ABSTRACT:
Despite common occurrence and importance of canine distemper disease the majority of tests currently available for diagnosis are hampered by either low sensitivity or specificity. In this study it was evaluated antigenic and immunogenic characteristics of a conserved region of nucleocapsid protein of canine distemper virus (rCDV NP) expressed in Escherichia coli employing a codon optimized synthetic gene. The expression of rCDVNP in Star strain (mean 300μg/mL, purified) was confirmed by SDS-PAGE and Western blot analysis by using His-Tag monoclonal antibodies. Western blot and ELISA, employing positive and negative control dog sera, demonstrated the rCDVNP antigenicity. The rCDVNP was inoculated in hens and immunoglobulin Y (IgY) was purified from the egg yolk. The mean yield of IgY was 28.55mg/mL. IgY reacted with the recombinant protein as demonstrated by Western blot and ELISA assays. In summary, our findings demonstrated that rCDVNP is antigenic since CDV positive dog sera recognized the protein in vitro. Additionally, the rCDVNP proved to be immunogenic in hens being possible to isolate a high concentration of specific IgY antibodies from the egg yolk. Taken together, these results indicate that the rCDVNP along with the specific IgY could be useful tools for development of the canine distemper immunodiagnostic assays.
INDEX TERMS:
Dogs; antigenic; immunogenic; canine distemper virus; nucleocapsid protein; Escherichia coli; codon; synthetic gene; expression; CDV; NP; IgY; viroses
RESUMO:
Apesar da ocorrência comum e importância da cinomose canina, a maioria dos testes atualmente disponíveis para diagnóstico são prejudicados pela baixa sensibilidade ou especificidade. Neste estudo foram avaliadas características antigênicas e imunogênicas de uma região conservada da proteína do nucleocapsídeo do virus da cinomose canina (rCDV NP) expressa em Escherichia coli empregando um gene sintético e codons otimizados. A expressão na cepa Star (média de 300μg/mL, purificada) foi confirmada por SDS-PAGE e Western blot utilizando anticorpos monoclonais anti-His-Tag. A antigenicidade da rCDVNP foi demonstrada por western blot e ELISA empregando soros de cães positivos e negativos. A rCDVNP foi inoculada em galinhas e imunoglobulina Y (gY) foi obtida e purificada a partir da gema. A produção média de IgY foi 28.55mg/mL. Anticorpos IgY reagiram com a proteína recombinante, quando analisados por Western blot e ELISA. Em resumo, nossos achados demonstram que a rCDVNP produzida é antigênica, uma vez que os anticorpos de soro de cães positivos para CDV reconheceram a proteína in vitro. Além disso, a rCDVNP foi imunogênica em galinhas, sendo possível isolar anticorpos IgY específicos a partir da gema do ovo em altas concentrações. Tomados em conjunto, estes resultados indicam que a rCDVNP juntamente com a IgY específica podem ser ferramentas úteis para elaborar ensaios de imunodiagnóstico de cinomose canina.
TERMOS DE INDEXAÇÃO:
Cães; antigenia; imunogenia; proteína do nucleocapsídeo; cinomose canina; Escherichia coli; gene sintético; códon; expressão; CDV; NP; IgY; viroses
Introduction
Canine distemper virus (CDV) causes a disease known as canine distemper (CD), characterized in acute systemic or chronic nervous infection (Beineke et al. 2009Beineke A., Puff C., Seehusen F. & Baumgärtner W. 2009. Pathogenesis and immunopathology of systemic and nervous canine distemper. Vet. Immunol. Immunop. 127(1/2):1-18. <http://dx.doi.org/10.1016/j.vetimm.2008.09.023> <PMid:19019458>
https://doi.org/10.1016/j.vetimm.2008.09...
). CD is a worldwide problem and it is associated with high morbidity and mortality (Elia et al. 2015Elia G., Camero M., Losurdo M., Lucente M.S., Larocca V., Martella V., Decaro N. & Buonavoglia C. 2015. Virological and serological findings in dogs with naturally occurring distemper. J. Virol. Methods 213:127-130. <http://dx.doi.org/10.1016/j.jviromet.2014.12.004> <PMid:25512131>
https://doi.org/10.1016/j.jviromet.2014....
), being considered the second highest fatality rate of any infectious disease, after rabies, in domestic dogs around the world (Deem at al. 2000Deem S.L., Spelman L.H., Yates R.A. & Montali R.J. 2000. Canine distemper in terrestrial carnivores: a review. J. Zoo Wildl. Med. 31(4):441-451. <http://dx.doi.org/10.1638/1042-7260(2000)031[0441:CDITCA]2.0.CO;2> <PMid:11428391>
https://doi.org/10.1638/1042-7260(2000)0...
). In Brazil, previous studies have shown that CD is the most important cause of early death or euthanasia in dogs (Bentubo et al. 2007Bentubo H.D.L., Tomaz M.A., Bondan E.F. & Lallo M.A. 2007. Expectativa de vida e causas de morte em cães na área metropolitana de São Paulo (Brasil). Ciência Rural 37(4):1021-1026. <http://dx.doi.org/10.1590/S0103-84782007000400016>
https://doi.org/10.1590/S0103-8478200700...
, Fighera et al. 2008Fighera R.A., Souza T.M., Silva M.C., Brum J.C., Graça D.G., Kommers G.D., Irigoyen L.F. & Barros C.S.L. 2008. Causas de morte e razões para eutanásia de cães da Mesorregião do Centro Ocidental Rio-Grandense (1965-2004). Pesq. Vet. Bras. 28(4):223-230. <http://dx.doi.org/10.1590/S0100-736X2008000400005>
https://doi.org/10.1590/S0100-736X200800...
). The disease affects various organs, resulting in respiratory, nervous and gastrointestinal disorders (Martella et al. 2008Martella V., Elia G. & Buonavoglia C. 2008. Canine distemper virus. Vet. Clin. N. Am., Small Anim. Pract. 38(4):787-797. <http://dx.doi.org/10.1016/j.cvsm.2008.02.007> <PMid:18501278>
https://doi.org/10.1016/j.cvsm.2008.02.0...
). It is also associated with severe leukopenia and immunosuppression, favoring opportunistic infections (Beineke et al. 2009Beineke A., Puff C., Seehusen F. & Baumgärtner W. 2009. Pathogenesis and immunopathology of systemic and nervous canine distemper. Vet. Immunol. Immunop. 127(1/2):1-18. <http://dx.doi.org/10.1016/j.vetimm.2008.09.023> <PMid:19019458>
https://doi.org/10.1016/j.vetimm.2008.09...
).
CDV is a Morbillivirus and belongs to the family Paramyxoviridae (ICTV 2014ICTV 2014. International Committee on Taxonomy of Viruses. Available at <Available at http://www.ictvonline.org/virusTaxonomy.asp
> Access on Dec. 15, 2016.
http://www.ictvonline.org/virusTaxonomy....
). The genomic RNA of the CDV is single stranded, negative-sense, and encodes six structural proteins: fusion protein (F), hemagglutinin (H), matrix (M), phosphoprotein (P), viral polymerase protein (L) and the nucleocapsid protein (NP) (Elia et al. 2006Elia G., Decaro N., Martella V., Cirone F., Lucente M.S., Lorusso E., Di Trani L. & Buonavoglia C. 2006. Detection of canine distemper virus in dogs by real-time RT-PCR. J. Virol. Methods 136(1/2):171-176. <http://dx.doi.org/10.1016/j.jviromet.2006.05.004> <PMid:16750863>
https://doi.org/10.1016/j.jviromet.2006....
). The NP has been separated in three regions, the variable N-terminus, the variable C-terminus and the highly conserved middle region (Yoshida et al. 1998Yoshida E., Iwatsuki K., Miyashita N., Gemma T., Kai C. & Mikami T. 1998. Molecular analysis of the nucleocapsid protein of recent isolates of canine distemper virus in Japan. Vet. Microbiol. 59(2/3):237-244. <http://dx.doi.org/10.1016/S0378-1135(97)00194-6> <PMid:9549863>
https://doi.org/10.1016/S0378-1135(97)00...
). It is produced in abundant amounts by host cells (Stettler & Zurbriggen 1995Stettler M. & Zurbriggen A. 1995. Nucleotide and deduced amino acid sequences of the nucleocapsid protein of the virulent A75/17-CDV strain of canine distemper virus. Vet. Microbiol. 44(2/4):211-217. <http://dx.doi.org/10.1016/0378-1135(95)00014-2> <PMid:8588315>
https://doi.org/10.1016/0378-1135(95)000...
) being expressed early during the viral replication cycle (Latha et al. 2007bLatha D., Geetha M., Ramadass P. & Narayanan R.B. 2007b. Evalution of ELISA based on the conserved and functional middle region of nucleocapsid protein to detect distemper infection in dogs. Vet. Microbiol. 120(3/4):251-260. <http://dx.doi.org/10.1016/j.vetmic.2006.11.019> <PMid:17224247>
https://doi.org/10.1016/j.vetmic.2006.11...
).
A variety of clinical parameters and immunodiagnostic assays can be performed for definitive ante mortem diagnosis of CD (Elia et al. 2015Elia G., Camero M., Losurdo M., Lucente M.S., Larocca V., Martella V., Decaro N. & Buonavoglia C. 2015. Virological and serological findings in dogs with naturally occurring distemper. J. Virol. Methods 213:127-130. <http://dx.doi.org/10.1016/j.jviromet.2014.12.004> <PMid:25512131>
https://doi.org/10.1016/j.jviromet.2014....
). However, due to the unpredictable and variable course of CD, many times the final diagnosis is inconclusive (Frisk et al. 1999Frisk A.L., König M., Moritz A. & Baumgärtner W. 1999. Detection of canine distemper virus nucleoprotein RNA by reverse transcription-PCR using serum, whole blood, and cerebrospinal fluid from dogs with distemper. J. Clin. Microbiol. 37(11):3634-3643. <PMid:10523566>) or only based on the clinical manifestations of the dogs (Latha et al. 2007bLatha D., Geetha M., Ramadass P. & Narayanan R.B. 2007b. Evalution of ELISA based on the conserved and functional middle region of nucleocapsid protein to detect distemper infection in dogs. Vet. Microbiol. 120(3/4):251-260. <http://dx.doi.org/10.1016/j.vetmic.2006.11.019> <PMid:17224247>
https://doi.org/10.1016/j.vetmic.2006.11...
). The recombinant DNA technology and its application facilitate routine diagnosis of infectious diseases (Balamurugan et al. 2010Balamurugan V., Venkatesan G., Sen A., Annamalai L., Bhanuprakash V. & Singh R.K. 2010. Recombinant protein-based viral disease diagnostics in veterinary medicine. Expert Rev. Mol. Diagn. 10(6):731-753. <http://dx.doi.org/10.1586/erm.10.61> <PMid:20843198>
https://doi.org/10.1586/erm.10.61...
). Using the recombinant technology, some studies have demonstrated the detection of the NP or antibodies against NP for the CD diagnosis (Barben et al. 1999Barben G., Stettler M., Jaggy A., Vandevelde M. & Zurbriggen A. 1999. Detection of IgM antibodies against a recombinant nucleocapsid protein of canine distemper virus in dog sera using a dot-blot assay. J. Vet. Med. A 46(2):115-122. <http://dx.doi.org/10.1046/j.1439-0442.1999.00198.x> <PMid:10216448>
https://doi.org/10.1046/j.1439-0442.1999...
, Von Messling et al. 1999Von Messling V., Harder T.C., Moennig V., Rautenberg P., Nolte I. & Haas L. 1999. Rapid and sensitive detection of Immunoglobulin M (IgM) and IgG antibodies against canine distemper virus by a new recombinant nucleocapsid protein-based enzyme-linked immunosorbent assay. J. Clin. Microbiol. 37(4):1049-1056. <PMid:10074525>, Latha et al. 2007aLatha D., Geetha M., Ramadass P. & Narayanan R.B. 2007a. Development of recombinant nucleocapsid protein based IgM-ELISA for the early detection of distemper infection in dogs. Vet. Immunol. Immunop. 119(3/4):278-286. <http://dx.doi.org/10.1016/j.vetimm.2007.06.006> <PMid:17659785>
https://doi.org/10.1016/j.vetimm.2007.06...
, 2007bLatha D., Geetha M., Ramadass P. & Narayanan R.B. 2007b. Evalution of ELISA based on the conserved and functional middle region of nucleocapsid protein to detect distemper infection in dogs. Vet. Microbiol. 120(3/4):251-260. <http://dx.doi.org/10.1016/j.vetmic.2006.11.019> <PMid:17224247>
https://doi.org/10.1016/j.vetmic.2006.11...
).
There are many options to express recombinant proteins depending on the specific requirement (Spencer et al. 2007Spencer K., Osorio F.A. & Hiscox J.A. 2007. Recombinant viral proteins for use in diagnostic ELISAs to detect virus infection. Vaccine 25(30):5653-5659. <http://dx.doi.org/10.1016/j.vaccine.2007.02.053> <PMid:17478017>
https://doi.org/10.1016/j.vaccine.2007.0...
), however, Escherichia coli expression dominates the expression systems and remains the first choice for laboratory investigations (Papaneophytou & Kontopidis 2014Papaneophytou C.P. & Kontopidis G. 2014. Statistical approaches to maximize recombinant protein expression in Escherichia coli: a general review. Protein Expr. Purif. 94:22-32. <http://dx.doi.org/10.1016/j.pep.2013.10.016> <PMid:24211770>
https://doi.org/10.1016/j.pep.2013.10.01...
). The expression in E. coli is relatively simple and a high yield of protein can be produced (LaVallie 2001LaVallie E.R. 2001. Production of recombinant proteins in Escherichia coli. Curr. Protoc. Protein Sci. Chapter 5:1. <http://dx.doi.org/10.1002/0471140864.ps0501s00> <PMid:18429175>
https://doi.org/10.1002/0471140864.ps050...
). In order to further optimize protein expression, synthetic DNA have been frequently used providing an advantageous way to obtain genes encoding target proteins. Besides, it is possible to modify the natural gene with the aim of enhancing expression in heterologous hosts (Welch et al. 2009Welch M., Govindarajan S., Ness J.E., Villalobos A., Gurney A., Minshull J. & Gustafsson C. 2009. Design parameters to control synthetic gene expression in Escherichia coli. Plos One 4(9):e7002. <http://dx.doi.org/10.1371/journal.pone.0007002> <PMid:19759823>
https://doi.org/10.1371/journal.pone.000...
).
In this study, we demonstrated the expression of a conserved region of CDV NP in E. coli employing a codon optimized synthetic gene and the evaluation of its antigenic and immunogenic properties in vitro and in vivo.
Materials and Methods
All experiments were conducted in accordance with protocols approved by the Comissão de Ética em Experimentação Animal (CEEA) of the Universidade Federal de Pelotas (Permit Number: 1251-2015).
Production of recombinant CDV NP (rCDVNP)
Construction of recombinant plasmid containing a codon optimized synthetic gene of CDV NP. All nucleotide sequences of the CDV nucleocapsid gene deposited in GenBank were analyzed and the conserved sequence located in the central region of NP gene was selected for this study (aa 222-316). The CDV NP nucleotide sequence was codon optimized for Escherichia coli expression and synthetized by GENONE®. Geneious Pro 4.8.5 and Vector NTI 8 softwares were used for analyses, edition, alignment and optimization of the sequence (Kearse et al. 2012Kearse M., Moir R., Wilson A., Stones-Havas S., Cheung M., Sturrock S., Buxton S., Cooper A., Markowitz S., Duran C., Thierer T., Ashton B., Meintjes P. & Drummond A. 2012. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28(12):1647-1649. <http://dx.doi.org/10.1093/bioinformatics/bts199> <PMid:22543367>
https://doi.org/10.1093/bioinformatics/b...
, Lu & Moriyama 2004Lu G. & Moriyama E.N. 2004. Vector NTI, a balanced all-in-one sequence analysis suite. Brief. Bioinform. 5(4):378-388. <http://dx.doi.org/10.1093/bib/5.4.378> <PMid:15606974>
https://doi.org/10.1093/bib/5.4.378...
). The synthetized gene was inserted into a pAE vector (Ramos et al. 2004Ramos C.R.R., Abreu P.A.E., Nascimento A.L.T.O. & Ho P.L. 2004. A high copy T7 Escherichia coli expression vector for the production of recombinant proteins with a minimal N-terminal His-tagged fusion peptide. Braz. J. Med. Biol. Res. 37(8):1103-1109. <http://dx.doi.org/10.1590/S0100-879X2004000800001> <PMid:15273812>
https://doi.org/10.1590/S0100-879X200400...
) between restriction enzymes BamHI and HindIII resulting in the pAE-NP recombinant plasmid.
Cloning and expression of the rCDVNP.E. coli TOP10F strain was transformed with pAE-NP using the heat shock method according Froger & Hall (2007Froger A. & Hall J.E. 2007. Transformation of plasmid DNA into E. coli using the heat shock method. JoVE (6):253. <PMid:18997900>). Screening of colonies by electrophoresis in 1% agarose gel was carried out in order to identify the pAE-NP. The recombinant clones were selected and amplified in LB medium (Kasvi®, Brasil) with ampicillin (100μg/mL). Thereafter, extraction of plasmid DNA by alkaline lysis with SDS was performed (Sambrook & Russell 2006Sambrook J. & Russell D.W. 2006. Preparation of Plasmid DNA by Alkaline Lysis with SDS: minipreparation. Cold Spring Harbor Protocols. 2006(1). <http://dx.doi.org/10.1101/pdb.prot4084> <PMid:22485489>
https://doi.org/10.1101/pdb.prot4084...
). Subsequently, E. coli Star strain was transformed with the recombinant pAE-NP (Froger & Hall 2007) aiming protein expression. After, transformed cells were immersed into a tube containing LB medium (Kasvi®) with ampicillin (100μg/mL) and then the culture was incubated overnight at 37°C with agitation. The overnight culture was diluted 1:10 in LB medium (Kasvi®) containing ampicillin (100μg/mL) and incubated at 37°C until to attain the optical density (OD600=0.6-0.8). The expression was induced with 0.5mM of Isopropyl β-D-1-thiogalactopyranoside (IPTG - Sigma USA) for 3 hours at 37°C with agitation for the production of rCDVNP.
Purification and analysis of the rCDVNP. The IPTG induced culture was centrifuged at 10,000 g for 10 min at 4°C. The precipitate was suspended in buffer (50mM NaH2PO4, 300mM NaCl and 20mM Imidazole) with lysozyme (50mg/mL) and sonicated seven times for 20 s with 60 Hz. The lysate was centrifuged at 10,000 g for 15 min at 4°C and then the precipitate was washed three times with phosphate buffered saline (PBS) (pH 7.4). The precipitate was suspended in 0.2% N-Lauroylsarcosine buffer (50mM NaH2PO4, 300mM NaCl, 20mM Imidazole and 0.2% (w/v) of N-Lauroylsarcosine) and remained 48 hours at 4°C. After that, the solution was centrifuged at 10,000g for 15min at 4°C and then the supernatant was collected into a new tube. The recombinant protein was purified with Ni2+ affinity chromatography column (HisTrap™ FF, GE Healthcare®). A step gradient of Imidazole was used to elute the bound proteins. Dialysis was carried out in PBS for 24 hours at 4°C. The final yield of purified protein was estimated using Qubit™ fluorometric quantitation (Life Technologies®).
Aliquots, obtained at each stage of rCDVNP expression and purification, were analyzed by 20% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot. Proteins were transferred to 0.45μm nitrocellulose membrane (BioRad®) which was blocked overnight with 5% skimmed milk in phosphate buffered saline containing Tween 20 (PBS-T20). After five washes with PBS-T, the membrane was incubated with anti His-Tag monoclonal antibodies (Sigma®, USA) at 1:10,000 for 1h at 37°C. After removal the unbound antibodies, the membrane was incubated with rabbit anti-mouse IgG conjugated with HRPO (Sigma®, USA) at 1:5,000 for 1h at 37°C. After five washing steps, 3,3’,5,5’-tetramethylbenzidine (TMB) (Sigma®, USA) substrate was added for 5min at room temperature.
Antigenic evaluation of rCDVNP
Serum samples. Blood samples were collected from the jugular vein via venopuncture from 10 dogs and kept at -20°C until use. Five serum samples were collected from Bulldog young pups prior to vaccination and used as negative controls. The other five samples were collected from undefined breed dogs (>1year-old) after vaccination and used as positive controls. The positive or negative antibody status of all serum samples were confirmed by serum neutralization (data not shown).
Reactivity of the rCDVNP. In order to confirm the reactivity of the rCDVNP with polyclonal sera against CDV, Western blot was carried out under the same conditions previously described. Positive and negative dog sera were used as primary antibody at 1:20 dilution, overnight at 4°C. Rabbit anti-dog IgG conjugated with HRPO (Sigma®, USA) was used as secondary antibody at 1:1,000 for 1h at 37°C.
Antigenic properties of rCDVNP by Indirect ELISA. Optimal concentrations of rCDVNP, serum samples and conjugated antibodies dilution were obtained by checkboard titrations. Except as otherwise indicated, all incubation steps were performed at 37°C. Three washings were performed with PBS-T20 between each step. Briefly, microtiter 96-well plates (Costar®, USA) were coated with 100ng/well of rCDVNP in 0.05M carbonate buffer, pH9.6, at 4°C overnight. Nonspecific binding sites were blocked with 100μL/well of PBS-T with 5% skimmed milk for 1h. Serial dilutions (1:2,560-1:40) of dog serum samples were incubated for 2 hours (100μL/well). Later, rabbit anti-dog IgG conjugated with HRPO (Sigma®, USA) at 1:7,500 was incubated for 1h (100μL/well). The reaction was visualized by using of o-phenylenediamine dihydrochloride (OPD) in phosphate citrate buffer (pH 5) containing 0.2% hydrogen peroxide (50μL/well) as substrate after incubation for 15min at room temperature. The reaction was stopped by adding 2.5 M H2SO4 and the absorbance was read at 492nm by a microplate reader. Analyses were performed in triplicate.
Immunogenic evaluation of rCDVNP
Inoculation of rCDVNP in hens. Three laying hens New Hampshire Red 20-weeks-old were kept in individual cages with food and water ad libitum throughout the study. The inoculations were performed according Wen et al. (2012)Wen J., Zhao S., He D., Yang Y., Li Y. & Zhu S. 2012. Preparation and characterization of egg yolk immunoglobulin Y specific to influenza B virus. Antiviral Res. 93(1):154-159. <http://dx.doi.org/10.1016/j.antiviral.2011.11.005> <PMid:22127067>
https://doi.org/10.1016/j.antiviral.2011...
with modifications. Briefly, 100μg of rCDVNP diluted in PBS were emulsified with an equal volume of incomplete Freund’s adjuvant (Sigma®, USA) and inoculated in the pectoral muscles with a final volume of 1mL distributed at two points. Animals were inoculated three times at intervals of two weeks. Eggs were collected and stored at 4°C.
Purification of IgY. IgY was isolated according to the water solution method described by Akita & Nakai (1992)Akita E.M. & Nakai S. 1992. Immunoglobulins from egg yolk: Isolation and purification. J. Food Sci. 57(3):629-634. <http://dx.doi.org/10.1111/j.1365-2621.1992.tb08058.x>
https://doi.org/10.1111/j.1365-2621.1992...
with modifications. Briefly, the yolk was separated from the white and 5mL of yolk were mixed with fresh and cold distilled water 9:1 (distilled water pH 5-5.5). This mixture was incubated for 6 hours at 4°C and centrifuged at 10,000g for 25min at 4°C. The supernatant was collected through sterile gauzes. Three-step salt precipitation was performed to purify the IgY in the supernatant in turn. First, ammonium sulfate was added to 50% saturation, this solution was stirred at 4°C for 30min. Precipitate was collected by centrifugation at 10,000g for 15min at 4°C and dissolved to the original volume of yolk in PBS. Ammonium sulfate was added to 33% saturation and this solution was incubated 15min and centrifuged as before. This step was repeated once and the precipitate was solubilized in 1mL of PBS. Dialysis was carried out in PBS for 24 hours at 4°C. Concentration of purified IgY was measured by Qubit™ fluorometric quantitation (Life Technologies®). The polyclonal antibodies were stored at -20°C. The purification of IgY were analyzed by 15% SDS-PAGE.
Immunogenicity of rCDVNP. Western blot was carried out under the same conditions employed to confirm the presence of the rCDVNP. Representative samples of IgY isolated from egg yolks (0 and 7 weeks after first inoculation) were used as primary antibody at 1:20 overnight at 4°C. Rabbit anti-chicken IgY conjugated with HRPO (Sigma®, USA) was used as secondary antibody at 1:5,000 for 2 hours at 37°C.
Evaluation of IgY anti-rCDVNP production by Indirect ELISA. Optimal concentrations of rCDVNP, IgY and conjugated antibodies were obtained by checkboard titration. The evaluation of IgY anti-rCDVNP production was carried out under the same conditions employed to evaluate the antigenic properties of the rCDVNP. Briefly, microtiter 96-well plate (Costar®, USA) were coated with 100ng/well of rCDVNP. IgY isolated from yolks at 0, 4, and 7 weeks after first inoculation were used as primary antibodies (1:50) and the secondary antibody used was rabbit anti-chicken IgY conjugated with HRPO (Sigma®, USA) at 1:10,000. Analyses were performed in triplicate.
Results
Production of rCDVNP
Recombinant plasmid pAE-NP. Two hundred and ninety-four base pairs (bp) corresponding to the middle nucleotide sequence of CDV NP were codon optimized, synthesized and directionally cloned into the plasmid pAE, generating the recombinant pAE-NP. Recombinant pAE-NP was successfully amplified in Escherichia coli TOP10F. This synthetized region resulted in a deduced amino acid sequence with 100% homology with all others CDV NP deposited in GenBank.
Analysis of rCDVNP expression. The expression of the synthetic gene resulted in a protein of approximately 12kDa, molecular weight as expected. Poly-histidine tag (6-His tag) was expressed fused to the recombinant protein, which facilitated its purification and it was used for the expression confirmation by anti His-Tag MAb (Fig.1). After purification, the protein yield was approximately 300μg/mL.
Analysis of rCDVNP expression. (A) 20% SDS-PAGE: Lane 1 = Pierce™ Unstained Protein MW Marker Thermo Scientific™, Lane 2 = Escherichia coli star negative control, Lane 3 = E. coli star transformed with pAE-NP non induced, Lane 4 = E. coli star transformed with pAE-N induced, Lane 5 = rCDVNP solubilized in 0.2% N-laurolylsarcosyne, Lane 6 = purified rCDVNP. (B) Western blot: Lane 1 = PageRuler™ Prestained Protein Ladder Thermo Scientific™, Lane 2 = E. coli star negative control, Lane 3 = E. coli star transformed with pAE-NP non induced, Lane 4 = E. coli star transformed with pAE-N induced, Lane 5 = rCDVNP solubilized in 0.2% N-laurolylsarcosyne, Lane 6 = purified rCDVNP (indicated by arrow).
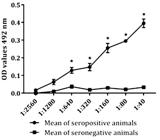
Antigenic evaluation of rCDVNP. In order to evaluate the antigenic properties of rCDVNP, Western blot and ELISA were performed. Antibodies from seropositive dogs recognized the rCDVNP by Western blot as shown in Figure 2, while no reactivity was observed with sera from the negative control. Reactivity was also observed in indirect ELISA using serial dilutions of positive control sera. Low absorbance values at all dilutions were detected with negative control (Fig.3).
Reactivity of the rCDVNP with polyclonal serum against CDV. 20% SDS-PAGE: Lane 1 = prestained SDS-PAGE Standards Bio-Rad®, Lane 2 = purified rCDVNP. Western blot: Lane 3 = dog sera (negative control), Lane 4 = dog sera (positive control) (rCDVNP is indicated by arrow).
Antigenic properties of rCDVNP by indirect ELISA. Interaction of antigen-antibody has been evaluated by ELISA using serial dilutions of serum derived from five seropositive and five seronegative dogs against canine distemper. The lanes represent the means of the optical density (OD) values of the samples. All tests were performed in triplicate.
Immunogenic evaluation of rCDVNP
Purification of IgY. The presence of IgY was confirmed by 15% SDS-PAGE which revealed two bands of approximately 68 and 27kDa, respectively, corresponding to the heavy and light chains of IgY (data not show). The mean yield of IgY was 28.55mg/mL.
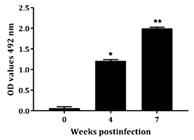
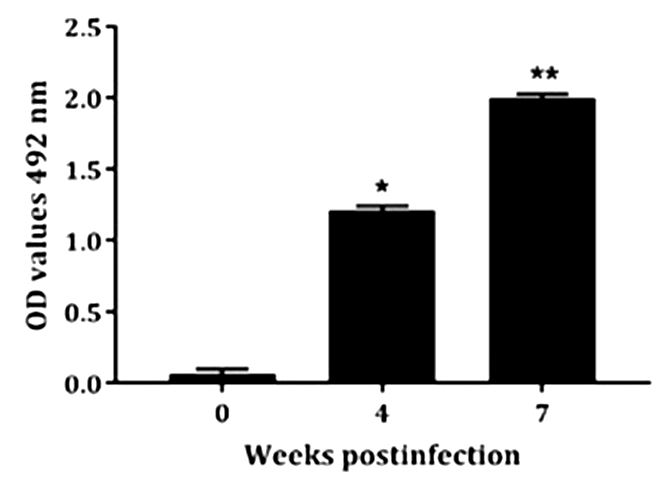
Immunogenic response to rCDVNP in hens. Inoculation of laying hens with rCDVNP resulted in a substantial production of specific IgY antibodies. The presence of specific IgY anti-rCDVNP in egg yolks were determined by Western blot analysis, in which a strong protein band of the molecular weight protein (~12 kDa) was identified. This band was detected in egg yolks of hens 7 weeks after the first inoculation, while remained undetectable in egg yolks derived from hens before the inoculations (Fig.4). In Figure 5, it is possible to visualize an increasing production of specific IgY anti rCDVNP after inoculations as assayed by indirect ELISA. These findings indicated that the recombinant CDV NP was highly immunogenic in hens.
Immunogenicity of rCDVNP. 20% SDS-PAGE: Lane 1 = Pre-stained SDS-PAGE Standards Bio-Rad®, Lane 2 = purified rCDVNP. Western blot: Lane 3 = representative sample of IgY purified before the inoculations, Lane 4 = representative sample of IgY purified 7 weeks after first inoculation (rCDVNP is indicated by arrow).
Evaluation of IgY anti-rCDVNP production by indirect ELISA. ELISA analysis of IgY anti-rCDVNP production with samples isolated at 0, 4 and 7 weeks after the first inoculation.
Discussion
Recombinant protein expression technology has the potential to produce a reliable source of antigens for use in diagnostic assays (Spencer et al. 2007Spencer K., Osorio F.A. & Hiscox J.A. 2007. Recombinant viral proteins for use in diagnostic ELISAs to detect virus infection. Vaccine 25(30):5653-5659. <http://dx.doi.org/10.1016/j.vaccine.2007.02.053> <PMid:17478017>
https://doi.org/10.1016/j.vaccine.2007.0...
). Proteins of CDV, such as H protein (Chan et al. 2009Chan K., Hsieh H., Wang H., Lee Y., Sung M., Wong M. & Hsu W. 2009. Identification, expression and antigenic analysis of recombinant hemagglutinin proteins of canine distemper virus. J. Virol. Methods 155(1):18-24. <http://dx.doi.org/10.1016/j.jviromet.2008.09.024> <PMid:18951919>
https://doi.org/10.1016/j.jviromet.2008....
, Cho et al. 2014Cho K., Kim J., Yoo H., Kim D., Park S., Song C., Choi I. & Lee J. 2014. Use of hydrophilic extra-viral domain of canine distemper virus H protein for enzyme-linked immunosorbent assay development. J. Vet. Sci. 15(4):503-509. <http://dx.doi.org/10.4142/jvs.2014.15.4.503> <PMid:25234325>
https://doi.org/10.4142/jvs.2014.15.4.50...
) and NP (Barben et al. 1999Barben G., Stettler M., Jaggy A., Vandevelde M. & Zurbriggen A. 1999. Detection of IgM antibodies against a recombinant nucleocapsid protein of canine distemper virus in dog sera using a dot-blot assay. J. Vet. Med. A 46(2):115-122. <http://dx.doi.org/10.1046/j.1439-0442.1999.00198.x> <PMid:10216448>
https://doi.org/10.1046/j.1439-0442.1999...
, Latha et al. 2007aLatha D., Geetha M., Ramadass P. & Narayanan R.B. 2007a. Development of recombinant nucleocapsid protein based IgM-ELISA for the early detection of distemper infection in dogs. Vet. Immunol. Immunop. 119(3/4):278-286. <http://dx.doi.org/10.1016/j.vetimm.2007.06.006> <PMid:17659785>
https://doi.org/10.1016/j.vetimm.2007.06...
, 2007bLatha D., Geetha M., Ramadass P. & Narayanan R.B. 2007b. Evalution of ELISA based on the conserved and functional middle region of nucleocapsid protein to detect distemper infection in dogs. Vet. Microbiol. 120(3/4):251-260. <http://dx.doi.org/10.1016/j.vetmic.2006.11.019> <PMid:17224247>
https://doi.org/10.1016/j.vetmic.2006.11...
, Von Messling et al. 1999Von Messling V., Harder T.C., Moennig V., Rautenberg P., Nolte I. & Haas L. 1999. Rapid and sensitive detection of Immunoglobulin M (IgM) and IgG antibodies against canine distemper virus by a new recombinant nucleocapsid protein-based enzyme-linked immunosorbent assay. J. Clin. Microbiol. 37(4):1049-1056. <PMid:10074525>), have been expressed to be used in immunodiagnostic tests. However, the H protein has shown antigenic alterations, since it is most likely to undergo changes under immunological pressure because it plays a role in the attachment of the virus to host cells (Shin et al. 1997bShin Y.S., Miyashita N., Hirayama N., Gemma T., Mikami T., Mori T., Iwatsuki K., Kai C. & Yoshida E. 1997b. Molecular and phylogenetic analyses of the haemagglutinin (H) proteins of field isolates of canine distemper virus from naturally infected dogs. J. Gen. Virol. 78(2):373-380. <http://dx.doi.org/10.1099/0022-1317-78-2-373> <PMid:9018060>
https://doi.org/10.1099/0022-1317-78-2-3...
). NP is one of the most abundant proteins in CDV infections and the most conserved among morbilliviruses (Stettler & Zurbriggen 1995Stettler M. & Zurbriggen A. 1995. Nucleotide and deduced amino acid sequences of the nucleocapsid protein of the virulent A75/17-CDV strain of canine distemper virus. Vet. Microbiol. 44(2/4):211-217. <http://dx.doi.org/10.1016/0378-1135(95)00014-2> <PMid:8588315>
https://doi.org/10.1016/0378-1135(95)000...
). Due its characteristics, NP has been used as viral antigen for diagnosis tests of morbillivirus (Yadav et al. 2009Yadav V., Balamurugan V., Bhanuprakash V., Sen A., Bhanot V., Venkatesan G., Riyesh T. & Singh R.K. 2009. Expression of peste des petits ruminants virus nucleocapsid protein in prokaryotic system and its potential use as a diagnostic antigen or immunogen. J. Virol. Methods 162(1/2):56-63. <http://dx.doi.org/10.1016/j.jviromet.2009.07.014> <PMid:19646481>
https://doi.org/10.1016/j.jviromet.2009....
) and also other viruses like influenza virus (Zhang et al. 2014Zhang R.H., Li C.H., He W.X., Wang C.L., Xu T., Jin M.L. & Chen H.C. 2014. Development of latex agglutination test with nucleoprotein as antigen for detection of antibodies to swine influenza virus. Int. Immunopharmacol. 19(2):201-205. <http://dx.doi.org/10.1016/j.intimp.2014.01.026> <PMid:24508548>
https://doi.org/10.1016/j.intimp.2014.01...
), coronavirus (Chen et al. 2015Chen Y., Chan K.H., Kang Y., Chen H., Luk H.K., Poon R.W., Chan J.F., Yuen K.Y., Xia N., Lau S.K. & Woo P.C. 2015. A sensitive and specific antigen detection assay for Middle East respiratory syndrome coronavirus. Emerg. Microbes Infect. 4(4):e26. <http://dx.doi.org/10.1038/emi.2015.26> <PMid:26421268>
https://doi.org/10.1038/emi.2015.26...
) and bunyavirus (Lee et al. 2016Lee H., Kim E.J., Song J.Y., Choi J.S., Lee J.Y., Cho I.S. & Shin Y.K. 2016. 2015Development and evaluation of a competitive enzyme-linked immunosorbent assay using a monoclonal antibody for diagnosis of severe fever with thrombocytopenia syndrome virus in bovine sera. J. Vet. Sci. 17(3):307-314. <http://dx.doi.org/10.4142/jvs.2016.17.3.307> <PMid:26435543>
https://doi.org/10.4142/jvs.2016.17.3.30...
).
In the present study, we cloned, expressed and purified a conserved region of CDV NP (amino acids # 222-316) from a synthetic and codon optimized gene for Escherichia coli expression. Recombinant CDV NP has already been expressed in different systems such as baculovirus (Von Messling et al. 1999Von Messling V., Harder T.C., Moennig V., Rautenberg P., Nolte I. & Haas L. 1999. Rapid and sensitive detection of Immunoglobulin M (IgM) and IgG antibodies against canine distemper virus by a new recombinant nucleocapsid protein-based enzyme-linked immunosorbent assay. J. Clin. Microbiol. 37(4):1049-1056. <PMid:10074525>) and VERO cells (Shin et al. 1997aShin Y., Mori T., Tomonaga K., Iwatsuki K., Kai C. & Mikami T. 1997a. Expression of the nucleocapsid protein gene of the canine distemper virus. J. Vet. Med. Sci. 59(1):51-53. <http://dx.doi.org/10.1292/jvms.59.51> <PMid:9035079>
https://doi.org/10.1292/jvms.59.51...
). The CDV NP expressed either in baculovirus or mammalian cell expression systems were found to be contaminated with cellular or baculovirus proteins resulting in nonspecific reactions (Shin et al. 1997aShin Y., Mori T., Tomonaga K., Iwatsuki K., Kai C. & Mikami T. 1997a. Expression of the nucleocapsid protein gene of the canine distemper virus. J. Vet. Med. Sci. 59(1):51-53. <http://dx.doi.org/10.1292/jvms.59.51> <PMid:9035079>
https://doi.org/10.1292/jvms.59.51...
, Von Messling et al. 1999Von Messling V., Harder T.C., Moennig V., Rautenberg P., Nolte I. & Haas L. 1999. Rapid and sensitive detection of Immunoglobulin M (IgM) and IgG antibodies against canine distemper virus by a new recombinant nucleocapsid protein-based enzyme-linked immunosorbent assay. J. Clin. Microbiol. 37(4):1049-1056. <PMid:10074525>). In this study, the rCDVNP was expressed in E. coli and the resulting protein was purified successfully.
It has been reported that the production of recombinant proteins in bacteria is simple and more economical when compared to other systems (Yadav et al. 2009Yadav V., Balamurugan V., Bhanuprakash V., Sen A., Bhanot V., Venkatesan G., Riyesh T. & Singh R.K. 2009. Expression of peste des petits ruminants virus nucleocapsid protein in prokaryotic system and its potential use as a diagnostic antigen or immunogen. J. Virol. Methods 162(1/2):56-63. <http://dx.doi.org/10.1016/j.jviromet.2009.07.014> <PMid:19646481>
https://doi.org/10.1016/j.jviromet.2009....
). Despite the E. coli lack of ability to make post-translational modifications in the expressed protein (Spencer et al. 2007Spencer K., Osorio F.A. & Hiscox J.A. 2007. Recombinant viral proteins for use in diagnostic ELISAs to detect virus infection. Vaccine 25(30):5653-5659. <http://dx.doi.org/10.1016/j.vaccine.2007.02.053> <PMid:17478017>
https://doi.org/10.1016/j.vaccine.2007.0...
), in this study it seemed do not interfere with the antigen specificity. Using codon optimization for E. coli, CDV NP was expressed from a synthetic gene, thus reducing time and costs when compared to the expression using the native gene (Newcomb et al. 2007Newcomb J., Carlson R. & Aldrich S.C. 2007. Genome Synthesis and Design Futures: Implications for the US Economy. Bio Economic Research Associates, Cambridge, MA. Available at <http://www.bio-era.net/reports/genome.html>.
http://www.bio-era.net/reports/genome.ht...
). Other studies have also expressed CDV NP efficiently in E. coli, but using the native gene cloned from virus isolated from the studied region (Barben et al. 1999Barben G., Stettler M., Jaggy A., Vandevelde M. & Zurbriggen A. 1999. Detection of IgM antibodies against a recombinant nucleocapsid protein of canine distemper virus in dog sera using a dot-blot assay. J. Vet. Med. A 46(2):115-122. <http://dx.doi.org/10.1046/j.1439-0442.1999.00198.x> <PMid:10216448>
https://doi.org/10.1046/j.1439-0442.1999...
, Latha et al. 2007bLatha D., Geetha M., Ramadass P. & Narayanan R.B. 2007b. Evalution of ELISA based on the conserved and functional middle region of nucleocapsid protein to detect distemper infection in dogs. Vet. Microbiol. 120(3/4):251-260. <http://dx.doi.org/10.1016/j.vetmic.2006.11.019> <PMid:17224247>
https://doi.org/10.1016/j.vetmic.2006.11...
, Yi & Cheng 2014Yi L. & Cheng S. 2014. A monoclonal antibody against truncated N protein (aa 277-471) of canine distemper virus. Monoclon. Antib. Immunodiagn. Immunother. 33(1):52-56. <http://dx.doi.org/10.1089/mab.2013.0066> <PMid:24555938>
https://doi.org/10.1089/mab.2013.0066...
).
The rCDVNP demonstrated to be antigenic, since CD positive dog sera recognized the protein both in its native and denatured form, without showing nonspecific reactions. In ELISA, antibodies from seropositive dogs recognized rCDVNP even at higher dilutions (Fig.3). A similar study evaluating the antigenicity of the CDV NP expressed in E. coli was demonstrated by Barben et al. (1999Barben G., Stettler M., Jaggy A., Vandevelde M. & Zurbriggen A. 1999. Detection of IgM antibodies against a recombinant nucleocapsid protein of canine distemper virus in dog sera using a dot-blot assay. J. Vet. Med. A 46(2):115-122. <http://dx.doi.org/10.1046/j.1439-0442.1999.00198.x> <PMid:10216448>
https://doi.org/10.1046/j.1439-0442.1999...
) and Latha et al. (2007aLatha D., Geetha M., Ramadass P. & Narayanan R.B. 2007a. Development of recombinant nucleocapsid protein based IgM-ELISA for the early detection of distemper infection in dogs. Vet. Immunol. Immunop. 119(3/4):278-286. <http://dx.doi.org/10.1016/j.vetimm.2007.06.006> <PMid:17659785>
https://doi.org/10.1016/j.vetimm.2007.06...
, 2007bLatha D., Geetha M., Ramadass P. & Narayanan R.B. 2007b. Evalution of ELISA based on the conserved and functional middle region of nucleocapsid protein to detect distemper infection in dogs. Vet. Microbiol. 120(3/4):251-260. <http://dx.doi.org/10.1016/j.vetmic.2006.11.019> <PMid:17224247>
https://doi.org/10.1016/j.vetmic.2006.11...
), in which epitopes of CDV NP also were recognized by antibodies present in dogs by Western blot, ELISA and dot-blot assays. NP can be the protein of choice to be used for research of the serological status of recent infections because it is the protein that induces higher antibodies titer in Morbillivirus infections (Elia et al. 2015Elia G., Camero M., Losurdo M., Lucente M.S., Larocca V., Martella V., Decaro N. & Buonavoglia C. 2015. Virological and serological findings in dogs with naturally occurring distemper. J. Virol. Methods 213:127-130. <http://dx.doi.org/10.1016/j.jviromet.2014.12.004> <PMid:25512131>
https://doi.org/10.1016/j.jviromet.2014....
, Von Messling et al. 1999Von Messling V., Harder T.C., Moennig V., Rautenberg P., Nolte I. & Haas L. 1999. Rapid and sensitive detection of Immunoglobulin M (IgM) and IgG antibodies against canine distemper virus by a new recombinant nucleocapsid protein-based enzyme-linked immunosorbent assay. J. Clin. Microbiol. 37(4):1049-1056. <PMid:10074525>).
The use of IgY to detect viral NP in immunodiagnostics assays is an efficient approach, described in studies with other viruses (Kammila et al. 2008Kammila S., Das D., Bhatnagar P.K., Sunwoo H.H., Zayas-Zamora G., King M. & Suresh M.R. 2008. A rapid point of care immunoswab assay for SARS-CoV detection. J. Virol. Methods 152(1/2):77-84. <http://dx.doi.org/10.1016/j.jviromet.2008.05.023> <PMid:18620761>, Veerasami et al. 2008Veerasami M., Singanallur N.B., Thirumeni N., Rana S.K., Shanmugham R., Ponsekaran S., Muthukrishnan M. & Villuppanoor S.A. 2008. Serotyping of foot-and-mouth disease virus by antigen capture-ELISA using monoclonal antibodies and chicken IgY. New Microbiol. 31(4):549-554. <PMid:19123312>). Although the production of IgY against the whole particle of CDV has already been demonstrated (Schmidt et al. 1989Schmidt P., Hafner A., Reubel G.H., Wanke R., Franke V., Lösch U. & Dahme E. 1989. Production of antibodies to canine distemper virus in chicken eggs for immunohistochemistry. J. Vet. Med. B 36(1/10):661-668. <http://dx.doi.org/10.1111/j.1439-0450.1989.tb00659.x> <PMid:2609804>
https://doi.org/10.1111/j.1439-0450.1989...
, Guimarães et al. 2009Guimarães M.C.C., Amaral L.G., Borges F.V., Vieira H.P.L., Matta C.G.F. & Matta M.F.R. 2009. Characterization of an IgY polyclonal antibodies directed against the canine distemper virus. Revta Ciênc. Méd. Biol. 8(1):18-25.), the methodology described in our work shows some improvements when compared to the study performed by Schmidt et al. (1989)Schmidt P., Hafner A., Reubel G.H., Wanke R., Franke V., Lösch U. & Dahme E. 1989. Production of antibodies to canine distemper virus in chicken eggs for immunohistochemistry. J. Vet. Med. B 36(1/10):661-668. <http://dx.doi.org/10.1111/j.1439-0450.1989.tb00659.x> <PMid:2609804>
https://doi.org/10.1111/j.1439-0450.1989...
, as we detected the presence of specific IgY in a shorter period of time. However, the amount of specific IgY obtained in these studies cannot be compared, since the techniques employed in the experiments were not the same. In addition, it is well established that the use of recombinant proteins to immunize animals is safer, faster and more specific when compared to native viral antigens (Spencer et al. 2007Spencer K., Osorio F.A. & Hiscox J.A. 2007. Recombinant viral proteins for use in diagnostic ELISAs to detect virus infection. Vaccine 25(30):5653-5659. <http://dx.doi.org/10.1016/j.vaccine.2007.02.053> <PMid:17478017>
https://doi.org/10.1016/j.vaccine.2007.0...
). Notably, our results demonstrated that the rCDVNP expressed in E. coli was highly immunogenic in hens and it was possible to obtain high concentrations of specific IgY antibodies from the egg yolk. This methodology represents a promising tool for the production of specific polyclonal antibodies since yolk antibodies are an alternative to mammalian antibodies and desirable from the perspective of animal welfare (Karlsson et al. 2004Karlsson M., Kollberg H. & Larsson A. 2004. Chicken IgY: utilizing the evolutionary advantage. World Poultry Sci. J. 60(3):341-348. <http://dx.doi.org/10.1079/WPS200422>
https://doi.org/10.1079/WPS200422...
). Besides, it is economically viable, because the production of IgY is relatively fast and of high yield (Polson et al. 1980Polson A., Von Wechmar M.B. & Van Regenmortel M.H. 1980. Isolation of viral IgY antibodies from yolks of immunized hens. Immunol. Commun. 9(5):475-493. <http://dx.doi.org/10.3109/08820138009066010> <PMid:7429529>
https://doi.org/10.3109/0882013800906601...
), as observed in this study mainly after the second inoculation in hens.
Further studies need to be performed in order to evaluate the reagents here produced as tools for diagnostic. However, our findings indicated that both rCDVNP and specific anti-CDV antibodies might be useful for several immunodiagnostic assays. Recombinant proteins have been used in indirect ELISAs either for monitoring levels of antibodies or detection of recent infections (Von Messling et al. 1999Von Messling V., Harder T.C., Moennig V., Rautenberg P., Nolte I. & Haas L. 1999. Rapid and sensitive detection of Immunoglobulin M (IgM) and IgG antibodies against canine distemper virus by a new recombinant nucleocapsid protein-based enzyme-linked immunosorbent assay. J. Clin. Microbiol. 37(4):1049-1056. <PMid:10074525>). As well, specific anti-CDV IgY can be used to confirm the presence of the antigen by direct ELISA (Vasconcellos et al. 2010Vasconcellos F.A., Coutinho M.L., da Silva E.F., Fernandes C.P., Monte L.G., Seyffert N., Dellagostin O.A. & Aleixo J.A. 2010. Testing different antigen capture ELISA formats for detection of Leptospira spp. in human blood serum. Trans. R. Soc. Trop. Med. Hyg. 104(4):259-264. <http://dx.doi.org/10.1016/j.trstmh.2009.10.005> <PMid:19942245>
https://doi.org/10.1016/j.trstmh.2009.10...
, Zhang et al. 2016Zhang X., Diraviyam T., Li X., Yao G. & Michael A. 2016. Preparation of chicken IgY against recombinant E2 protein of bovine viral diarrhea virus (BVDV) and development of ELISA and ICA for BVDV detection. Biosci. Biotechnol. Biochem. 80(12):2467-2472. <http://dx.doi.org/10.1080/09168451.2016.1217144> <PMid:27484991>
https://doi.org/10.1080/09168451.2016.12...
), for fast detection of the viral antigen by immunochromatographic tests (Zhang et al. 2016), and also to confirm the presence of the virus in tissues, as the main antibody in immunochemistry (Schmidt et al. 1989Schmidt P., Hafner A., Reubel G.H., Wanke R., Franke V., Lösch U. & Dahme E. 1989. Production of antibodies to canine distemper virus in chicken eggs for immunohistochemistry. J. Vet. Med. B 36(1/10):661-668. <http://dx.doi.org/10.1111/j.1439-0450.1989.tb00659.x> <PMid:2609804>
https://doi.org/10.1111/j.1439-0450.1989...
).
Conclusions
In summary, the synthetic gene encoding a conserved region of the nucleocapsid protein of canine distemper virus (rCDV NP) was successfully cloned and expressed in Escherichia coli resulting in a recombinant NP protein highly antigenic and immunogenic.
Moreover, our results indicated that this approach may be a practical strategy for the large-scale production of specific anti-CDV antibodies to be used in diagnostic tests of CDV.
Acknowledgements
Financial support was provided by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo a Pesquisa do Estado do Rio Grande do Sul (FAPERGS) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
References
- Akita E.M. & Nakai S. 1992. Immunoglobulins from egg yolk: Isolation and purification. J. Food Sci. 57(3):629-634. <http://dx.doi.org/10.1111/j.1365-2621.1992.tb08058.x>
» https://doi.org/10.1111/j.1365-2621.1992.tb08058.x - Balamurugan V., Venkatesan G., Sen A., Annamalai L., Bhanuprakash V. & Singh R.K. 2010. Recombinant protein-based viral disease diagnostics in veterinary medicine. Expert Rev. Mol. Diagn. 10(6):731-753. <http://dx.doi.org/10.1586/erm.10.61> <PMid:20843198>
» https://doi.org/10.1586/erm.10.61 - Barben G., Stettler M., Jaggy A., Vandevelde M. & Zurbriggen A. 1999. Detection of IgM antibodies against a recombinant nucleocapsid protein of canine distemper virus in dog sera using a dot-blot assay. J. Vet. Med. A 46(2):115-122. <http://dx.doi.org/10.1046/j.1439-0442.1999.00198.x> <PMid:10216448>
» https://doi.org/10.1046/j.1439-0442.1999.00198.x - Beineke A., Puff C., Seehusen F. & Baumgärtner W. 2009. Pathogenesis and immunopathology of systemic and nervous canine distemper. Vet. Immunol. Immunop. 127(1/2):1-18. <http://dx.doi.org/10.1016/j.vetimm.2008.09.023> <PMid:19019458>
» https://doi.org/10.1016/j.vetimm.2008.09.023 - Bentubo H.D.L., Tomaz M.A., Bondan E.F. & Lallo M.A. 2007. Expectativa de vida e causas de morte em cães na área metropolitana de São Paulo (Brasil). Ciência Rural 37(4):1021-1026. <http://dx.doi.org/10.1590/S0103-84782007000400016>
» https://doi.org/10.1590/S0103-84782007000400016 - Chan K., Hsieh H., Wang H., Lee Y., Sung M., Wong M. & Hsu W. 2009. Identification, expression and antigenic analysis of recombinant hemagglutinin proteins of canine distemper virus. J. Virol. Methods 155(1):18-24. <http://dx.doi.org/10.1016/j.jviromet.2008.09.024> <PMid:18951919>
» https://doi.org/10.1016/j.jviromet.2008.09.024 - Chen Y., Chan K.H., Kang Y., Chen H., Luk H.K., Poon R.W., Chan J.F., Yuen K.Y., Xia N., Lau S.K. & Woo P.C. 2015. A sensitive and specific antigen detection assay for Middle East respiratory syndrome coronavirus. Emerg. Microbes Infect. 4(4):e26. <http://dx.doi.org/10.1038/emi.2015.26> <PMid:26421268>
» https://doi.org/10.1038/emi.2015.26 - Cho K., Kim J., Yoo H., Kim D., Park S., Song C., Choi I. & Lee J. 2014. Use of hydrophilic extra-viral domain of canine distemper virus H protein for enzyme-linked immunosorbent assay development. J. Vet. Sci. 15(4):503-509. <http://dx.doi.org/10.4142/jvs.2014.15.4.503> <PMid:25234325>
» https://doi.org/10.4142/jvs.2014.15.4.503 - Deem S.L., Spelman L.H., Yates R.A. & Montali R.J. 2000. Canine distemper in terrestrial carnivores: a review. J. Zoo Wildl. Med. 31(4):441-451. <http://dx.doi.org/10.1638/1042-7260(2000)031[0441:CDITCA]2.0.CO;2> <PMid:11428391>
» https://doi.org/10.1638/1042-7260(2000)031[0441:CDITCA]2.0.CO;2 - Elia G., Decaro N., Martella V., Cirone F., Lucente M.S., Lorusso E., Di Trani L. & Buonavoglia C. 2006. Detection of canine distemper virus in dogs by real-time RT-PCR. J. Virol. Methods 136(1/2):171-176. <http://dx.doi.org/10.1016/j.jviromet.2006.05.004> <PMid:16750863>
» https://doi.org/10.1016/j.jviromet.2006.05.004 - Elia G., Camero M., Losurdo M., Lucente M.S., Larocca V., Martella V., Decaro N. & Buonavoglia C. 2015. Virological and serological findings in dogs with naturally occurring distemper. J. Virol. Methods 213:127-130. <http://dx.doi.org/10.1016/j.jviromet.2014.12.004> <PMid:25512131>
» https://doi.org/10.1016/j.jviromet.2014.12.004 - Fighera R.A., Souza T.M., Silva M.C., Brum J.C., Graça D.G., Kommers G.D., Irigoyen L.F. & Barros C.S.L. 2008. Causas de morte e razões para eutanásia de cães da Mesorregião do Centro Ocidental Rio-Grandense (1965-2004). Pesq. Vet. Bras. 28(4):223-230. <http://dx.doi.org/10.1590/S0100-736X2008000400005>
» https://doi.org/10.1590/S0100-736X2008000400005 - Frisk A.L., König M., Moritz A. & Baumgärtner W. 1999. Detection of canine distemper virus nucleoprotein RNA by reverse transcription-PCR using serum, whole blood, and cerebrospinal fluid from dogs with distemper. J. Clin. Microbiol. 37(11):3634-3643. <PMid:10523566>
- Froger A. & Hall J.E. 2007. Transformation of plasmid DNA into E. coli using the heat shock method. JoVE (6):253. <PMid:18997900>
- Guimarães M.C.C., Amaral L.G., Borges F.V., Vieira H.P.L., Matta C.G.F. & Matta M.F.R. 2009. Characterization of an IgY polyclonal antibodies directed against the canine distemper virus. Revta Ciênc. Méd. Biol. 8(1):18-25.
- ICTV 2014. International Committee on Taxonomy of Viruses. Available at <Available at http://www.ictvonline.org/virusTaxonomy.asp > Access on Dec. 15, 2016.
» http://www.ictvonline.org/virusTaxonomy.asp - Kammila S., Das D., Bhatnagar P.K., Sunwoo H.H., Zayas-Zamora G., King M. & Suresh M.R. 2008. A rapid point of care immunoswab assay for SARS-CoV detection. J. Virol. Methods 152(1/2):77-84. <http://dx.doi.org/10.1016/j.jviromet.2008.05.023> <PMid:18620761>
- Karlsson M., Kollberg H. & Larsson A. 2004. Chicken IgY: utilizing the evolutionary advantage. World Poultry Sci. J. 60(3):341-348. <http://dx.doi.org/10.1079/WPS200422>
» https://doi.org/10.1079/WPS200422 - Kearse M., Moir R., Wilson A., Stones-Havas S., Cheung M., Sturrock S., Buxton S., Cooper A., Markowitz S., Duran C., Thierer T., Ashton B., Meintjes P. & Drummond A. 2012. Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28(12):1647-1649. <http://dx.doi.org/10.1093/bioinformatics/bts199> <PMid:22543367>
» https://doi.org/10.1093/bioinformatics/bts199 - Latha D., Geetha M., Ramadass P. & Narayanan R.B. 2007a. Development of recombinant nucleocapsid protein based IgM-ELISA for the early detection of distemper infection in dogs. Vet. Immunol. Immunop. 119(3/4):278-286. <http://dx.doi.org/10.1016/j.vetimm.2007.06.006> <PMid:17659785>
» https://doi.org/10.1016/j.vetimm.2007.06.006 - Latha D., Geetha M., Ramadass P. & Narayanan R.B. 2007b. Evalution of ELISA based on the conserved and functional middle region of nucleocapsid protein to detect distemper infection in dogs. Vet. Microbiol. 120(3/4):251-260. <http://dx.doi.org/10.1016/j.vetmic.2006.11.019> <PMid:17224247>
» https://doi.org/10.1016/j.vetmic.2006.11.019 - LaVallie E.R. 2001. Production of recombinant proteins in Escherichia coli Curr. Protoc. Protein Sci. Chapter 5:1. <http://dx.doi.org/10.1002/0471140864.ps0501s00> <PMid:18429175>
» https://doi.org/10.1002/0471140864.ps0501s00 - Lee H., Kim E.J., Song J.Y., Choi J.S., Lee J.Y., Cho I.S. & Shin Y.K. 2016. 2015Development and evaluation of a competitive enzyme-linked immunosorbent assay using a monoclonal antibody for diagnosis of severe fever with thrombocytopenia syndrome virus in bovine sera. J. Vet. Sci. 17(3):307-314. <http://dx.doi.org/10.4142/jvs.2016.17.3.307> <PMid:26435543>
» https://doi.org/10.4142/jvs.2016.17.3.307 - Lu G. & Moriyama E.N. 2004. Vector NTI, a balanced all-in-one sequence analysis suite. Brief. Bioinform. 5(4):378-388. <http://dx.doi.org/10.1093/bib/5.4.378> <PMid:15606974>
» https://doi.org/10.1093/bib/5.4.378 - Martella V., Elia G. & Buonavoglia C. 2008. Canine distemper virus. Vet. Clin. N. Am., Small Anim. Pract. 38(4):787-797. <http://dx.doi.org/10.1016/j.cvsm.2008.02.007> <PMid:18501278>
» https://doi.org/10.1016/j.cvsm.2008.02.007 - Newcomb J., Carlson R. & Aldrich S.C. 2007. Genome Synthesis and Design Futures: Implications for the US Economy. Bio Economic Research Associates, Cambridge, MA. Available at <http://www.bio-era.net/reports/genome.html>.
» http://www.bio-era.net/reports/genome.html - Papaneophytou C.P. & Kontopidis G. 2014. Statistical approaches to maximize recombinant protein expression in Escherichia coli: a general review. Protein Expr. Purif. 94:22-32. <http://dx.doi.org/10.1016/j.pep.2013.10.016> <PMid:24211770>
» https://doi.org/10.1016/j.pep.2013.10.016 - Polson A., Von Wechmar M.B. & Van Regenmortel M.H. 1980. Isolation of viral IgY antibodies from yolks of immunized hens. Immunol. Commun. 9(5):475-493. <http://dx.doi.org/10.3109/08820138009066010> <PMid:7429529>
» https://doi.org/10.3109/08820138009066010 - Ramos C.R.R., Abreu P.A.E., Nascimento A.L.T.O. & Ho P.L. 2004. A high copy T7 Escherichia coli expression vector for the production of recombinant proteins with a minimal N-terminal His-tagged fusion peptide. Braz. J. Med. Biol. Res. 37(8):1103-1109. <http://dx.doi.org/10.1590/S0100-879X2004000800001> <PMid:15273812>
» https://doi.org/10.1590/S0100-879X2004000800001 - Sambrook J. & Russell D.W. 2006. Preparation of Plasmid DNA by Alkaline Lysis with SDS: minipreparation. Cold Spring Harbor Protocols. 2006(1). <http://dx.doi.org/10.1101/pdb.prot4084> <PMid:22485489>
» https://doi.org/10.1101/pdb.prot4084 - Schmidt P., Hafner A., Reubel G.H., Wanke R., Franke V., Lösch U. & Dahme E. 1989. Production of antibodies to canine distemper virus in chicken eggs for immunohistochemistry. J. Vet. Med. B 36(1/10):661-668. <http://dx.doi.org/10.1111/j.1439-0450.1989.tb00659.x> <PMid:2609804>
» https://doi.org/10.1111/j.1439-0450.1989.tb00659.x - Shin Y., Mori T., Tomonaga K., Iwatsuki K., Kai C. & Mikami T. 1997a. Expression of the nucleocapsid protein gene of the canine distemper virus. J. Vet. Med. Sci. 59(1):51-53. <http://dx.doi.org/10.1292/jvms.59.51> <PMid:9035079>
» https://doi.org/10.1292/jvms.59.51 - Shin Y.S., Miyashita N., Hirayama N., Gemma T., Mikami T., Mori T., Iwatsuki K., Kai C. & Yoshida E. 1997b. Molecular and phylogenetic analyses of the haemagglutinin (H) proteins of field isolates of canine distemper virus from naturally infected dogs. J. Gen. Virol. 78(2):373-380. <http://dx.doi.org/10.1099/0022-1317-78-2-373> <PMid:9018060>
» https://doi.org/10.1099/0022-1317-78-2-373 - Spencer K., Osorio F.A. & Hiscox J.A. 2007. Recombinant viral proteins for use in diagnostic ELISAs to detect virus infection. Vaccine 25(30):5653-5659. <http://dx.doi.org/10.1016/j.vaccine.2007.02.053> <PMid:17478017>
» https://doi.org/10.1016/j.vaccine.2007.02.053 - Stettler M. & Zurbriggen A. 1995. Nucleotide and deduced amino acid sequences of the nucleocapsid protein of the virulent A75/17-CDV strain of canine distemper virus. Vet. Microbiol. 44(2/4):211-217. <http://dx.doi.org/10.1016/0378-1135(95)00014-2> <PMid:8588315>
» https://doi.org/10.1016/0378-1135(95)00014-2 - Vasconcellos F.A., Coutinho M.L., da Silva E.F., Fernandes C.P., Monte L.G., Seyffert N., Dellagostin O.A. & Aleixo J.A. 2010. Testing different antigen capture ELISA formats for detection of Leptospira spp. in human blood serum. Trans. R. Soc. Trop. Med. Hyg. 104(4):259-264. <http://dx.doi.org/10.1016/j.trstmh.2009.10.005> <PMid:19942245>
» https://doi.org/10.1016/j.trstmh.2009.10.005 - Veerasami M., Singanallur N.B., Thirumeni N., Rana S.K., Shanmugham R., Ponsekaran S., Muthukrishnan M. & Villuppanoor S.A. 2008. Serotyping of foot-and-mouth disease virus by antigen capture-ELISA using monoclonal antibodies and chicken IgY. New Microbiol. 31(4):549-554. <PMid:19123312>
- Von Messling V., Harder T.C., Moennig V., Rautenberg P., Nolte I. & Haas L. 1999. Rapid and sensitive detection of Immunoglobulin M (IgM) and IgG antibodies against canine distemper virus by a new recombinant nucleocapsid protein-based enzyme-linked immunosorbent assay. J. Clin. Microbiol. 37(4):1049-1056. <PMid:10074525>
- Welch M., Govindarajan S., Ness J.E., Villalobos A., Gurney A., Minshull J. & Gustafsson C. 2009. Design parameters to control synthetic gene expression in Escherichia coli Plos One 4(9):e7002. <http://dx.doi.org/10.1371/journal.pone.0007002> <PMid:19759823>
» https://doi.org/10.1371/journal.pone.0007002 - Wen J., Zhao S., He D., Yang Y., Li Y. & Zhu S. 2012. Preparation and characterization of egg yolk immunoglobulin Y specific to influenza B virus. Antiviral Res. 93(1):154-159. <http://dx.doi.org/10.1016/j.antiviral.2011.11.005> <PMid:22127067>
» https://doi.org/10.1016/j.antiviral.2011.11.005 - Yadav V., Balamurugan V., Bhanuprakash V., Sen A., Bhanot V., Venkatesan G., Riyesh T. & Singh R.K. 2009. Expression of peste des petits ruminants virus nucleocapsid protein in prokaryotic system and its potential use as a diagnostic antigen or immunogen. J. Virol. Methods 162(1/2):56-63. <http://dx.doi.org/10.1016/j.jviromet.2009.07.014> <PMid:19646481>
» https://doi.org/10.1016/j.jviromet.2009.07.014 - Yi L. & Cheng S. 2014. A monoclonal antibody against truncated N protein (aa 277-471) of canine distemper virus. Monoclon. Antib. Immunodiagn. Immunother. 33(1):52-56. <http://dx.doi.org/10.1089/mab.2013.0066> <PMid:24555938>
» https://doi.org/10.1089/mab.2013.0066 - Yoshida E., Iwatsuki K., Miyashita N., Gemma T., Kai C. & Mikami T. 1998. Molecular analysis of the nucleocapsid protein of recent isolates of canine distemper virus in Japan. Vet. Microbiol. 59(2/3):237-244. <http://dx.doi.org/10.1016/S0378-1135(97)00194-6> <PMid:9549863>
» https://doi.org/10.1016/S0378-1135(97)00194-6 - Zhang R.H., Li C.H., He W.X., Wang C.L., Xu T., Jin M.L. & Chen H.C. 2014. Development of latex agglutination test with nucleoprotein as antigen for detection of antibodies to swine influenza virus. Int. Immunopharmacol. 19(2):201-205. <http://dx.doi.org/10.1016/j.intimp.2014.01.026> <PMid:24508548>
» https://doi.org/10.1016/j.intimp.2014.01.026 - Zhang X., Diraviyam T., Li X., Yao G. & Michael A. 2016. Preparation of chicken IgY against recombinant E2 protein of bovine viral diarrhea virus (BVDV) and development of ELISA and ICA for BVDV detection. Biosci. Biotechnol. Biochem. 80(12):2467-2472. <http://dx.doi.org/10.1080/09168451.2016.1217144> <PMid:27484991>
» https://doi.org/10.1080/09168451.2016.1217144
Publication Dates
-
Publication in this collection
Aug 2018
History
-
Received
06 June 2017 -
Accepted
19 July 2017