoso central foram observados no dia 20. Histologicamente, o tecido cerebral indiferenciado foi observado como uma massa de tecido mesenquimal entre os dias 18 e 20 e no 25º dia o tecido no interior do canal medular apresentou maior densidade. No dia 30 o tecido cerebral apresentou-se diferenciado, período no qual a medula espinhal preenchia todo o canal vertebral e foi possível observar os estratos cerebral e cerebelar. No dia 45 as intumescências cervical e lombar foram visualizadas e os hemisférios cerebrais estavam divididos, com uma clara distinção entre substância branca e cinzenta no cérebro e cerebelo. O sulco mediano dorsal da medula espinhal e a cauda equina foram evidentes apenas no dia 50. Não houve diferenças estruturais significativas em fetos de 50 e 60 dpc e animais a termo eram todos lisencefálicos. Estudos morfológicos do sistema nervoso em guinea pig podem fornecer informações importantes para estudos clínicos em seres humanos devido ao alto grau de maturidade neurológica em relação ao seu período de gestação curto, fato que servir como excelente ferramenta em estudos neurológicos.
Termos de Indexação:
Sistema nervoso; embriologia; modelos animais; histologia.
Introduction
Cavia porcellus, popularly known as guinea pig, is a hystricomorph rodent from South America. Like other exotic species, the guinea pig has become popular as a pet due to their small size and docility (Endersby 2009Endersby J. 2009. A Guinea Pig's History of Biology. Harvard University Press, Cambridge. 544p.). This popularity has increased the interest in its behavior, physiology and management, leading it to be used as an experimental animal (Taylor & Lee 2012Taylor D.K. & Lee V.K. 2012. Guinea pigs as experimental models, p.705-744. In: Suckow M.A., Stevens K.A. & Wilson R.P. (Eds), The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents. Academic Press, Boston ., Dumitrascu et al. 2013Dumitrascu R.J., Heitmann W., Seeger W., Weissmann N. & Schulz R. 2013. Obstructive sleep apnea, oxidative stress and cardiovascular disease: lessons from animal studies. Oxid. Med. Cell. Longev. 2013:234631.). Due to anatomical and physiological similarities, related to pregnancy and placentation (Miglino et al. 2004Miglino M.A., Carter A.M., Ambrósio C.E., Bonatelli M., De Oliveira M.F., Dos Santos F., Rodrigues R.F. & Santos T.C. 2004. Vascular organization of the histricomorph placenta: a comparative study in the agouti, capybara, guinea pig, paca and rock cavy. Placenta 25:438-448., Mess 2007Mess A. 2007. The guinea pig placenta: model of placental growth dynamics. Placenta 28:812-815., Oliveira et al. 2012Oliveira M.F., Do Vale A.M., Favaron P.O., Vasconcelos B.G., De Oliveira B.G., Miglino M.A. & Mess A. 2012. Development of yolk sac inversion in Galea spixii and Cavia porcellus (Rodentia, Caviidae). Placenta 33:878-881., Vasconcelos et al. 2013Vasconcelos B.G., Favaron P.O., Miglino M.A. & Mess A. 2013. Development and morphology of the inverted yolk sac in the guinea pig (Cavia porcellus). Theriogenology 80:636-641.), this species became extremely important as an experimental model for human placentation, as well as for respiratory diseases (Canning & Chou 2008Canning B.J. & Chou Y. 2008. Using guinea pigs in studies relevant to asthma and COPD. Pulm. Pharmacol. Ther. 21:702-720., Skerry et al. 2013Skerry C., Pokkali S., Pinn M., Be N.A., Harper J., Karakousis P.C. & Jain S.K. 2013. Vaccination with recombinant Mycobacterium tuberculosis PknD attenuates bacterial dissemination to the brain in guinea pigs. Plos One 8:e66310.), cardiovascular problems (Zaragoza et al. 2011Zaragoza C., Gomez-Guerrero C., Martin-Ventura J.L., Blanco-Colio L., Lavin B., Mallavia B., Tarin C., Mas S., Ortiz A. & Egido J. 2011. Animal models of cardiovascular diseases. J. Biomed. Biotechnol. 2011:497841.), and bacterial, viral and fungal infections (Pica et al. 2012Pica N., Chou Y. , Bouvier N.M. & Palese P. 2012. Transmission of Influenza B Viruses in the guinea pig. J. Virol. 86:4279-4287., Zhang et al. 2012Zhang Y., Lou X., Yang H., Guo X., Zhang X., He P. & Jiang X. 2012. Establishment of a leptospirosis mo del in guinea pigs using an epicutaneous inoculations route. BMC Infect. Dis. 12:20.).
Murine rodents (rats and mice) have a period of intrauterine development relatively short. Newborns are immature both somatic and behaviorally for some time after birth (Dobbing & Sands 1970Dobbing J. & Sands J. 1970. Growth and development of the brain and spinal cord of the guinea pig. Brain Res. 17:115-123., 1973)Dobbing J. & Sands J. 1973. Quantitative growth and development of human brain. Arch. Dis. Child. 48:757-767. and their brain grow considerably from birth to maturity. The guinea pig is considered exceptional due to its high degree of neurological maturity at birth when compared to other rodents (Draper 1920Draper R.L. 1920. The prenatal growth of the guinea pig. Anat. Rec. 18:369-392., Altman & Das 1967Altman J. & Das G.D. 1967. Postnatal neurogenesis in the Guinea pig. Nature 214:1098-1101., Hargaden & Singer 2012Hargaden M. & Singer L. 2012. Anatomy, Physiology and Behavior, p.575-602. In: Suckow M.A., Stevens K.A. & Wilson R.P. (Eds), The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents. Academic Press, Boston.).
In the guinea pig, cell proliferation events are complete at birth, unlike that seen in other rodents, where it persists for up to 1 month after birth (Lossi et al. 1997Lossi L., Marroni P. & Merighi P. 1997. Cell Proliferation in the Cerebellar Cortex of the Rabbit and Guinea Pig. XXIst Congress of the European Association of Veterinary Anatomists. Anat. Histol. Embryol. 26:257.). The postnatal cell recruitment may be related to factors such as gestational short period and immature cell in the cerebellum at birth (Tveden-Nyborg et al. 2012Tveden-Nyborg P. , Vogt L., Schjoldager J.G., Jeannet N., Hasselholt S., Paidi M.D., Christen S. & Lykkesfeldt J. 2012. Maternal Vitamin C Deficiency during Pregnancy Persistently Impairs Hippocampal Neurogenesis in Offspring of Guinea Pigs. PLoS One 7:e48488.).
Rodents have been widely used in studies of neurological disorders such as dystonia (Oleas et al. 2013Oleas J., Yokoi F., Deandrade M.P., Pisani A. & Li Y. 2013: Engineering animal models of dystonia. Mov. Disord. 28:990-1000.) and Alzheimer's disease (Epis et al. 2010Epis R., Gardoni F., Marcello E., Genazzani A., Canonico P.L. & Di Luca M. 2010. Searching for new animal models of Alzheimer's disease. Eur. J. Pharmacol. 626:57-63.). Accordingly, mouse models have played an important role in the mechanisms involving neurological diseases, especially in clinical studies related to development (Haramoto et al. 2008Haramoto M., Tatemoto H. & Muto N. 2008: Essential role of ascorbic acid in neural differentiation and development: High levels of ascorbic acid 2-glucoside effectively enhance nerve growth factor-induced neurite formation and elongation in PC12 cells. Glob. J. Health Sci. 54:43-49.). However, for diseases such as Alzheimer's, these same rodent models have been discarded, especially in some studies on the role of cholesterol in this disease (Ledesma & Dotti 2005Ledesma M.D. & Dotti C.G. 2005. The conflicting role of brain cholesterol in Alzheimer's disease: lessons from the brain plasminogen system. Biochem. Soc. Symp. 72:129-38.). In contrast, the guinea pig has been chosen as reference for the development of animal studies by having peptide sequences identical to human.
Due to an increasing use of the guinea pig as an experimental model and the absence of studies on organogenesis and development of the central nervous system in this species, it is necessary a better and greater knowledge of embryonic development of this system, contributing to studies regarding to drug testing and its short and long-term effects in the nerve tissue, malformations and anatomical and pathological changes, thus consolidating this species as an ideal model for neurological diseases. Herein, we described the development of components of the central nervous system in guinea pigs, from day 12 post conception until birth.
Materials and Methods
Animals and gross examination
Forty-one embryos and fetuses of Cavia porcellus at 12, 14, 18, 20, 22, 25, 30, 45, 50, 60 days post conception (dpc) and at term were used (Table 1). To obtain the animals, females were paired with males and vaginal cytology was performed by Panoptic-fast method (Laborclin®) to detect copulation, after which pregnant females were euthanized. For this, animals were pre-anesthetized with xylazine 2% (40mg/kg/IM) and ketamine hydrochloride 1% (60mg/kg/IM) and euthanized by the administration of thiopental sodium 2.5% (60mg/kg/IV).
Biometric data related to gestational age, weight and size (Crown-rump) of embryos and fetuses of Cavia porcellus
Embryos and fetuses were removed from the uterus and primarily examined macroscopically in order to examine their external characteristics. Age groups were determined based on animals' weight (g) and size (crown-rump, cm; Evans & Sack 1973Evans H.E. & Sack W.O. 1973. Prenatal development of domestic and laboratory mammals: growth curves, external features and selected references. Anat. Histol. Embryol. 2:11-45.), obtained by a digital scale (Marte® AL500) and a caliper (Pantec® 150mm), respectively. Animals were dissected and all organs composing the central nervous system were exposed and collected.
All procedures followed the guidelines of the Ethics Committee for the Use of Animals at the School of Veterinary Medicine and Animal Science at the University of São Paulo. Samples used were remnants of the study conducted by Vasconcelos et al. (2013Vasconcelos B.G., Favaron P.O., Miglino M.A. & Mess A. 2013. Development and morphology of the inverted yolk sac in the guinea pig (Cavia porcellus). Theriogenology 80:636-641.; Animal Bioethics Protocol No. 2521/2012).
Microscopy analysis
Samples for light microscopy were fixed in 10% paraformaldehyde. After fixation, they were rinsed in tap water, dehydrated in increasing ethanol solutions (70-100%), cleared in xylene and embedded in paraffin (Paraplast®). 6μm sections were obtained in an automatic microtome (Leica RM2155) and stained with Hematoxylin-eosin, Masson's Trichrome and Toluidine blue. Slides were analyzed using a photomicroscope (Nikon Eclipse E-800).
Samples for scanning electron microscopy were fixed in 2.5% glutaraldehyde solution and thereafter rinsed in distilled water under rotation (3x10min). Then, samples were dehydrated in increasing series of ethanol under rotation, [70% (1x15min), 80% (1x15min), 90% (1x15min) and 100% (3x30 min)], critical point dried (Balzers CPD 020) and subsequently mounted on aluminum metal bases (stub) using carbon glue and coated with gold by sputting. Samples were analyzed in a scanning electron microscope (LEO 435 VP).
Results
Gross anatomy
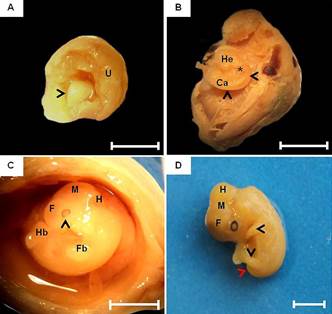
Due the undifferentiated stage of the tissues, in the early stage of development (12th dpc), it was not possible to observe any defined structure. On day 14, the embryo appeared as a mass of mesenchymal tissue, completely surrounded by extra-embryonic membranes in close contact with the uterine lumen (Fig.1A). On 18 dpc, after the membrane dissection, the first indication of body formation was noted, being able to distinguish cephalic and caudal regions and somites. At this stage, the embryo had a pronounced cervical curvature (Fig.1B).
Macroscopic characteristics of embryonic development of the guinea pig. (A) Embryo at 14 days post conception (dpc), comprising a mass of mesenchymal tissue (arrowhead). Note the uterine wall (U). Bar: 0.5cm. (B) Embryo at 18 dpc. Cervical curvature (arrowheads). Head (He), somites (*) and caudal region (Ca). Bar: 0.5cm. (C) Embryo at 20 dpc. Rudimentary optic vesicle with slight pigmentation of the retina (arrowhead), early differentiation of primary brain vesicles: forebrain (F), midbrain (M) and hindbrain (H). Forelimb (Fb) and hindlimbs (Hb) buds. Bar: 1cm. (D) After 22 days, notice the development and growth of the body of the embryo, forelimbs and hindlimbs (arrowheads) and tail (arrow) and brain vesicle: forebrain (F), midbrain (M) and hindbrain (H). Bar: 1cm.
By day 20, rudimentary optical vesicles were identified in the cephalic region with a slight retinal pigmentation and the beginning of the primitive brain vesicles differentiation (forebrain, midbrain and hindbrain) (Fig.1C). In addition, the brain had an irregular shape and it was not possible to observed nor the division into two hemispheres or even the structures of the brainstem. Still, there is no cerebral and cerebellar structures division by the transverse fissure. Between the 22nd and 25 dpc, there is a significant development of the embryo, especially in relation to the increased size of the head and body and development of the forelimbs and hindlimbs (Fig.1D).
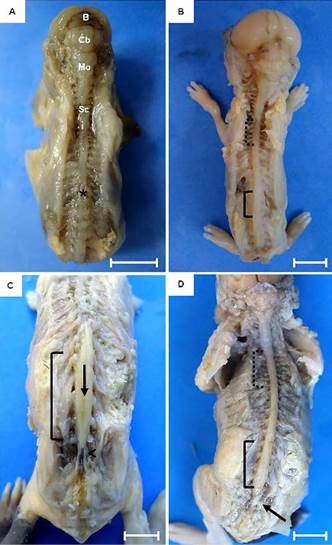
Although the brain can be evidenced in fetus with 30 days of gestation, it was still undifferentiated. The nervous tissue without macroscopic differentiation of cerebral sulci and gyri, was observed, as well as the white and gray matter. At this stage, brain and brainstem were still present as an aggregate of nervous tissue without macroscopic differences between structures. The spinal cord was present throughout the spinal canal without thickenings (intumescentiae) and the cauda equina was absent (Fig.2A). At 45th dpc, the onset of spinal cord after the medulla oblongata was observed, extending to the sacrococcygeal articulation. Cervical and lumbar intumescences were noted only as dilations due to the increase of nervous tissue, not being evident either the median sulcus of the dorsal spinal cord or the cauda equina (Fig.2B). Next to the fore and hindlimbs, dilatations of the spinal cord were noted, forming the cervical and lumbar intumescences only in 50 days' fetuses, in which the cauda equina and dural sac were evident (Fig.2C and 2D). In animals at term the medulla oblongata was well developed and it was possible to observe a defined dorsal median grove of the spinal cord and the cauda equina in its most caudal portion.
Fetal development of the central nervous system of guinea pigs. (A) Fetus of 30 days post conception (dpc). Dorsal view of the brain (B), cerebellum (Cb), medulla oblongata (Mo) and spinal cord (Sc). Note the absence of dilatation or intumescences (*). (B) Fetus of 45 dpc. Notice a mild dilatation of the spinal cord in the cervical (dotted line) and lumbar (solid line) regions. (C,D). Fetus at 60 dpc. Dorsal median grove of the spinal cord (arrow) and cauda equina (arrow head). Bars: 1cm.
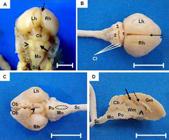
After 45 dpc the brain was fully developed and the division of left and right cerebral hemispheres could be visualized, marked by the dorsal median groove. Cerebellar hemispheres had cerebellar leaves, easily visualized and separated by fissures; and a cerebellar vermis, more developed at this stage, with a clearly observed medulla oblongata (Fig.3A and 3B). Ventrally, it was possible to identify the olfactory bulb, pons, pyramids and pituitary infundibulum (Fig.3C). In sagittal median section, telencephalic and diencephalic structures were observed, with distinction between white and gray matter, ventricle, corpus callosum and interthalamic adherence (Fig.3D).
Central nervous system of guinea pigs at 45 days post conception. (A,B) Dorsal view of the brain. (A) Left (Lh) and right (Rh) cerebral hemispheres, after removal of the dura matter. Observe the absence of sulci and gyri. Dorsal median groove (dotted arrow), cerebellum (Cb) with cerebellar hemispheres (arrowhead) and vermis (solid arrow), medulla oblongata (Mo) and spinal cord (Sc). (B) Left (Lh) and right (Rh) cerebral hemispheres. Cerebellar leaves (Cl) in evidence, rostral lobe (1), vermis (2), and cerebellar hemisphere (3). (C) Ventral view. Left (Lh) and right (Rh) cerebral hemispheres. Olfactory bulb (Ob), pons (Po), pyramids (highlighted), pituitary infundibulum (arrowhead - the pituitary gland was removed). Medulla oblongata (Mo) and Spinal cord (Sc). (D) Sagittal view. Gray matter (Gm) and white matter (Wm), third ventricle (solid arrow), corpus callosum (dotted arrow) and interthalamic adherence (arrowhead). Cerebellum (Cb), pons (Po), medulla oblongata (Mo) and spinal cord (Sc). Bars: In A = 0.5cm and B-D =1cm.
There were no significant structural differences at 50 and 60 dpc. During this phase there was only an increase in the size of anatomical structures due to the early fetal development and the onset of appendices to the cornea and outer covering of the body (fur). At this stage the sulci and gyri were not yet present in cerebral hemispheres (Fig.3A).
Microscopy
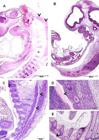
Histologically, undifferentiated brain tissue was observed as a mass of mesenchymal tissue between the 18 and 20 dpc (Fig.4A and 4B). Neural tube closure was observed between 14 and 18 days post conception.
Photomicrography of guinea pig embryos, focusing on the development of the central nervous system. (A,B) Embryos at 18 and 20 days post conception (dpc), respectively (same sagittal level). Undifferentiated brain tissue, presented as a mass of mesenchymal tissue (highlighted). Vertebral bodies in formation (*) and developing spinal cord (arrowheads). (C) Embryo at 22 dpc. Note the filled medullary canal (arrowheads). (D,E) Embryo at 25 dpc. Vertebral discs formed by hyaline cartilage (*), bone marrow filling the entire spinal canal (arrowhead). (E) Development of pelvic bones (*) highlighted. Hematoxylin-eosin.
On 22 dpc the primordium of the spinal cord was observed, with a spinal canal well defined and populated by cells (Fig.4C). Vertebral bodies were in formation (Fig.4A and 4B). After 22 days, despite the ossification of the vertebral bodies, these were still presented as a diffuse tissue, since fetal chondroblasts were dispersed and the tissue had not yet consolidated (Fig.4C). At 25 dpc, the tissue within the medullary canal had its density higher than in the previous stage (Fig.4D and 4E). At this stage it was already possible to observe the formation of pelvic bones (Fig.4E).
At 30 dpc the brain tissue was differentiated and it was already possible to observe the meninges. The dura mater (outermost layer) was composed of a thick layer of dense connective tissue fibers, followed the arachnoid, a more delicate connective tissue. The pia mater (innermost layer) was equally delicate and adheres directly to the surfaces of the brain and spinal cord.
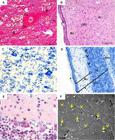
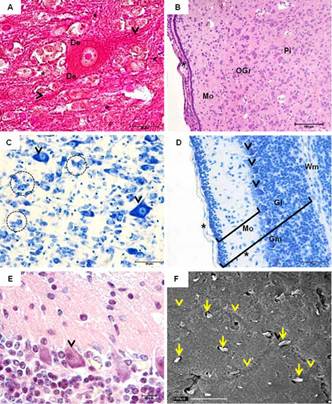
At 45th dpc, tissues were more differentiated. The white matter was predominant in the most central parts of the brain. White matter was devoid of neuronal cell bodies and consisted primarily of myelinated axons, which impart a white color to this region of the CNS. The gray matter (GM). It consisted of neurons, their dendrites, and the supportive cells called neuroglia, nonneuronal cells surrounding neurons and their axons and dendrites (Fig.5A). The size, shape, and mode of branching of these neurons are highly variable according to the CNS region examined.
Central nervous system of guinea pigs fetuses at 45 (A-D) and 50 (E,F) days post conception. (A) Neuron with evident nucleus and nucleolus. Dendrites (De) and axon (arrowheads) entering the neuroglia (*). Masson's Trichrome. (B) Pia mater (arrowhead) followed by the Molecular layer (Mo), Outer granular layer (OGr) and Pyramidal cell layer (Pi). Hematoxylin-Eosin (HE). (C) Glia cells (highlighted) and pyramidal cells (indicated by arrowheads). Toluidine blue (TB). (D) Cerebellum - Gray matter (Gm) and white (Wm). Note the molecular layer (Mo), Purkinje cell layer (arrowheads), granular layer (Gl) and pia mater (*). TB. (F) Purkinje cells with evident dendrites (arrowhead). HE. (A) Scanning electron microscopy of the brain. Absence of grooves, with a slight impression of gyri (arrows). Nerve bundles (arrowheads).
At this stage, both cerebral and cerebellar stratums were observed. The cerebral cortex was divided into six layers, which were classified according to quantity, size and shape of neurons, with one or more cell types predominant in each layer. The pia mater covered the most superficial layer, the molecular layer (1st layer), which has its peripheral portion composed predominantly of neuroglia. The external (outer) granular layer (2nd) contained mainly different types of neuroglial cells and small pyramidal cells. In the external pyramidal layer (3rd), medium-sized pyramidal cells predominated. The internal granular layer (4th) is a thin layer and contains mainly small granule cells. The internal pyramidal layer (5th) contained numerous neuroglial cells and the larger pyramidal cells. The multiform layer was the last and deepest, being adjacent to the white matter of the cerebral cortex, containing cells of varying shapes and sizes (Fig.5B and 5C).
Moreover, the cerebellar cortex had only 3 layers, particularly distinct from each other: the molecular layer, outer and basically composed of neuroglia; a middle layer characterized by the presence of piriform neurons called Purkinje cells; and the innermost layer namely granular layer, formed by small neurons compactly organized (Fig.5D). Purkinje cells were easily identified from day 50, having large size and well developed dendrites resembling roots entering the molecular layer, causing it to have more sparse cells (Fig.5E). Animals presented a lissencephalic brain at birth (Fig.5F).
In the spinal cord, the white and gray matter had an inverse location to that observed in the brain and cerebellum. The white matter, outer, along with the gray matter, inner, were arranged in an "H" shape (Fig.6A), which had an orifice, i.e. central canal of the spinal cord, coated by ependymal cells (Fig.6B). In early stages of development, this canal represented the lumen of the embryonic neural tube. This "H" region was divided into dorsal and ventral horn or columns (Fig.6A).
Spinal cord of guinea pigs fetuses at 60 days of development. (A) Lumbar intumescence. White and Grey matter (Wm and Gm, respectively). Central canal or central commissure of the medullary H (arrowhead), ventral horn (Vh) and dorsal horn (Dh). Toluidine blue. (B) Cervical spinal cord. High magnification of the medullary H central canal (*) lined by ependymal cells. HE.
Discussion
The nervous system is extensively studied by morphologists for being the most complex system of the body, although conservative in terms of changes. Comparative and developmental studies enable researchers to build phylogeny sketches of this system. An increasing knowledge about the macroscopic, microscopic and ultrastructure of the system provides a better contribution in applied sciences since the nervous system neurogenesis is a very active field of research (Hildebrand 2006Hildebrand M. & Goslow Jr. G.E. 2006. Análise da estrutura dos vertebrados. Atheneu, São Paulo. 637p., Ming & Song 2011Ming G.L. & Song H. 2011. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70:687-702., Konefal et al. 2013Konefal S., Elliot M. & Crespi B. 2013. The adaptive significance of adult neurogenesis: an integrative approach. Front Neuroanat. 7:21.).
The evolution of the nervous system can be explained by Romero (2000)Romero S.M.B. 2000. Fundamentos de Neurofisiologia Comparada. Holos, Ribeirão Preto, 170p., who made a broad analogy of the brain and spinal cord evolutionary process, which corroborate with the findings in this study for the guinea pig. Hargaden & Singer (2012)Hargaden M. & Singer L. 2012. Anatomy, Physiology and Behavior, p.575-602. In: Suckow M.A., Stevens K.A. & Wilson R.P. (Eds), The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents. Academic Press, Boston. observed a basic organization of the spinal cord, with a degree of complexity similar to other mammals, having dorsolateral and ventrolateral grooves with the dorsal and ventral roots of spinal nerves joining the spinal cord along these grooves.
The development of the nervous system is characterized by changes usually considered as evidence of plasticity. Oda et al. (2002)Oda J.Y., Goncales-Sant'ana D.M. & Carvalho J. 2002. Plasticidade neural e regeneração funcional do sistema nervoso: contribuição ao estudo de revisão. Arq. Cienc. Unipar 6:171-176. state that during embryogenesis, an excessive number of neurons is generated, and therefore a portion of them dies by a mechanism of programmed cell death - apoptosis, resulting in a fine-adjustment of neuronal population. This may explain the absence of differentiated nerve tissue until day 30 of gestation, when the neuronal population is evident in this study.
The nervous system has its origin in the neural plate (ectoderm) of the embryonic disc. The last parts of the neural tube that remain open are anterior and posterior neuropores. The neural tube closure occurs around 10.5 to 11.0 days of development in rats, between 9.0 to 9.5 days in mice, 9.5 to 10.5 days in rabbits, 8.25 to 8.5 days in hamsters and about 14.5 days of gestation in Oligoryzomys (Favaron et al. 2012Favaron P.O., Rodrigues M.N., Oliveira M.F., Biasi C.M. & Miglino M.A. 2012. Embryonic and Fetal Development in - Pigmy Rice Rat Oligoryzomys sp. (Rodentia, Sigmodontinae) and its Significance for Being a new Experimental Model. Anat. Histol. Embryol. 41:286-299.). According to Monie (1976)Monie I.W. 1976. Comparative development of the nervous, respiratory, and cardiovascular systems. Environ. Health Perspect. 18:55-60. in the guinea pig the neural tube closure occurs at a more advanced gestational period when compared to these other species, around 15.25 to 16.5 days, similar to our study, where this closure was observed between 14 and 18 days post conception.
Macroscopically, during the initial embryonic development of the guinea pig, the cerebral structures were not visualized up to 35 days post conception, stage of development that refers to approximately half of the gestational period. Due to this fact, we may infer that, in terms of central nervous system, this species has a late embryonic development, since these structures were observed in their full development, both macroscopically and microscopically, only after 50 days of gestation.
These findings corroborate those found by Dobbing & Sands (1970Dobbing J. & Sands J. 1970. Growth and development of the brain and spinal cord of the guinea pig. Brain Res. 17:115-123., 1973)Dobbing J. & Sands J. 1973. Quantitative growth and development of human brain. Arch. Dis. Child. 48:757-767., who reported these characteristics to murine models (rats and mice), i.e., they claim that such models are immature at birth and their CNS develop exponentially after birth. Hargaden & Singer (2012)Hargaden M. & Singer L. 2012. Anatomy, Physiology and Behavior, p.575-602. In: Suckow M.A., Stevens K.A. & Wilson R.P. (Eds), The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents. Academic Press, Boston., affirm that the guinea pig has a high degree of neurological maturity at birth, where its brain is well developed by day 30 and its cerebral hemispheres is completely divided by day 45.
From day 45 post conception the presence of several neuronal bodies was observed during the microscopy analysis. However, the neuronal regulation cited by authors above could not be evidenced since our study was based only on morphological analysis during the fetal period. In addition to this information, Tapia (1997)Tapia R. 1997. Mecanismos celulares y moleculares de la neurodegeneracion. Gac. Med. Mex. 134:685-703., Amarante-Mendes & Green (1999)Amarante-Mendes G.P. & Green D.R. 1999: The regulation of apoptotic cell death. Braz. J. Med. Biol. Res. 32:1053-1061. and Ferrari et al. (2001)Ferrari E.A.M., Toyoda M.S.S., Faleiros L. & Cerutti S.M. 2001. Plasticidade Neural: relações com o comportamento e abordagens experimentais. Psic. Teor. Pesq. 17:187-194. reported that for mammals in general, there is a regulation on the number of neurons in the CNS after birth.
Microscopically, undifferentiated brain tissue is observed as a mass of mesenchymal tissue between the 18 and 20 dpc. After this period, the primordium of the spinal cord can be observed, with a spinal canal well defined and populated by cells. At 30 dpc the brain tissue was differentiated, and from 45 dpc the difference between the white and gray matter was possible to be observed. The CNS structure development after the middle third of gestation is similar to the observed by Dobbing & Sands (1970Dobbing J. & Sands J. 1970. Growth and development of the brain and spinal cord of the guinea pig. Brain Res. 17:115-123., 1973)Dobbing J. & Sands J. 1973. Quantitative growth and development of human brain. Arch. Dis. Child. 48:757-767. in rats and mice, but different to that reported by Hargaden & Singer (2012)Hargaden M. & Singer L. 2012. Anatomy, Physiology and Behavior, p.575-602. In: Suckow M.A., Stevens K.A. & Wilson R.P. (Eds), The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents. Academic Press, Boston., who affirm that the brain is well developed by day 30.
The interest in using guinea pigs as experimental models in neurological diseases studies is mainly due to their high degree of maturity at birth, verified in our study by the high level of neuronal differentiation from day 45 post conception. Van Kan et al. (2009)Van Kan C.M., De Vries J.I.P., Lüchinger A.B., Mulder E.J.H. & Taverne M.A.M. 2009. Ontogeny of fetal movements in the guinea pig. Physiol. Behav. 98:338-344. evaluated the integrity and development of the nervous system in young animals through the evaluation of fetal movement patterns and found a striking similarity in patterns of humans and guinea pigs, suggesting this animal as a promising model for evaluating the effects of physical, chemical and biological external agents in the nervous system during fetal development.
However, the knowledge about this species embryonic development is still considered scarce. The guinea pig is a species considered premature when compared to other rodents once its brain is in a more advanced stage of development when compared to human being even these being lissencephalic at birth, as seen in our study. However, due to the similarities observed in placental structure (Vasconcelos et al. 2013Vasconcelos B.G., Favaron P.O., Miglino M.A. & Mess A. 2013. Development and morphology of the inverted yolk sac in the guinea pig (Cavia porcellus). Theriogenology 80:636-641.) in comparison to the human placentation, this animal is an excellent model to understand both role and effects of hormonal and environmental factors during the development of the central nervous system in the uterine environment.
Murine rodents (rats and mice) have a period of intrauterine development relatively short and newborns are immature, both somatic and behaviorally for some time after birth. Dobbing & Sands (1970Dobbing J. & Sands J. 1970. Growth and development of the brain and spinal cord of the guinea pig. Brain Res. 17:115-123., 1973)Dobbing J. & Sands J. 1973. Quantitative growth and development of human brain. Arch. Dis. Child. 48:757-767. claim that murine models are immature at birth and their CNS develop exponentially after birth. In contrast, the guinea pig has a high degree of neurological maturity at birth (Hargaden & Singer 2012Hargaden M. & Singer L. 2012. Anatomy, Physiology and Behavior, p.575-602. In: Suckow M.A., Stevens K.A. & Wilson R.P. (Eds), The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents. Academic Press, Boston.), which is corroborated by our study, where its cerebral hemispheres is completely divided by day 30 and its brain structures as well defined by day 45. This makes them excellent experimental models to direct neurological studies, for diseases such as dystonia (Oleas et al. 2013Oleas J., Yokoi F., Deandrade M.P., Pisani A. & Li Y. 2013: Engineering animal models of dystonia. Mov. Disord. 28:990-1000.) and Alzheimer's (Epis et al. 2010Epis R., Gardoni F., Marcello E., Genazzani A., Canonico P.L. & Di Luca M. 2010. Searching for new animal models of Alzheimer's disease. Eur. J. Pharmacol. 626:57-63.), or indirect, such as nutritional studies on the role of vitamin C during pregnancy (Tveden-Nyborg et al. 2012Tveden-Nyborg P. , Vogt L., Schjoldager J.G., Jeannet N., Hasselholt S., Paidi M.D., Christen S. & Lykkesfeldt J. 2012. Maternal Vitamin C Deficiency during Pregnancy Persistently Impairs Hippocampal Neurogenesis in Offspring of Guinea Pigs. PLoS One 7:e48488.).
Animal models of dystonia may be classified as phenotypic, which mimic signals seen in human patients, or genotyping, which simulate gene mutations obtained by directed mutagenesis or transgenic techniques insertion. Oleas et al. (2013)Oleas J., Yokoi F., Deandrade M.P., Pisani A. & Li Y. 2013: Engineering animal models of dystonia. Mov. Disord. 28:990-1000. suggest the use of this rodent in pathophysiology studies of the disease and suggest new therapeutic since the guinea pig neuronal cell proliferation is complete at birth (Lossi et al. 2002Lossi L., Coli A., Giannessi E., Stornelli M.R. & Marroni P. 2002. Cell proliferation and apoptosis during histogenesis of the guinea pig and rabbit cerebellar cortex. Ital. J. Anat. Embryol. 107:117-125.). Our findings suggest that the absence of nervous tissue differentiation until day 25 post conception makes the guinea pig an important model for molecular studies regarding the brain and spinal cord development mechanisms.
Studies have shown that substances such as vitamin C are essential in the regulation of neuronal differentiation and maturation (Norkus & Rosso 1981Norkus E.P. & Rosso P. 1981. Effects of maternal intake of ascorbic acid on the postnatal metabolism of this vitamin in the guinea pig. J. Nutr. 111:624-630., Lykkesfeldt & Moos 2005Lykkesfeldt J. & Moos T. 2005. Age-dependent change in Vitamin C status: a phenomenon of maturation rather than of ageing. Mech. Ageing Dev. 126:892-898., Qiu et al. 2007Qiu S., Li L., Weeber E.J. & May J.M. 2007. Ascorbate transport by primary cultured neurons and its role in neuronal function and protection against excitotoxicity. J. Neurosci. Res. 85:1046-1056.) and its efficiency during pregnancy implies a negative impact on fetal development (Tveden-Nyborg et al. 2009Tveden-Nyborg P. & Lykkesfeldt J. 2009. Does vitamin C deficiency result in impaired brain development in infants? Redox Rep. 14:2-6., 2012Tveden-Nyborg P. , Vogt L., Schjoldager J.G., Jeannet N., Hasselholt S., Paidi M.D., Christen S. & Lykkesfeldt J. 2012. Maternal Vitamin C Deficiency during Pregnancy Persistently Impairs Hippocampal Neurogenesis in Offspring of Guinea Pigs. PLoS One 7:e48488.). Once we showed that in the first third of pregnancy the spinal cord and peripheral nervous system segments are being developed, the ingestion of this vitamin should be monitored during this period in order to demonstrate its impact during the development.
Our study verified that the medullary canal was fully populated by cells at day 30. In the study of Alzheimer's disease, currently an incurable degenerative disease, the guinea pig overlaps in terms of degree of relevance to other murine models by being a non-transgenic animal. This fact enables a more detailed study of the disease, particularly regarding to cholesterol, a substance proven important in the production of a protein which destroys neurons. This feature also allows the use of this rodent for evaluation of traumatic injuries to the spinal cord (Ouyang et al. 2008Ouyang H., Galle B., Li J., Nauman E. & Shi R. 2008. Biomechanics of spinal cord injury: a multimodal investigation using ex vivo guinea pig spinal cord white matter. J. Neurotrauma 25:19-29., Sun et al. 2012Sun W., Fu Y., Shi Y., Cheng J.K., Cao P. & Shi R. 2012. Paranodal myelin damage after acute stretch in Guinea pig spinal cord. J. Neurotrauma 29:611-619.) and development of effective therapeutic and clinical interventions in pathologies associated with acute spinal cord injuries.
The morphological study of the central nervous system in guinea pig can provide important information for neurological studies in humans, since this animal has a high degree of neurological maturity in relation to its short gestation period, resembling the human and serving as an excellent experimental model in neurological studies.
Acknowledgements
Our researchers greatly appreciates the grant provided by FAPEMA (Process Fapema BD 00190/11). We appreciate the cooperation of the staff from Mantenedor da Fauna Silvestre Marinovic- Gonçalves/MG, Brazil, which provided the animals used in this study and the contribution of researchers Claudia Marinovic de Oliveira and Bruno Gomes Vasconcelos.
References
- Altman J. & Das G.D. 1967. Postnatal neurogenesis in the Guinea pig Nature 214:1098-1101.
- Amarante-Mendes G.P. & Green D.R. 1999: The regulation of apoptotic cell death. Braz. J. Med. Biol. Res. 32:1053-1061.
- Canning B.J. & Chou Y. 2008. Using guinea pigs in studies relevant to asthma and COPD. Pulm. Pharmacol. Ther. 21:702-720.
- Dobbing J. & Sands J. 1970. Growth and development of the brain and spinal cord of the guinea pig. Brain Res. 17:115-123.
- Dobbing J. & Sands J. 1973. Quantitative growth and development of human brain. Arch. Dis. Child. 48:757-767.
- Draper R.L. 1920. The prenatal growth of the guinea pig. Anat. Rec. 18:369-392.
- Dumitrascu R.J., Heitmann W., Seeger W., Weissmann N. & Schulz R. 2013. Obstructive sleep apnea, oxidative stress and cardiovascular disease: lessons from animal studies. Oxid. Med. Cell. Longev. 2013:234631.
- Endersby J. 2009. A Guinea Pig's History of Biology. Harvard University Press, Cambridge. 544p.
- Epis R., Gardoni F., Marcello E., Genazzani A., Canonico P.L. & Di Luca M. 2010. Searching for new animal models of Alzheimer's disease. Eur. J. Pharmacol. 626:57-63.
- Evans H.E. & Sack W.O. 1973. Prenatal development of domestic and laboratory mammals: growth curves, external features and selected references. Anat. Histol. Embryol. 2:11-45.
- Favaron P.O., Rodrigues M.N., Oliveira M.F., Biasi C.M. & Miglino M.A. 2012. Embryonic and Fetal Development in - Pigmy Rice Rat Oligoryzomys sp. (Rodentia, Sigmodontinae) and its Significance for Being a new Experimental Model. Anat. Histol. Embryol. 41:286-299.
- Ferrari E.A.M., Toyoda M.S.S., Faleiros L. & Cerutti S.M. 2001. Plasticidade Neural: relações com o comportamento e abordagens experimentais. Psic. Teor. Pesq. 17:187-194.
- Haramoto M., Tatemoto H. & Muto N. 2008: Essential role of ascorbic acid in neural differentiation and development: High levels of ascorbic acid 2-glucoside effectively enhance nerve growth factor-induced neurite formation and elongation in PC12 cells. Glob. J. Health Sci. 54:43-49.
- Hargaden M. & Singer L. 2012. Anatomy, Physiology and Behavior, p.575-602. In: Suckow M.A., Stevens K.A. & Wilson R.P. (Eds), The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents. Academic Press, Boston.
- Hildebrand M. & Goslow Jr. G.E. 2006. Análise da estrutura dos vertebrados. Atheneu, São Paulo. 637p.
- Konefal S., Elliot M. & Crespi B. 2013. The adaptive significance of adult neurogenesis: an integrative approach. Front Neuroanat. 7:21.
- Ledesma M.D. & Dotti C.G. 2005. The conflicting role of brain cholesterol in Alzheimer's disease: lessons from the brain plasminogen system. Biochem. Soc. Symp. 72:129-38.
- Lossi L., Marroni P. & Merighi P. 1997. Cell Proliferation in the Cerebellar Cortex of the Rabbit and Guinea Pig. XXIst Congress of the European Association of Veterinary Anatomists. Anat. Histol. Embryol. 26:257.
- Lossi L., Coli A., Giannessi E., Stornelli M.R. & Marroni P. 2002. Cell proliferation and apoptosis during histogenesis of the guinea pig and rabbit cerebellar cortex. Ital. J. Anat. Embryol. 107:117-125.
- Lykkesfeldt J. & Moos T. 2005. Age-dependent change in Vitamin C status: a phenomenon of maturation rather than of ageing. Mech. Ageing Dev. 126:892-898.
- Mess A. 2007. The guinea pig placenta: model of placental growth dynamics. Placenta 28:812-815.
- Miglino M.A., Carter A.M., Ambrósio C.E., Bonatelli M., De Oliveira M.F., Dos Santos F., Rodrigues R.F. & Santos T.C. 2004. Vascular organization of the histricomorph placenta: a comparative study in the agouti, capybara, guinea pig, paca and rock cavy. Placenta 25:438-448.
- Ming G.L. & Song H. 2011. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70:687-702.
- Monie I.W. 1976. Comparative development of the nervous, respiratory, and cardiovascular systems. Environ. Health Perspect. 18:55-60.
- Norkus E.P. & Rosso P. 1981. Effects of maternal intake of ascorbic acid on the postnatal metabolism of this vitamin in the guinea pig. J. Nutr. 111:624-630.
- Oda J.Y., Goncales-Sant'ana D.M. & Carvalho J. 2002. Plasticidade neural e regeneração funcional do sistema nervoso: contribuição ao estudo de revisão. Arq. Cienc. Unipar 6:171-176.
- Oleas J., Yokoi F., Deandrade M.P., Pisani A. & Li Y. 2013: Engineering animal models of dystonia. Mov. Disord. 28:990-1000.
- Oliveira M.F., Do Vale A.M., Favaron P.O., Vasconcelos B.G., De Oliveira B.G., Miglino M.A. & Mess A. 2012. Development of yolk sac inversion in Galea spixii and Cavia porcellus (Rodentia, Caviidae). Placenta 33:878-881.
- Ouyang H., Galle B., Li J., Nauman E. & Shi R. 2008. Biomechanics of spinal cord injury: a multimodal investigation using ex vivo guinea pig spinal cord white matter. J. Neurotrauma 25:19-29.
- Pica N., Chou Y. , Bouvier N.M. & Palese P. 2012. Transmission of Influenza B Viruses in the guinea pig. J. Virol. 86:4279-4287.
- Qiu S., Li L., Weeber E.J. & May J.M. 2007. Ascorbate transport by primary cultured neurons and its role in neuronal function and protection against excitotoxicity. J. Neurosci. Res. 85:1046-1056.
- Romero S.M.B. 2000. Fundamentos de Neurofisiologia Comparada. Holos, Ribeirão Preto, 170p.
- Skerry C., Pokkali S., Pinn M., Be N.A., Harper J., Karakousis P.C. & Jain S.K. 2013. Vaccination with recombinant Mycobacterium tuberculosis PknD attenuates bacterial dissemination to the brain in guinea pigs. Plos One 8:e66310.
- Sun W., Fu Y., Shi Y., Cheng J.K., Cao P. & Shi R. 2012. Paranodal myelin damage after acute stretch in Guinea pig spinal cord. J. Neurotrauma 29:611-619.
- Taylor D.K. & Lee V.K. 2012. Guinea pigs as experimental models, p.705-744. In: Suckow M.A., Stevens K.A. & Wilson R.P. (Eds), The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents. Academic Press, Boston .
- Tapia R. 1997. Mecanismos celulares y moleculares de la neurodegeneracion. Gac. Med. Mex. 134:685-703.
- Tveden-Nyborg P. & Lykkesfeldt J. 2009. Does vitamin C deficiency result in impaired brain development in infants? Redox Rep. 14:2-6.
- Tveden-Nyborg P. , Vogt L., Schjoldager J.G., Jeannet N., Hasselholt S., Paidi M.D., Christen S. & Lykkesfeldt J. 2012. Maternal Vitamin C Deficiency during Pregnancy Persistently Impairs Hippocampal Neurogenesis in Offspring of Guinea Pigs. PLoS One 7:e48488.
- Van Kan C.M., De Vries J.I.P., Lüchinger A.B., Mulder E.J.H. & Taverne M.A.M. 2009. Ontogeny of fetal movements in the guinea pig. Physiol. Behav. 98:338-344.
- Vasconcelos B.G., Favaron P.O., Miglino M.A. & Mess A. 2013. Development and morphology of the inverted yolk sac in the guinea pig (Cavia porcellus). Theriogenology 80:636-641.
- Zaragoza C., Gomez-Guerrero C., Martin-Ventura J.L., Blanco-Colio L., Lavin B., Mallavia B., Tarin C., Mas S., Ortiz A. & Egido J. 2011. Animal models of cardiovascular diseases. J. Biomed. Biotechnol. 2011:497841.
- Zhang Y., Lou X., Yang H., Guo X., Zhang X., He P. & Jiang X. 2012. Establishment of a leptospirosis mo del in guinea pigs using an epicutaneous inoculations route. BMC Infect. Dis. 12:20.
Publication Dates
-
Publication in this collection
Aug 2016
History
-
Received
06 May 2015 -
Accepted
18 Jan 2016