Abstract:
The aim was to study the effects of different gamete coincubation times on porcine in vitro fertilization (IVF), and to verify whether efficiency could be improved by reducing oocyte exposure time to spermatozoa during IVF. In groups of 50, a total of 508 immature cumulus-oocyte complexes (COCs) were matured in NCSU-37 medium. The COCs were cultured for 44 hours and then inseminated with in natura semen (2,000 spermatozoa/oocyte). The sperm and oocytes were coincubated according to the following treatments (T): T1 = oocytes exposed to spermatozoa for one hour (173 oocytes), T2 = oocytes exposed to spermatozoa for two hours (170 oocytes), and T3 = oocytes exposed to spermatozoa for three hours (165 oocytes). After these coincubation periods, the oocytes were washed in fertilization medium (TALP medium) to remove spermatozoa not bound to the zona pellucida and cultured in another similar medium (containing no sperm). Eighteen to twenty hours after fertilization, the putative zygotes were stained in Hoechst-33342 to evaluate the IVF results. The penetration rate was higher (P<0.05) after two hours of coincubation time than it was for one or three hours. Furthermore, 68.60% of the ova coincubated with the spermatozoa for two hours were monospermic. The oocytes exposed to spermatozoa for one hour (T1) presented a higher (P<0.01) rate of polyspermy than those in T2 and T3. Fertilization performance (%) did not differ (P>0.05) between oocytes exposed to spermatozoa for one (T1) and three hours (T3). However, optimum (P=0.048) results were obtained after two hours of coincubation, when the rate of fertilization performance was 50.16±8.52%. The number of penetrated sperm per oocyte, as well as male pronucleus formation, did not differ (P>0.05) between the treatments evaluated. Under these assay conditions, especially in relation to the sperm concentration used, gamete coincubation for a period of two hours appears to be optimal for monospermy and fertilization performance. Thus, it is the optimal time period for obtaining a large number of pig embryos capable of normal development.
Index Terms:
Coincubation time; fertilization performance; in vitro fertilization; oocyte, pig
Resumo:
Esse estudo foi realizado para avaliar os efeitos de diferentes tempos de coincubação dos gametas sobre a fertilização in vitro (FIV) de suínos e se a eficiência dessa técnica poderia ser melhorada pela redução no período que os ovócitos são expostos aos espermatozoides durante a FIV. Um total de 508 (em grupos de 50) complexos cumulus-ovócito (COCs) imaturos foram maturados no meio NCSU-37. Os COCs foram cultivados por 40-44 horas e então inseminados com sêmen in natura (2.000 espermatozoides/ovócito). Os espermatozoides e ovócitos foram coincubados de acordo com os seguintes tratamentos (T): T1 = ovócitos expostos aos espermatozoides por uma hora (173 ovócitos); T2 = ovócitos expostos aos espermatozoides por duas horas (170 ovócitos) e T3 = ovócitos expostos aos espermatozoides por três horas (165 ovócitos). Após esses períodos de coincubação, os ovócitos foram lavados em meio de fertilização (meio TALP) para remoção dos espermazoides não ligados a zona pelúcida e cultivados em outro mesmo meio (não contendo espermatozoides). Após 18-20 horas de fertilização, os prováveis zigotos foram corados com Hoechst-33342 para avaliação dos resultados da FIV. A taxa de penetração foi maior (P<0,05) após o tempo de coincubação de duas horas em comparação a uma e três horas. Além disso, 68,60% dos ovócitos coincubados com os espermatozoides por duas horas foram monospérmicos. Os ovócitos expostos aos espermatozoides por uma hora (T1) apresentaram elevada (P<0,01) taxa de polispermia em comparação com o T2 e T3. A eficiência da fertilização (%) não diferiu (P>0,05) entre os ovócitos expostos aos espermatozoides por uma (T1) e três horas (T3). Entretanto, ótimos (P=0,048) resultados foram obtidos após duas horas de coincubação, quando a taxa da eficiência da fertilização foi 50,16 ± 8,52%. O número de espermatozoides penetrados por ovócito e a formação de pro-núcleo masculino não diferiu (P>0,05) entre os tratamentos avaliados. Sob as condições de ensaio realizadas, especialmente em relação à concentração espermática utilizada, a coincubação dos gametas por um período de duas horas parece ser ótima para as taxas de monospermia e eficiência da fertilização. Portanto, um tempo provavelmente ótimo para obter um elevado número de embriões suínos capazes de ter um desenvolvimento normal.
Termos de Indexação:
Eficiência da fertilização; fertilização in vitro; ovócito; suíno; tempo de coincubação
Introduction
For many years, studies on in vitro fertilization (IVF) in pigs have been conducted as an important tool for reproduction (Cheng et al. 1986Cheng W.T.K., Polge C. & Moor R.M. 1986. In vitro fertilization of pig and sheep oocytes. Theriogenology 25:146., Mattioli et al. 1989Mattioli M., Bacci M.L., Galeati G. & Seren E. 1989. Developmental competence of pig oocytes matured and fertilized in vitro. Theriogenology 31:1201-1207., Zhang et al. 2012Zhang W., Yi K., Yan H. & Zhou X. 2012. Advances on in vitro production and cryopreservation of porcine embryos. Anim. Reprod. Sci. 132:115-122., Ballester et al. 2014Ballester L., Romero-Aguirregomezcorta J., Soriano-Úbeda C., Matás C., Romar R. & Coy P. 2014. Timing of oviductal fluid collection, steroid concentrations, and sperm preservation method affect porcine in vitro fertilization efficiency. Fertil. Steril. 102:1762-1768.). However, the procedures currently used for in vitro maturation (IVM) and IVF for in vitro production (IVP) of porcine embryos frequently result in low rates of embryonic development (Grupen et al. 1997Grupen C.G. , Nagashima H. & Nottle M.B. 1997. Role of epidermal growth factor and insulin-like growth factor-I on porcine oocyte maturation and embryonic development in vitro. Reprod. Fertil. Dev. 9:571-575., Coy & Romar 2002Coy P. & Romar R. 2002. In vitro production of pig embryos: a point of view. Reprod. Fertil. Dev. 14:275-286.).
Among the factors that affect the efficiency of this technique, we can highlight the high rates of polyspermy, which occur due to several factors, such as sperm concentration, the fertilization of immature and old oocytes, and, as a main factor, the spermatic coincubation period (Coy & Romar 2002Coy P. & Romar R. 2002. In vitro production of pig embryos: a point of view. Reprod. Fertil. Dev. 14:275-286., Wang et al. 2003Wang W.H., Day B.N. & Wu G.M. 2003. How does polyspermy happen in mammalian oocytes? Micr. Res. Tech. 61:335-341., Oberlender et al. 2012Oberlender G., Murgas L.D.S., Zangeronimo M.G., Silva A.C., Menezes T.A., Pontelo T.P. & Pereira L.J. 2012. Aspectos relacionados à ocorrência da polispermia durante a fertilização in vitro (FIV) em suínos. Rev. Cient. Eletr. Med. Vet. 19:1-28., Romar et al. 2016Romar R., Funahashi H. & Coy P. 2016. In vitro fertilization in pigs: New molecules and protocols to consider in the forthcoming years. Theriogenology 85:125-134.). According to Coy et al. (1993)Coy P., Martinez E., Ruiz S., Vazquez J.M., Roca J., Matas C. & Pellicer M.T. 1993. In vitro fertilization of pig oocytes after different coincubation intervals. Theriogenology 39:1201-1208., the penetration rates in IVF of porcine oocytes are high, but are also accompanied by high rates of polyspermic fertilization. Thus, polyspermy during IVF represents the main obstacle in the IVF of porcine embryos (Gil et al. 2004Gil M.A., Ruiz M., Vazquez J.M., Roca J., Day B.N. & Martinez E.A. 2004. Effect of short periods of sperm-oocyte coincubation during in vitro fertilization on embryo development in pigs. Theriogenology 62:544-552., Tokeshi et al. 2007Tokeshi I., Yoshimoto T., Muto N., Nakamura S., Ashizawa K., Nakada T. & Tatemoto H. 2007. Antihyaluronidase action of ellagic acid effectively prevents polyspermy as a result of suppression of the acrosome reaction induced by sperm-zona interaction during in vitro fertilization of porcine oocytes. J. Reprod. Dev. 53:755-764., Oberlender et al. 2013bOberlender G., Murgas L.D.S., Zangeronimo M.G., Pontelo T.P., Menezes T.A. & Silva A.C. 2013b. Porcine follicular fluid concentration of free insulin-like growth factor-I collected from different diameter ovarian follicles. Pesq. Vet. Bras. 33:1269-1274.). According Coy & Romar (2002)Coy P. & Romar R. 2002. In vitro production of pig embryos: a point of view. Reprod. Fertil. Dev. 14:275-286., while the rate of polyspermy can achieve rates ranging from 40% to 60%, the percentage of ova penetrated is close to 100%.
Many studies have been conducted in an attempt to decrease the rate of polyspermy in IVF of porcine oocytes (Oberlender et al. 2013aOberlender G., Murgas L.D.S., Zangeronimo M.G., Silva A.C., Menezes T.A., Pontelo T.P. & Vieira L.A. 2013a. Role of insulin-like growth factor-I and follicular fluid from ovarian follicles with different diameters on porcine oocyte maturation and fertilization in vitro. Theriogenology 80:319-327., Ballester et al. 2014Ballester L., Romero-Aguirregomezcorta J., Soriano-Úbeda C., Matás C., Romar R. & Coy P. 2014. Timing of oviductal fluid collection, steroid concentrations, and sperm preservation method affect porcine in vitro fertilization efficiency. Fertil. Steril. 102:1762-1768.). In vivo, the incidence of polyspermy is less than 5% (Funahashi et al. 2000Funahashi H. , Ekwall H. & Rodriguez-Martinez H. 2000. Zona reaction in porcine oocytes fertilized in vivo and in vitro as seen with scanning electron microscopy. Biol. Reprod. 63:1437-1442.). Polyspermy increases when a high number of spermatozoa are deposited directly into the oviduct (Hunter 1973Hunter R.H.F. 1973. Polyspermic fertilization in pig after tubal deposition of excessive numbers of spermatozoa. J. Exp. Zool. 183:57-64., 1991Hunter R.H.F. 1991. Oviduct function in pigs, with particular reference to the pathological condition of polyspermy. Mol. Reprod. Dev. 29:385-391., Gil et al. 2004Gil M.A., Ruiz M., Vazquez J.M., Roca J., Day B.N. & Martinez E.A. 2004. Effect of short periods of sperm-oocyte coincubation during in vitro fertilization on embryo development in pigs. Theriogenology 62:544-552.). Likewise, the reduction of the number of spermatozoa during in vitro coincubation with the oocytes increases monospermy rates. However, this is accompanied by a low penetration rate (Abeydeera & Day 1997Abeydeera L.R. & Day B.N. 1997. Fertilization and subsequent development in vitro of pig oocytes inseminated in a modified Tris-buffered medium with frozen-thawed ejaculated spermatozoa. Biol. Reprod. 57:729-734., Gil et al. 2004Gil M.A., Ruiz M., Vazquez J.M., Roca J., Day B.N. & Martinez E.A. 2004. Effect of short periods of sperm-oocyte coincubation during in vitro fertilization on embryo development in pigs. Theriogenology 62:544-552.).
Several different studies (Coy et al. 1993Coy P., Martinez E., Ruiz S., Vazquez J.M., Roca J., Matas C. & Pellicer M.T. 1993. In vitro fertilization of pig oocytes after different coincubation intervals. Theriogenology 39:1201-1208., Ocampo et al. 1994Ocampo M.B., Ocampo L.C., Mori T., Ueda J. & Kanagawa H. 1994. Timing of sequential changes in chromosome configurations during the second meiotic division and cytoplasmic events of pig oocytes matured and fertilized in vitro. Anim. Reprod. Sci. 34:281-288., Gil et al. 2004Gil M.A., Ruiz M., Vazquez J.M., Roca J., Day B.N. & Martinez E.A. 2004. Effect of short periods of sperm-oocyte coincubation during in vitro fertilization on embryo development in pigs. Theriogenology 62:544-552.) have found that reducing the amount of time that oocytes are exposed to spermatozoa increases monospermy rates. Most current IVF systems use a three- to six-hour gamete coincubation period (Abeydeera & Day 1997Abeydeera L.R. & Day B.N. 1997. Fertilization and subsequent development in vitro of pig oocytes inseminated in a modified Tris-buffered medium with frozen-thawed ejaculated spermatozoa. Biol. Reprod. 57:729-734., Funahashi et al. 1999Funahashi H. , Mcintush E.W., Smith M.F. & Day B.N. 1999. The presence of Tissue Inhibitor of Matrix Metalloproteinase-1 (TIMP-1) during meiosis improves porcine ''oocyte competence'' as determined by early embryonic development after in vitro fertilization. J. Reprod. Dev. 45:265-271., Wang et al. 1999Wang W.H., Abeydeera L.R., Han Y., Prather R.S. & Day B.N. 1999. Morphologic evaluation and actin filament distribution in porcine embryos produced in vitro and in vivo. Biol. Reprod. 60:1020-1028., Abeydeera et al. 2000Abeydeera L.R. , Wang W.H., Cantley T.C., Rieke A., Murphy C.N., Prather R.S. & Day B.N. 2000. Development and viability of pig oocytes matured in a protein-free medium containing epidermal growth factor. Theriogenology 54:787-797., Gil et al. 2003Gil M.A., Abeydeera L.R. , Day B.N. , Vazquez J.M., Roca J. & Martinez E.A. 2003. Effect of the volume of medium and number of oocytes during in vitro fertilization in embryo development in pigs. Theriogenology 60:767-776., Oberlender et al. 2013aOberlender G., Murgas L.D.S., Zangeronimo M.G., Silva A.C., Menezes T.A., Pontelo T.P. & Vieira L.A. 2013a. Role of insulin-like growth factor-I and follicular fluid from ovarian follicles with different diameters on porcine oocyte maturation and fertilization in vitro. Theriogenology 80:319-327., Nguyen et al. 2015Nguyen B.X., Kikuchi K., Uoc N.T., Dang-Nguyen T.Q., Linh N.V., Men N.T., Nguyen T.T. & Nagai T. 2015. Production of Ban miniature pig embryos by in vitro fertilization: a comparative study with Landrace. Anim. Sci. J. 86:487-493.), compared with the coincubation times used in the first IVF systems (Iritani et al. 1978Iritani A., Niwa K. & Imai H. 1978. Sperm penetration in vivo of pig follicular oocytes matured in culture. J. Reprod. Fertil. 54:379-383.). In other studies (Coy et al. 1993Coy P., Martinez E., Ruiz S., Vazquez J.M., Roca J., Matas C. & Pellicer M.T. 1993. In vitro fertilization of pig oocytes after different coincubation intervals. Theriogenology 39:1201-1208., Grupen & Nottle 2000Grupen C.G. & Nottle M.B. 2000. A simple modification of the in vitro fertilization procedure. Theriogenology 53:422., Gil et al. 2004Gil M.A., Ruiz M., Vazquez J.M., Roca J., Day B.N. & Martinez E.A. 2004. Effect of short periods of sperm-oocyte coincubation during in vitro fertilization on embryo development in pigs. Theriogenology 62:544-552.), penetration rates were demonstrably improved when oocytes were exposed to spermatozoa for 10 minutes rather than three to six hours. Thus, the modification of gamete coincubation time during porcine IVF has been investigated to decrease the rate of polyspermy (Coy et al. 1993Coy P., Martinez E., Ruiz S., Vazquez J.M., Roca J., Matas C. & Pellicer M.T. 1993. In vitro fertilization of pig oocytes after different coincubation intervals. Theriogenology 39:1201-1208., Abeydeera & Day 1997Abeydeera L.R. & Day B.N. 1997. Fertilization and subsequent development in vitro of pig oocytes inseminated in a modified Tris-buffered medium with frozen-thawed ejaculated spermatozoa. Biol. Reprod. 57:729-734.).
Considering the above, our study was designed to evaluate the effects of different gamete coincubation times on porcine IVF and if the efficiency of this technique could be improved by a reduction (three hours to one) in the period of time that oocytes were exposed to spermatozoa during IVF.
Materials and Methods
Place of investigation and location of animals. The study was performed in the Department of Physiology at Faculty of Veterinary Science, University of Murcia in Murcia, Spain. All biological materials (ovaries) were obtained from an abattoir (Matadero de "El Pozo Alimentación") in Alhama de Murcia, in the city of Murcia, Spain. Ovaries were collected from five- to six-month-old prepubertal Landrace × Large White crossbred gilts with an average weight of 90 to 100 kg.
Culture media and reagents. Unless otherwise indicated, all the chemicals and reagents used in this study were purchased from Sigma-Aldrich Química S.A. (Madrid, Spain).
The basic medium for oocyte IVM was North Carolina State University-37 (NCSU-37) (Petters & Wells 1993Petters R.M. & Wells K.D. 1993. Culture of pig embryos. J. Reprod. Fertil. Suppl. 48:61-73.) supplemented with 0.57mᴍ cysteine, 50μᴍ β-mercaptoethanol, 5.0mg/L insulin, 1.0mᴍ dibutyryl cAMP (dbAMPc), 10 IU/mL hCG (Chorulon®, Intervet International B.V., Boxmeer, Holland), 10 IU/mL eCG (Folligon®, Intervet International B.V., Boxmeer, Holland), and 10% (vol/vol) porcine follicular fluid (3-8mm in diameter) (Coy et al. 2008aCoy P., Cánovas S., Mondéjar I., Saavedra M.D., Romar R., Grullón L., Matás C. & Avilés M. 2008a. Oviduct-specific glycoprotein and heparin modulate sperm-zona pellucida interaction during fertilization and contribute to the control of polyspermy. Proc. Natl Acad. Sci. USA 105:15809-15814., 2008bCoy P. , Grullón L. , Cánovas S. , Romar R. , Matás C. & Avilés M. 2008b. Hardening of the zona pellucida of unfertilized eggs can reduce polyspermic fertilization in the pig and cow. Reproduction 135:19-27., Oberlender et al. 2013aOberlender G., Murgas L.D.S., Zangeronimo M.G., Silva A.C., Menezes T.A., Pontelo T.P. & Vieira L.A. 2013a. Role of insulin-like growth factor-I and follicular fluid from ovarian follicles with different diameters on porcine oocyte maturation and fertilization in vitro. Theriogenology 80:319-327.).
The basic medium for IVF, designated as TALP medium (Tyrode's albumin lactate pyruvate), was the same as the one used by Rath et al. (1999)Rath D., Long C.R., Dobrinsky J.R., Welch G.R., Schreier L.L. & Johnson L.A. 1999. In vitro production of sexed embryos for gender preselection: high-speed sorting of X-chromosome-bearing sperm to produce pigs after embryo transfer. J. Anim. Sci. 77:3346-3352.; it was supplemented with 1.1mᴍ sodium pyruvate and 0.3% fatty acid-free bovine serum albumin (BSA-FAF) (Coy et al. 2008aCoy P., Cánovas S., Mondéjar I., Saavedra M.D., Romar R., Grullón L., Matás C. & Avilés M. 2008a. Oviduct-specific glycoprotein and heparin modulate sperm-zona pellucida interaction during fertilization and contribute to the control of polyspermy. Proc. Natl Acad. Sci. USA 105:15809-15814., 2008bCoy P. , Grullón L. , Cánovas S. , Romar R. , Matás C. & Avilés M. 2008b. Hardening of the zona pellucida of unfertilized eggs can reduce polyspermic fertilization in the pig and cow. Reproduction 135:19-27.).
All culture media employed were prepared using ultrapure water (Milli-Q, 18.2 ᴍΩ cm-1; Direct-Q 5, Millipore, Darmstadt, Germany) and equilibrated in an incubator at 38.5°C under 5% CO2 and 100% humidity for three hours before use.
Oocyte collection and preparation. Ovaries from prepubertal gilts were collected immediately after slaughter and transported to the laboratory in a thermal container with 0.9% (wt/vol) NaCl solution containing 0.1% (wt/vol) of kanamycin sulfate at 38°C. The time elapsed from animal slaughter to oocyte recovery was ≤ 2 hours.
Following delivery to the laboratory as previously described, ovaries were washed twice in 0.04% (vol/vol) cetrimide solution and twice more in saline, both at 38°C. Cumulus-oocyte complexes (COCs) from follicles 3-8 mm in diameter were aspirated using an 18-ga needle attached to a 10-mL disposable syringe. Follicular contents obtained were stored in 15-mL sterile tubes, and COCs were allowed to sediment for 10-15 minutes under a warming plate at 38°C. Afterward, the supernatant was discarded and the pellets obtained were deposited into 90 × 15 mm petri dishes for the process of selecting COCs to be used for IVM.
Oocyte selection was performed with a stereoscopic microscope (Nikon). Only intact COCs obtained within two hours of slaughter (Matás et al. 1996Matás C. , Martinez E., Vázquez J.M., Roca J. & Gadea J. 1996. In vitro penetration assay of boar sperm fertility: effect of various factors on the penetrability of immature pig oocytes. Theriogenology 46:503-513.) with a homogeneous cytoplasm an compact cumulus oophorus were used. Morphologically abnormal oocytes (brown-colored, shrunken, or granulated cytoplasm or indistinguishable cytoplasmic membranes) were excluded (Hong et al. 2004Hong J.Y., Yong H.Y., Lee B.C., Hwang W.S., Lim J.M. & Lee E.S. 2004. Effects of amino acids on maturation, fertilization and embryo development of pig follicular oocytes in two IVM media. Theriogenology 62:1473-1482.). After selection, the oocytes were washed twice in Dulbecco's phosphate buffer saline supplemented with 0.001% (wt/vol) polyvinyl alcohol and 0.0005% (wt/vol) phenol red as the pH indicator. Following this, the COCs were washed twice more in previously equilibrated NCSU-37 maturation medium.
Oocyte in vitro maturation (IVM). Groups of 50 COCs each were transferred into four-well culture multidishes and cultured in 500μL of the preequilibrated supplemented NCSU-37 medium for 20-22 hours at 38.5°C under 5% CO2 in air (Matás et al. 2003Matás C. , Coy P. , Romar R. , Marco M., Gadea J. & Ruiz S. 2003. Effect of sperm preparation method on in vitro fertilization in pigs. Reproduction 125:133-141.). After culture, the oocytes were washed twice with fresh IVM medium and transferred to fresh IVM medium without dibutyryl cAMP, eCG, or hCG; they were then cultured for an additional 20-22 hours (Funahashi & Day 1993Funahashi H. & Day B.N. 1993. Effects of the duration of exposure to supplemental hormones on cytoplasmic maturation of pig oocytes in vitro. J. Reprod. Fertil. 98:179-185., Park et al. 2009Park C.H., Lee S.G., Choi D.H. & Lee C.K. 2009. A modified swim-up method reduces polyspermy during in vitro fertilization of porcine oocytes. Anim. Reprod. Sci. 115:169-181.).
Semen collection and sperm preparation. Semen samples for IVF were collected using the gloved hand technique (Hancock & Howell 1959Hancock J.L. & Howell G.J.R. 1959. The collection of boar semen. Vet. Record. 71:664-665.) from mature Pietrain boars of known fertility selected from the "Dalland Hybrid España, S.A." Artificial Insemination Center in Murcia, Spain. In each collection, a sperm-rich fraction was retained in a pre-warmed thermos (Larsen 1986Larsen R. 1986. Semen collection from the boar, p.969-972. In: Morrow D. (Ed.), Current Therapy in Theriogenology. W.B. Saunders, Philadelphia., Gadea 2002Gadea J. 2002. Manual básico del laboratorio de semen. Universidad de Murcia, Murcia, España. 39p.), whereas the gel fraction was retained on a gauze tissue that covered the thermos opening.
Afterwards, the ejaculate was diluted 1:1 with isothermal Beltsville thawing solution (BTS) extender (MINITUB Abfüll- und Labortechnik GmbH & Co. KG, Tiefenbach, Germany) (Pursel & Johnson 1975Pursel V.G. & Johnson L.A. 1975. Freezing of boar spermatozoa; fertilizing capacity with concentrated semen and a new thawing procedure. J. Anim. Sci. 40:99-102.) and immediately transported to the laboratory and protected from light. Only semen samples with normal physiological parameters suitable for use in artificial insemination were used for IVF.
For sperm preparation for IVF, sperm were separated and selected from extended semen by sedimentation/washing through a two-step (45% and 90% vol/vol) Percoll® gradient (Satake et al. 2006Satake N., Elliott R.M., Watson P.F. & Holt W.V. 2006. Sperm selection and competition in pigs may be mediated by the differential motility activation and suppression of sperm subpopulations within the oviduct. J. Exp. Biol. 209:1560-1572.). For this purpose, 2mL of 45% Percoll® were layered on top of 2 mL of 90% Percoll® in a 15-mL conic centrifuge tube. Finally, 0.5 mL of diluted semen were added, with care taken to avoid mixing the solutions (Parrish et al. 1995Parrish J.J., Krogenaes A. & Susko-Parrish J.L. 1995. Effect of bovine sperm separation by either swim-up or Percoll method on success of in vitro fertilization and early embryonic development. Theriogenology 44:859-869.).
The samples were then centrifuged at 800g for 30 minutes at 24°C. The supernatant layers were then removed by aspiration, after which the resultant sperm pellet was resuspended in 10mL of preequilibrated TALP medium (Rath et al. 1999Rath D., Long C.R., Dobrinsky J.R., Welch G.R., Schreier L.L. & Johnson L.A. 1999. In vitro production of sexed embryos for gender preselection: high-speed sorting of X-chromosome-bearing sperm to produce pigs after embryo transfer. J. Anim. Sci. 77:3346-3352.) and washed by centrifugation at 800g for 10 minutes at 24°C (Matás et al. 2011Matás C. , Vieira L., García-Vázquez F.A., Avilés-López K., López-Úbeda R., Carvajal J.A. & Gadea J. 2011. Effects of centrifugation through three different discontinuous Percoll gradients on boar sperm function. Anim. Reprod. Sci. 127:62-72.). Finally, the pellet was resuspended and diluted in preequilibrated TALP medium to give a final adjusted concentration of 4×105 sperm/mL (Coy et al. 2010Coy P. , Lloyd R., Romar R. , Satake N., Matás C. , Gadea J. & Holt W.V. 2010. Effects of porcine pre-ovulatory oviductal fluid on boar sperm function. Theriogenology 74:632-642.), as determined by a SpermaCue® photometer (MINITÜB Abfüll-und Labortechnik GmbH & Co. KG, Tiefenbach, Germany).
Oocyte in vitro fertilization (IVF). After IVM (40-44 hours of culture) and before insemination, oocytes were mechanically stripped of their enclosing cumulus cells by gentle aspiration with a pipette until they were completely denuded. The cumulus-free oocytes were then washed twice in TALP medium, at which point groups of 40-50 oocytes were transferred to each well of four-well culture multidishes containing 250μL of IVF medium (TALP), which had been previously equilibrated at 38.5°C under 5% CO2. This process was carried out simultaneously with the first semen centrifugation using a Percoll® gradient.
For fertilization, 250μL of the sperm suspension were added to each well containing oocytes at a final ratio of 2,000 sperm per oocyte (1×105 sperm/well) (Malo et al. 2010Malo C., Gil L., Gonzalez N., Martínez F., Cano R., De Blas I. & Espinosa E. 2010. Antioxidant supplementation improves boar sperm characteristics and fertility after cryopreservation: comparison between cysteine and rosemary (Rosmarinus officinalis). Cryobiology 61:142-147.). Afterward, the sperm and oocytes were coincubated at 38.5°C in 5% CO2 in air according to the following treatments (T): treatment 1 (T1) = oocytes were exposed to spermatozoa for one hour, treatment 2 (T2) = oocytes were exposed to spermatozoa for two hours, and treatment 3 (T3) = oocytes were exposed to spermatozoa for three hours. After these coincubation periods, adherent spermatozoa and cumulus mass were removed from the zona pellucida by pipetting (Mattioli et al. 1989Mattioli M., Bacci M.L., Galeati G. & Seren E. 1989. Developmental competence of pig oocytes matured and fertilized in vitro. Theriogenology 31:1201-1207.); the oocytes were then washed twice in previously equilibrated fresh TALP medium and cultured for 18-20 hours until fixation.
Assessment of IVM and IVF. Eighteen to twenty hours after fertilization, the putative zygotes of the three different experimental groups were fixed in 0.5% glutaraldehyde in phosphate buffer saline (PBS). They were then stained in 1% Hoechst-33342 in PBS for 30 minutes, washed in PBS, and mounted on glass slides and examined under an epifluorescence microscope (Leica DMLS) at ×200 and ×400 magnification and 495nm wavelength ultraviolet filter for evidence of maturation and sperm penetration.
Oocyte IVM rate, percentage of degenerated oocytes, penetration rate, monospermy rate, fertilization performance, number of penetrated sperm per oocyte, and pronuclear formation rate were assessed (Algriany et al. 2004Algriany O., Bevers M., Schoevers E., Colenbrander B. & Dieleman S. 2004. Follicle size-dependent effects of sow follicular fluid on in vitro cumulus expansion, nuclear maturation and blastocyst formation of sow cumulus oocytes complexes. Theriogenology 62:1483-1497., Coy et al. 2010Coy P. , Lloyd R., Romar R. , Satake N., Matás C. , Gadea J. & Holt W.V. 2010. Effects of porcine pre-ovulatory oviductal fluid on boar sperm function. Theriogenology 74:632-642., Malo et al. 2010Malo C., Gil L., Gonzalez N., Martínez F., Cano R., De Blas I. & Espinosa E. 2010. Antioxidant supplementation improves boar sperm characteristics and fertility after cryopreservation: comparison between cysteine and rosemary (Rosmarinus officinalis). Cryobiology 61:142-147., Oberlender et al. 2013aOberlender G., Murgas L.D.S., Zangeronimo M.G., Silva A.C., Menezes T.A., Pontelo T.P. & Vieira L.A. 2013a. Role of insulin-like growth factor-I and follicular fluid from ovarian follicles with different diameters on porcine oocyte maturation and fertilization in vitro. Theriogenology 80:319-327.). The IVM rate was evaluated as the percentage of oocytes with a nuclear morphology corresponding to metaphases II (MII), which was considered "mature" (Bijttebier et al. 2008Bijttebier J., Van Soom A., Meyer E., Mateusen B. & Maes D. 2008. Preovulatory follicular fluid during in vitro maturation decreases polyspermic fertilization of cumulus-intact porcine oocytes in vitro maturation of porcine oocytes. Theriogenology 70:715-724.). Degenerate oocytes were discarded and the proportion of MII oocytes was calculated from the nondegenerate oocytes. The penetration rate was assessed as the percentage of mature oocytes penetrated by one or more sperm. The monospermy rate was evaluated as the percentage of oocytes with two pronuclei or with one pronucleus together with one decondensed sperm head; fertilization performance was the percentage of monospermic oocytes with two pronuclei in relation to the total number of fertilized oocytes. The rate of pronucleus formation was defined as the percentage of monospermic oocytes that showed both a male and a female pronucleus.
Experimental design. To assess the effects of different gamete coincubation times on porcine IVF, a randomized block design (RBD) with three treatments (oocytes exposed to spermatozoa for one hour - T1, two hours - T2, and three hours - T3) was used. The blocks consisted of fertilization days. A total of three replicates per treatment were performed, and each experimental plot was represented by 50 oocytes.
Statistical analysis. Data are presented as mean ± standard deviation (SD). All variables obtained were modeled according to the binomial model of parameters (Coy et al. 2008bCoy P. , Grullón L. , Cánovas S. , Romar R. , Matás C. & Avilés M. 2008b. Hardening of the zona pellucida of unfertilized eggs can reduce polyspermic fertilization in the pig and cow. Reproduction 135:19-27., 2010Coy P. , Lloyd R., Romar R. , Satake N., Matás C. , Gadea J. & Holt W.V. 2010. Effects of porcine pre-ovulatory oviductal fluid on boar sperm function. Theriogenology 74:632-642., Romar et al. 2012Romar R., Coy P. & Rath D. 2012. Maturation conditions and boar affect timing of cortical reaction in porcine oocytes. Theriogenology 78:1126-1139., Oberlender et al. 2013aOberlender G., Murgas L.D.S., Zangeronimo M.G., Silva A.C., Menezes T.A., Pontelo T.P. & Vieira L.A. 2013a. Role of insulin-like growth factor-I and follicular fluid from ovarian follicles with different diameters on porcine oocyte maturation and fertilization in vitro. Theriogenology 80:319-327.). A normality test (Shapiro-Wilk) was performed and data were analyzed by analysis of variance (ANOVA). When ANOVA results were significant, the IVM rate and IVF data at different spermatic coincubation times (one hour - T1, two hours - T2, and three hours - T3) were compared using Tukey's test.
For variables that were not normally distributed (percentage of degenerated oocytes, rate of male pronucleus formation, and number of penetrated sperm per oocyte), an arcsine transformation was performed to achieve a normal distribution (Coy et al. 2008aCoy P., Cánovas S., Mondéjar I., Saavedra M.D., Romar R., Grullón L., Matás C. & Avilés M. 2008a. Oviduct-specific glycoprotein and heparin modulate sperm-zona pellucida interaction during fertilization and contribute to the control of polyspermy. Proc. Natl Acad. Sci. USA 105:15809-15814., Oberlender et al. 2013aOberlender G., Murgas L.D.S., Zangeronimo M.G., Silva A.C., Menezes T.A., Pontelo T.P. & Vieira L.A. 2013a. Role of insulin-like growth factor-I and follicular fluid from ovarian follicles with different diameters on porcine oocyte maturation and fertilization in vitro. Theriogenology 80:319-327.). A significance level of 5% was considered to indicate a statistically meaningful difference. All statistical analyses were performed using the statistical package IBM® SPSS for Windows, version 20.07.
Results
A total of 508 oocytes were collected and examined from prepubertal gilts. From these, 173 were in T1 (sperm and oocytes coincubated for one hour), 170 in T2 (sperm and oocytes coincubated for two hours), and 165 in T3 (sperm and oocytes coincubated for three hours).
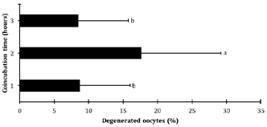
Regardless of the treatment used, the oocyte maturation rate was 88.59±9.84%. The maturation rate was higher (P=0.023) after coincubation time for 1 and 3 hours than it was for 2 hours (Fig.1). Out of all evaluated oocytes, 24.78% reached the stage of metaphase II, but were not penetrated; no differences were found (P=0.330) between the groups for this variable (Table 1). The percentage of degenerated oocytes was higher (P=0.023) in the ova of T2 oocytes (those exposed to spermatozoa for 2 hours) with mean of 17.65% (n=30 degenerated oocytes of the total examined), compared to 8.67% in T1 oocytes (n=15 of 173) and 8.48% in T3 oocytes (n=14 of 165), which did not differ from each other (P>0.05) (Fig.2).
Evolution of nuclear maturation, penetration and monospermy rate in oocytes coincubated with spermatozoa for 1, 2, and 3 hours.
Percentage of degenerated oocytes after 1, 2, and 3 hours of coincubation with spermatozoa. a,b Different letters denote significant differences according to Tukey's test (P=0.023).
The effects of different coincubation time on porcine oocytes IVF are shown in Table 2 and Figure 1. The penetration rate was higher (P<0.05) after two hours of coincubation time (75.20±10.42%) versus 1 or 3 hours (69.78 ± 11.45% and 66.57±22.68%, respectively). Furthermore, 68.60% of the ova coincubated with the spermatozoa for 2 hours were monospermic. The percentage of monospermically fertilized ova after 1 and 3 hours of coincubation was 42.67% and 66.35%, respectively (Fig.1). Despite having a lower rate (P<0.05) of sperm penetration, the oocytes exposed to spermatozoa for 3 hours presented a similar rate of monospermic fertilization after 1 hour of incubation.
The percentage of polyspermy ranged from 29.49 to 57.33% in all three groups. The oocytes exposed to spermatozoa for 1 hour (T1) presented a higher (P<0.01) rate of polyspermy than those in T2 and T3.
Fertilization performance (%) did not differ (P>0.05) between oocytes exposed to spermatozoa for 1 (T1) or 3 hours (T3). However, the ova coincubated with the spermatozoa for 2 hours (T2) presented the best (P=0.048) rate of fertilization performance (50.16 ± 8.52%) (Fig.3).
In vitro fertilization (IVF) results. T1 = oocytes exposed to spermatozoa for 1 hour (polyspermic oocyte); T2 = oocytes exposed to spermatozoa for 2 hours (monospermic oocyte), and T3 = oocytes exposed to spermatozoa for three hours (polyspermic oocyte). Dashed arrow = decondensed sperm head; straight arrow = sperm adhered to the zona pellucida and * = pronucleus formation.
Significant differences were not found (P>0.05) between the treatments evaluated for either the number of penetrated sperm per oocyte or the male pronucleus formation. The average number of penetrated sperm per oocyte and the mean of male pronucleus formation regardless of the treatment were 1.75±0.70 and 91.79±11.72%, respectively.
Discussion
The reduction of coincubation time from 3 to 2 hours resulted in an efficient increase in sperm penetration, monospermic fertilizations, and, most importantly, fertilization performance. On the other hand, the increase in coincubation time reduced the number of polyspermic fertilizations. The percentage of penetration remained at about 70%, and monospermy was considerably higher with 2 and 3 hours of gamete coincubations. In the short coincubation time (1 hour), the rate of monospermic fertilization was low, even with a penetration rate similar to the long incubation time (3 hours). The number of penetrated sperm per oocyte and the male pronucleus formation after 1, 2, and 3 hours remained constant, with average values of 1.48 to 1.93 and 86.83 to 96.88%, respectively. With fewer than two hours of coincubation, monospermy decreased significantly (P<0.01), affecting fertilization performance. Thus, we demonstrated that penetration, monospermy, and fertilization performance have a positive relation with spermatic coincubation time.
The modification of gamete coincubation time during porcine IVF to decrease the rate of polyspermy in vitro has been previously investigated (Cheng et al. 1986Cheng W.T.K., Polge C. & Moor R.M. 1986. In vitro fertilization of pig and sheep oocytes. Theriogenology 25:146., Mattioli et al. 1989Mattioli M., Bacci M.L., Galeati G. & Seren E. 1989. Developmental competence of pig oocytes matured and fertilized in vitro. Theriogenology 31:1201-1207., Coy et al. 1993Coy P., Martinez E., Ruiz S., Vazquez J.M., Roca J., Matas C. & Pellicer M.T. 1993. In vitro fertilization of pig oocytes after different coincubation intervals. Theriogenology 39:1201-1208., Abeydeera & Day 1997Abeydeera L.R. & Day B.N. 1997. Fertilization and subsequent development in vitro of pig oocytes inseminated in a modified Tris-buffered medium with frozen-thawed ejaculated spermatozoa. Biol. Reprod. 57:729-734., Gil et al. 2004Gil M.A., Ruiz M., Vazquez J.M., Roca J., Day B.N. & Martinez E.A. 2004. Effect of short periods of sperm-oocyte coincubation during in vitro fertilization on embryo development in pigs. Theriogenology 62:544-552., 2007Gil M.A., Almiñana C., Cuello C., Parrilla I., Roca J., Vazquez J.M. & Martinez E.A. 2007. Brief coincubation of gametes in porcine in vitro fertilization: role of sperm:oocyte ratio and post-coincubation medium. Theriogenology 67:620-626., Almiñana et al. 2005Almiñana C., Gil M.A., Cuello C., Roca J., Vazquez J.M., Rodriguez-Martinez H. & Martinez E.A. 2005. Adjustments in IVF system for individual boars: value of additives and time of sperm-oocyte co-incubation. Theriogenology 64:1783-1796.).
According to Coy et al. (1993)Coy P., Martinez E., Ruiz S., Vazquez J.M., Roca J., Matas C. & Pellicer M.T. 1993. In vitro fertilization of pig oocytes after different coincubation intervals. Theriogenology 39:1201-1208., the longer the coincubation time, the greater the number of collisions between spermatozoa and zona pellucida; thus, the greater the risk that polyspermy will occur. However, this was not observed in our study, as we obtained a higher rate of polyspermy when the oocytes were exposed to spermatozoa for one hour.
The penetration and monospermy rates obtained in this study differed from the data presented by Coy et al. (1993)Coy P., Martinez E., Ruiz S., Vazquez J.M., Roca J., Matas C. & Pellicer M.T. 1993. In vitro fertilization of pig oocytes after different coincubation intervals. Theriogenology 39:1201-1208.. For all data, our study showed an average higher than those obtained by the authors mentioned above. This difference can be explained by the fact that, in our study, we used oocytes obtained from the ovaries of prepubertal gilts after slaughter. In the study of these authors, the oocytes were recovered from the oviducts of prepubertal gilts. Furthermore, their medium of sperm capacitation and IVF was different from what was used in our study.
Regarding the percentage of degenerated oocytes at the end of the IVM period, the results of our study were in agreement with other experiments reporting averages ranging from 8% to 23% (Illera et al. 1998Illera M.J., Lorenzo P.L., Illera J.C. & Petters R.M. 1998. Developmental competence of immature pig oocytes under the influence of EGF, IGF-I, follicular fluid and gonadotropins during IVM-IVF processes. Int. J. Dev. Biol. 42:1169-1172., Suzuki et al. 2003Suzuki H., Saito Y., Kagawa N. & Yang X. 2003. In vitro fertilization and polyspermy in the pig: factors affecting fertilization rates and cytoskeletal reorganization of the oocyte. Microsc. Res. Tech. 61:327-334., Kim et al. 2010Kim J., You J., Hyun S.H., Lee G., Lim J. & Lee E. 2010. Developmental competence of morphologically poor oocytes in relation to follicular size and oocyte diameter in the pig. Mol. Reprod. Dev. 77:330-339., Oberlender et al. 2013aOberlender G., Murgas L.D.S., Zangeronimo M.G., Silva A.C., Menezes T.A., Pontelo T.P. & Vieira L.A. 2013a. Role of insulin-like growth factor-I and follicular fluid from ovarian follicles with different diameters on porcine oocyte maturation and fertilization in vitro. Theriogenology 80:319-327.).
In the present study, regardless of the time of coincubation (oocytes exposed to spermatozoa), maturation rates were within the range reported by various studies (Kikuchi et al. 2009Kikuchi K., Somfai T., Nakai M. & Nagai T. 2009. Appearance, fate and utilization of abnormal porcine embryos produced by in vitro maturation and fertilization. Soc. Reprod. Fertil. Suppl. 66:135-147., Zhang et al. 2012Zhang W., Yi K., Yan H. & Zhou X. 2012. Advances on in vitro production and cryopreservation of porcine embryos. Anim. Reprod. Sci. 132:115-122., and Oberlender et al. 2013aOberlender G., Murgas L.D.S., Zangeronimo M.G., Silva A.C., Menezes T.A., Pontelo T.P. & Vieira L.A. 2013a. Role of insulin-like growth factor-I and follicular fluid from ovarian follicles with different diameters on porcine oocyte maturation and fertilization in vitro. Theriogenology 80:319-327.); during IVM, approximately 10% to 30% of oocytes did not reach metaphase II of the second meiotic division. In our study, approximately 7.51% to 17.57% of IVM failure was due to oocyte degeneration.
The numbers of penetrated sperm per oocyte obtained in our study were similar to those observed by Matás et al. (2011)Matás C. , Vieira L., García-Vázquez F.A., Avilés-López K., López-Úbeda R., Carvajal J.A. & Gadea J. 2011. Effects of centrifugation through three different discontinuous Percoll gradients on boar sperm function. Anim. Reprod. Sci. 127:62-72., Romar et al. (2012)Romar R., Coy P. & Rath D. 2012. Maturation conditions and boar affect timing of cortical reaction in porcine oocytes. Theriogenology 78:1126-1139., and Oberlender et al. (2013a)Oberlender G., Murgas L.D.S., Zangeronimo M.G., Silva A.C., Menezes T.A., Pontelo T.P. & Vieira L.A. 2013a. Role of insulin-like growth factor-I and follicular fluid from ovarian follicles with different diameters on porcine oocyte maturation and fertilization in vitro. Theriogenology 80:319-327.; in those studies, gamete coincubation was performed for one to three hours. Conversely, Coy et al. (2008b)Coy P. , Grullón L. , Cánovas S. , Romar R. , Matás C. & Avilés M. 2008b. Hardening of the zona pellucida of unfertilized eggs can reduce polyspermic fertilization in the pig and cow. Reproduction 135:19-27. obtained an average of 5.2 to 12.7 penetrated sperm per oocyte, values higher than those found in our study (1.75±0.70). This result may have been due to time that the oocytes were exposed to spermatozoa, which was 4 hours in their study.
Regarding male pronucleus formation, results from our study were in agreement with those of Romar et al. (2012)Romar R., Coy P. & Rath D. 2012. Maturation conditions and boar affect timing of cortical reaction in porcine oocytes. Theriogenology 78:1126-1139. and Oberlender et al. (2013a)Oberlender G., Murgas L.D.S., Zangeronimo M.G., Silva A.C., Menezes T.A., Pontelo T.P. & Vieira L.A. 2013a. Role of insulin-like growth factor-I and follicular fluid from ovarian follicles with different diameters on porcine oocyte maturation and fertilization in vitro. Theriogenology 80:319-327., who observed that male pronucleus formation was in excess of 85% of oocytes by 18 hours after fertilization. In our study, the rate of male pronucleus formation ranged from 86.83% to 96.88%.
Conclusions
Under these assay conditions, especially in relation to the sperm concentration used, gamete coincubation for a period of two hours appears to be optimal for monospermy and fertilization performance. Thus, this is the optimal time period for obtaining a large number of pig embryos capable of normal development.
In addition, as described by other researchers, the examination of other parameters, such as medium volume, sperm concentration, and physiological environment, may further improve the efficiency of porcine IVF.
Acknowledgements
To the staff of the slaughterhouse "ElPozo Alimentación", Alhama de Murcia, Murcia, Spain for supplying the biological samples. This work was supported by Federal Institute of Education, Science and Technology of South of Minas Gerais (IFSuldeMinas, Muzambinho Campus ("Instituto Federal de Educação, Ciência e Tecnologia do Sul de Minas Gerais (IFSuldeMinas, Campus Muzambinho") and in part by FAPEMIG (Research Support Foundation of the State of Minas Gerais), CNPq (National Council for Scientific and Technological Development) and CAPES (Coordination of Improvement of Higher Education Personnel), Brazil.
References
- Abeydeera L.R. & Day B.N. 1997. Fertilization and subsequent development in vitro of pig oocytes inseminated in a modified Tris-buffered medium with frozen-thawed ejaculated spermatozoa. Biol. Reprod. 57:729-734.
- Abeydeera L.R. , Wang W.H., Cantley T.C., Rieke A., Murphy C.N., Prather R.S. & Day B.N. 2000. Development and viability of pig oocytes matured in a protein-free medium containing epidermal growth factor. Theriogenology 54:787-797.
- Algriany O., Bevers M., Schoevers E., Colenbrander B. & Dieleman S. 2004. Follicle size-dependent effects of sow follicular fluid on in vitro cumulus expansion, nuclear maturation and blastocyst formation of sow cumulus oocytes complexes. Theriogenology 62:1483-1497.
- Almiñana C., Gil M.A., Cuello C., Roca J., Vazquez J.M., Rodriguez-Martinez H. & Martinez E.A. 2005. Adjustments in IVF system for individual boars: value of additives and time of sperm-oocyte co-incubation. Theriogenology 64:1783-1796.
- Ballester L., Romero-Aguirregomezcorta J., Soriano-Úbeda C., Matás C., Romar R. & Coy P. 2014. Timing of oviductal fluid collection, steroid concentrations, and sperm preservation method affect porcine in vitro fertilization efficiency. Fertil. Steril. 102:1762-1768.
- Bijttebier J., Van Soom A., Meyer E., Mateusen B. & Maes D. 2008. Preovulatory follicular fluid during in vitro maturation decreases polyspermic fertilization of cumulus-intact porcine oocytes in vitro maturation of porcine oocytes. Theriogenology 70:715-724.
- Cheng W.T.K., Polge C. & Moor R.M. 1986. In vitro fertilization of pig and sheep oocytes. Theriogenology 25:146.
- Coy P. & Romar R. 2002. In vitro production of pig embryos: a point of view. Reprod. Fertil. Dev. 14:275-286.
- Coy P., Cánovas S., Mondéjar I., Saavedra M.D., Romar R., Grullón L., Matás C. & Avilés M. 2008a. Oviduct-specific glycoprotein and heparin modulate sperm-zona pellucida interaction during fertilization and contribute to the control of polyspermy. Proc. Natl Acad. Sci. USA 105:15809-15814.
- Coy P. , Grullón L. , Cánovas S. , Romar R. , Matás C. & Avilés M. 2008b. Hardening of the zona pellucida of unfertilized eggs can reduce polyspermic fertilization in the pig and cow. Reproduction 135:19-27.
- Coy P. , Lloyd R., Romar R. , Satake N., Matás C. , Gadea J. & Holt W.V. 2010. Effects of porcine pre-ovulatory oviductal fluid on boar sperm function. Theriogenology 74:632-642.
- Coy P., Martinez E., Ruiz S., Vazquez J.M., Roca J., Matas C. & Pellicer M.T. 1993. In vitro fertilization of pig oocytes after different coincubation intervals. Theriogenology 39:1201-1208.
- Funahashi H. & Day B.N. 1993. Effects of the duration of exposure to supplemental hormones on cytoplasmic maturation of pig oocytes in vitro J. Reprod. Fertil. 98:179-185.
- Funahashi H. , Ekwall H. & Rodriguez-Martinez H. 2000. Zona reaction in porcine oocytes fertilized in vivo and in vitro as seen with scanning electron microscopy. Biol. Reprod. 63:1437-1442.
- Funahashi H. , Mcintush E.W., Smith M.F. & Day B.N. 1999. The presence of Tissue Inhibitor of Matrix Metalloproteinase-1 (TIMP-1) during meiosis improves porcine ''oocyte competence'' as determined by early embryonic development after in vitro fertilization. J. Reprod. Dev. 45:265-271.
- Gadea J. 2002. Manual básico del laboratorio de semen. Universidad de Murcia, Murcia, España. 39p.
- Gil M.A., Abeydeera L.R. , Day B.N. , Vazquez J.M., Roca J. & Martinez E.A. 2003. Effect of the volume of medium and number of oocytes during in vitro fertilization in embryo development in pigs. Theriogenology 60:767-776.
- Gil M.A., Almiñana C., Cuello C., Parrilla I., Roca J., Vazquez J.M. & Martinez E.A. 2007. Brief coincubation of gametes in porcine in vitro fertilization: role of sperm:oocyte ratio and post-coincubation medium. Theriogenology 67:620-626.
- Gil M.A., Ruiz M., Vazquez J.M., Roca J., Day B.N. & Martinez E.A. 2004. Effect of short periods of sperm-oocyte coincubation during in vitro fertilization on embryo development in pigs. Theriogenology 62:544-552.
- Grupen C.G. & Nottle M.B. 2000. A simple modification of the in vitro fertilization procedure. Theriogenology 53:422.
- Grupen C.G. , Nagashima H. & Nottle M.B. 1997. Role of epidermal growth factor and insulin-like growth factor-I on porcine oocyte maturation and embryonic development in vitro Reprod. Fertil. Dev. 9:571-575.
- Hancock J.L. & Howell G.J.R. 1959. The collection of boar semen. Vet. Record. 71:664-665.
- Hong J.Y., Yong H.Y., Lee B.C., Hwang W.S., Lim J.M. & Lee E.S. 2004. Effects of amino acids on maturation, fertilization and embryo development of pig follicular oocytes in two IVM media. Theriogenology 62:1473-1482.
- Hunter R.H.F. 1973. Polyspermic fertilization in pig after tubal deposition of excessive numbers of spermatozoa. J. Exp. Zool. 183:57-64.
- Hunter R.H.F. 1991. Oviduct function in pigs, with particular reference to the pathological condition of polyspermy. Mol. Reprod. Dev. 29:385-391.
- Illera M.J., Lorenzo P.L., Illera J.C. & Petters R.M. 1998. Developmental competence of immature pig oocytes under the influence of EGF, IGF-I, follicular fluid and gonadotropins during IVM-IVF processes. Int. J. Dev. Biol. 42:1169-1172.
- Iritani A., Niwa K. & Imai H. 1978. Sperm penetration in vivo of pig follicular oocytes matured in culture. J. Reprod. Fertil. 54:379-383.
- Kikuchi K., Somfai T., Nakai M. & Nagai T. 2009. Appearance, fate and utilization of abnormal porcine embryos produced by in vitro maturation and fertilization. Soc. Reprod. Fertil. Suppl. 66:135-147.
- Kim J., You J., Hyun S.H., Lee G., Lim J. & Lee E. 2010. Developmental competence of morphologically poor oocytes in relation to follicular size and oocyte diameter in the pig. Mol. Reprod. Dev. 77:330-339.
- Larsen R. 1986. Semen collection from the boar, p.969-972. In: Morrow D. (Ed.), Current Therapy in Theriogenology. W.B. Saunders, Philadelphia.
- Malo C., Gil L., Gonzalez N., Martínez F., Cano R., De Blas I. & Espinosa E. 2010. Antioxidant supplementation improves boar sperm characteristics and fertility after cryopreservation: comparison between cysteine and rosemary (Rosmarinus officinalis). Cryobiology 61:142-147.
- Matás C. , Coy P. , Romar R. , Marco M., Gadea J. & Ruiz S. 2003. Effect of sperm preparation method on in vitro fertilization in pigs. Reproduction 125:133-141.
- Matás C. , Martinez E., Vázquez J.M., Roca J. & Gadea J. 1996. In vitro penetration assay of boar sperm fertility: effect of various factors on the penetrability of immature pig oocytes. Theriogenology 46:503-513.
- Matás C. , Vieira L., García-Vázquez F.A., Avilés-López K., López-Úbeda R., Carvajal J.A. & Gadea J. 2011. Effects of centrifugation through three different discontinuous Percoll gradients on boar sperm function. Anim. Reprod. Sci. 127:62-72.
- Mattioli M., Bacci M.L., Galeati G. & Seren E. 1989. Developmental competence of pig oocytes matured and fertilized in vitro Theriogenology 31:1201-1207.
- Nguyen B.X., Kikuchi K., Uoc N.T., Dang-Nguyen T.Q., Linh N.V., Men N.T., Nguyen T.T. & Nagai T. 2015. Production of Ban miniature pig embryos by in vitro fertilization: a comparative study with Landrace. Anim. Sci. J. 86:487-493.
- Oberlender G., Murgas L.D.S., Zangeronimo M.G., Pontelo T.P., Menezes T.A. & Silva A.C. 2013b. Porcine follicular fluid concentration of free insulin-like growth factor-I collected from different diameter ovarian follicles. Pesq. Vet. Bras. 33:1269-1274.
- Oberlender G., Murgas L.D.S., Zangeronimo M.G., Silva A.C., Menezes T.A., Pontelo T.P. & Pereira L.J. 2012. Aspectos relacionados à ocorrência da polispermia durante a fertilização in vitro (FIV) em suínos. Rev. Cient. Eletr. Med. Vet. 19:1-28.
- Oberlender G., Murgas L.D.S., Zangeronimo M.G., Silva A.C., Menezes T.A., Pontelo T.P. & Vieira L.A. 2013a. Role of insulin-like growth factor-I and follicular fluid from ovarian follicles with different diameters on porcine oocyte maturation and fertilization in vitro Theriogenology 80:319-327.
- Ocampo M.B., Ocampo L.C., Mori T., Ueda J. & Kanagawa H. 1994. Timing of sequential changes in chromosome configurations during the second meiotic division and cytoplasmic events of pig oocytes matured and fertilized in vitro Anim. Reprod. Sci. 34:281-288.
- Park C.H., Lee S.G., Choi D.H. & Lee C.K. 2009. A modified swim-up method reduces polyspermy during in vitro fertilization of porcine oocytes. Anim. Reprod. Sci. 115:169-181.
- Parrish J.J., Krogenaes A. & Susko-Parrish J.L. 1995. Effect of bovine sperm separation by either swim-up or Percoll method on success of in vitro fertilization and early embryonic development. Theriogenology 44:859-869.
- Petters R.M. & Wells K.D. 1993. Culture of pig embryos. J. Reprod. Fertil. Suppl. 48:61-73.
- Pursel V.G. & Johnson L.A. 1975. Freezing of boar spermatozoa; fertilizing capacity with concentrated semen and a new thawing procedure. J. Anim. Sci. 40:99-102.
- Rath D., Long C.R., Dobrinsky J.R., Welch G.R., Schreier L.L. & Johnson L.A. 1999. In vitro production of sexed embryos for gender preselection: high-speed sorting of X-chromosome-bearing sperm to produce pigs after embryo transfer. J. Anim. Sci. 77:3346-3352.
- Romar R., Coy P. & Rath D. 2012. Maturation conditions and boar affect timing of cortical reaction in porcine oocytes. Theriogenology 78:1126-1139.
- Romar R., Funahashi H. & Coy P. 2016. In vitro fertilization in pigs: New molecules and protocols to consider in the forthcoming years. Theriogenology 85:125-134.
- Satake N., Elliott R.M., Watson P.F. & Holt W.V. 2006. Sperm selection and competition in pigs may be mediated by the differential motility activation and suppression of sperm subpopulations within the oviduct. J. Exp. Biol. 209:1560-1572.
- Suzuki H., Saito Y., Kagawa N. & Yang X. 2003. In vitro fertilization and polyspermy in the pig: factors affecting fertilization rates and cytoskeletal reorganization of the oocyte. Microsc. Res. Tech. 61:327-334.
- Tokeshi I., Yoshimoto T., Muto N., Nakamura S., Ashizawa K., Nakada T. & Tatemoto H. 2007. Antihyaluronidase action of ellagic acid effectively prevents polyspermy as a result of suppression of the acrosome reaction induced by sperm-zona interaction during in vitro fertilization of porcine oocytes. J. Reprod. Dev. 53:755-764.
- Wang W.H., Abeydeera L.R., Han Y., Prather R.S. & Day B.N. 1999. Morphologic evaluation and actin filament distribution in porcine embryos produced in vitro and in vivo Biol. Reprod. 60:1020-1028.
- Wang W.H., Day B.N. & Wu G.M. 2003. How does polyspermy happen in mammalian oocytes? Micr. Res. Tech. 61:335-341.
- Zhang W., Yi K., Yan H. & Zhou X. 2012. Advances on in vitro production and cryopreservation of porcine embryos. Anim. Reprod. Sci. 132:115-122.
Publication Dates
-
Publication in this collection
June 2016
History
-
Received
27 Sept 2015 -
Accepted
13 Apr 2016