ABSTRACT:
To evaluate the outcome of acute lesions in the brains of sheep that completely clinically recover from acute polioencephalomalacia (PEM), ten sheep were used in this experiment. Eight of those sheep received varying doses of amprolium to induce PEM. Four sheep were treated intramuscularly with 40mg/kg/body weight with thiamine to allow recovery and four sheep were left untreated. Two control sheep did not receive either amprolium or thiamine and were kept along with the other eight sheep for the duration of the experiment. Except for the two drugs, the diet and water source were the same for the ten sheep. Two sheep receiving high daily doses of amprolium and one sheep receiving a lower dose had acute deaths and developed acute brain lesions consisting of neuronal laminar cortical necrosis (red neurons), edema, reactive astrocytes, swollen endothelial cells and gitter cells infiltration. Four sheep that recovered from lower doses of amprolium-induced PEM after being treated with thiamine and another one that recovered spontaneously were euthanatized six months after clinical recovery and had gross changes consisting of segmental absence of cortical tissue. Histologically these segmental cortex-deprived areas corresponded to quasi-empty spaces where only vessels and gitter cells existed. No changes were seen in the brains of the two control sheep.
INDEX TERMS:
Small ruminant diseases; sheep; brain; polioencephalomalacia; healing of central nervous tissue; patology
RESUMO:
Para avaliar a evolução das lesões agudas no cérebro de ovinos que se recuperam clinicamente de polioencefalomalacia aguda (PEM), dez ovinos foram usados neste experimento. Oito desses ovinos receberam doses variáveis de amprólio para induzir PEM. Quatro ovinos foram tratados intramuscularmente com 40mg/kg/peso corporal de tiamina para permitir a recuperação, e outros quatro ficaram sem tratamento. Dois ovinos controles não receberam amprólio nem tiamina e foram mantidos com os outros oito ovinos durante a duração do experimento. Exceto pelas duas drogas, a dieta e a fonte de água eram as mesmas para os dez ovinos. Dois ovinos que receberam doses diárias altas de amprólio, e um que recebeu doses menores, tiveram mortes agudas e desenvolveram lesões cerebrais constituídas por necrose neuronal laminar cortical (neurônios vermelhos), edema, tumefação de células endoteliais, astrócitos reativos, tumefação de células endoteliais e infiltração por células gitter. Quatro ovinos que se recuperam da PEM induzida por amprólio, após tratamento com tiamina, e outro que se recuperou espontaneamente, permaneceram clinicamente normais e foram submetidos a eutanásia seis meses após a recuperação clínica. Na necropsia, apresentavam alterações macroscópicas caracterizadas por ausência segmentar de tecido corticocerebral. Histologicamente, essas áreas privadas de tecido cortical consistiam de espaços praticamente vazios onde apenas vasos e células gitter eram vistos. Não foram encontradas alterações no encéfalo das duas ovelhas controle.
TERMOS DE INDEXAÇÃO:
Doenças de pequenos ruminantes; ovinos; cérebro; polioencefalomalacia; cicatrização do tecido do sistema nervoso; patologia
Introduction
Polioencephalomalacia (PEM) is a morphologic descriptive term for necrosis with softening of the encephalic gray matter (Vandevelde et al. 2012Vandevelde M., Higgins R.J. & Oevermann A. 2012. Polioencephalomacia (PE) or cerebrocortical necrosis (CCN), p.108-112. In: Ibid. (Eds), Veterinary Neuropathology. Wiley-Blackwell, Iowa.). Although several causes have been described for this condition in several species, the term is commonly used to refer a disease in cattle and sheep attributed mainly to sulfur poisoning (Cunha et al. 2010Cunha P.H.J., Bandarra P.M., Dias M.M., Borges A.S. & Driemeier D. 2010. Surto de polioencefalomalacia por ingestão excessiva de enxofre na dieta em bezerros no Rio Grande do Sul. Pesq. Vet. Bras. 30(8):613-617. http://dx.doi.org/10.1590/S0100-736X2010000800001.
https://doi.org/10.1590/S0100-736X201000...
) and/or thiamin deficiency (Sant’Ana & Barros 2010Sant’Ana F.J.F. & Barros C.S.L. 2010. Polioencephalomalacia in ruminants in Brazil. Braz. J. Vet Pathol. 3:70-79.) and that has been successfully treated with parenteral thiamine (Daly 1968Daly F.J. 1968. Polioencephalomalacia: response to thiamine treatment in sheep and a cow. Aust. Vet. J. 44(11):525. http://dx.doi.org/10.1111/j.1751-0813.1968.tb09015.x. PMid:5749411.
https://doi.org/10.1111/j.1751-0813.1968...
, Mendes et al. 2007Mendes L.C.N., Borges A.S., Peiró J.R., Feitosa F.L.F. & Anhesini C.R. 2007. Estudo retrospectivo de 19 casos de polioencefalomalacia, em bovinos, responsivos ao tratamento com tiamina. Arq. Bras. Med. Vet. Zootec. 59(1):239-241. http://dx.doi.org/10.1590/S0102-09352007000100038.
https://doi.org/10.1590/S0102-0935200700...
). Sulphur poisoning related PEM may have a relationship with thiamine deficiency through the process of sulfite cleaving thiamine (Miller & Zachary 2017Miller A.D. & Zachary J.F. 2017. Nervous system: degenerative diseases, p.805-907. In: Zachary J.F. (Ed.), Pathologic Basis of Veterinary Disease. 6th ed. Elsevier, St Louis . http://dx.doi.org/10.1016/B978-0-323-35775-3.00014-X.
https://doi.org/10.1016/B978-0-323-35775...
).
Amprolium is a thiamine analog coccidiostat drug that blocks thiamine uptake in coccidian and has been used also as an experimental tool in the induction of PEM in ruminants (Morgan 1974Morgan K.T. 1974. Amprolium poisoning of preruminant lambs: an ultrastructural study of the cerebral malacia and the nature of the inflammatory response. J. Pathol. 112(4):229-236. http://dx.doi.org/10.1002/path.1711120407. PMid:4835271.
https://doi.org/10.1002/path.1711120407...
, Spicer & Horton 1981Spicer E.M. & Horton B.J. 1981. Biochemistry of natural and amprolium-induced polioencephalomalacia in sheep. Aust. Vet. J. 57(5):230-235. http://dx.doi.org/10.1111/j.1751-0813.1981.tb02667.x. PMid:7295240.
https://doi.org/10.1111/j.1751-0813.1981...
, Sant’Ana et al. 2009Sant’Ana F.J.F. , Nogueira A.P.A., Souza R.I.C., Cardinal S.G., Lemos R.A.A. & Barros C.S.L. 2009. Polioencefalomalacia experimental induzida por amprólio em ovinos. Pesq. Vet. Bras. 29(9):747-752. http://dx.doi.org/10.1590/S0100-736X2009000900012.
https://doi.org/10.1590/S0100-736X200900...
).
Although there are reports on the residual brain lesions of ruminants recovering from spontaneous PEM (Adams et al. 1956Adams O.R., Griner L.A. & Jensen R. 1956. Polioencephalomalacia of cattle and sheep. J. Am. Vet. Med. Assoc. 129(7):311-321. PMid:13366824.) there are no detailed observations of the evolution of the lesions, as the brains of recovered animals are, for obvious regions, rarely examined.
This study examined the acute lesions in sheep with amprolium-induced PEM, and subsequently correlates them with those of sheep recovered from the acute phase of amprolium-induced PEM. The recovery in our cases was either thiamine-induced or spontaneous and sheep were examined six months after recovery.
Materials and Methods
Ten sheep were used in this experiment. Their mean age and weight was respectively 6-month-old and 19kg. At the beginning of the experiment, they were dewormed and identified by sequential numbers from 1-10.
During a seven-day adaptation period and throughout the experiment the ten sheep were kept indoors in five stalls of 1.5x2m (two sheep per stall). They were offered free access to water and 2% of their body weight in dry matter consisting of corn silage and ovine commercial ration. A clinical exam was performed daily in each sheep.
In order to induce PEM, two sheep - Sheep 1 and 2 - received amprolium4 4 Amprolbase, Saúde Animal Ltda, Av. Emílio Marconato 100, Galpão A3, Jaraguaína, SP 13820-000, Brazil. www.farmabase.com.br at daily doses of 1,000mg/kg/body weight (bw) for 5 and 10 days respectively. As this high dosage of amprolium induced a disease too acute to be treated which included gastrointestinal disturbances, additionally six sheep - Sheep 3-8 - were given amprolium at daily doses of 500mg/kg/bw for respectively 49, 20, 22, 28, 30, and 31 days. Sheep 4-6 were treated with on single intramuscular (IM) dose of 40mg/kg/bw of thiamine5 5 Monovin B1, uso veterinário, Laboratório Bravet Ltda, Rua Visconde de Santa Cruz 276, Rio de Janeiro, RJ 20950-340, Brazil. and Sheep 8 received three IM doses of 40mg/kg/bw of thiamine. Sheep 3 and 7 were left untreated. The criterium to determine the time of implementation of the thiamine treatment was the day the sheep showed unequivocal neurological signs including blindness, opisthotonus, and recumbency. Two control sheep did not receive either amprolium or thiamine and were kept along with the other eight sheep for the duration of the experiment. Except for the two drugs, the diet and water source were the same for the ten sheep. Sheep either died spontaneously or were euthanatized (including the two controls) at the end of the experiment. All ten sheep were necropsied and fragments of several organs were processed for histopathology. Data of the experiment are summarized in Table 1. The experiment was approved by Ethics Committee of the Federal University of Mato Grosso do Sul that regulates the use of experimental animals, and registered under the protocol no. 780/2016.
Results
Sheep 1 and 2 were found dead respectively 5 and 10 days after the start of the experiment. They did not develop clear-cut neurological signs and died too acutely preventing the implementation of treatment. The only clinical signs observed were anorexia and marked diarrhea.
Sheep 3-8 developed neurological clinical signs compatible with PEM that develop 11-47 days after the start of the experiment (Table 1). Those signs included tremors, opisthotonus (Fig.1), blindness, recumbency, and paddling. The four thiamine-treated sheep (Sheep 4-6, and 8) recovered from clinical signs within 6-10 hours of treatment and remained clinically normal for 6 months after recovery when they were euthanatized for necropsy. One untreated sheep (Sheep 7) recovered spontaneously after a neurological clinical disease of two-week duration and was euthanatized 6 months after recovery. Controls (Sheep 9 and 10) were euthanatized at the end of the experiment immediately after the last experimental sheep was euthanatized.
Significant necropsy findings were restricted to the cerebral cortex of Sheep 1-8. No significant necropsy findings were present in the two control sheep.
The brains of Sheep 1-3 were moderately swollen as detected by flattened gyri and narrowing of sulci (Fig.2). Additionally Sheep 3 had hemorrhages visible at cut surface of the brain stem Sheep 4-8 that survived for longer periods had similar gross lesions in the brain which consisted of segmental loss of cerebrocortical tissue. These areas of cortical loss were characterized by cavitations (Fig.3) in the cortical gray matter.
Brain of Sheep 4, affected by amprolium-induced polioencephalomalacia. Died on day 5 of the experiment. There is edema of the cerebral cortex evidenced by flattening of gyri and narrowing of sulci.
Brain of Sheep 6, recovered from amprolium-induced polioencephalomalacia. Cut surface at the level of basal nuclei. Six-month-old lesion. Loss of cortical tissue can be seen as cavitations (arrows) in the cortical gray matter. CN= caudate nucleus, IC = internal capsule.
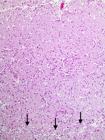
Microscopic examination of the brain of Sheep 1-3 revealed acute lesions characterized by edema, acidophilic (red) neuronal laminar necrosis and hypertrophy of capillary endothelial cells, infiltration of foamy macrophages in the neuropil and perivascular spaces, and cleavage of the deeper lamina of the cortical brain (Fig.4). The red neurons developed and angular shrunk silhouette and dark pyknotic nucleus. Five Sheep (Sheep 4-8) had similar changes of segmental decortication. In these segmental areas, the normal cortical tissue has vanished (Fig.5) and was replaced by only two elements: blood vessels (and some associated connective tissue) and gitter cells (Fig.6). No histological lesions were observed in the controls (Sheep 9 and 10).
Severe widespread neuronal necrosis in Sheep 3, with infiltration by foamy macrophages (gitter cells) and cleavage (arrows) of cerebrocortical lamina resulting in laminar separation, affected by amprolium-induced polioencephalomalacia. Died after an acute clinical course of two days. There is also striking prominence of the capillaries due to endothelial cell hyperplasia and hypertrophy. HE, obj.10x.
Histopathology of the telencephalic cortex of Sheep 5, recovered from amprolium-induced polioencephalomalacia. Six-month-old lesion. The cortex has disappeared leaving a quasi-empty space between the pia-arachnoid (pa) and the subcortical white matter (wm). Within this quasi-empty one can see only remaining vessels and gitter cells. HE, obj.10x.
Histopathology of the telencephalic cortex of Sheep 8, recovered from amprolium-induced polioencephalomalacia.. Six-month-old lesion. Remaining vessels and gitter cells after the dead cortical tissue have been removed. HE, obj.20x. In the inset, one can see the details of the lesion showing vessels (arrows) and gitter cells (arrowheads). HE, obj.40x.
Discussion
Sheep with amprolium-induced PEM in this experiment either died or were euthanatized in two periods after the onset of clinical signs: 2-5 days (three cases) and six months (5 cases). Hereafter we shall refer to these stages as acute and healing (cured). Gross lesions were limited to cerebral cortex. The histopathological examination of the brains of affected sheep at these two periods allows us to speculate on the time frame of lesion development.
The time of development and morphological constituents of acute PEM lesions in the three sheep of this study conforms with which is described in a recent edition of a veterinary pathology reference book (Miller & Zachary 2017Miller A.D. & Zachary J.F. 2017. Nervous system: degenerative diseases, p.805-907. In: Zachary J.F. (Ed.), Pathologic Basis of Veterinary Disease. 6th ed. Elsevier, St Louis . http://dx.doi.org/10.1016/B978-0-323-35775-3.00014-X.
https://doi.org/10.1016/B978-0-323-35775...
): two days after onset of disease, the surface of the brain was edematous with flattening of cerebrocortical gyri and narrowing of sulci. The microscopic lesions consisted of laminar necrosis (red neurons) of the deeper lamina of the cerebral cortex, edema, and prominence of small blood vessels, infiltration of foamy macrophages in the neuropil and perivascular spaces and separation of the deeper lamina of the cortical brain. The resolution (cure, healing) of this lesions could be appreciated in the brain of five sheep examined after they recovered either spontaneously or due to thiamine treatment. The typical neurological clinical signs of PEM developed by these sheep allow us to infer that they developed acute disease and lesions as the ones just described above, and that the lesion characterized by a segmental absence of brain cortex found grossly and microscopically in these cases represents the healing process of the active lesions. The dead neurons, their processes, and dead glial elements are phagocytosed by monocytes coming from the blood (mostly) and by resident microglial cells which engorge becoming foamy macrophages, known as gitter cells, which find their way out of the brain carrying their load of dead material mainly through perivascular space (Vandevelde et al. 2012Vandevelde M., Higgins R.J. & Oevermann A. 2012. Polioencephalomacia (PE) or cerebrocortical necrosis (CCN), p.108-112. In: Ibid. (Eds), Veterinary Neuropathology. Wiley-Blackwell, Iowa.). The healed lesions of these five sheep were of cavities within which only gitter cells and vascular remains could be observed. Most likely, the decline and disappearance of brain edema was determinant in improving the clinical status.
Although it is stated that ruminants recovering from PEM “become partially decorticate and remain blind and stupid” (Cantile & Youssef 2009Cantile C. & Youssef S. 2009. Nervous system: polioencephalomalacia of ruminants, p.309-312. In: Maxie M.G. (Ed.), Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol.1. 6th ed. Elsevier, St Louis.) this was not the case with the five recovered sheep in the current experiment: a fortnight or less after treatment, in spite of loss of extensive areas o cerebral cortex, sheep were clinically normal and remained so for the six months before euthanasia. Although this is difficult event to equate, it is not unheard of regarding domestic animals. Sometimes, quite large chronic brain lesions will be present but be clinically silent whereas smaller but acute lesions do often have a clinical expression (Mayhew 2009Mayhew J. 2009. Interpretation of signs of brain and cranial nerve disease, p.18. In: Ibid. (Ed.), Large Animal Neurology. 2nd ed. Wiley Blackwell, Iowa.). This fact is more likely related to the anatomical site of the lesions and probably sheep can do well even with extensive loss of their cerebral cortex but would succumb to smaller lesions in the brain stem.
Our results seem to point out that 500mg/kg/bw is an adequate dose to systematically induce PEM in sheep without the risk of developing a too acute disease complicated with gastrointestinal signs, such as diarrhea (Morgan et al. 1975Morgan K.T., Coop R.L. & Doxey D.L. 1975. Amprolium poisoning of preruminant lambs: an investigation of the encephalopathy and the haemorrhagic and diarrhoeic syndromes. J. Pathol. 116(2):73-81. http://dx.doi.org/10.1002/path.1711160203. PMid:1151527.
https://doi.org/10.1002/path.1711160203...
). This information could be useful for further experiments. On the other hand, although the number of cases is in this experiment are too limited to be conclusive, the treatment with thiamine (40mg/kg/bw) seem to be adequate to halt the progression of the disease. More studies though are necessary to establish a model for disease production and treatment.
This study demonstrated that healed cerebrocortical lesions of PEM although extensive are compatible with life and clinical normalcy in sheep and that spontaneous recovery may occur. This may have some importance in the routine diagnosis of brain diseases in ruminants, since the adequate significance can be attributed to residual lesion such as these when found at necropsy.
Acknowledgements
This study was funded by Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia do Estado de Mato Grosso do Sul (Fundect/CNPq - Grant 15/2014, Pronem/MS - Grant 59/300.126/2015 e Fundect/CAPES 05/2014, PVMS 59/300.032/2015).
References
- Adams O.R., Griner L.A. & Jensen R. 1956. Polioencephalomalacia of cattle and sheep. J. Am. Vet. Med. Assoc. 129(7):311-321. PMid:13366824.
- Cantile C. & Youssef S. 2009. Nervous system: polioencephalomalacia of ruminants, p.309-312. In: Maxie M.G. (Ed.), Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol.1. 6th ed. Elsevier, St Louis.
- Cunha P.H.J., Bandarra P.M., Dias M.M., Borges A.S. & Driemeier D. 2010. Surto de polioencefalomalacia por ingestão excessiva de enxofre na dieta em bezerros no Rio Grande do Sul. Pesq. Vet. Bras. 30(8):613-617. http://dx.doi.org/10.1590/S0100-736X2010000800001.
» https://doi.org/10.1590/S0100-736X2010000800001 - Daly F.J. 1968. Polioencephalomalacia: response to thiamine treatment in sheep and a cow. Aust. Vet. J. 44(11):525. http://dx.doi.org/10.1111/j.1751-0813.1968.tb09015.x. PMid:5749411.
» https://doi.org/10.1111/j.1751-0813.1968.tb09015.x - Mayhew J. 2009. Interpretation of signs of brain and cranial nerve disease, p.18. In: Ibid. (Ed.), Large Animal Neurology. 2nd ed. Wiley Blackwell, Iowa.
- Mendes L.C.N., Borges A.S., Peiró J.R., Feitosa F.L.F. & Anhesini C.R. 2007. Estudo retrospectivo de 19 casos de polioencefalomalacia, em bovinos, responsivos ao tratamento com tiamina. Arq. Bras. Med. Vet. Zootec. 59(1):239-241. http://dx.doi.org/10.1590/S0102-09352007000100038.
» https://doi.org/10.1590/S0102-09352007000100038 - Miller A.D. & Zachary J.F. 2017. Nervous system: degenerative diseases, p.805-907. In: Zachary J.F. (Ed.), Pathologic Basis of Veterinary Disease. 6th ed. Elsevier, St Louis . http://dx.doi.org/10.1016/B978-0-323-35775-3.00014-X.
» https://doi.org/10.1016/B978-0-323-35775-3.00014-X - Morgan K.T. 1974. Amprolium poisoning of preruminant lambs: an ultrastructural study of the cerebral malacia and the nature of the inflammatory response. J. Pathol. 112(4):229-236. http://dx.doi.org/10.1002/path.1711120407. PMid:4835271.
» https://doi.org/10.1002/path.1711120407 - Morgan K.T., Coop R.L. & Doxey D.L. 1975. Amprolium poisoning of preruminant lambs: an investigation of the encephalopathy and the haemorrhagic and diarrhoeic syndromes. J. Pathol. 116(2):73-81. http://dx.doi.org/10.1002/path.1711160203. PMid:1151527.
» https://doi.org/10.1002/path.1711160203 - Sant’Ana F.J.F. & Barros C.S.L. 2010. Polioencephalomalacia in ruminants in Brazil. Braz. J. Vet Pathol. 3:70-79.
- Sant’Ana F.J.F. , Nogueira A.P.A., Souza R.I.C., Cardinal S.G., Lemos R.A.A. & Barros C.S.L. 2009. Polioencefalomalacia experimental induzida por amprólio em ovinos. Pesq. Vet. Bras. 29(9):747-752. http://dx.doi.org/10.1590/S0100-736X2009000900012.
» https://doi.org/10.1590/S0100-736X2009000900012 - Spicer E.M. & Horton B.J. 1981. Biochemistry of natural and amprolium-induced polioencephalomalacia in sheep. Aust. Vet. J. 57(5):230-235. http://dx.doi.org/10.1111/j.1751-0813.1981.tb02667.x. PMid:7295240.
» https://doi.org/10.1111/j.1751-0813.1981.tb02667.x - Vandevelde M., Higgins R.J. & Oevermann A. 2012. Polioencephalomacia (PE) or cerebrocortical necrosis (CCN), p.108-112. In: Ibid. (Eds), Veterinary Neuropathology. Wiley-Blackwell, Iowa.
-
4
Amprolbase, Saúde Animal Ltda, Av. Emílio Marconato 100, Galpão A3, Jaraguaína, SP 13820-000, Brazil. www.farmabase.com.br
-
5
Monovin B1, uso veterinário, Laboratório Bravet Ltda, Rua Visconde de Santa Cruz 276, Rio de Janeiro, RJ 20950-340, Brazil.
Publication Dates
-
Publication in this collection
May 2018
History
-
Received
23 May 2017 -
Accepted
14 June 2017