ABSTRACT:
The aim of the present study was to evaluate the intracytoplasmic lipid content, development and cryotolerance of in vitro-produced bovine embryos treated with different concentrations of forskolin before vitrification. Embryos were produced from abattoir-derived ovaries and allocated into four groups. In the treatment groups, forskolin was added to the in vitro culture medium on Day 6 and incubated for 24 hours in one of the following concentrations: 2.5μM (Forsk 2.5 group), 5.0μM (Forsk 5.0 group) or 10.0μM (Forsk 10.0 group). Embryos from the control group were cultured without forskolin. On Day 7 of culture, the expanded blastocysts were stained with the lipophilic dye Sudan Black B for determination of the intracytoplasmic lipid content or were cryopreserved via the Vitri-Ingá® procedure. Although there were no significant differences (P>0.05) in the blastocyst rates between the Control group (44.9%) and the other treatments, the embryo production was lower (P<0.05) in Forsk 10.0 group (38.8%) compared to Forsk 2.5 (50.5%) and Forsk 5.0 (54.7%) groups. The intracytoplasmic lipid content (expressed in arbitrary units of pixels) in blastocysts from the Control group (1.00±0.03) was similar (P>0.05) to that found in Forsk 2.5 (0.92±0.03) and Forsk 10.0 groups (1.06±0.03) groups; however the lipid accumulation in blastocysts from Forsk 5.0 group (0.82±0.04) was lower than in the Control group (P<0.05). Based on these results, Forsk 5.0 treatment was tested for cryotolerance and it was observed that the blastocoel re-expansion rate evaluated 24 hours after warming was greater (P<0.05) in Forsk 5.0 group (72.2%) compared to the Control group (46.2%). In conclusion, pre-treatment with forskolin at a concentration of 5.0 μM for 24 hours before vitrification is effective in reducing the intracytoplasmic lipid content and, consequently, improves cryotolerance of IVP bovine embryos.
INDEX TERMS:
Lipid content; cryotolerance; forskolin; vitrification; in vitro production; embryo; bovine; intracytoplasmic lipid accumulation
RESUMO:
Os embriões foram produzidos a partir de ovários obtidos em abatedouro e foram alocados em quatro grupos experimentais. Nos grupos tratados, o forskolin foi adicionado ao meio de cultivo in vitro no dia 6 do cultivo e os embriões foram incubados durante 24 horas com uma das seguintes concentrações: 2,5μM (grupo Forsk 2,5), 5,0μM (grupo Forsk 5,0) ou 10,0μM (grupo Forsk 10,0). Os embriões do grupo controle foram cultivados na ausência de forskolin. No dia 7 do cultivo, os blastocistos expandidos foram corados com o corante lipofílico Sudan Black B para a determinação do teor de lípidos intracitoplasmáticos ou foram criopreservados através do protocolo Vitri-Ingá®. Não foi observada diferença significativa (P>0,05) na taxa de produção de blastocistos entre o grupo Controle (44,9%) e os demais tratamentos, todavia observou-se menor produção de embriões (P<0,05) no grupo Forsk 10,0 (38,8%) em comparação com os grupos Forsk 2,5 (50,5%) e Forsk 5,0 (54,7%). A quantidade de lipídeos intracitoplasmáticos do grupo Controle (1,00±0,03) foi semelhante (P>0,05) a dos grupos Forsk 2,5 (0,92±0,03) e Forsk 10,0 (1,06±0,03); no entanto, o acúmulo de lípidos nos blastocistos do grupo Forsk 5.0 (0,82 ± 0,04) foi menor do que no grupo controle (P<0,05). A partir destes resultados, o grupo Forsk 5,0 foi testado quanto à criotolerância e foi observado que a taxa de re-expansão da blastocele 24 horas após o aquecimento foi maior (P<0,05) no grupo Forsk 5,0 (72,2%) quando comparado ao grupo Controle (46,2%). Em conclusão, o pré-tratamento com forskolin na concentração de 5,0 μM durante 24 horas antes da vitrificação foi eficiente para promover a redução da quantidade de lipídeos intracitoplasmáticos e, consequentemente, melhorou a criotolerância de embriões bovinos produzidos in vitro.
TERMOS DE INDEXAÇÃO:
Lipídios intracitoplasmáticos; criotolerância; forskolin; vitrificação; produção in vitro; embrião; bovino; acúmulo lipídico intracitoplasmático
Introduction
In the last decades, the introduction of innovating reproductive biotechnologies in agriculture allowed this field to grow significantly and increased the efficiency and profitability of livestock (Blondin 2015Blondin P. 2015. Status of embryo production in the world. Anim. Reprod. 12(3):356-358.). Currently, the global production of bovine embryos produced in vitro (IVP) exceeded the half million mark, with a total of 517,587 embryos produced from oocytes recovered from females by transvaginal ovum pick-up (OPU). Of this total of IVP embryos, 70.8% were produced in Brazil and most of them were freshly transferred (301,552 embryos were transferred in 2013, of which 95% were fresh and 5% were frozen) (Perry 2015Perry G. 2015. 2013. Statistics of embryo collection and transfer in domestic farm animals. Embryo Transfer Newsletter 33:14-26.).
On the other hand, embryos produced in vivo are less sensitive to cryopreservation than those produced in vitro (Abe et al., 2002Abe H., Yamashita S., Satoh T. & Hoshi H. 2002. Accumulation of cytoplasmic lipid droplets in bovine embryos and cryotolerance of embryos developed in different culture systems using serum-free or serum containing media. Molec. Reprod. Develop. 61:57-66., Rizos et al. 2003Rizos D., Guitiérrez-Adán A., Pérez-Garnelo S., De La Fuente J., Boland M.P. & Lonergan N.P. 2003. Bovine embryo culture in the presence or absence of serum: implications for blastocyst development, cryotolerance, and messenger RNA expression. Biol. Reprod. 68:236-243., Pereira et al. 2008Pereira R.M., Carvalhais I., Pimenta J., Baptista M.C., Vasques M.I., Horta A.E.M., Santos I.C., Marques M.R., Reis A., Silva Pereira M. & Marques C.C. 2008. Biopsied and vitrified bovine embryos viability is improved by trans10, cis12 conjugated linoleic acid supplementation during in vitro embryo culture. Anim. Reprod. Sci. 106:322-332.). Currently, the cryopreservation of in vivo-produced embryos is routinely used in commercial programs of embryo transfer (ET). According to the International Embryo Transfer Society (IETS), in 2013 almost 60% of transfers of in vivo-derived bovine embryos were carried out after thawing of cryopreserved embryos (Perry 2015Perry G. 2015. 2013. Statistics of embryo collection and transfer in domestic farm animals. Embryo Transfer Newsletter 33:14-26.). Thus, cryopreservation of embryos is a key tool for storage and exchange of genetic resources of farm animals (Almiñana & Cuello 2015Almiñana C. & Cuello C. 2015. What is new in the cryopreservation of embryos? Anim. Reprod. 12(3):418-427.).
The reduced resistance of IVP embryos to cryopreservation (Abe et al. 2002Abe H., Yamashita S., Satoh T. & Hoshi H. 2002. Accumulation of cytoplasmic lipid droplets in bovine embryos and cryotolerance of embryos developed in different culture systems using serum-free or serum containing media. Molec. Reprod. Develop. 61:57-66., Rizos et al. 2003Rizos D., Guitiérrez-Adán A., Pérez-Garnelo S., De La Fuente J., Boland M.P. & Lonergan N.P. 2003. Bovine embryo culture in the presence or absence of serum: implications for blastocyst development, cryotolerance, and messenger RNA expression. Biol. Reprod. 68:236-243., Pereira et al. 2008Pereira R.M., Carvalhais I., Pimenta J., Baptista M.C., Vasques M.I., Horta A.E.M., Santos I.C., Marques M.R., Reis A., Silva Pereira M. & Marques C.C. 2008. Biopsied and vitrified bovine embryos viability is improved by trans10, cis12 conjugated linoleic acid supplementation during in vitro embryo culture. Anim. Reprod. Sci. 106:322-332.) can be partially attributed to the effects of some components of the in vitro culture (IVC) medium, especially fetal cow serum (FCS). The FCS may interfere with embryonic lipid metabolism in three different ways: increasing the synthesis of lipids (especially triacylglycerides) in embryos (Abd El Razek et al. 2000Abd El Razek I.M., Charpigny G., Kodja S., Marquant-Leguienne B., Mermillod P. & Guyader-Joly C. 2000. Differences in lipids composition between in vivo and in vitro produced bovine embryos. Theriogenology 53:12-19.); increasing the lipid content via internalization of lipoproteins present in serum (Ferguson & Leese 1999Ferguson E.M. & Leese H.J. 1999. Triglyceride content of bovine oocytes and early embryos. J. Reprod. Fert. 116:373-378., Sata et al. 1999Sata R., Tsujii H., Abe H., Yamashita S. & Hochi H. 1999. Fatty acid composition of bovine embryos cultured in serum-free and serum-containing médium during early embryonic development. J. Reprod. Develop. 45:97-103.); or altering lipid metabolism in the mitochondria, mainly by increasing the intracellular lipid stocks (Abe & Hoshi 2003Abe H. & Hoshi H. 2003. Evaluation of bovine embryos produced in high performance serum-free media. J. Reprod. Develop. 49:193-202.). The large amount of lipid droplets in the cytoplasm of in vitro produced embryos, especially in the early stages of development, makes the embryos more sensitive to chilling injuries (Almiñana & Cuello 2015Almiñana C. & Cuello C. 2015. What is new in the cryopreservation of embryos? Anim. Reprod. 12(3):418-427.).
To overcome the problem of excessive lipid accumulation and thus improve the embryo survival after cryopreservation, two strategies can be pursued: developing better cryopreservation techniques or modifying the molecular composition of the embryos during IVC to render them more cryotolerant (Massip et al. 1995Massip A., Mermillod P. & Dinnyes A. 1995. Morphology and biochemistry of in vitro produced bovine embryos: implications for their cryopreservation. Hum. Reprod. 10(11):3004-3011., Seidel 2006Seidel Jr G.E. 2006. Modifying oocytes and embryos to improve their cryopreservation. Theriogenology 65:228-235., Gómez et al. 2008Gómez E., Rodríguez A., Muñoz M., Caamaño J.N., Hidalgo C.O., Morán E., Facal N. & Díez C. 2008. Serum free embryo culture medium improves in vitro survival of bovine blastocysts to vitrification. Theriogenology 69:1013 1021.). In this regard, some strategies have been initially proposed to prevent the excessive lipid accumulation in the in vitro-produced embryos, such as mechanical removal of lipids before cryopreservation (Nagashima et al. 1995Nagashima H., Kashiwazaki N., Ashman R.J., Grupen C.G. & Nottlen M. 1995. Cryopreservation of porcine embryos. Nature 1995:374-416.). However, this invasive method changes the developmental potential of blastocysts transferred to recipients (Diez et al. 2001Diez C., Heyman Y., Le Bourhis D., Guyader-Joly C., Degrouard J. & Renard J.P. 2001. Delipidating in vitro-produced bovine zygotes: effect on further development and consequences for freezability. Theriogenology 55:923-936.). Moreover, the mechanical delipidation substantially increases the chance of transmission of pathogens because of the zona pellucida damage induced by micromanipulation (Stringfellow 1998Stringfellow D.A. 1998. Recommendations for the sanitary handling of in vivo derived embryos, p.79-84. In: Stringfellow D.A. & Seidel S.M. (Eds), Manual of the International Embryo Transfer Society, Savoy, IL.).
A partial delipidation induced by chemical agents has been proposed as a more appropriate strategy. Several drugs have lipolytic effect, including norepinhephrine, dibutyryl cyclic adenosine monophosphate (dbcMP), isoproterenol, forskolin and theophyline. Forskolin has been reported to increase the cryosurvival of porcine blastocysts produced in vitro by reducing their intracellular lipid content, especially triacylglycerols (Men et al. 2006Men H., Agca Y., Riley L.K. & Critser J.K. 2006. Improved survival of vitrified porcine embryos after partial delipation through chemically stimulated lipolysis and inhibition of apoptosis. Theriogenology 66:2008-2016.). Although data concerning the effect of forskolin on bovine IVP embryos are more scarce than for pig, recent studies demonstrated an improvement in bovine embryo survival and quality after vitrification (Paschoal et al. 2012Paschoal D.M., Sudano M.J., Guastali M.D., Maziero R.R.D., Crocomo L.F., Magalhães L.C.O., Rascado T.S., Martins Jr A. & Landim-Alvarenga F.C. 2012. Forskolin effect on the cryosurvival of in vitro-produced bovine embryos in the presence or absence of fetal calf serum. Zygote 22:146-157., Sanches et al. 2013Sanches B.V., Marinho L.S.R., Oliveira Filho B.D., Pontes J.H.F., Basso A.C., Meirinhos M.L.G., Silva-Santos K.C., Ferreira C.R. & Seneda M.M. 2013. Cryosurvival and pregnancy rates after exposure of IVF-derived Bos indicus embros to forskolin before vitrification. Theriogenology 80:372-377.).
Forskolin acts by activating the adenylate cyclase that regulates cyclic adenosine monophosphate (cAMP) levels in cells (Seamon et al. 1981Seamon K.B., Padgett W. & Daly J.W. 1981. Forskolin: unique diterpene activator of adenylate cyclase in membranes in intact cells. Proc. Natl Acad. Sci. USA 78:3363-3367., Ho & Shi 1982Ho R.J. & Shi Q.H. 1982. Forskolin as a novel lipolytic agent. Biochem. Biophys. Res. Commun. 107:157-164., Ek et al. 1997Ek I., Arner P., Bergqvist A., Carlström K. & Wahrenberg H. 1997. Impaired adipocyte lipolysis in nonobese women with the polycystic ovary syndrome: a possible link to insulin resistance? J. Clin. Endocrinol. Metabol. 82:1147-1153., Arrese et al. 1999Arrese E.L., Matthew T.F., Gazard J.L. & Wells M.A. 1999. Calcium and cAMP are second messengers in the adipokinetic hormone-induced lipolysis of triacylglycerols in Manduca sexta fat body. J. Lip. Res. 40:556-64.). CAMP is an important signaling molecule that regulates the physiological functions of various systems (Seamon et al. 1981Seamon K.B., Padgett W. & Daly J.W. 1981. Forskolin: unique diterpene activator of adenylate cyclase in membranes in intact cells. Proc. Natl Acad. Sci. USA 78:3363-3367., Ho & Shi 1982Ho R.J. & Shi Q.H. 1982. Forskolin as a novel lipolytic agent. Biochem. Biophys. Res. Commun. 107:157-164., Ek et al. 1997Ek I., Arner P., Bergqvist A., Carlström K. & Wahrenberg H. 1997. Impaired adipocyte lipolysis in nonobese women with the polycystic ovary syndrome: a possible link to insulin resistance? J. Clin. Endocrinol. Metabol. 82:1147-1153., Arrese et al. 1999Arrese E.L., Matthew T.F., Gazard J.L. & Wells M.A. 1999. Calcium and cAMP are second messengers in the adipokinetic hormone-induced lipolysis of triacylglycerols in Manduca sexta fat body. J. Lip. Res. 40:556-64.), including the regulation of lipolysis (Lafontan & Berlan 1993Lafontan M. & Berlan M. 1993. Fat cell adrenergic receptors and the control of white and brown fat cell function. J. Lip. Res. 34:1057-1091.). Although the delipidation induced by forskolin have improved cryotolerance of bovine embryos (Paschoal et al. 2012Paschoal D.M., Sudano M.J., Guastali M.D., Maziero R.R.D., Crocomo L.F., Magalhães L.C.O., Rascado T.S., Martins Jr A. & Landim-Alvarenga F.C. 2012. Forskolin effect on the cryosurvival of in vitro-produced bovine embryos in the presence or absence of fetal calf serum. Zygote 22:146-157., Sanches et al. 2013Sanches B.V., Marinho L.S.R., Oliveira Filho B.D., Pontes J.H.F., Basso A.C., Meirinhos M.L.G., Silva-Santos K.C., Ferreira C.R. & Seneda M.M. 2013. Cryosurvival and pregnancy rates after exposure of IVF-derived Bos indicus embros to forskolin before vitrification. Theriogenology 80:372-377.), the effects of increasing cAMP levels are unspecific on the metabolism (Seamon et al. 1981Seamon K.B., Padgett W. & Daly J.W. 1981. Forskolin: unique diterpene activator of adenylate cyclase in membranes in intact cells. Proc. Natl Acad. Sci. USA 78:3363-3367., Ho & Shi 1982Ho R.J. & Shi Q.H. 1982. Forskolin as a novel lipolytic agent. Biochem. Biophys. Res. Commun. 107:157-164., Ek et al. 1997Ek I., Arner P., Bergqvist A., Carlström K. & Wahrenberg H. 1997. Impaired adipocyte lipolysis in nonobese women with the polycystic ovary syndrome: a possible link to insulin resistance? J. Clin. Endocrinol. Metabol. 82:1147-1153., Arrese et al. 1999Arrese E.L., Matthew T.F., Gazard J.L. & Wells M.A. 1999. Calcium and cAMP are second messengers in the adipokinetic hormone-induced lipolysis of triacylglycerols in Manduca sexta fat body. J. Lip. Res. 40:556-64.), so it is possible that forskolin can induce effects on embryonic cells that are still unknown (Sanches et al. 2013Sanches B.V., Marinho L.S.R., Oliveira Filho B.D., Pontes J.H.F., Basso A.C., Meirinhos M.L.G., Silva-Santos K.C., Ferreira C.R. & Seneda M.M. 2013. Cryosurvival and pregnancy rates after exposure of IVF-derived Bos indicus embros to forskolin before vitrification. Theriogenology 80:372-377.). For this reason, it is necessary to establish the lowest effective dose of this drug for purpose of inducing lipolysis in IVP embryos.
Therefore, the aim of this study was to evaluate the effects of different concentrations of forskolin added during in vitro culture on the total amount of intracellular lipids and cryotolerance of IVP bovine embryos after vitrification.
Materials and Methods
Chemicals, reagents and media. Chemicals were purchased from Sigma Chemical Co. (St Louis, MO, USA) unless otherwise stated. Plastic materials were obtained from Corning Inc. (Acton, MA, USA). The medium for in vitro maturation (IVM) consisted of TCM199 (Gibco®, Invitrogen Co., Grand Island, NY, USA) supplemented with 10% (v:v) FCS (Gibco®, Invitrogen Co.), 0.2mM sodium pyruvate, 25mM sodium bicarbonate, 50μg/mL amikacin, 0.5μg/mL FSH (Folltropin-V, Bioniche Animal Health, Ontario, Canada), and 100 IU/mL hCG (Vetecor®, Hertape Calier, Juatuba, MG, Brazil). The medium for in vitro fertilization (IVF) consisted of Tyrode’s albumin lactate pyruvate (TALP) containing 0.2mM Na-pyruvate, 6mg/mL fraction V fatty acid-free bovine serum albumin (BSA), 25mM sodium bicarbonate, 13mM Na-lactate, 50μg/mL amikacin, 40μL/mL PHE solution (1mM hypotaurine, 2mM penicillamine and 250μM epinephrine) and 10μg/mL heparin, as previously described (Parrish et al. 1988Parrish J.J., Susko-Parrish J.L. & First N.L. 1988. Capacitation of bovine sperm by heparin. Biol. Reprod. 38:1171-1180.). The medium for IVC of embryos consisted of modified synthetic oviductal fluid (SOF), as previously described (Vajta et al. 1999Vajta G., Rindom N., Peura T.T., Holm P., Greve T. & Callesen H. 1999. The effect of media, serum and temperature on in vitro survival of bovine blastocysts after open pulled straw (OPS) vitrification. Theriogenology 52:939-948.), supplemented with 50μg/mL amikacin, 5mg/mL BSA and 2.5% (v:v) FCS. Milimolar stock concentrations of forskolin were stored at 20oC dissolved in anydrous dimethylsulphoxide (DMSO) solutions and were freshly diluted each day of the experiment to obtain a final concentration of 10μM, 5μM and 2.5μM. The maximum final concentration of DMSO in the cultures was 0.08% (according to previous results from Stinshoff et al. (2014)Stinshoff H., Wilkening S., Hanstedt A., Bollwein H. & Wrenzycki C. 2014. Dimethylsulfoxide and conjugated linoleic acids affect bovine embryo development in vitro. Reprod. Fertil. Dev. 26(4):502-510., the presence of 0.2% DMSO in the culture medium exerted no toxic effects on the developing embryos compared to control).
Oocyte collection and maturation. Ovaries from slaughtered cows were obtained in a local abattoir and transported to the laboratory. Intact cumulus-oocyte complexes (COCs) were aspirated from antral follicles (3 to 8mm in diameter), and oocytes with at least four layers of cumulus cells with homogenous cytoplasm were selected for the experiments. Selected COCs (n=848) were washed and cultured in microdrops of 100μL of IVM medium (20 oocytes/microdrop), covered with mineral oil for 22 hours at 38.5oC in an atmosphere of 5% CO2 in air with maximum humidity.
In vitro fertilization. Following IVM, oocytes were subjected to fertilization with frozen semen from a single batch from a Nellore bull (Bos taurus indicus). Motile spermatozoa were obtained by centrifugation of the frozen-thawed semen on a Percoll (GE Healthcare, Uppsala, Sweden) discontinuous density gradient (250μL of 45% Percoll over 250μL of 90% Percoll in a 1.5mL microtube) for 7 min at 2500×g at room temperature. Sperm cells were added to the fertilization droplet at 2×106 cells/mL. The COCs (20 per 90μL droplet overlaid with mineral oil) and spermatozoa were coincubated for 18 hours at 38.5oC in an atmosphere of 5% CO2 in air, with maximum humidity. The day of fertilization was defined as Day 0 (D0).
Embryo culture. Following fertilization, the presumptive zygotes were partially stripped from the cumulus cells by gentle pipetting and subsequently washed three times in TALP medium and twice in IVC medium. Zygotes were cultured in 500μL of IVC medium (control group), supplemented on D6 with forskolin in one of the following concentrations: (A) 2.5μM (group Forsk 2.5); (B) 5μM (group Forsk 5.0); or (C) 10.0μM (group Forsk 10.0). Embryos were cultured without mineral oil (20 embryos per well) on a monolayer of cumulus cells in a 4-well cell culture plate. The culture was carried out at 38.5oC in an atmosphere of 5% CO2 in air, with maximum humidity. Cleavage rates were assessed under stereoscopic microscopy at 40× magnification at 72 hours post-insemination (h.p.i.), and blastocyst development rates were recorded at 168 h.p.i. (Day 7).
Quantification of intracellular lipid content by Sudan Black B staining. The intracellular lipid content in D7-expanded blastocysts (n=99) was quantified using the lipophilic dye Sudan Black B. Embryos were fixed in 1ml 4% paraformaldehyde in PBS, pH 7.4, for 2 hours at room temperature. Then, they were washed in PBS containing 0.5% PVP and then transferred to drops of 50% ethanol in distilled water. After 2 min, embryos were stained in drops of 1% Sudan Black B (w/v) in 70% ethanol for 2 min, then they were washed 3 times with 50% ethanol, 5 min each, followed by a 5 min wash in 0.5% PVP in distilled water. Prepared embryos were mounted in 10-μL drop of glycerol on cover slips and examined under a light microscope at at 40× magnification. Stained embryos were imaged immediately using an Olympus, IX51 inverted microscope running image Q-Capture Pro Image software (Media Cybernetics, Inc., Version 5.0.1.26) to quantify the area and intensity of pixels from embryos. Images were converted to gray scale and the background signal intensity was subtracted from the measured values of the treatment micrographs. The control group was chosen as a calibrator and the measured value of each treatment micrograph was divided by the mean of the calibrator to generate the relative expression levels of pixels (expressed in arbitrary units). The means of the relative expression values were plotted graphically with error bars representing the standard error of the mean (SEM).
Vitrification, warming and subsequent embryo culture. All materials used for the vitrification-warming procedures were supplied by Ingámed (Perobal, Brazil), including the vitrification solutions (VI-I and VI-II), warming solutions (DV-I, DV-II and DV-III), vitrification strips (Vitri-Ingá) and their plastic sheaths. Expanded blastocysts (grades 1 and 2, classified according to the IETS Manual (Wright, 2009Wright J.M. 2009. Apêndice D: ilustrações fotográficas do estágio de desenvolvimento embrionário e códigos de qualidade, p.165-168. In: Ibid. (Ed.), Manual da Sociedade Internacional de Transferência de Embriões. 4ª ed. Savoy) were washed twice in holding medium (SOF supplemented with 250 mM HEPES and 20% of FCS) and transferred to VI-I for 5 min at 37oC. Subsequently, the embryos were transferred to a 10-μL drop of VI-II for 60s at room temperature. Then, the embryos were placed in the hole in the tip of a Vitri-Ingá strip with a minimum amount of vitrification solution and immersed directly in liquid nitrogen (N2). The plastic sheaths, that had been cooled previously in N2 liquid vapour for 1 min, were immersed vertically in the liquid N2. The Vitri-Ingá strips were then inserted into the plastic sheaths for safe storage. For the warming procedure, the Vitri-Ingá strips were withdrawn from the liquid N2 and transferred to the DV-I warming solution for 1 min at 37oC. Subsequently, embryos were transferred to DV-II for 3 min at room temperature and washed twice in DV-III for 5 min each time. The embryos were next washed twice in holding medium and cultured in SOF supplemented with 2.5% (v:v) FCS and 5mg/mL BSA under saturated humidity and 5% CO2 for 24 hours. Thereafter, embryos were evaluated to determine the blastocoel re-expansion rates.
Statistical analysis. The experiments were replicated at least four times, on different days. The percentage of embryos at specific stages of development was estimated by considering the total number of oocytes in each maturation well. Blastocysts rates and re-expansion frequency were analysed with a Chi-squared test. Intracellular lipid content was compared by ANOVA. When a statistically significant effect was found, Tukey’s test was applied for multiple comparisons of means. The data were analyzed using JMP statistical software version 5.0.1a (SAS Inst. Inc., Cary, NC, USA). The level of statistical significance was set at P<0.05.
Results
The effects of different concentrations of forskolin during IVC on cleavage and blastocyst rates are given in Table 1. There was no effect (P>0.05) of forskolin on cleavage rates (mean 81.7%). Embryo development to the blastocyst stage was higher (P<0.05) when forskolin was added to IVC medium at 2.5μM (50.5%) and 5.0μM (54.7%) than at 10.0μM (38.8%). However, none of these treatments differ (P>0.05) from the control group (44.9%).
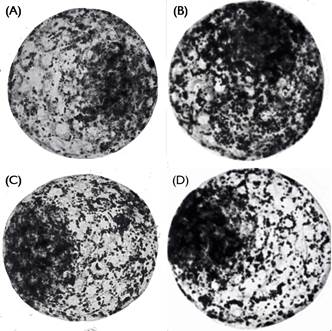
Intracellular lipid content of expanded blastocysts (expressed as expressed in arbitrary units of pixels; Fig.1) was similar (P>0.05) between the control group (1.00±0.03) and Forsk 2.5 (0.92±0.03) and Forsk 10.0 (1.06±0.03) groups. However, the intracellular lipid content was decreased (P<0.05) in Forsk 5.0 group (0.82±0.04) compared to the others. Representative photomicrographs of bovine embryos stained with Sudan Black B are shown in Figure 2.
Effect of forskolin supplementation during in vitro culture on intracellular lipid content of expanded blastocysts. Control: embryos (n=31) were cultured in vitro in IVC medium (SOF suplemented with 2.5% SFB and 2.5% BSA); Forsk 2.5: embryos (n=23) were cultured in vitro in IVC medium suplemented with 2.5μM forskolin from the 6th day (D6) to the end of the culture (D7); Forsk 5.0: embryos (n=22) were cultured in vitro in IVC medium suplemented with 5.0μM forskolin from D6 to D7; Forsk 10.0: embryos (n=23) were cultured in vitro in IVC medium suplemented with 10.0μM forskolin from D6 to D7. Treatments were repeated in four replicates. The data represent the mean±SEM. abc Different letters indicate significant differences between treatments (P<0.05, Tukey’s test).
Representative photomicrographs of bovine embryos stained with Sudan Black B for evaluation of intracellular lipid content. (A) Control, (B) Forskolin 2.5μM; (C) Forskolin 5.0μM, and (D) Forskolin 10.0μM, 40× magnification. Images converted to gray scale.
As the treatment with 5.0μM forskolin was more effective to reduce the lipid content in the embryos, this group was tested for cryotolerance. It was observed that the re-expansion rates evaluated after 24 hours of in vitro culture was higher (P<0.05) in Forsk 5.0 group (71.4%) compared to control group (46.2%). This results are given in Table 2.
Discussion
There is growing evidence suggesting that the intrinsic quality of the oocyte is the key factor in determining the proportion of oocytes that develop to the blastocyst stage (Rizos et al. 2003Rizos D., Guitiérrez-Adán A., Pérez-Garnelo S., De La Fuente J., Boland M.P. & Lonergan N.P. 2003. Bovine embryo culture in the presence or absence of serum: implications for blastocyst development, cryotolerance, and messenger RNA expression. Biol. Reprod. 68:236-243., Lonergan et al. 2003Lonergan P., Rizos D., Gutierrez-Adan A., Fair T. & Boland M.P. 2003. Oocyte and embryo quality: effect of origin, culture conditions and gene expression patterns. Reprod. Dom. Anim. 38:259-267.). In this study, only oocytes of best quality (according to International Embryo Transfer Society standards) were selected for the experiments, and how the treatments were performed only in the embryos but not in the oocytes, it was expected that the blastocyst yields were unaffected by supplementation of IVC medium with forskolin in various concentrations. These results are in agreement with data from Paschoal et al. (2012)Paschoal D.M., Sudano M.J., Guastali M.D., Maziero R.R.D., Crocomo L.F., Magalhães L.C.O., Rascado T.S., Martins Jr A. & Landim-Alvarenga F.C. 2012. Forskolin effect on the cryosurvival of in vitro-produced bovine embryos in the presence or absence of fetal calf serum. Zygote 22:146-157., which did not observe differences in blastocyst yield of embryos treated with (46.3% and 34.2% in medium supplemented with or without FCS, respectively) or without 10μM forskolin (46.8% and 33.3% in medium supplemented with or without FCS, respectively) during 24 hours of IVC. Likewise, Sanches et al. (2013)Sanches B.V., Marinho L.S.R., Oliveira Filho B.D., Pontes J.H.F., Basso A.C., Meirinhos M.L.G., Silva-Santos K.C., Ferreira C.R. & Seneda M.M. 2013. Cryosurvival and pregnancy rates after exposure of IVF-derived Bos indicus embros to forskolin before vitrification. Theriogenology 80:372-377. found no differences in blastocysts production rates after treatment of IVC embryos with 10μM forskolin for 48 hours (45.3%) compared to untreated embryos (39.9%).
On the other hand, growing evidence indicates that the post-fertilization culture environment, rather than the origin of the oocytes, is the main factor determining blastocyst quality (Rizos et al. 2003Rizos D., Guitiérrez-Adán A., Pérez-Garnelo S., De La Fuente J., Boland M.P. & Lonergan N.P. 2003. Bovine embryo culture in the presence or absence of serum: implications for blastocyst development, cryotolerance, and messenger RNA expression. Biol. Reprod. 68:236-243., Lonergan et al. 2003Lonergan P., Rizos D., Gutierrez-Adan A., Fair T. & Boland M.P. 2003. Oocyte and embryo quality: effect of origin, culture conditions and gene expression patterns. Reprod. Dom. Anim. 38:259-267.). Although the addition of forskolin did not affect embryonic development rates in the present study, the intracellular lipid content was lowered and, consequently, the cryoresistence was increased in embryos treated with 5.0μM forskolin during 24 hours before vitrification. Such observations highlight the importance of the post-fertilization culture environment for the quality of the resulting blastocyst. Although the term "quality" is very unspecific and the parameters that allow the classification of embryos in different quality grades were not well established until the present time, several studies have evaluated the quality in terms of specifics aspects like apoptosis rates, cytoplasmic accumulation of reactive oxygen species, mitochondrial membrane potential, lipid accumulation, resistance to freezing and even pregnancy rates after transfer to synchronized recipients (Accorsi et al. 2016Accorsi M.F., Leão B.C.S., Rocha-Frigoni N.A.S., Perri S.H.V. & Mingoti G.Z. 2016. Reduction in cytoplasmic lipid content in bovine embryos cultured in vitro with linoleic acid in semi-defined medium is correlated with increases in cryotolerance. Zygote 24(4):485-494., Rocha-Frigoni et al. 2014Rocha-Frigoni N.A.S., Leão B.C.S., Nogueira É., Accorsi M.F. & Mingoti G.Z. 2014. Reduced levels of intracellular reactive oxygen species and apoptotic status are not correlated with increases in cryotolerance of bovine embryos produced in vitro in the presence of antioxidants. Reprod. Fertil. Dev. 26:797-805., Sanches et al. 2013Sanches B.V., Marinho L.S.R., Oliveira Filho B.D., Pontes J.H.F., Basso A.C., Meirinhos M.L.G., Silva-Santos K.C., Ferreira C.R. & Seneda M.M. 2013. Cryosurvival and pregnancy rates after exposure of IVF-derived Bos indicus embros to forskolin before vitrification. Theriogenology 80:372-377., Paschoal et al. 2012Paschoal D.M., Sudano M.J., Guastali M.D., Maziero R.R.D., Crocomo L.F., Magalhães L.C.O., Rascado T.S., Martins Jr A. & Landim-Alvarenga F.C. 2012. Forskolin effect on the cryosurvival of in vitro-produced bovine embryos in the presence or absence of fetal calf serum. Zygote 22:146-157., Pereira et al. 2008Pereira R.M., Carvalhais I., Pimenta J., Baptista M.C., Vasques M.I., Horta A.E.M., Santos I.C., Marques M.R., Reis A., Silva Pereira M. & Marques C.C. 2008. Biopsied and vitrified bovine embryos viability is improved by trans10, cis12 conjugated linoleic acid supplementation during in vitro embryo culture. Anim. Reprod. Sci. 106:322-332.). Thus, the present results demonstrated an increase in the quality of in vitro-produced bovine embryos treated with forskolin, measured in terms of cryotolerance.
The addition of FCS in the culture medium is one of the main factors responsible for causing morphological and physiological differences (Abe et al. 1999Abe H., Yamashita S., Itoh T., Satoh T. & Hoshi H. 1999. Ultrastructure of bovine embryos developed from in vitro-matured and -fertilized oocytes: comparative morphological evaluation of embryos cultured either in serum-free medium or in serum-supplemented medium. Mol. Reprod. Dev. 53:325-335., Pereira et al. 2008Pereira R.M., Carvalhais I., Pimenta J., Baptista M.C., Vasques M.I., Horta A.E.M., Santos I.C., Marques M.R., Reis A., Silva Pereira M. & Marques C.C. 2008. Biopsied and vitrified bovine embryos viability is improved by trans10, cis12 conjugated linoleic acid supplementation during in vitro embryo culture. Anim. Reprod. Sci. 106:322-332.) in IVC embryos when compared to embryos produced in the absence of FCS (Rizos et al. 2003Rizos D., Guitiérrez-Adán A., Pérez-Garnelo S., De La Fuente J., Boland M.P. & Lonergan N.P. 2003. Bovine embryo culture in the presence or absence of serum: implications for blastocyst development, cryotolerance, and messenger RNA expression. Biol. Reprod. 68:236-243.) or embryos produced in vivo (Paschoal et al. 2012Paschoal D.M., Sudano M.J., Guastali M.D., Maziero R.R.D., Crocomo L.F., Magalhães L.C.O., Rascado T.S., Martins Jr A. & Landim-Alvarenga F.C. 2012. Forskolin effect on the cryosurvival of in vitro-produced bovine embryos in the presence or absence of fetal calf serum. Zygote 22:146-157.). Some of these differences include the increased number and size of lipid droplets (Abe et al. 1999Abe H., Yamashita S., Itoh T., Satoh T. & Hoshi H. 1999. Ultrastructure of bovine embryos developed from in vitro-matured and -fertilized oocytes: comparative morphological evaluation of embryos cultured either in serum-free medium or in serum-supplemented medium. Mol. Reprod. Dev. 53:325-335., Rizos et al. 2003Rizos D., Guitiérrez-Adán A., Pérez-Garnelo S., De La Fuente J., Boland M.P. & Lonergan N.P. 2003. Bovine embryo culture in the presence or absence of serum: implications for blastocyst development, cryotolerance, and messenger RNA expression. Biol. Reprod. 68:236-243.) and differences in the viability of embryos, evaluated by the percentage of damaged cells (Paschoal et al. 2012Paschoal D.M., Sudano M.J., Guastali M.D., Maziero R.R.D., Crocomo L.F., Magalhães L.C.O., Rascado T.S., Martins Jr A. & Landim-Alvarenga F.C. 2012. Forskolin effect on the cryosurvival of in vitro-produced bovine embryos in the presence or absence of fetal calf serum. Zygote 22:146-157..). To avoid the undesirable intracellular accumulation of lipids, several studies have tried to replace serum in the culture medium (Mingoti et al. 2009Mingoti G.Z., Caiado Castro V.S., Méo S.C., Barretto L.S. & Garcia J.M. 2009. The effect of interaction between macromolecule supplement and oxy- gen tension on bovine oocytes and embryos cultured in vitro. Zygote 17:321-382.). However, despite their potential adverse effects on embryo quality (Rizos et al. 2003Rizos D., Guitiérrez-Adán A., Pérez-Garnelo S., De La Fuente J., Boland M.P. & Lonergan N.P. 2003. Bovine embryo culture in the presence or absence of serum: implications for blastocyst development, cryotolerance, and messenger RNA expression. Biol. Reprod. 68:236-243.), the serum contains substances which are beneficial to the embryo development, such as growth factors and chelators of heavy metals (Stein 2007Stein A. 2007. Decreasing variability in your cell culture. Biotechniques 43:228-229.). For this reason, it has been difficult to avoid or find a suitable replacement for serum in media formulated for in vitro cultures, particularly for laboratories working with commercial purpose.
As the lipid accumulation has often been associated with loss of embryo viability and the increase in injuries caused by cryopreservation, this study investigated the effect of chemical lipolysis with the use of various concentrations of forskolin in an attempt to find the lower efficient concentration able to reduce the intracellular lipid accumulation in embryos produced in vitro in the presence of FCS, and results demonstrated a significant reduction in the cytoplasmic lipid content at the dose of 5.0μM forskolin. Although previous studies have been developed to evaluate the lipolytic effects of forskolin in bovine IVC embryos, it is not yet established the best protocol, including the most effective dose and duration of treatment. In a preliminary communication, Paschoal et al. (2015Paschoal D.M., Sudano M.J., Maziero R.R.D., Guastali M.D., Magalhães L.C.O., Landim-Alvarenga F.C., Martins Jr A. & Leal C.L.V. 2015. Decreased lipid granules of in vitro produced bovine embryos with low concentrations of forskolin. Anim. Reprod. 12:686.) found effective lipolytic activity when treating bovine embryos with low doses of forskolin (lipid measurement in control: 50.6 vs 2.5μM forskolin: 46.2), however these authors did not evaluate whether this low concentration of forskolin improved embryo quality in terms of cryotolerance. Paschoal et al. (2012)Paschoal D.M., Sudano M.J., Guastali M.D., Maziero R.R.D., Crocomo L.F., Magalhães L.C.O., Rascado T.S., Martins Jr A. & Landim-Alvarenga F.C. 2012. Forskolin effect on the cryosurvival of in vitro-produced bovine embryos in the presence or absence of fetal calf serum. Zygote 22:146-157. found similar re-expansion rates after warming of vitrified in vivo-derived embryos and embryos produced in vitro in the presence of forskolin at a dose of 10.0μM for 24 hours. Likewise, Sanches et al. (2013Sanches B.V., Marinho L.S.R., Oliveira Filho B.D., Pontes J.H.F., Basso A.C., Meirinhos M.L.G., Silva-Santos K.C., Ferreira C.R. & Seneda M.M. 2013. Cryosurvival and pregnancy rates after exposure of IVF-derived Bos indicus embros to forskolin before vitrification. Theriogenology 80:372-377.) found no differences in the rates of blastocoel re-expansion and hatching of embryos exposed to 10.0μM forskolin for 48 hours before vitrification (87.5% and 70.5%, respectively) compared with non-treated embryos (79.2% and 63.3%, respectively). Thus, the condition established in the present study (dose of 5.0μM forskolin for 24 hours before vitrification) seems to be more effective than those proposed in earlier studies.
As previously mentioned, the reduction of the effective dose of forskolin is desirable because this drug stimulates lipolysis via activation of adenylate cyclase with consequent increase in cAMP levels (Honnor et al. 1985Honnor R.C., Dhillon G.S. & Londos C. 1985. cAMP-dependent protein kinase and lipolysis in rat adipocytes. II. Definition of steadystate relationship with lipolytic and antilipolytic modulators. J. Biol. Chem. 260:15130-15138., Lafontan & Berlan 1993Lafontan M. & Berlan M. 1993. Fat cell adrenergic receptors and the control of white and brown fat cell function. J. Lip. Res. 34:1057-1091.). Since the effects of cAMP are unspecific, as this signaling molecule regulates several physiological functions (Seamon et al. 1981Seamon K.B., Padgett W. & Daly J.W. 1981. Forskolin: unique diterpene activator of adenylate cyclase in membranes in intact cells. Proc. Natl Acad. Sci. USA 78:3363-3367., Ho & Shi 1982Ho R.J. & Shi Q.H. 1982. Forskolin as a novel lipolytic agent. Biochem. Biophys. Res. Commun. 107:157-164., Ek et al. 1997Ek I., Arner P., Bergqvist A., Carlström K. & Wahrenberg H. 1997. Impaired adipocyte lipolysis in nonobese women with the polycystic ovary syndrome: a possible link to insulin resistance? J. Clin. Endocrinol. Metabol. 82:1147-1153., Arrese et al. 1999Arrese E.L., Matthew T.F., Gazard J.L. & Wells M.A. 1999. Calcium and cAMP are second messengers in the adipokinetic hormone-induced lipolysis of triacylglycerols in Manduca sexta fat body. J. Lip. Res. 40:556-64.), it is possible that forskolin can induce effects on embryonic cells that are still unknown (Sanches et al. 2013Sanches B.V., Marinho L.S.R., Oliveira Filho B.D., Pontes J.H.F., Basso A.C., Meirinhos M.L.G., Silva-Santos K.C., Ferreira C.R. & Seneda M.M. 2013. Cryosurvival and pregnancy rates after exposure of IVF-derived Bos indicus embros to forskolin before vitrification. Theriogenology 80:372-377.). When compared to previous studies (Sanches et al. 2013Sanches B.V., Marinho L.S.R., Oliveira Filho B.D., Pontes J.H.F., Basso A.C., Meirinhos M.L.G., Silva-Santos K.C., Ferreira C.R. & Seneda M.M. 2013. Cryosurvival and pregnancy rates after exposure of IVF-derived Bos indicus embros to forskolin before vitrification. Theriogenology 80:372-377., Paschoal et al. 2012Paschoal D.M., Sudano M.J., Guastali M.D., Maziero R.R.D., Crocomo L.F., Magalhães L.C.O., Rascado T.S., Martins Jr A. & Landim-Alvarenga F.C. 2012. Forskolin effect on the cryosurvival of in vitro-produced bovine embryos in the presence or absence of fetal calf serum. Zygote 22:146-157.), the results of the present study demonstrated that lower concentrations of forskolin was still effective in induction of cellular lipolysis, but more studies are needed in order to assess the effects of this drug on the metabolism, gene expression and embryonic proteome. However, as expected, the use of forskolin improved embryo survival after vitrification-warming as a result of reduction of intracytoplasmic lipid content. These findings confirm that the use of lipolytic agents is a valuable strategy to improve the quality of bovine embryos produced in vitro, aiming to produce blastocysts with higher rates of post-cryopreservation survival.
Conclusion
In conclusion, pre-treatment with 5.0uM forskolin for 24 hours before vitrification decreases the intracytoplasmic lipid content and improves the cryotolerance of IVP bovine embryos
Acknowledgements
Fapesp (#2013/07382-6), CAPES and CNPq (#306746/2012-3)
References
- Abd El Razek I.M., Charpigny G., Kodja S., Marquant-Leguienne B., Mermillod P. & Guyader-Joly C. 2000. Differences in lipids composition between in vivo and in vitro produced bovine embryos. Theriogenology 53:12-19.
- Abe H. & Hoshi H. 2003. Evaluation of bovine embryos produced in high performance serum-free media. J. Reprod. Develop. 49:193-202.
- Abe H., Yamashita S., Itoh T., Satoh T. & Hoshi H. 1999. Ultrastructure of bovine embryos developed from in vitro-matured and -fertilized oocytes: comparative morphological evaluation of embryos cultured either in serum-free medium or in serum-supplemented medium. Mol. Reprod. Dev. 53:325-335.
- Abe H., Yamashita S., Satoh T. & Hoshi H. 2002. Accumulation of cytoplasmic lipid droplets in bovine embryos and cryotolerance of embryos developed in different culture systems using serum-free or serum containing media. Molec. Reprod. Develop. 61:57-66.
- Accorsi M.F., Leão B.C.S., Rocha-Frigoni N.A.S., Perri S.H.V. & Mingoti G.Z. 2016. Reduction in cytoplasmic lipid content in bovine embryos cultured in vitro with linoleic acid in semi-defined medium is correlated with increases in cryotolerance. Zygote 24(4):485-494.
- Almiñana C. & Cuello C. 2015. What is new in the cryopreservation of embryos? Anim. Reprod. 12(3):418-427.
- Arrese E.L., Matthew T.F., Gazard J.L. & Wells M.A. 1999. Calcium and cAMP are second messengers in the adipokinetic hormone-induced lipolysis of triacylglycerols in Manduca sexta fat body. J. Lip. Res. 40:556-64.
- Blondin P. 2015. Status of embryo production in the world. Anim. Reprod. 12(3):356-358.
- Diez C., Heyman Y., Le Bourhis D., Guyader-Joly C., Degrouard J. & Renard J.P. 2001. Delipidating in vitro-produced bovine zygotes: effect on further development and consequences for freezability. Theriogenology 55:923-936.
- Ek I., Arner P., Bergqvist A., Carlström K. & Wahrenberg H. 1997. Impaired adipocyte lipolysis in nonobese women with the polycystic ovary syndrome: a possible link to insulin resistance? J. Clin. Endocrinol. Metabol. 82:1147-1153.
- Ferguson E.M. & Leese H.J. 1999. Triglyceride content of bovine oocytes and early embryos. J. Reprod. Fert. 116:373-378.
- Gómez E., Rodríguez A., Muñoz M., Caamaño J.N., Hidalgo C.O., Morán E., Facal N. & Díez C. 2008. Serum free embryo culture medium improves in vitro survival of bovine blastocysts to vitrification. Theriogenology 69:1013 1021.
- Ho R.J. & Shi Q.H. 1982. Forskolin as a novel lipolytic agent. Biochem. Biophys. Res. Commun. 107:157-164.
- Honnor R.C., Dhillon G.S. & Londos C. 1985. cAMP-dependent protein kinase and lipolysis in rat adipocytes. II. Definition of steadystate relationship with lipolytic and antilipolytic modulators. J. Biol. Chem. 260:15130-15138.
- Lafontan M. & Berlan M. 1993. Fat cell adrenergic receptors and the control of white and brown fat cell function. J. Lip. Res. 34:1057-1091.
- Lonergan P., Rizos D., Gutierrez-Adan A., Fair T. & Boland M.P. 2003. Oocyte and embryo quality: effect of origin, culture conditions and gene expression patterns. Reprod. Dom. Anim. 38:259-267.
- Massip A., Mermillod P. & Dinnyes A. 1995. Morphology and biochemistry of in vitro produced bovine embryos: implications for their cryopreservation. Hum. Reprod. 10(11):3004-3011.
- Men H., Agca Y., Riley L.K. & Critser J.K. 2006. Improved survival of vitrified porcine embryos after partial delipation through chemically stimulated lipolysis and inhibition of apoptosis. Theriogenology 66:2008-2016.
- Mingoti G.Z., Caiado Castro V.S., Méo S.C., Barretto L.S. & Garcia J.M. 2009. The effect of interaction between macromolecule supplement and oxy- gen tension on bovine oocytes and embryos cultured in vitro. Zygote 17:321-382.
- Nagashima H., Kashiwazaki N., Ashman R.J., Grupen C.G. & Nottlen M. 1995. Cryopreservation of porcine embryos. Nature 1995:374-416.
- Parrish J.J., Susko-Parrish J.L. & First N.L. 1988. Capacitation of bovine sperm by heparin. Biol. Reprod. 38:1171-1180.
- Paschoal D.M., Sudano M.J., Guastali M.D., Maziero R.R.D., Crocomo L.F., Magalhães L.C.O., Rascado T.S., Martins Jr A. & Landim-Alvarenga F.C. 2012. Forskolin effect on the cryosurvival of in vitro-produced bovine embryos in the presence or absence of fetal calf serum. Zygote 22:146-157.
- Paschoal D.M., Sudano M.J., Maziero R.R.D., Guastali M.D., Magalhães L.C.O., Landim-Alvarenga F.C., Martins Jr A. & Leal C.L.V. 2015. Decreased lipid granules of in vitro produced bovine embryos with low concentrations of forskolin. Anim. Reprod. 12:686.
- Pereira R.M., Carvalhais I., Pimenta J., Baptista M.C., Vasques M.I., Horta A.E.M., Santos I.C., Marques M.R., Reis A., Silva Pereira M. & Marques C.C. 2008. Biopsied and vitrified bovine embryos viability is improved by trans10, cis12 conjugated linoleic acid supplementation during in vitro embryo culture. Anim. Reprod. Sci. 106:322-332.
- Perry G. 2015. 2013. Statistics of embryo collection and transfer in domestic farm animals. Embryo Transfer Newsletter 33:14-26.
- Rizos D., Guitiérrez-Adán A., Pérez-Garnelo S., De La Fuente J., Boland M.P. & Lonergan N.P. 2003. Bovine embryo culture in the presence or absence of serum: implications for blastocyst development, cryotolerance, and messenger RNA expression. Biol. Reprod. 68:236-243.
- Rocha-Frigoni N.A.S., Leão B.C.S., Nogueira É., Accorsi M.F. & Mingoti G.Z. 2014. Reduced levels of intracellular reactive oxygen species and apoptotic status are not correlated with increases in cryotolerance of bovine embryos produced in vitro in the presence of antioxidants. Reprod. Fertil. Dev. 26:797-805.
- Sanches B.V., Marinho L.S.R., Oliveira Filho B.D., Pontes J.H.F., Basso A.C., Meirinhos M.L.G., Silva-Santos K.C., Ferreira C.R. & Seneda M.M. 2013. Cryosurvival and pregnancy rates after exposure of IVF-derived Bos indicus embros to forskolin before vitrification. Theriogenology 80:372-377.
- Sata R., Tsujii H., Abe H., Yamashita S. & Hochi H. 1999. Fatty acid composition of bovine embryos cultured in serum-free and serum-containing médium during early embryonic development. J. Reprod. Develop. 45:97-103.
- Seamon K.B., Padgett W. & Daly J.W. 1981. Forskolin: unique diterpene activator of adenylate cyclase in membranes in intact cells. Proc. Natl Acad. Sci. USA 78:3363-3367.
- Seidel Jr G.E. 2006. Modifying oocytes and embryos to improve their cryopreservation. Theriogenology 65:228-235.
- Stein A. 2007. Decreasing variability in your cell culture. Biotechniques 43:228-229.
- Stinshoff H., Wilkening S., Hanstedt A., Bollwein H. & Wrenzycki C. 2014. Dimethylsulfoxide and conjugated linoleic acids affect bovine embryo development in vitro. Reprod. Fertil. Dev. 26(4):502-510.
- Stringfellow D.A. 1998. Recommendations for the sanitary handling of in vivo derived embryos, p.79-84. In: Stringfellow D.A. & Seidel S.M. (Eds), Manual of the International Embryo Transfer Society, Savoy, IL.
- Vajta G., Rindom N., Peura T.T., Holm P., Greve T. & Callesen H. 1999. The effect of media, serum and temperature on in vitro survival of bovine blastocysts after open pulled straw (OPS) vitrification. Theriogenology 52:939-948.
- Wright J.M. 2009. Apêndice D: ilustrações fotográficas do estágio de desenvolvimento embrionário e códigos de qualidade, p.165-168. In: Ibid. (Ed.), Manual da Sociedade Internacional de Transferência de Embriões. 4ª ed. Savoy
Publication Dates
-
Publication in this collection
Apr 2017
History
-
Received
02 Feb 2016 -
Accepted
11 Aug 2016