ABSTRACT:
Clostridial diseases are important causes of livestock losses in the southern Rio Grande do Sul. Since 1978 annual surveys conducted at the “Laboratório Regional de Diagnóstico” of the “Universidade Federal de Pelotas” (LRD-UFPel) have shown that clostridial diseases represent 10.40% of the bacterial diseases diagnosed in cattle and 1.65% of all diseases diagnosis in cattle over a 40-year period. The purpose of this study is to review the clinical, epidemiological and pathological aspects of the clostridial diseases diagnosed in cattle from January 1978 to December 2018 at the LRD-UFPel in the hopes that it will constitute a useful guide for field veterinary practitioners and interested farmers. We assessed and review the necropsy protocols of 6,736 cattle; these necropsies were performed either by LRD-UFPel faculty or by field veterinary practitioners; 111 outbreaks (1.65%) were diagnosed as clostridial disease, distributed as follows: 35 outbreaks of tetanus, 34 of blackleg, 23 of bacillary hemoglobinuria, 11 of malignant edema (gas gangrene), and eight of botulism. Approximately 904, from a total of 42,480 cattle at risk, died in these outbreaks.
INDEX TERMS:
Clostridial diseases; diagnosis; cattle; Rio Grande do Sul; Brazil; blackleg; botulism; malignant edema; gas gangrene; bacillary hemoglobinuria; tetanus; Clostridium spp
RESUMO:
Clostridioses são doenças produzidas por alguma das espécies do gênero Clostridium e são importantes causas de perdas pecuárias no sul do Rio Grande do Sul. Pesquisas anuais realizadas no Laboratório Regional de Diagnóstico da Faculdade de Veterinária da Universidade Federal de Pelotas (LRD-UFPel) desde 1978 demonstraram que as clostridioses representaram 11,1% das doenças bacterianas diagnosticadas em bovinos e 1,65% de todos os diagnósticos de doenças em bovinos ao longo de 40 anos. O objetivo deste estudo é revisar os aspectos clínicos, epidemiológicos e patológicos das clostridioses diagnosticadas de janeiro de 1978 a dezembro de 2018, pelo LRD/UFPel com a intenção de que esse trabalho possa servir de guia útil para os veterinários de campo e fazendeiros interessados. Foram avaliados e revisados os protocolos de necropsia de 6.736 bovinos; essas necropsias foram realizadas pelo pessoal do LRD/UFPel ou por veterinários de campo. Cento e quatro (1,16%) casos foram diagnosticados como clostridioses, distribuídos da seguinte forma: 35 surtos de tétano, 34 de cartbúnculo sintomático, 23 de hemoglobinúria bacilar, 11 de edema maligno (gangrena gasosa) e oito de botulismo. Aproximadamente 904, de um total de 42.480 bovinos sob-risco, morreram nesses surtos.
TERMOS DE INDEXAÇÃO:
Clostridiose; diagnóstico; bovinos; Rio Grande do Sul; Brasil; carbúnculo sintomático; botulismo; edema maligno; gangrene gasosa; hemoglobinúria bacilar; tétano; Clostridium spp
Introduction
Clostridial diseases are bacterial diseases caused by one or more of the several species of Clostridium and their potent toxins. Members of the genus Clostridium are gram-positive anaerobic rods that form heat-resistant endospores (Rood 2016Rood J.I. 2016. General Physiological and virulence properties of the pathogenic clostridia, p.7-12. In: Uzal F., Prescott J., Songer G. & Popoff M. (Eds), Clostridial Diseases of Animals. John Wiley and Sons. Ames. <http://dx.doi.org/10.1002/9781118728291.ch2>.
https://doi.org/10.1002/9781118728291.ch...
).
Although the pathogenesis of clostridial diseases always involves the action of specific toxins produced by the bacteria (with only one exception) they are truly infectious diseases. The infectious agent needs to establish it in the host and overcome its immune defenses so it can grow, multiply and elaborate the toxins (Rood 2016Rood J.I. 2016. General Physiological and virulence properties of the pathogenic clostridia, p.7-12. In: Uzal F., Prescott J., Songer G. & Popoff M. (Eds), Clostridial Diseases of Animals. John Wiley and Sons. Ames. <http://dx.doi.org/10.1002/9781118728291.ch2>.
https://doi.org/10.1002/9781118728291.ch...
). The exception is botulism, which is usually a true toxemia, being caused by the ingestion of botulinum neurotoxin (BoNT) preformed in food (Guizelini et al. 2019Guizelini C.C., Lemos R.A.A., de Paula J.L.P., Pupin R.C., Gomes D.C., Barros C.S.L., Neves D.A., Alcântara L.O.B., Silva R.O.S., Lobato F.C.F. & Martins T.B. 2019. Type C botulism outbreak in feedlot cattle fed contaminated corn silage. Anaerobe 55:103-106. <http://dx.doi.org/10.1016/j.anaerobe.2018.11.003> <PMid:30408576>
https://doi.org/10.1016/j.anaerobe.2018....
).
Clostridial diseases can be divided into three major types: 1) neurotoxic diseases (Böhmel & Gessler 2010Böhmel H. & Gessler F. 2010. Neurotoxigenic clostridia, p.189-202. In: Gyles C.L., Prescott J.F., Songer J.G. & Thoen C.O. (Eds), Pathogenesis of Bacterial Disease in Animals. 4th ed. Wiley Blackwell, Ames. <http://dx.doi.org/10.1002/9780470958209.ch11>.
https://doi.org/10.1002/9780470958209.ch...
), 2) histotoxic diseases (Songer 2010aSonger J.G. 2010a. Histotoxic clostridia, p.203-209. In: Gyles C.L., Prescott J.F., Songer J.G. & Thoen C.O. (Eds), Pathogenesis of Bacterial Disease in Animals. 4th ed. Wiley Blackwell, Ames. <http://dx.doi.org/10.1002/9780470958209.ch12>.
https://doi.org/10.1002/9780470958209.ch...
) and 3) enteric diseases (Songer 2010bSonger J.G. 2010b. Enteric clostridia, p.211-229. In: Gyles C.L., Prescott J.F., Songer J.G. & Thoen C.O. (Eds), Pathogenesis of Bacterial Disease in Animals. 4th ed. Wiley Blackwell, Ames. <http://dx.doi.org/10.1002/9780470958209.ch13>.
https://doi.org/10.1002/9780470958209.ch...
). In cattle, the neurotoxic group includes botulism, due to the ingestion of BoNTs; and tetanus, caused by the infection of Clostridium tetani and related neurotoxins (TeNT). The histotoxic group includes diseases such as blackleg (Clostridium chauvoei), malignant edema (Clostridium novyi type A, Clostridium perfringens type A, Clostridium sordelli, Clostridium septicum), and bacillary hemoglobinuria (Clostridium haemolyticum). The group of enteric diseases includes several types of necrotizing and hemorrhagic enteritis (Clostridium difficile in calves, C. perfringens type C, C. perfringens type E) and enterotoxemia (C. perfringens type D) which occurs in small ruminants and possibly in cattle (Uzal et al. 2002Uzal F.A., Kelly W.R., Morris W.E. & Assis R.A. 2002. Effects of intravenous injection of Clostridium perfringens type D epsilon toxin in calves. J. Comp. Pathol. 126(1):71-75. <http://dx.doi.org/10.1053/jcpa.2001.0514> <PMid:11814324>
https://doi.org/10.1053/jcpa.2001.0514...
, Lobato et al. 2006Lobato F.C.F., Assis R.A., Abreu V.L.V., Souza Junior M.F., Lima C.G.R.D. & Salvarani F.M. 2006. Enterotoxemia em bovino. Arqs Bras. Med. Vet. Zootec. 58(5):952-954. <http://dx.doi.org/10.1590/S0102-09352006000500037>
https://doi.org/10.1590/S0102-0935200600...
, Filho et al. 2009Filho E.J.F., Carvalho A.U., Assis R.A., Lobato F.F., Rachid M.A., Carvalho A.A., Ferreira P.M., Nascimento R.A., Fernandes A.A., Vidal J.E. & Uzal F.A. 2009. Clinicopathologic features of experimental Clostridium perfringens type D enterotoxemia in cattle. Vet. Pathol. 46(6):1213-1220. <http://dx.doi.org/10.1354/vp.08-VP-0304-U-FL> <PMid:19605912>
https://doi.org/10.1354/vp.08-VP-0304-U-...
, Mete et al. 2013Mete A., Garcia J., Ortega J., Lane M., Scholes S. & Uzal F.A. 2013. Brain lesions associated with Clostridium perfringens type D epsilon toxin in a Holstein heifer calf. Vet. Pathol. 50(5):765-768. <http://dx.doi.org/10.1177/0300985813476058> <PMid:23381925>
https://doi.org/10.1177/0300985813476058...
).
Clostridial diseases usually occur with high rates of lethality causing significant economic losses in cattle in the region of influence of the “Laboratório Regional de Diagnóstico” of the “Universidade Federal de Pelotas” (LRD-UFPel), which is located in the South of the state of Rio Grande do Sul (latitude 31o45’48 “South, longitude 52o29’02” West, altitude 21m), Brazil.
We aimed to review the clinical, epidemiological and pathological aspects of the clostridial diseases diagnosed in cattle from January 1978 to December 2018 at the LRD-UFPel in the hopes that it will constitute a useful guide for field veterinary practitioners and interested farmers.
Materials and Methods
We reviewed the necropsy protocols of 6,736 necropsies of cattle performed from January 1978 to December 2018 and filed at the LRD-UFPel. These necropsies were performed either by LRD-UFPel faculty or by field veterinary practitioners from the LRD-UFPel influence area, who subsequently submitted material for histological evaluation at the LRD-UFPel. We selected cases of clostridial diseases found in these 6,736 necropsies. From each outbreak, we annotated the following data: municipality of origin, year of occurrence, age of affected cattle, type of breeding, morbidity, mortality and lethality rates. The authors did not make any changes in the final diagnosis that appeared in each protocol.
We also made a brief review on the epidemiology, clinical signs, necropsy findings, histopathology and methods of diagnosis and prophylaxis of each clostridial disease reported in this study.
Results and Adiscussion
From the results of the 6,736 bovine necropsies reviewed here, 1,000 (14.85%) were of bacterial diseases. A hundred and four of those (11.1%) were clostridial diseases. In this review, out of a total of 42,480 cattle at risk, approximately 904 died, reaching a mortality rate of 2.13%. Five specific clostridial diseases were found. In descending order of frequency, there were 35 outbreaks of tetanus (31.5% of the clostridial disease), 34 outbreaks of blackleg (30.6% of the cases), 23 outbreaks of bacillary hemoglobinuria (20.7% of the cases), 11 outbreaks of malignant edema (gas gangrene) (9.9% of the cases), and 8 outbreaks of botulism (7.2% of the cases). Epidemiological data on the outbreaks of each disease are in Table 1. With the exception of botulism, in all clostridial diseases outbreaks of this study, a lethality rate of approximately 100% was observed, which shows the importance of this category of diseases as causes of cattle losses.
There was an unusual frequency of cases of bovine tetanus, especially if we consider that cattle is relatively resistant to the disease (Popoff 2016Popoff M.R. 2016. Tetanus, p.295-302. In: Uzal F., Prescott J., Songer G. & Popoff M. (Eds), Clostridial Diseases of Animals. John Wiley and Sons, Ames. <http://dx.doi.org/10.1002/9781118728291.ch25>.
https://doi.org/10.1002/9781118728291.ch...
) when compared for example, with horses, and that the disease tends to be sporadic in cattle (Raposo 2007Raposo J.B. 2007. Tétano, p.425-432. In: Riet-Correa F., Schild A.L., Lemos R.A.A & Borges J.R.J. (Eds), Doenças de Ruminantes e Equídeos. Vol.1. 3rd ed. Pallotti, Santa Maria.). However the following unusual epidemiological conditions explain the high incidence of tetanus in this study: in 2001 large outbreaks of tetanus occurred in five farms in the area of influence of the LRD-UFPel. In these outbreaks at least 297 beef cattle and 50 sheep died. Injections with an anthelmintic contaminated with Clostridium tetani was applied to a large number of cattle and caused unusually the large outbreaks (Driemeier et al. 2007Driemeier D., Schild A.L., Fernandes J.C., Colodel E.M., Correa A.M., Cruz C.E. & Barros C.S.L. 2007. Outbreaks of tetanus in beef cattle and sheep in Brazil associated with disophenol injection. J. Vet. Med. A, Physiol. Pathol. Clin. Med. 54(6):333-335. <http://dx.doi.org/10.1111/j.1439-0442.2007.00922.x> <PMid:17650154>
https://doi.org/10.1111/j.1439-0442.2007...
). In 2009, 24 outbreaks of tetanus occurred in the southern RS. In this particular outbreak there was a history of drug and/or vaccine application in most of the herds (Quevedo et al. 2011Quevedo P.S., Ladeira S.R.L., Soares M.P., Marcolongo-Pereira C., Sallis E.S.V., Grecco F.B., Estima-Silva P. & Schild A.L. 2011. Tétano em bovinos no sul do Rio Grande do Sul: estudo de 24 surtos. Pesq. Vet. Bras. 31(12):1066-1070. <http://dx.doi.org/10.1590/S0100-736X2011001200005>
https://doi.org/10.1590/S0100-736X201100...
).
Tetanus is a neurological disease characterized by spastic paralysis. It results from the contamination of wounds with spores of Clostridium tetani acquired from the environment (Popoff 2016Popoff M.R. 2016. Tetanus, p.295-302. In: Uzal F., Prescott J., Songer G. & Popoff M. (Eds), Clostridial Diseases of Animals. John Wiley and Sons, Ames. <http://dx.doi.org/10.1002/9781118728291.ch25>.
https://doi.org/10.1002/9781118728291.ch...
). Deep wounds with little exposure to air and the presence of necrotic tissue provide anaerobic conditions favoring spore germination (Popoff 2016Popoff M.R. 2016. Tetanus, p.295-302. In: Uzal F., Prescott J., Songer G. & Popoff M. (Eds), Clostridial Diseases of Animals. John Wiley and Sons, Ames. <http://dx.doi.org/10.1002/9781118728291.ch25>.
https://doi.org/10.1002/9781118728291.ch...
).
Spastic paralysis results from the action of TeNT produced by C. tetani at the synapses of the interneurons in the central nervous system. When the TeNT reaches the neuromuscular end plate, becomes internalized at the presynaptic terminal (Mayhew 2009Mayhew J. 2009. Toxic diseases, p.321-359 In: Ibid. (Ed), Large Animal Neurology. Wiley Blackwell. Ames.). Through retrograde transport (Bizzini 1989Bizzini B. 1989. Axoplasmic transport and transynaptic movement of tetanus toxins, p.203-229. In: Simpson L.L. (Ed), Botulism Neurotoxin and Tetanus Toxin. Academic Press, New York. <http://dx.doi.org/10.1016/B978-0-12-644445-2.50015-5>
https://doi.org/10.1016/B978-0-12-644445...
), it reaches the body of the motor neuron, from where it is transferred to the inhibitory neurons within the spinal cord and brain stem (Fig.1). TeNT compromises the release of neurotransmitter at the synapse, preventing the inhibitory neuron from disrupting the action triggered by the motor neuron, then resulting in spasticity (Barros et al. 2006bBarros C.S.L., Driemeier D., Dutra I.S. & Lemos R.A.A. 2006b. Tétano, p.59-64. In: Ibid. (Eds), Doenças do Sistema Nervoso de Bovinos no Brasil. Valée, Montes Claros ., Mayhew 2009Mayhew J. 2009. Toxic diseases, p.321-359 In: Ibid. (Ed), Large Animal Neurology. Wiley Blackwell. Ames.).
Pathogenesis of tetanus. Tetanus neurotoxin (TetNT, yellow asterisks) is directed by retrograde transport (shown as a purple line) into motor neurons by the action of microtubules and actin microfilaments. TetNT inhibits the exocytosis of specific synaptic vesicles at inhibitory neuronal terminals. Thus the action of the inhibitory neuron is not exerted on the motor neuron, resulting in spasticity. (Modified from Mayhew 2009Mayhew J. 2009. Toxic diseases, p.321-359 In: Ibid. (Ed), Large Animal Neurology. Wiley Blackwell. Ames.)
The potential risk for tetanus in cattle exists in almost all rural properties due to the ubiquitous occurrence of C. tetani in the soil, water, and feces of animals and human beings, and poor husbandry practices that create favorable conditions for the development of the disease. The port of entry is usually a perforating or cutting wound, contaminated by soil, feces or other material containing bacterial spores and anaerobic microorganisms. These wounds may be related to castration, umbilical stump, calving (Odendaal & Kriek 2004Odendaal M.W. & Kriek N.P.J. 2004. Tetanus, p.1878-1884. In: Coetzer J.A.W. & Tustin R.C. (Eds), Infectious Diseases of Livestock. Vol.3. 3rd ed. Oxford Press, Cape Town.) or may be caused by scarification of the intestinal mucosa by plant fibers (Raposo 2007Raposo J.B. 2007. Tétano, p.425-432. In: Riet-Correa F., Schild A.L., Lemos R.A.A & Borges J.R.J. (Eds), Doenças de Ruminantes e Equídeos. Vol.1. 3rd ed. Pallotti, Santa Maria.). The bacterium has limited capacity to invade the host, restricting itself to the inoculation site (Raposo 2007Raposo J.B. 2007. Tétano, p.425-432. In: Riet-Correa F., Schild A.L., Lemos R.A.A & Borges J.R.J. (Eds), Doenças de Ruminantes e Equídeos. Vol.1. 3rd ed. Pallotti, Santa Maria.).
In Brazil, the occurrence of tetanus is generally sporadic but, as was already mentioned outbreaks with high morbidity, mortality and lethality have occurred in cattle handled in pens for some kind of procedure, the use of unhygienic equipment or contamination of the skin by dust or mud during vaccination and de-worming practices (Salvador & Freire 1998Salvador S.C. & Freire C.A. 1998. Ocorrência de tétano epizoótico em bovinos no Estado de Minas Gerais. Arqs Inst. Biológico, São Paulo, 65:95., Barros et al. 2006bBarros C.S.L., Driemeier D., Dutra I.S. & Lemos R.A.A. 2006b. Tétano, p.59-64. In: Ibid. (Eds), Doenças do Sistema Nervoso de Bovinos no Brasil. Valée, Montes Claros ., Driemeier et al. 2007Driemeier D., Schild A.L., Fernandes J.C., Colodel E.M., Correa A.M., Cruz C.E. & Barros C.S.L. 2007. Outbreaks of tetanus in beef cattle and sheep in Brazil associated with disophenol injection. J. Vet. Med. A, Physiol. Pathol. Clin. Med. 54(6):333-335. <http://dx.doi.org/10.1111/j.1439-0442.2007.00922.x> <PMid:17650154>
https://doi.org/10.1111/j.1439-0442.2007...
).
The incubation period is 18 hours to 4 weeks in most cases 7-15 days (Driemeier et al. 2007Driemeier D., Schild A.L., Fernandes J.C., Colodel E.M., Correa A.M., Cruz C.E. & Barros C.S.L. 2007. Outbreaks of tetanus in beef cattle and sheep in Brazil associated with disophenol injection. J. Vet. Med. A, Physiol. Pathol. Clin. Med. 54(6):333-335. <http://dx.doi.org/10.1111/j.1439-0442.2007.00922.x> <PMid:17650154>
https://doi.org/10.1111/j.1439-0442.2007...
). The lower the incubation period and progression, the more severe is tetanus. There is also a correlation with the port of entry: lesions close to the head usually result in more severe disease. In cases with a long incubation period, it may not be possible to detect the portal of entry (Barros et al. 2006bBarros C.S.L., Driemeier D., Dutra I.S. & Lemos R.A.A. 2006b. Tétano, p.59-64. In: Ibid. (Eds), Doenças do Sistema Nervoso de Bovinos no Brasil. Valée, Montes Claros .).
In cattle, early signs include mild or moderate bloat tremors, mandibular trismus, muscular rigidity (Fig.2) and prolapse of the third eyelid and opisthotonus (Fig.3). Affected cattle are alert and anxious with erect ears. Asymmetric contractions induce spinal curvature and lateral deviation of the tail (Bleck 1989Bleck T.P. 1989. Clinical aspects of tetanus, p.379-398. In: Simpson L.L. (Ed.), Botulism Neurotoxin and Tetanus Toxin. Academic Press, New York . <http://dx.doi.org/10.1016/B978-0-12-644445-2.50025-8>
https://doi.org/10.1016/B978-0-12-644445...
, Driemeier et al. 2007Driemeier D., Schild A.L., Fernandes J.C., Colodel E.M., Correa A.M., Cruz C.E. & Barros C.S.L. 2007. Outbreaks of tetanus in beef cattle and sheep in Brazil associated with disophenol injection. J. Vet. Med. A, Physiol. Pathol. Clin. Med. 54(6):333-335. <http://dx.doi.org/10.1111/j.1439-0442.2007.00922.x> <PMid:17650154>
https://doi.org/10.1111/j.1439-0442.2007...
).
No specific morphological changes are observed. Gray matter hemorrhages, decubitus pneumonia and asphyxia lesions reported by some authors (Valette & Petermann 1988Valette L. & Petermann H.G. 1988. Clostridium tetani, p.667-688. In: Blobel H. & Schliesser T. (Eds), Handbuch der bakteriellen Infektionen bei Tieren. Band II. Gustav Verlag Fischer, Sttutgart.) are secondary changes or artifacts. The diagnosis of tetanus is primarily clinical and epidemiological. Prophylaxis includes the application of two doses of vaccine with an interval of four weeks between applications.
Of the 34 outbreaks or sporadic cases of blackleg diagnosed in cattle, 15 occurred in the winter, nine in the spring, seven in the fall, and three in the summer. As has already been observed (Heckler et al. 2018Heckler R.F., Lemos R.A.A., Gomes D.C., Dutra I.S., Silva R.O.S., Lobato F.C.F., Ramos C.A.N. & Brumatti R.C. 2018. Blackleg in cattle in the state of Mato Grosso do Sul, Brazil: 59 cases. Pesq. Vet. Bras. 38(1):6-14. <http://dx.doi.org/10.1590/1678-5150-pvb-4964>
https://doi.org/10.1590/1678-5150-pvb-49...
) blackleg cases tend to be distributed throughout the year without showing specific seasonality (Table 1). However, it is also described that the prevalence of the disease was higher during the fall (Riet-Correa 2007aRiet-Correa F. 2007a. Carbúnculo sintomático, p.264-288. In: Riet-Correa F., Schild A.L., Lemos R.A.A & Borges J.R.J. (Eds), Doenças de Ruminantes e Equídeos. Vol.1. 3rd ed. Pallotti, Santa Maria .). The age of affected cattle in this study ranged from four months to two years and exceptionally, in one outbreak a 4-year old cow was affected. Usually 6-24 month-old well nourish cattle are affected (Useh et al. 2006Useh N.M., Ibrahim N.D.G., Nok A.J. & Esievo K.A.N. 2006. Relationship between outbreaks of blackleg of cattle and annual rainfall in Zaria, Nigeria. Vet. Rec. 158(3):100-101. <http://dx.doi.org/10.1136/vr.158.3.100> <PMid:16428667>
https://doi.org/10.1136/vr.158.3.100...
), but cases were already reported in a 3-day calf (Sojka et al. 1992Sojka J.E., Bowersock T.L., Parker J.E., Blevins W.G. & Irigoyen L. 1992. Clostridium chauvoei myositis infection in a neonatal calf. J. Vet. Diagn. Invest. 4(2):201-203. <http://dx.doi.org/10.1177/104063879200400219> <PMid:1616991>
https://doi.org/10.1177/1040638792004002...
) and a bovine fetus transplacentally infected (Abreu et al. 2017Abreu C.C., Edwards E.E., Edwards J.F., Gibbons P.M., Leal de Araújo J., Rech R.R. & Uzal F.A. 2017. Blackleg in cattle: a case report of fetal infection and a literature review. J. Vet. Diagn. Invest. 29(5):612-621. <http://dx.doi.org/10.1177/1040638717713796> <PMid:28599620>
https://doi.org/10.1177/1040638717713796...
). In Mato Grosso do Sul state the disease has been diagnosed in cattle of 2.5-3 years old, not vaccinated or vaccinated long time before the outbreak (Riet-Correa 2007aRiet-Correa F. 2007a. Carbúnculo sintomático, p.264-288. In: Riet-Correa F., Schild A.L., Lemos R.A.A & Borges J.R.J. (Eds), Doenças de Ruminantes e Equídeos. Vol.1. 3rd ed. Pallotti, Santa Maria ., Heckler et al. 2018Heckler R.F., Lemos R.A.A., Gomes D.C., Dutra I.S., Silva R.O.S., Lobato F.C.F., Ramos C.A.N. & Brumatti R.C. 2018. Blackleg in cattle in the state of Mato Grosso do Sul, Brazil: 59 cases. Pesq. Vet. Bras. 38(1):6-14. <http://dx.doi.org/10.1590/1678-5150-pvb-4964>
https://doi.org/10.1590/1678-5150-pvb-49...
). Roughly our data are similar. Surveys of the prevalence of clostridial diseases in Brazil generally indicate blackleg as the most frequent disease (Correa et al. 1980Correa M.W., Correa C.N.M., Lopes C.A.M., Langoni H. & Modolo J.R. 1980. Enfermidades por clostrídios, 1969-1978. Arqs Bras. Med. Vet. Zootec. 32:369-374., Riet-Correa 2007aRiet-Correa F. 2007a. Carbúnculo sintomático, p.264-288. In: Riet-Correa F., Schild A.L., Lemos R.A.A & Borges J.R.J. (Eds), Doenças de Ruminantes e Equídeos. Vol.1. 3rd ed. Pallotti, Santa Maria ., Heckler et al. 2018Heckler R.F., Lemos R.A.A., Gomes D.C., Dutra I.S., Silva R.O.S., Lobato F.C.F., Ramos C.A.N. & Brumatti R.C. 2018. Blackleg in cattle in the state of Mato Grosso do Sul, Brazil: 59 cases. Pesq. Vet. Bras. 38(1):6-14. <http://dx.doi.org/10.1590/1678-5150-pvb-4964>
https://doi.org/10.1590/1678-5150-pvb-49...
). In our study, this is also a trend. However for reasons already explained there were more cases of tetanus, which is unusual.
The pathogenesis of blackleg is relatively well established (Useh et al. 2006Useh N.M., Ibrahim N.D.G., Nok A.J. & Esievo K.A.N. 2006. Relationship between outbreaks of blackleg of cattle and annual rainfall in Zaria, Nigeria. Vet. Rec. 158(3):100-101. <http://dx.doi.org/10.1136/vr.158.3.100> <PMid:16428667>
https://doi.org/10.1136/vr.158.3.100...
, Abreu & Uzal 2016Abreu C.C. & Uzal F.A. 2016. Blackleg, p.231-242. In: Uzal F., Prescott J., Songer G. & Popoff M. (Eds), Clostridial Diseases of Animals. John Wiley and Sons, Ames. <http://dx.doi.org/10.1002/9781118728291.ch19>
https://doi.org/10.1002/9781118728291.ch...
, Abreu et al. 2017Abreu C.C., Edwards E.E., Edwards J.F., Gibbons P.M., Leal de Araújo J., Rech R.R. & Uzal F.A. 2017. Blackleg in cattle: a case report of fetal infection and a literature review. J. Vet. Diagn. Invest. 29(5):612-621. <http://dx.doi.org/10.1177/1040638717713796> <PMid:28599620>
https://doi.org/10.1177/1040638717713796...
). Spores of Clostridium chauvoei are ingested from the contaminated soil and absorbed by the intestinal mucosa gaining access to the blood circulation. The spores are then distributed through various tissues, mainly skeletal muscles and myocardium, where they become dormant. When the occurrence of an injury (which can be trauma, injection, etc.) favors a local anaerobic environment, spore germination occurs and the bacteria produce potent exotoxins that cause necrotizing myositis. The proliferating bacteria and toxins then enter the bloodstream causing exotoxemia and death (Fig.4).
Blackleg Pathogenesis. Clostridium chauvoei spores are ingested from the contaminated soil (1) and absorbed through the intestinal mucosa into the blood circulation (2). The spores are then distributed through various tissues, mainly skeletal muscles, where they become dormant within macrophages (3). When a muscle injury occurs, a favorable anaerobic environment is created, the spores germinate, and the bacteria produce potent exotoxins that cause hemorrhagic necrotizing emphysematous myositis (4). The proliferating bacteria and toxins enter the bloodstream causing exotoxemia and death (5) (Modified from Abreu et al. 2017Abreu C.C., Edwards E.E., Edwards J.F., Gibbons P.M., Leal de Araújo J., Rech R.R. & Uzal F.A. 2017. Blackleg in cattle: a case report of fetal infection and a literature review. J. Vet. Diagn. Invest. 29(5):612-621. <http://dx.doi.org/10.1177/1040638717713796> <PMid:28599620>
https://doi.org/10.1177/1040638717713796... ).
The clinical manifestations of blackleg are often not observed. Due to the rapid clinical course, cattle are often found dead; sudden death may occur and are generally attributed to myocardial lesions (Williams 1977Williams B.M. 1977. Clostridial myositis in cattle: bacteriology and gross pathology. Vet. Rec. 100(5):90-91. <http://dx.doi.org/10.1136/vr.100.5.90> <PMid:190758>
https://doi.org/10.1136/vr.100.5.90...
, Glastonbury et al. 1988Glastonbury J.R., Searson J.E., Links I.J. & Tuckett L.M. 1988. Clostridial myocarditis in lambs. Aust. Vet. J. 65(7):208-209. <http://dx.doi.org/10.1111/j.1751-0813.1988.tb14459.x> <PMid:3421885>
https://doi.org/10.1111/j.1751-0813.1988...
, Helman et al. 1997Helman G., Welsh R.D., Stair E.L. & Ely R.W. 1997. Diagnosing visceral blackleg as a cause of sudden death in cattle. Vet. Med. 92:914-918., Uzal et al. 2003Uzal F.A., Paramidani M., Assis R., Morris W. & Miyakawa M.F. 2003. Outbreak of clostridial myocarditis in calves. Vet. Rec. 152(5):134-136. <http://dx.doi.org/10.1136/vr.152.5.134> <PMid:12585599>
https://doi.org/10.1136/vr.152.5.134...
). The clinical picture is acute or hyperacute and clinical signs consists of lameness, swelling, muscle crepitation and fever. Death invariably occurs within 24 to 36 hours from the onset of the clinical signs (Barros 2016bBarros C.S.L. 2016b. Sistema muscular, p.663-702. In: Santos R.L. & Alessi A.C. (Eds), Patologia Veterinária. Roca, São Paulo .).
The cadaver of a bovine that dies from blackleg quickly swells. Significant macroscopic changes include localized swelling and crepitus of the muscles in the hind limbs and chest (Barros 2016bBarros C.S.L. 2016b. Sistema muscular, p.663-702. In: Santos R.L. & Alessi A.C. (Eds), Patologia Veterinária. Roca, São Paulo .). On the cutting surface, the affected muscle is dark-red and contains numerous gas bubbles (Fig.5). In the periphery, the muscle is red and moist due to edema (early lesions). At the center of the lesion, the affected muscle is dark-red, dry and friable (mature lesions) (Barros 2016bBarros C.S.L. 2016b. Sistema muscular, p.663-702. In: Santos R.L. & Alessi A.C. (Eds), Patologia Veterinária. Roca, São Paulo ., Heckler et al. 2018Heckler R.F., Lemos R.A.A., Gomes D.C., Dutra I.S., Silva R.O.S., Lobato F.C.F., Ramos C.A.N. & Brumatti R.C. 2018. Blackleg in cattle in the state of Mato Grosso do Sul, Brazil: 59 cases. Pesq. Vet. Bras. 38(1):6-14. <http://dx.doi.org/10.1590/1678-5150-pvb-4964>
https://doi.org/10.1590/1678-5150-pvb-49...
). Muscle affected by blackleg often has a butyric odor, such as rancid butter (Cooper & Valentine 2016Cooper B.J. & Valentine B.A. 2016. Malignant edema and gas gangrene, p.232. In: Maxie M.G. (Ed.), Jubb, Kennedy and Palmer’s Pathology of Domestic Animals. Vol.1. 6th ed. Elsevier, St Louis.). In addition to skeletal muscle, heart and tongue may be affected (Fig.6). Myocardial lesions are similar to those of skeletal muscles and associated with fibrinous pericarditis and pleuritis (Williams 1977Williams B.M. 1977. Clostridial myositis in cattle: bacteriology and gross pathology. Vet. Rec. 100(5):90-91. <http://dx.doi.org/10.1136/vr.100.5.90> <PMid:190758>
https://doi.org/10.1136/vr.100.5.90...
, Abreu & Uzal 2016Abreu C.C. & Uzal F.A. 2016. Blackleg, p.231-242. In: Uzal F., Prescott J., Songer G. & Popoff M. (Eds), Clostridial Diseases of Animals. John Wiley and Sons, Ames. <http://dx.doi.org/10.1002/9781118728291.ch19>
https://doi.org/10.1002/9781118728291.ch...
). Histologically, necrotic fibers appear dissociated by gas bubbles and hemorrhage (Fig.7), and there is meager neutrophilic infiltrate (Barros 2016bBarros C.S.L. 2016b. Sistema muscular, p.663-702. In: Santos R.L. & Alessi A.C. (Eds), Patologia Veterinária. Roca, São Paulo .). In some cases, one can observe intralesional gram-positive rods. Vaccination is the preferred method of control (Abreu & Uzal 2016Abreu C.C. & Uzal F.A. 2016. Blackleg, p.231-242. In: Uzal F., Prescott J., Songer G. & Popoff M. (Eds), Clostridial Diseases of Animals. John Wiley and Sons, Ames. <http://dx.doi.org/10.1002/9781118728291.ch19>
https://doi.org/10.1002/9781118728291.ch...
)
Clostridial diseases. Blackleg. Gross lesions at the hidlimb muscles. The muscle to the right is moist (early lesion). The dark, dry muscle to the left represents a more advanced phase of the lesion. Observe the well-developed gas bubbles in the muscle at the top right.
Clostridial diseases. Blackleg. Schematic representation of the anatomical sites where muscle lesions are more commonly seen: muscles of the thigh, diaphragm, heart, and tongue.
Clostridial diseases. Blackleg. Hemorrhagic necrotizing myositis. A histopathological aspect of the striated muscle lesion. There is hyaline necrosis of myofibers and edema and hemorrhage between myofibers. HE, obj.40x.
The results of this survey demonstrate that the most critical clostridial diseases in cattle in the southern area of Rio Grande do Sul (RS) state are blackleg and bacillary hemoglobinuria (BH), with 34 and 23 outbreaks respectively. To make this assumption one have to consider the conditions in which many tetanus outbreaks occurred in the period of the current study, as explained previously. In the outbreaks of BH the morbidity, mortality and lethality ratios were the same (Table 1).
BH is an acute or subacute disease, usually fatal, affecting mainly cattle and, more rarely, other species (Schild 2007Schild A.L. 2007. Hemoglobinúria bacilar, p.305-308. In: Riet-Correa F., Schild A.L., Lemos R.A.A & Borges J.R.J. (Eds), Doenças de Ruminantes e Equídeos. Vol.1. 3rd ed. Pallotti, Santa Maria .). It is caused by toxins of Clostridium haemolyticum referred to, also by some authors as Clostridium novyi type D (Kriek & Odendaal 2004Odendaal M.W. & Kriek N.P.J. 2004. Tetanus, p.1878-1884. In: Coetzer J.A.W. & Tustin R.C. (Eds), Infectious Diseases of Livestock. Vol.3. 3rd ed. Oxford Press, Cape Town.), a rod, sporulated, gram-negative anaerobic (Schild 2007Schild A.L. 2007. Hemoglobinúria bacilar, p.305-308. In: Riet-Correa F., Schild A.L., Lemos R.A.A & Borges J.R.J. (Eds), Doenças de Ruminantes e Equídeos. Vol.1. 3rd ed. Pallotti, Santa Maria .).
BH may be sporadic or endemic. Morbidity is 0.25%-12% (Schild 2007Schild A.L. 2007. Hemoglobinúria bacilar, p.305-308. In: Riet-Correa F., Schild A.L., Lemos R.A.A & Borges J.R.J. (Eds), Doenças de Ruminantes e Equídeos. Vol.1. 3rd ed. Pallotti, Santa Maria .) and the mortality is 80-100% (Navarro et al. 2016Navarro M., Dutra Quintela F. & Uzal F.A. 2016. Bacillary hemoglobinuria, p.265-274. In: Uzal F., Prescott J., Songer G. & Popoff M. (Eds), Clostridial Diseases of Animals. John Wiley and Sons, Ames. <http://dx.doi.org/10.1002/9781118728291.ch22>.
https://doi.org/10.1002/9781118728291.ch...
). High incidence of BH occurs in flooded regions where the occurrence of hepatic trematodes, mainly Fasciola hepatica exist (Barros 2016aBarros C.S.L. 2016a. Fígado, vias biliares e pâncreas exócrino, p.181-265. In: Santos R.L. & Alessi A.C. (Eds), Patologia Veterinária. 2ª ed. Roca, São Paulo.). In other countries Fascioloides magna and Dicrocoelium dendriticum may also be associated with the disease (Navarro et al. 2016Navarro M., Dutra Quintela F. & Uzal F.A. 2016. Bacillary hemoglobinuria, p.265-274. In: Uzal F., Prescott J., Songer G. & Popoff M. (Eds), Clostridial Diseases of Animals. John Wiley and Sons, Ames. <http://dx.doi.org/10.1002/9781118728291.ch22>.
https://doi.org/10.1002/9781118728291.ch...
). The main determinant of the incidence of the disease is the amount of hepatic trematodes in the cattle grazing area (Navarro et al. 2016Navarro M., Dutra Quintela F. & Uzal F.A. 2016. Bacillary hemoglobinuria, p.265-274. In: Uzal F., Prescott J., Songer G. & Popoff M. (Eds), Clostridial Diseases of Animals. John Wiley and Sons, Ames. <http://dx.doi.org/10.1002/9781118728291.ch22>.
https://doi.org/10.1002/9781118728291.ch...
). However in some cases of the outbreaks reported in this study, no evidence of parasitism was observed in the liver. This might indicate that the parasitic lesions where masqueraded or that other factors may be involved in triggering the lesions. Liver biopsy was already inculpated as one of these factors (Monaghan & Sheahan 1987Monaghan M.L. & Sheahan B.J. 1987. Liver biopsy in ragwort poisoning. Vet. Rec. 120(15):374. <http://dx.doi.org/10.1136/vr.120.15.374> <PMid:3590594>
https://doi.org/10.1136/vr.120.15.374...
), but is improbable that cattle in these outbreaks had been submitted to this procedure.
In the current study BH was the second most frequent clostridial disease observed in four municipalities in RS, all of which had low terrains and soils used mainly for planting rice. In these regions there are snails of the genus Lymnea which are intermediate hosts for F. hepatica. Accordingly in the current study BH occurred throughout all seasons of the year and all outbreaks were observed in municipalities in the southern part of the state, where there is flat and low lands mainly used for rice crops. F. hepatica can cause liver lesions that determine an anaerobic environment favoring multiplication of C. haemotyticum.
In RS, BH affects well-nourished cattle older than two years pasturing in low fields such as those on the coast (Schild 2007Schild A.L. 2007. Hemoglobinúria bacilar, p.305-308. In: Riet-Correa F., Schild A.L., Lemos R.A.A & Borges J.R.J. (Eds), Doenças de Ruminantes e Equídeos. Vol.1. 3rd ed. Pallotti, Santa Maria ., Barros 2016aBarros C.S.L. 2016a. Fígado, vias biliares e pâncreas exócrino, p.181-265. In: Santos R.L. & Alessi A.C. (Eds), Patologia Veterinária. 2ª ed. Roca, São Paulo.). Outbreaks of the disease have also been observed after periods of flood when conditions for increasing the population of F. hepatica producing overwhelming numbers of metacercariae (Schild 2007Schild A.L. 2007. Hemoglobinúria bacilar, p.305-308. In: Riet-Correa F., Schild A.L., Lemos R.A.A & Borges J.R.J. (Eds), Doenças de Ruminantes e Equídeos. Vol.1. 3rd ed. Pallotti, Santa Maria .). In these cases BH may become endemic. Paradoxically, in these regions, dry conditions induce cattle to concentrate in parts of the field where there is some water and consequently high populations of F. hepatica (Navarro et al. 2016Navarro M., Dutra Quintela F. & Uzal F.A. 2016. Bacillary hemoglobinuria, p.265-274. In: Uzal F., Prescott J., Songer G. & Popoff M. (Eds), Clostridial Diseases of Animals. John Wiley and Sons, Ames. <http://dx.doi.org/10.1002/9781118728291.ch22>.
https://doi.org/10.1002/9781118728291.ch...
).
Affected cattle can eliminate spores through feces and urine, thus contaminating pastures. Although it is described as a major occurrence in the summer and autumn (Schild 2007Schild A.L. 2007. Hemoglobinúria bacilar, p.305-308. In: Riet-Correa F., Schild A.L., Lemos R.A.A & Borges J.R.J. (Eds), Doenças de Ruminantes e Equídeos. Vol.1. 3rd ed. Pallotti, Santa Maria .), in our study BH occurred with the same frequency throughout the year.
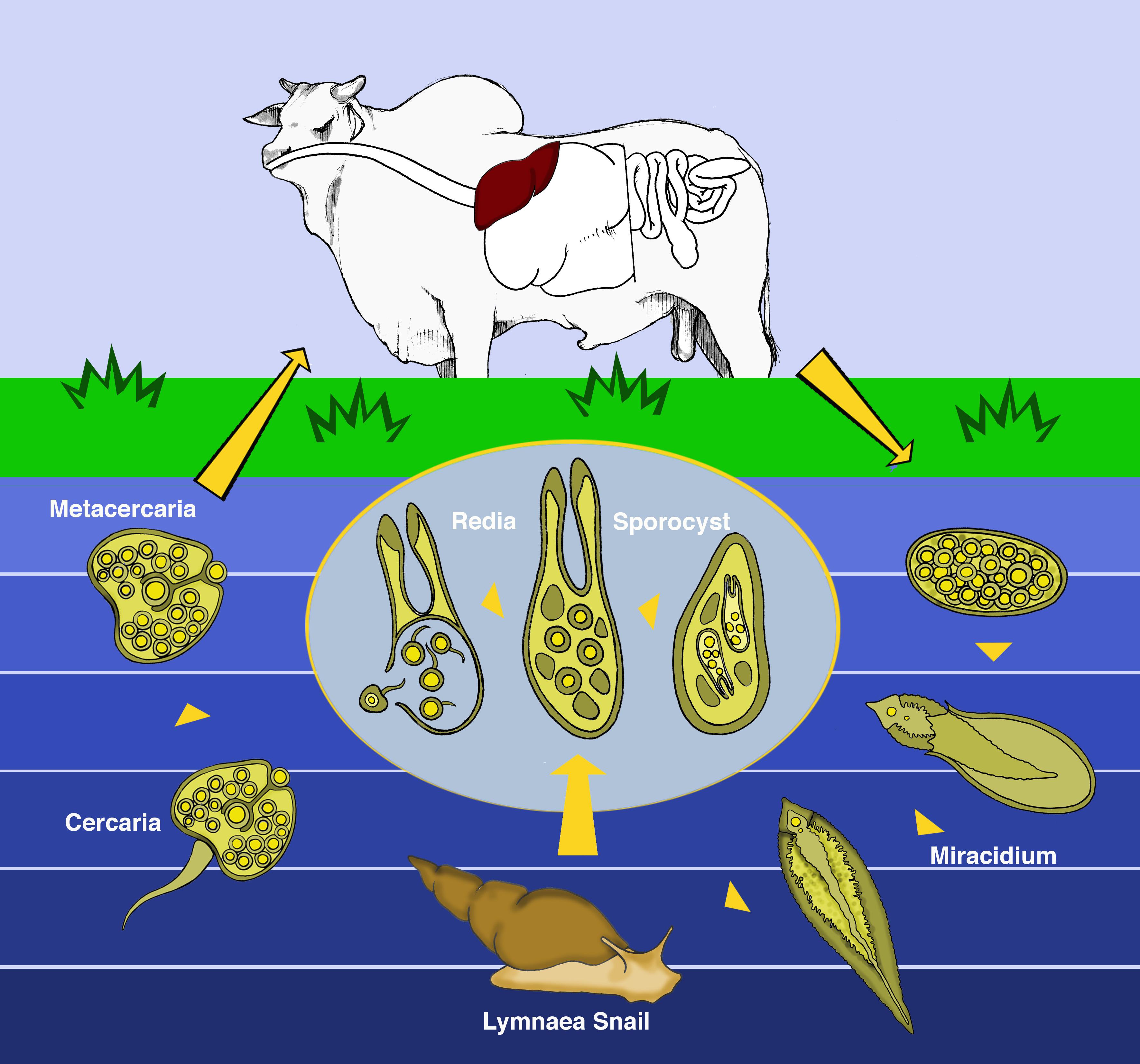
Essential aspects of pathogenesis are as follows (Kriek & Odendaal 2004Odendaal M.W. & Kriek N.P.J. 2004. Tetanus, p.1878-1884. In: Coetzer J.A.W. & Tustin R.C. (Eds), Infectious Diseases of Livestock. Vol.3. 3rd ed. Oxford Press, Cape Town.): as previously state BH occurs only in regions where both F. hepatica and C. haemolyticum are endemic. In the life cycle (Fig.8) of F. hepatica the final host ingests the metacercariae which are attached to the grass (Tessele et al. 2013Tessele B., Brum J.S. & Barros C.S.L. 2013. Lesões parasitárias encontradas em bovinos abatidos para consumo humano. Pesq. Vet. Bras. 33(7):873-889. <http://dx.doi.org/10.1590/S0100-736X2013000700008>
https://doi.org/10.1590/S0100-736X201300...
). The ingested metacercariae penetrate the duodenum of the definitive host, cross the intestinal wall, migrate through the coelom and penetrate the capsule of the liver, migrating through the hepatic parenchyma to reach the hepatic ducts. The migration of the metacercariae through the liver harboring latent C. haemolyticum spores (within Kuppfer cells) induces hepatic lesions (Fig.9) and those create the necessary conditions for bacterial growth and toxin production (Kriek & Odendaal 2004Odendaal M.W. & Kriek N.P.J. 2004. Tetanus, p.1878-1884. In: Coetzer J.A.W. & Tustin R.C. (Eds), Infectious Diseases of Livestock. Vol.3. 3rd ed. Oxford Press, Cape Town.).
The life cycle of Fasciola hepatica. Eggs are shed in the bile and feces. A hatching egg releases the miracidium that survives only in humid environments. The miracidium penetrates actively in the intermediate host (snail of the genus Lymnaea). Inside the snail, each miracidium gives rise 5-8 rediae which originate daughter rediae and cercariae. Cercariae leave the snail and set themselves on grass, just below the water level, turning into metacercariae. The final host ingests the metacercariae along with the grass. People and other domestic and wild mammals can also become infected. The ingested metacercariae cross the duodenum of the definitive host and penetrate the intestinal wall, wander through the coelom, penetrate the capsule of the liver, and migrate through the hepatic parenchyma to reach the hepatic ducts (Modified from Tessele et al. 2013Tessele B., Brum J.S. & Barros C.S.L. 2013. Lesões parasitárias encontradas em bovinos abatidos para consumo humano. Pesq. Vet. Bras. 33(7):873-889. <http://dx.doi.org/10.1590/S0100-736X2013000700008>
https://doi.org/10.1590/S0100-736X201300... ).
Clostridial diseases. Bacillary hemoglobinuria (BH). Liver, cut surface. The triangular single white area of coagulative necrosis seen to the right is a hallmark lesion of BH. The ducts are thick walled (chronic cholangitis) and some specimens of Fasciola hepatica are in their lumen. Migration of F. hepatica metacercariae through liver parenchyma favors sporulation of Clostridium haemolyticum, the causative agent of BH.
BH is a hemolytic disease and clinical signs are largely the result of intravascular hemolysis (Barros 2016aBarros C.S.L. 2016a. Fígado, vias biliares e pâncreas exócrino, p.181-265. In: Santos R.L. & Alessi A.C. (Eds), Patologia Veterinária. 2ª ed. Roca, São Paulo.). The disease may be acute (clinical course of 10-12 hours) or subacute (3-4 days), but under grazing conditions cattle are generally found dead (Navarro et al. 2016Navarro M., Dutra Quintela F. & Uzal F.A. 2016. Bacillary hemoglobinuria, p.265-274. In: Uzal F., Prescott J., Songer G. & Popoff M. (Eds), Clostridial Diseases of Animals. John Wiley and Sons, Ames. <http://dx.doi.org/10.1002/9781118728291.ch22>.
https://doi.org/10.1002/9781118728291.ch...
). The incubation period is 7-10 days (Barros 2016aBarros C.S.L. 2016a. Fígado, vias biliares e pâncreas exócrino, p.181-265. In: Santos R.L. & Alessi A.C. (Eds), Patologia Veterinária. 2ª ed. Roca, São Paulo.).
Clinical signs include hemoglobinuria, jaundice and fever (40-41°C), that tends to disappear with the progression of the disease, and blood in the feces (Kriek & Odendaal 2004Kriek N.P.J. & Odendaal M.W. 2004. Clostridium novyi type D infection, p.1686-1687. In: Coetzer J.A.W. & Tustin RC. (Eds), Infectious Diseases of Livestock. 3rd ed. Vol.3. Oxford Press, Cape Town., Schild 2007Schild A.L. 2007. Hemoglobinúria bacilar, p.305-308. In: Riet-Correa F., Schild A.L., Lemos R.A.A & Borges J.R.J. (Eds), Doenças de Ruminantes e Equídeos. Vol.1. 3rd ed. Pallotti, Santa Maria ., Barros 2016aBarros C.S.L. 2016a. Fígado, vias biliares e pâncreas exócrino, p.181-265. In: Santos R.L. & Alessi A.C. (Eds), Patologia Veterinária. 2ª ed. Roca, São Paulo., Navarro et al. 2016Navarro M., Dutra Quintela F. & Uzal F.A. 2016. Bacillary hemoglobinuria, p.265-274. In: Uzal F., Prescott J., Songer G. & Popoff M. (Eds), Clostridial Diseases of Animals. John Wiley and Sons, Ames. <http://dx.doi.org/10.1002/9781118728291.ch22>.
https://doi.org/10.1002/9781118728291.ch...
). Jaundice may not be very striking. Pregnant cows may abort. There is anemia (red blood cells 1-4 x 106/mm3 and hemoglobin 3-89mg/dl and leukogram 7,700-34,800 leukocytes/mm3) and glycemia can be 100-200mg/dl in some cases (Schild 2007Schild A.L. 2007. Hemoglobinúria bacilar, p.305-308. In: Riet-Correa F., Schild A.L., Lemos R.A.A & Borges J.R.J. (Eds), Doenças de Ruminantes e Equídeos. Vol.1. 3rd ed. Pallotti, Santa Maria ., Barros 2016aBarros C.S.L. 2016a. Fígado, vias biliares e pâncreas exócrino, p.181-265. In: Santos R.L. & Alessi A.C. (Eds), Patologia Veterinária. 2ª ed. Roca, São Paulo., Navarro et al. 2016Navarro M., Dutra Quintela F. & Uzal F.A. 2016. Bacillary hemoglobinuria, p.265-274. In: Uzal F., Prescott J., Songer G. & Popoff M. (Eds), Clostridial Diseases of Animals. John Wiley and Sons, Ames. <http://dx.doi.org/10.1002/9781118728291.ch22>.
https://doi.org/10.1002/9781118728291.ch...
).
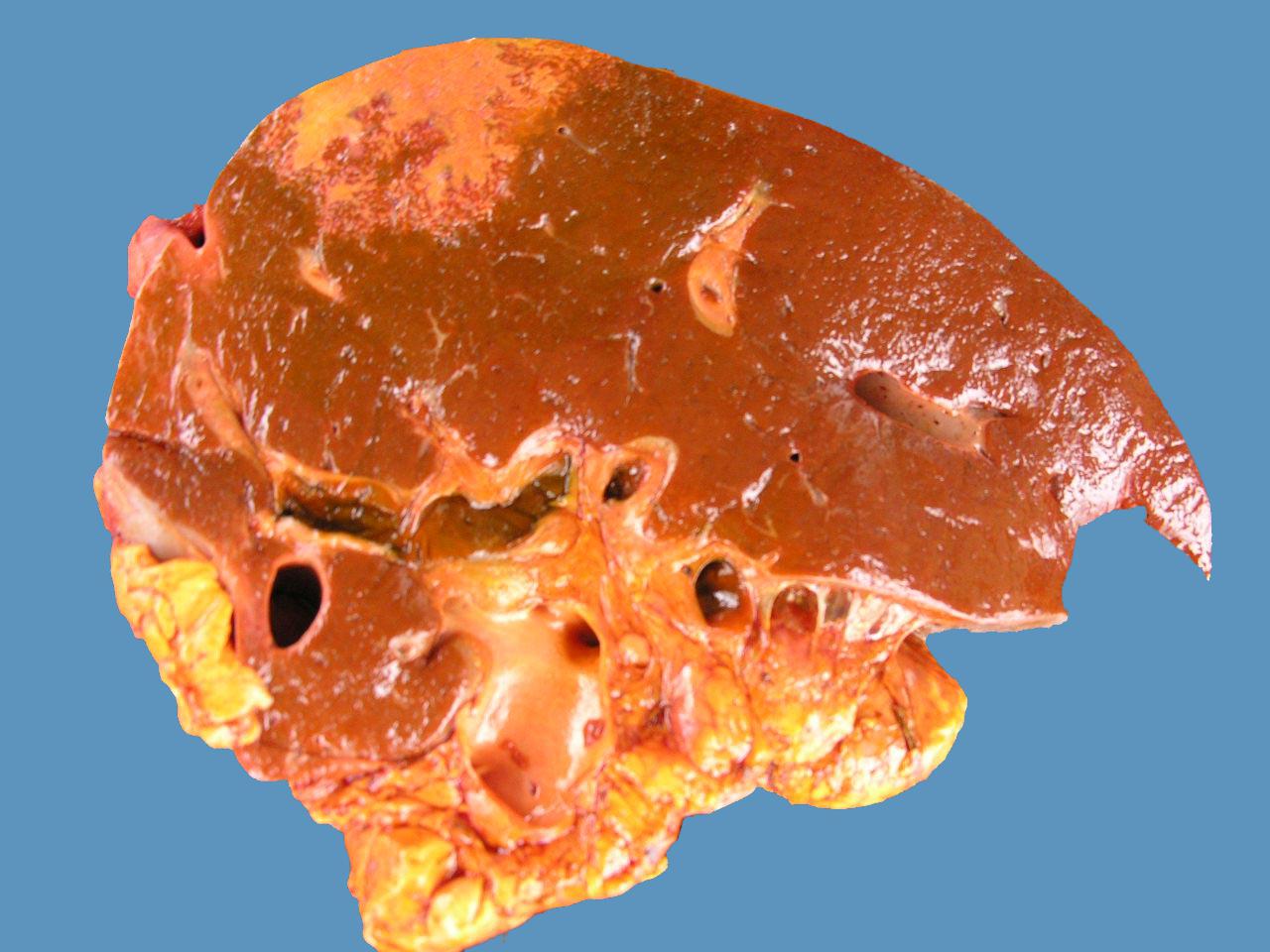
The main necropsy findings are single 5-20cm white to tan necrotic areas in the hepatic parenchyma which is orange due to pigmentation by bilirubin (Barros 2016aBarros C.S.L. 2016a. Fígado, vias biliares e pâncreas exócrino, p.181-265. In: Santos R.L. & Alessi A.C. (Eds), Patologia Veterinária. 2ª ed. Roca, São Paulo.). Such areas (Fig.10) may be ischemic resulting from thrombosis or may be the direct action of toxin (Barros 2016aBarros C.S.L. 2016a. Fígado, vias biliares e pâncreas exócrino, p.181-265. In: Santos R.L. & Alessi A.C. (Eds), Patologia Veterinária. 2ª ed. Roca, São Paulo., Navarro et al. 2016Navarro M., Dutra Quintela F. & Uzal F.A. 2016. Bacillary hemoglobinuria, p.265-274. In: Uzal F., Prescott J., Songer G. & Popoff M. (Eds), Clostridial Diseases of Animals. John Wiley and Sons, Ames. <http://dx.doi.org/10.1002/9781118728291.ch22>.
https://doi.org/10.1002/9781118728291.ch...
), or both. These areas of necrosis are considered pathognomonic when observed in conjunction with other clinical signs (Navarro et al. 2016Navarro M., Dutra Quintela F. & Uzal F.A. 2016. Bacillary hemoglobinuria, p.265-274. In: Uzal F., Prescott J., Songer G. & Popoff M. (Eds), Clostridial Diseases of Animals. John Wiley and Sons, Ames. <http://dx.doi.org/10.1002/9781118728291.ch22>.
https://doi.org/10.1002/9781118728291.ch...
). Other findings are the same as in any intravascular hemolytic disease and include jaundice, petechial hemorrhages in the subcutaneous tissue, dark kidneys, and coffee-colored urine (Schild 2007Schild A.L. 2007. Hemoglobinúria bacilar, p.305-308. In: Riet-Correa F., Schild A.L., Lemos R.A.A & Borges J.R.J. (Eds), Doenças de Ruminantes e Equídeos. Vol.1. 3rd ed. Pallotti, Santa Maria .).
Clostridial diseases. Bacillary hemoglobinuria. Liver, cut surface. Notice the large irregular bordered pale area of coagulative necrosis in the liver parenchyma. A red rim of hemorrhage and inflammation surrounds the necrotic focus.
Histopathologically, the area of coagulative necrosis is surrounded by an inflammatory border of neutrophils (Fig.11) and intralesional rods can be found within the liver sinusoids. Hemoglobinuric nephrosis are usually observed (Navarro et al. 2016Navarro M., Dutra Quintela F. & Uzal F.A. 2016. Bacillary hemoglobinuria, p.265-274. In: Uzal F., Prescott J., Songer G. & Popoff M. (Eds), Clostridial Diseases of Animals. John Wiley and Sons, Ames. <http://dx.doi.org/10.1002/9781118728291.ch22>.
https://doi.org/10.1002/9781118728291.ch...
).
Clostridial diseases. Bacillary hemoglobinuria. Histopathology. A large area of coagulative necrosis to the right is surrounded by a rim of leucocytes. HE, obj.20x.
The diagnosis is based on the epidemiology, characteristic necropsy findings, and can be confirmed by polymerase chain reaction, fluorescent antibody technique and immunohistochemistry. The indicated form of control is vaccination (Navarro et al. 2016Navarro M., Dutra Quintela F. & Uzal F.A. 2016. Bacillary hemoglobinuria, p.265-274. In: Uzal F., Prescott J., Songer G. & Popoff M. (Eds), Clostridial Diseases of Animals. John Wiley and Sons, Ames. <http://dx.doi.org/10.1002/9781118728291.ch22>.
https://doi.org/10.1002/9781118728291.ch...
).
We found eleven outbreaks of malignant edema (gas gangrene) in our survey. Interestingly in one of those, there was 100% of lethality induced by poor husbandry. Alternatively, good husbandry can prevent this clostridial disease.
Malignant edema is a clostridial infection that mainly affects ruminants (Odani et al. 2009Odani J.S., Blanchard P.C., Adaska J.M., Moeller R.B. & Uzal F.A. 2009. Malignant edema in postpartum dairy cattle. J. Vet. Diagn. Invest. 21(6):920-924. <http://dx.doi.org/10.1177/104063870902100631> <PMid:19901305>
https://doi.org/10.1177/1040638709021006...
), horses (Peek et al. 2003Peek S.F., Semrad S.D. & Perkins G.A. 2003. Clostridial myonecrosis in horses (37 cases 1985-2000). Equine Vet. J. 35(1):86-92. <http://dx.doi.org/10.2746/042516403775467513> <PMid:12553469>
https://doi.org/10.2746/0425164037754675...
, Raymundo et al. 2010Raymundo D.L., Pavarini S.P., Bezerra Junior P.S., Antoniassi N.A.B., Brecht B.S., Gomes M.J.P. & Driemeier D. 2010. Mionecrose aguda por Clostridium septicum em equinos. Pesq. Vet. Bras. 30(8):637-640. <http://dx.doi.org/10.1590/S0100-736X2010000800005>
https://doi.org/10.1590/S0100-736X201000...
, Farias et al. 2014Farias L.D., Azevedo Mda.S., Trost M.E., De La Côrte F.D., Irigoyen L.F. & Vargas A.C. 2014. Acute myonecrosis in horse caused by Clostridium novyi type A. Braz. J. Microbiol. 45(1):221-224. <http://dx.doi.org/10.1590/S1517-83822014005000023> <PMid:24948935>
https://doi.org/10.1590/S1517-8382201400...
) and other mammals and birds (Silva et al. 2016bSilva R.O.S., Uzal F.A., Oliveira Junior C.A. & Lobato F.C.F. 2016b. Gas gangrene (malignant edema), p.243-254. In: Uzal F., Prescott J., Songer G. & Popoff M. (Eds), Clostridial Diseases of Animals. John Wiley and Sons, Ames. <http://dx.doi.org/10.1002/9781118728291.ch20>.
https://doi.org/10.1002/9781118728291.ch...
). It is due to contamination of wounds by bacteria of the genus Clostridium including one or more of the following: Clostridium septicum, Clostridium chauvoei, Clostridium novyi type A, Clostridium perfringens type A, and Clostridium sordellii (Prescott 2016Prescott J.F. 2016. Brief description of animal pathogenic clostridia, p.13-19. In: Uzal F., Prescott J., Songer G. & Popoff M. (Eds), Clostridial Diseases of Animals. John Wiley and Sons. Ames. <http://dx.doi.org/10.1002/9781118728291.ch3>.
https://doi.org/10.1002/9781118728291.ch...
, Silva et al. 2016bSilva R.O.S., Uzal F.A., Oliveira Junior C.A. & Lobato F.C.F. 2016b. Gas gangrene (malignant edema), p.243-254. In: Uzal F., Prescott J., Songer G. & Popoff M. (Eds), Clostridial Diseases of Animals. John Wiley and Sons, Ames. <http://dx.doi.org/10.1002/9781118728291.ch20>.
https://doi.org/10.1002/9781118728291.ch...
). All of these microorganisms are ubiquitous and can be found in the environment and the intestines of animals and humans (Silva et al. 2016aSilva R.O.S., Oliveira Junior C.A., Gonçalves L.A. & Lobato F.C.F. 2016a. Botulism in ruminants in Brazil. Ciência Rural 46(8):1411-1417. <http://dx.doi.org/10.1590/0103-8478cr20151486>
https://doi.org/10.1590/0103-8478cr20151...
).
Many authors consider malignant edema and gas gangrene as synonyms (Silva et al. 2016bSilva R.O.S., Uzal F.A., Oliveira Junior C.A. & Lobato F.C.F. 2016b. Gas gangrene (malignant edema), p.243-254. In: Uzal F., Prescott J., Songer G. & Popoff M. (Eds), Clostridial Diseases of Animals. John Wiley and Sons, Ames. <http://dx.doi.org/10.1002/9781118728291.ch20>.
https://doi.org/10.1002/9781118728291.ch...
, Riet-Correa 2007aRiet-Correa F. 2007a. Carbúnculo sintomático, p.264-288. In: Riet-Correa F., Schild A.L., Lemos R.A.A & Borges J.R.J. (Eds), Doenças de Ruminantes e Equídeos. Vol.1. 3rd ed. Pallotti, Santa Maria .). Others separate them into two distinct diseases (Cooper & Valentine 2016Cooper B.J. & Valentine B.A. 2016. Malignant edema and gas gangrene, p.232. In: Maxie M.G. (Ed.), Jubb, Kennedy and Palmer’s Pathology of Domestic Animals. Vol.1. 6th ed. Elsevier, St Louis.) with the following explanation for the split: both diseases are associated with subcutaneous and muscular hemorrhagic edema and necrosis; when gas is produced in the lesion the term gas gangrene is appropriated. In the absence of gas and when edema predominates, the disease is referred as malignant edema. According to these authors (Cooper & Valentine 2016Cooper B.J. & Valentine B.A. 2016. Malignant edema and gas gangrene, p.232. In: Maxie M.G. (Ed.), Jubb, Kennedy and Palmer’s Pathology of Domestic Animals. Vol.1. 6th ed. Elsevier, St Louis.) C. septicum would be a more common cause of gas gangrene, and C. perfringens of malignant edema.
In this paper we consider malignant edema and gas gangrene as synonyms and we favor the use of the term “malignant edema” for the convenience of a single denomination for these conditions and because, in our experience, hemorrhagic edema in the subcutaneous tissue and between the muscles is the most frequent lesion at necropsy; more rarely, lesions occur in the muscles (myonecrosis). The typical lesion is acute cellulitis. An identical clostridial disease induced by C. novyi type A is observed in rams which, to increase confusion, is known yet by another sobriquet: “swollen head” (Cooper & Valentine 2016Cooper B.J. & Valentine B.A. 2016. Malignant edema and gas gangrene, p.232. In: Maxie M.G. (Ed.), Jubb, Kennedy and Palmer’s Pathology of Domestic Animals. Vol.1. 6th ed. Elsevier, St Louis., Prescott 2016Prescott J.F. 2016. Brief description of animal pathogenic clostridia, p.13-19. In: Uzal F., Prescott J., Songer G. & Popoff M. (Eds), Clostridial Diseases of Animals. John Wiley and Sons. Ames. <http://dx.doi.org/10.1002/9781118728291.ch3>.
https://doi.org/10.1002/9781118728291.ch...
).
Cases of malignant edema are observed sporadically. Outbreaks occur when there is collective trauma. It affects animals of any age. It can occur after dipping, shearing, tail docking, castration, deliverance (Odani et al. 2009Odani J.S., Blanchard P.C., Adaska J.M., Moeller R.B. & Uzal F.A. 2009. Malignant edema in postpartum dairy cattle. J. Vet. Diagn. Invest. 21(6):920-924. <http://dx.doi.org/10.1177/104063870902100631> <PMid:19901305>
https://doi.org/10.1177/1040638709021006...
), or injections with contaminated needles. When the disease is caused by the use of contaminated needles, mortality is very high in the first 48 hours, as occurred in an outbreak observed in this study, where outbreaks caused by syringes contaminated with C. septicum caused 100% mortality in calves. In Mato Grosso do Sul, outbreaks of malignant edema with tongue myonecrosis were apparently caused by contamination of wounds caused by grazing coarse grass (Lemos 1998Lemos R.A.A. 1998. Mionecrose causada por clostrídios, p.388-396. In: Ibid. (Ed), Principais Enfermidades de Bovinos de Corte do Mato Grosso do Sul. Universidade Federal do Mato Grosso do Sul, Campo Grande.).
For the diagnosis of malignant edema, one must search for recent injuries to the skin (which does not appear in blackleg) and for the characteristic necropsy lesions. The identification of the agent can be performed by immunofluorescence, immunohistochemistry (Assis et al. 2005Assis R.A., Lobato F.C.F., Serakidis R., Santos R.L., Dias G.R.C., Nascimento R.A.P., Abreu B.L.V., Parreiras P.M. & Uzal F.A. 2005. Immunohistochemical detection of clostridia species in paraffin-embedded tissues of experimentally inoculated guinea pigs. Pesq. Vet. Bras. 25(1):4-8. <http://dx.doi.org/10.1590/S0100-736X2005000100002>
https://doi.org/10.1590/S0100-736X200500...
), or isolation and characterization of the Clostridium spp. (Riet-Correa 2007bRiet-Correa F. 2007b. Edema maligno, p.286-265. In: Riet-Correa F., Schild A.L., Lemos R.A.A. & Borges J.R.J. (Eds), Doenças de Ruminantes e Equídeos. Vol.1. 3rd ed. Pallotti, Santa Maria .) and type. In young cattle (6 months to 2 years old) the disease should be differentiated from blackleg. In blackleg, there is no association with wounds and muscular lesions always predominate.
Affected animals may be treated with high doses of penicillin or broad-spectrum antibiotics. For prophylaxis, it is necessary to avoid contamination, especially with soil, of the instruments and syringes used in the herd. The animals should be vaccinated annually with vaccines containing mainly C. septicum, C. novyi type B, and C. sordelli.
In seven out of the eight botulism outbreaks (7.7% of all clostridial diseases), in the current study, cattle were in a pasture of native grass, and in only one outbreak cattle were supplemented with minerals. The majority of cases (5/8) occurred in the municipality of Rio Grande, RS, in flooded areas, which have not being used for crops since they were submerged for the most part of the year. In three outbreaks there was animal carrion in the pasture. Five outbreaks occurred in the summer (January-February), two in the fall and one in the spring. Morbidity, mortality and lethality were from 0.5-10.8 to 0.16-10.8 respectively and there was an average of 30% lethality (Table 1).
Botulism is a clostridial disease whose main characteristic is flaccid paralysis of various muscle groups. It is usually caused by the ingestion of botulinum neurotoxins (BoNTs) produced by Clostridium botulinum (an anaerobic gram-positive bacterium) found in the soil, water, food, and alimentary system of many animals (Le Marechal et al. 2016Le Marechal C., Woudstra C. & Fach P. 2016. Botulism, p.303-330. In: Uzal F., Prescott J., Songer G. & Popoff M. (Eds), Clostridial Diseases of Animals. John Wiley and Sons, Ames. <http://dx.doi.org/10.1002/9781118728291.ch26>.
https://doi.org/10.1002/9781118728291.ch...
).
There are seven potent distinct BoNTs identified by the letters A-G (Dover et al. 2014Dover N., Barash J.R., Hill K.K., Xie G. & Arnon S.S. 2014. Molecular characterization of a novel botulinum neurotoxin type H gene. J. Infect. Dis. 209(2):192-202. <http://dx.doi.org/10.1093/infdis/jit450> <PMid:24106295>
https://doi.org/10.1093/infdis/jit450...
, Maslanka et al. 2016Maslanka S.E., Luquez C., Dykes J.K., Tepp W.H., Pier C.L., Pellett S., Raphael B.H., Kalb S.R., Barr J.R., Rao A. & Johnson E.A. 2016. A novel botulinum neurotoxin, previously reported as serotype H, has a hybrid-like structure with regions of similarity to the structures of serotypes A and F and is neutralized with serotype A antitoxin. J. Infect. Dis. 213(3):379-385. <http://dx.doi.org/10.1093/infdis/jiv327> <PMid:26068781>
https://doi.org/10.1093/infdis/jiv327...
). BoNTs are the most toxic biological substances that exist (Lamanna 1959Lamanna C. 1959. The most poisonous poison. Science 130(3378):763-772. <http://dx.doi.org/10.1126/science.130.3378.763> <PMid:14413547>
https://doi.org/10.1126/science.130.3378...
); a gram of pure toxin evenly distributed in the feed can kill 400,000 adult cows (Galey et al. 2000Galey F.D., Terra R., Walker R., Adaska J., Etchebarne M.A., Puschner B., Fisher E., Whitlock R.H., Rocke T., Willoughby D. & Tor E. 2000. Type C botulism in dairy cattle from feed contaminated with a dead cat. Vet. Diagn. Invest. 12(3):204-209. <http://dx.doi.org/10.1177/104063870001200302> <PMid:10826832>
https://doi.org/10.1177/1040638700012003...
). After ingestion and absorption from the intestine, BoNT reaches the bloodstream, is disseminated in the body and exerts its effect on the peripheral somatic motor nerves that supply skeletal muscle. At the neuromuscular junction, the neurotoxin inhibits the exocytosis of acetylcholine, which compromises the stimulus for muscle contraction, resulting in flaccid paralysis, a hallmark of the disease (Fig.12). The central nervous system is spared because it is protected by the blood-brain barrier (Mayhew 2009Mayhew J. 2009. Toxic diseases, p.321-359 In: Ibid. (Ed), Large Animal Neurology. Wiley Blackwell. Ames.). There are no gross or histologic lesions associated with botulism (Barros et al. 2006aBarros C.S.L., Driemeier D., Dutra I.S. & Lemos R.A.A. 2006a. Botulismo, p.57-62. In: Ibid. (Eds), Doenças do Sistema Nervoso de Bovinos no Brasil. Valée, Montes Claros.).
Pathogenesis of botulism. Schematic view of a motor neuron and the neuromuscular junction, The botulinum neurotoxin (BoNTs, shown as red asterisks) are internalized at synapses in endosomal compartments, one of which might attach to synaptic vesicles (blue dots). At the neuromuscular junction, the BoNT inhibits the exocytosis of acetylcholine, which compromises the stimulus for muscle contraction, resulting in flaccid paralysis. The synaptic vesicles are synthesized in the endoplasmic reticulum and released by the Golgi apparatus. (Modified from Mayhew 2009Mayhew J. 2009. Toxic diseases, p.321-359 In: Ibid. (Ed), Large Animal Neurology. Wiley Blackwell. Ames.)
Botulism is endemic in Brazil, mainly in the Midwestern states (Soares et al. 2018Soares M.C., Gaspar A.O., Brumatti R.C., Gomes D.C., Neves D.A., Alcântara L.O.B., Leal P.V. & Lemos R.A.A. 2018. Economic impact of an outbreak of botulism in a cattle feedlot. Pesq. Vet. Bras. 38(7):1365-1370. <http://dx.doi.org/10.1590/1678-5150-pvb-5643>
https://doi.org/10.1590/1678-5150-pvb-56...
) and the BoNTs responsible for the outbreaks in cattle in our country are, mostly, types C and D (Barros et al. 2006aBarros C.S.L., Driemeier D., Dutra I.S. & Lemos R.A.A. 2006a. Botulismo, p.57-62. In: Ibid. (Eds), Doenças do Sistema Nervoso de Bovinos no Brasil. Valée, Montes Claros., Silva et al. 2016). In the last 15-20 years, botulism has emerged as a significant disease in Europe and a bioterrorism threat (Skarin et al. 2013Skarin H., Tevell Åberg A., Woudstra C., Hansen T., Löfström C., Koene M., Bano L., Hedeland M., Anniballi F., De Medici D. & Olsson Engvall E. 2013. The workshop on animal botulism in Europe. Biosecur Bioterror 11(Suppl.1):S183-S190. <http://dx.doi.org/10.1089/bsp.2012.0076> <PMid:23971805>
https://doi.org/10.1089/bsp.2012.0076...
).
Cattle become ingesting the BoNTs from carrion, drinking stagnant water contaminated with animal carrion (Dutra et al. 2001Dutra I.S., Döbereiner J., Rosa I.V., Souza L.A.A. & Nonato M. 2001. Surtos de botulismo em bovinos no Brasil associados a ingestão de água contaminada. Pesq. Vet. Bras. 21(2):43-48. <http://dx.doi.org/10.1590/S0100-736X2001000200002>
https://doi.org/10.1590/S0100-736X200100...
), degraded food, or food contaminated with carrion from rodent, birds, and cats (Galey et al. 2000Galey F.D., Terra R., Walker R., Adaska J., Etchebarne M.A., Puschner B., Fisher E., Whitlock R.H., Rocke T., Willoughby D. & Tor E. 2000. Type C botulism in dairy cattle from feed contaminated with a dead cat. Vet. Diagn. Invest. 12(3):204-209. <http://dx.doi.org/10.1177/104063870001200302> <PMid:10826832>
https://doi.org/10.1177/1040638700012003...
).
In cattle, the morbidity is variable, and the lethality is generally 100%, although there are cases of recovery of cattle that ingest small doses and develop mild clinical signs (Barros et al. 2006aBarros C.S.L., Driemeier D., Dutra I.S. & Lemos R.A.A. 2006a. Botulismo, p.57-62. In: Ibid. (Eds), Doenças do Sistema Nervoso de Bovinos no Brasil. Valée, Montes Claros., Constable et al. 2017Constable P.D., Hinchcliff K.W., Done S.H. & Greenberg W. 2017. Botulism, 1363-1367. In: Ibid (Eds), Veterinary Medicine a Textbook of the Diseases of Cattle, Horses, Sheep, Pigs, and Goats. Elsevier, Philadelphia.).
In an outbreak in Paraíba state, northeastern Brazil, 96.66% of sheep (145/150), 51.77% of goats (233\450), and 96.77% of cattle (30\31) died after the ingestion of chicken litter (Riet-Correa et al. 2003Riet-Correa F., Tabosa L.M., Azevedo E.O., Medeiros R.M.T., Simões S.V.D., Dantas A.F., Alves C.J., Nobre V.M.T., Athayde A.C., Gomes A.A. & Lima E.F. 2003. Doenças dos ruminantes e equinos no semi-árido da Paraíba. Semi-Árido em Foco 1:2-86.). In a feedlot of 1,700 cattle Mato Grosso do Sul, 1,090 steers died within four days (Guizelini et al. 2019Guizelini C.C., Lemos R.A.A., de Paula J.L.P., Pupin R.C., Gomes D.C., Barros C.S.L., Neves D.A., Alcântara L.O.B., Silva R.O.S., Lobato F.C.F. & Martins T.B. 2019. Type C botulism outbreak in feedlot cattle fed contaminated corn silage. Anaerobe 55:103-106. <http://dx.doi.org/10.1016/j.anaerobe.2018.11.003> <PMid:30408576>
https://doi.org/10.1016/j.anaerobe.2018....
).
Clinical signs of botulism may appear 1-17 days after ingestion of contaminated food (Barros et al. 2006aBarros C.S.L., Driemeier D., Dutra I.S. & Lemos R.A.A. 2006a. Botulismo, p.57-62. In: Ibid. (Eds), Doenças do Sistema Nervoso de Bovinos no Brasil. Valée, Montes Claros., Constable et al. 2017Constable P.D., Hinchcliff K.W., Done S.H. & Greenberg W. 2017. Botulism, 1363-1367. In: Ibid (Eds), Veterinary Medicine a Textbook of the Diseases of Cattle, Horses, Sheep, Pigs, and Goats. Elsevier, Philadelphia.). Although most cases are acute, the course of the disease may be hyperacute (less than 24 hours), acute (1-2 days), subacute (3-7 days), or chronic (7-30 days). In the chronic form affected cattle have better chances of survival (Barros et al. 2006aBarros C.S.L., Driemeier D., Dutra I.S. & Lemos R.A.A. 2006a. Botulismo, p.57-62. In: Ibid. (Eds), Doenças do Sistema Nervoso de Bovinos no Brasil. Valée, Montes Claros.). It is likely that the dose of toxin ingested determines the course of the disease. Larger doses would determine acute conditions, while lower doses would cause chronic disease.
The clinical signs of botulism are quite characteristic so much so as to allow a reliable clinical diagnosis (Barros et al. 2006aBarros C.S.L., Driemeier D., Dutra I.S. & Lemos R.A.A. 2006a. Botulismo, p.57-62. In: Ibid. (Eds), Doenças do Sistema Nervoso de Bovinos no Brasil. Valée, Montes Claros.). It is characterized by partial or complete flaccid paralysis of the muscles of locomotion, chewing and swallowing. The animals present a decrease, but never a complete absence, of the musculature of the limbs, with flaccid paralysis of two (Fig.13) or four limbs.
Clostridial diseases. Botulism. Flaccid paralysis in a cow. The lack of tonus in the muscle limbs (flaccidity) prevents the steer from standing.
The main clinical sign is the difficulty of locomotion, characterized by staggering gait, affecting mainly the hind limbs and evolving to the forelimbs, head and neck. The animal tends to lie in sternal decubitus with the head resting on the flank or with the chin on the ground. Flaccid paralysis affects the tongue (Fig.14) which is easily pulled from the mouth. There is also flaccidity of the tail, which remains the medial and distal portions apart from the body. Bradycardia occurs, and respiration is dyspneic, laborious, diaphragmatic (abdominal), with inspiration in two phases, the second being prolonged. There is flaccidity of chewing muscles, which is observed as inability to seize, chew and swallow the food (Barros et al. 2006aBarros C.S.L., Driemeier D., Dutra I.S. & Lemos R.A.A. 2006a. Botulismo, p.57-62. In: Ibid. (Eds), Doenças do Sistema Nervoso de Bovinos no Brasil. Valée, Montes Claros.).
Clostridial diseases. Botulism. Flaccid paralysis causes the tongue to protrude from the mouth.
Rarely, there are sensory abnormalities, which can be assessed by maintaining cutaneous, paravertebral and limb sensitivity. While cattle lied down for prolonged periods, they may develop ischemia of large muscle masses. In this case, there will be a loss of sensitivity due to muscle and nerve compression (Barros et al. 2006aBarros C.S.L., Driemeier D., Dutra I.S. & Lemos R.A.A. 2006a. Botulismo, p.57-62. In: Ibid. (Eds), Doenças do Sistema Nervoso de Bovinos no Brasil. Valée, Montes Claros.)
The diagnosis of the disease is based on the clinical signs, the history, and the absence of significant gross and histological lesions. Botulism should be suspected whenever cattle in good condition, are found in a permanent position. As botulism is a neurological peripheral disease there are no obvious changes in attitude and sensibility. Botulism usually affects cattle of various ages spectrum.
Other techniques can be employed to confirm a clinical diagnosis. Intraperitoneal inoculation in mice (biological assay) of 500ml of hepatic extract, blood serum, ruminal or intestinal contents is considered the most specific test but still has a very low sensitivity. When the test is positive, one should perform the serum neutralization test (or seroprotection), which is based on the neutralization of the botulinum toxin with the specific antitoxin.
Treatment for botulism in cattle is controversial, but some limited results have been obtained with hyperimmune serum (Guizelini et al. 2019Guizelini C.C., Lemos R.A.A., de Paula J.L.P., Pupin R.C., Gomes D.C., Barros C.S.L., Neves D.A., Alcântara L.O.B., Silva R.O.S., Lobato F.C.F. & Martins T.B. 2019. Type C botulism outbreak in feedlot cattle fed contaminated corn silage. Anaerobe 55:103-106. <http://dx.doi.org/10.1016/j.anaerobe.2018.11.003> <PMid:30408576>
https://doi.org/10.1016/j.anaerobe.2018....
). As an alternative, supportive treatment should be performed (Barros et al. 2006aBarros C.S.L., Driemeier D., Dutra I.S. & Lemos R.A.A. 2006a. Botulismo, p.57-62. In: Ibid. (Eds), Doenças do Sistema Nervoso de Bovinos no Brasil. Valée, Montes Claros.). The elimination of field carcasses is an important auxiliary measure. Vaccination of the herd is another important form of control and prophylaxis in areas where there is a high incidence of the disease. Animals can be vaccinated from four months of age and revaccinated within 30-40 days, and then annually.
Some final considerations are in order. The five clostridial diseases discussed here have characteristic clinical signs and epidemiological features. Three of them have necropsy findings and histopathology so characteristic that, when associated with clinical signs epidemiology, allow for a diagnosis. Two others do not present anatomopathological findings, but have almost unmistakable clinical signs (Table 2) and laboratory diagnostic techniques that might confirm them are suboptimal or nonexistent. In no way are we advocating against the use of appropriate laboratory diagnostic techniques. These considerations are made to explain that, even although, in the current study we made the diagnoses without the aid of such ancillary techniques, the diagnosis are sound. We admit that a few diagnostic errors may have been committed, but not to the point of damaging the trend of the data provided here. In Table 2, we suggest and approach for a field working diagnosis of these diseases.
Conclusions
Five clostridial diseases in cattle should be considered in the field diagnosis in southern Rio Grande do Sul, namely tetanus, blackleg, bacillary hemoglobinuria, malignant edema, and botulism.
Certain characteristic clinical, epidemiological, necropsy and histopathological findings (or instead, their absence) are fundamental for the diagnosis in the field.
Treatment is possible but, in most cases, unsuccessful due to the acute course of these five diseases. Vaccination is the best procedure to prevent any one of them.
Acknowledgements
The authors are grateful to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq: 305283/2015-4, 431659/2016-8), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (finance code 001) for scientific, financial support and student’s scholarship. The authors are also indebted to Dr. Mario Assis Neto for the art of the drawings Figure 1, 4, 6, 8, and 12. His excellence is very much appreciated.
References
- Abreu C.C. & Uzal F.A. 2016. Blackleg, p.231-242. In: Uzal F., Prescott J., Songer G. & Popoff M. (Eds), Clostridial Diseases of Animals. John Wiley and Sons, Ames. <http://dx.doi.org/10.1002/9781118728291.ch19>
» https://doi.org/10.1002/9781118728291.ch19 - Abreu C.C., Edwards E.E., Edwards J.F., Gibbons P.M., Leal de Araújo J., Rech R.R. & Uzal F.A. 2017. Blackleg in cattle: a case report of fetal infection and a literature review. J. Vet. Diagn. Invest. 29(5):612-621. <http://dx.doi.org/10.1177/1040638717713796> <PMid:28599620>
» https://doi.org/10.1177/1040638717713796 - Assis R.A., Lobato F.C.F., Serakidis R., Santos R.L., Dias G.R.C., Nascimento R.A.P., Abreu B.L.V., Parreiras P.M. & Uzal F.A. 2005. Immunohistochemical detection of clostridia species in paraffin-embedded tissues of experimentally inoculated guinea pigs. Pesq. Vet. Bras. 25(1):4-8. <http://dx.doi.org/10.1590/S0100-736X2005000100002>
» https://doi.org/10.1590/S0100-736X2005000100002 - Barros C.S.L. 2016a. Fígado, vias biliares e pâncreas exócrino, p.181-265. In: Santos R.L. & Alessi A.C. (Eds), Patologia Veterinária. 2ª ed. Roca, São Paulo.
- Barros C.S.L. 2016b. Sistema muscular, p.663-702. In: Santos R.L. & Alessi A.C. (Eds), Patologia Veterinária. Roca, São Paulo .
- Barros C.S.L., Driemeier D., Dutra I.S. & Lemos R.A.A. 2006a. Botulismo, p.57-62. In: Ibid. (Eds), Doenças do Sistema Nervoso de Bovinos no Brasil. Valée, Montes Claros.
- Barros C.S.L., Driemeier D., Dutra I.S. & Lemos R.A.A. 2006b. Tétano, p.59-64. In: Ibid. (Eds), Doenças do Sistema Nervoso de Bovinos no Brasil. Valée, Montes Claros .
- Bizzini B. 1989. Axoplasmic transport and transynaptic movement of tetanus toxins, p.203-229. In: Simpson L.L. (Ed), Botulism Neurotoxin and Tetanus Toxin. Academic Press, New York. <http://dx.doi.org/10.1016/B978-0-12-644445-2.50015-5>
» https://doi.org/10.1016/B978-0-12-644445-2.50015-5 - Bleck T.P. 1989. Clinical aspects of tetanus, p.379-398. In: Simpson L.L. (Ed.), Botulism Neurotoxin and Tetanus Toxin. Academic Press, New York . <http://dx.doi.org/10.1016/B978-0-12-644445-2.50025-8>
» https://doi.org/10.1016/B978-0-12-644445-2.50025-8 - Böhmel H. & Gessler F. 2010. Neurotoxigenic clostridia, p.189-202. In: Gyles C.L., Prescott J.F., Songer J.G. & Thoen C.O. (Eds), Pathogenesis of Bacterial Disease in Animals. 4th ed. Wiley Blackwell, Ames. <http://dx.doi.org/10.1002/9780470958209.ch11>.
» https://doi.org/10.1002/9780470958209.ch11 - Constable P.D., Hinchcliff K.W., Done S.H. & Greenberg W. 2017. Botulism, 1363-1367. In: Ibid (Eds), Veterinary Medicine a Textbook of the Diseases of Cattle, Horses, Sheep, Pigs, and Goats. Elsevier, Philadelphia.
- Cooper B.J. & Valentine B.A. 2016. Malignant edema and gas gangrene, p.232. In: Maxie M.G. (Ed.), Jubb, Kennedy and Palmer’s Pathology of Domestic Animals. Vol.1. 6th ed. Elsevier, St Louis.
- Correa M.W., Correa C.N.M., Lopes C.A.M., Langoni H. & Modolo J.R. 1980. Enfermidades por clostrídios, 1969-1978. Arqs Bras. Med. Vet. Zootec. 32:369-374.
- Dover N., Barash J.R., Hill K.K., Xie G. & Arnon S.S. 2014. Molecular characterization of a novel botulinum neurotoxin type H gene. J. Infect. Dis. 209(2):192-202. <http://dx.doi.org/10.1093/infdis/jit450> <PMid:24106295>
» https://doi.org/10.1093/infdis/jit450 - Driemeier D., Schild A.L., Fernandes J.C., Colodel E.M., Correa A.M., Cruz C.E. & Barros C.S.L. 2007. Outbreaks of tetanus in beef cattle and sheep in Brazil associated with disophenol injection. J. Vet. Med. A, Physiol. Pathol. Clin. Med. 54(6):333-335. <http://dx.doi.org/10.1111/j.1439-0442.2007.00922.x> <PMid:17650154>
» https://doi.org/10.1111/j.1439-0442.2007.00922.x - Dutra I.S., Döbereiner J., Rosa I.V., Souza L.A.A. & Nonato M. 2001. Surtos de botulismo em bovinos no Brasil associados a ingestão de água contaminada. Pesq. Vet. Bras. 21(2):43-48. <http://dx.doi.org/10.1590/S0100-736X2001000200002>
» https://doi.org/10.1590/S0100-736X2001000200002 - Farias L.D., Azevedo Mda.S., Trost M.E., De La Côrte F.D., Irigoyen L.F. & Vargas A.C. 2014. Acute myonecrosis in horse caused by Clostridium novyi type A. Braz. J. Microbiol. 45(1):221-224. <http://dx.doi.org/10.1590/S1517-83822014005000023> <PMid:24948935>
» https://doi.org/10.1590/S1517-83822014005000023 - Filho E.J.F., Carvalho A.U., Assis R.A., Lobato F.F., Rachid M.A., Carvalho A.A., Ferreira P.M., Nascimento R.A., Fernandes A.A., Vidal J.E. & Uzal F.A. 2009. Clinicopathologic features of experimental Clostridium perfringens type D enterotoxemia in cattle. Vet. Pathol. 46(6):1213-1220. <http://dx.doi.org/10.1354/vp.08-VP-0304-U-FL> <PMid:19605912>
» https://doi.org/10.1354/vp.08-VP-0304-U-FL - Galey F.D., Terra R., Walker R., Adaska J., Etchebarne M.A., Puschner B., Fisher E., Whitlock R.H., Rocke T., Willoughby D. & Tor E. 2000. Type C botulism in dairy cattle from feed contaminated with a dead cat. Vet. Diagn. Invest. 12(3):204-209. <http://dx.doi.org/10.1177/104063870001200302> <PMid:10826832>
» https://doi.org/10.1177/104063870001200302 - Glastonbury J.R., Searson J.E., Links I.J. & Tuckett L.M. 1988. Clostridial myocarditis in lambs. Aust. Vet. J. 65(7):208-209. <http://dx.doi.org/10.1111/j.1751-0813.1988.tb14459.x> <PMid:3421885>
» https://doi.org/10.1111/j.1751-0813.1988.tb14459.x - Guizelini C.C., Lemos R.A.A., de Paula J.L.P., Pupin R.C., Gomes D.C., Barros C.S.L., Neves D.A., Alcântara L.O.B., Silva R.O.S., Lobato F.C.F. & Martins T.B. 2019. Type C botulism outbreak in feedlot cattle fed contaminated corn silage. Anaerobe 55:103-106. <http://dx.doi.org/10.1016/j.anaerobe.2018.11.003> <PMid:30408576>
» https://doi.org/10.1016/j.anaerobe.2018.11.003 - Heckler R.F., Lemos R.A.A., Gomes D.C., Dutra I.S., Silva R.O.S., Lobato F.C.F., Ramos C.A.N. & Brumatti R.C. 2018. Blackleg in cattle in the state of Mato Grosso do Sul, Brazil: 59 cases. Pesq. Vet. Bras. 38(1):6-14. <http://dx.doi.org/10.1590/1678-5150-pvb-4964>
» https://doi.org/10.1590/1678-5150-pvb-4964 - Helman G., Welsh R.D., Stair E.L. & Ely R.W. 1997. Diagnosing visceral blackleg as a cause of sudden death in cattle. Vet. Med. 92:914-918.
- Kriek N.P.J. & Odendaal M.W. 2004. Clostridium novyi type D infection, p.1686-1687. In: Coetzer J.A.W. & Tustin RC. (Eds), Infectious Diseases of Livestock. 3rd ed. Vol.3. Oxford Press, Cape Town.
- Lamanna C. 1959. The most poisonous poison. Science 130(3378):763-772. <http://dx.doi.org/10.1126/science.130.3378.763> <PMid:14413547>
» https://doi.org/10.1126/science.130.3378.763 - Le Marechal C., Woudstra C. & Fach P. 2016. Botulism, p.303-330. In: Uzal F., Prescott J., Songer G. & Popoff M. (Eds), Clostridial Diseases of Animals. John Wiley and Sons, Ames. <http://dx.doi.org/10.1002/9781118728291.ch26>.
» https://doi.org/10.1002/9781118728291.ch26 - Lemos R.A.A. 1998. Mionecrose causada por clostrídios, p.388-396. In: Ibid. (Ed), Principais Enfermidades de Bovinos de Corte do Mato Grosso do Sul. Universidade Federal do Mato Grosso do Sul, Campo Grande.
- Lobato F.C.F., Assis R.A., Abreu V.L.V., Souza Junior M.F., Lima C.G.R.D. & Salvarani F.M. 2006. Enterotoxemia em bovino. Arqs Bras. Med. Vet. Zootec. 58(5):952-954. <http://dx.doi.org/10.1590/S0102-09352006000500037>
» https://doi.org/10.1590/S0102-09352006000500037 - Maslanka S.E., Luquez C., Dykes J.K., Tepp W.H., Pier C.L., Pellett S., Raphael B.H., Kalb S.R., Barr J.R., Rao A. & Johnson E.A. 2016. A novel botulinum neurotoxin, previously reported as serotype H, has a hybrid-like structure with regions of similarity to the structures of serotypes A and F and is neutralized with serotype A antitoxin. J. Infect. Dis. 213(3):379-385. <http://dx.doi.org/10.1093/infdis/jiv327> <PMid:26068781>
» https://doi.org/10.1093/infdis/jiv327 - Mayhew J. 2009. Toxic diseases, p.321-359 In: Ibid. (Ed), Large Animal Neurology. Wiley Blackwell. Ames.
- Mete A., Garcia J., Ortega J., Lane M., Scholes S. & Uzal F.A. 2013. Brain lesions associated with Clostridium perfringens type D epsilon toxin in a Holstein heifer calf. Vet. Pathol. 50(5):765-768. <http://dx.doi.org/10.1177/0300985813476058> <PMid:23381925>
» https://doi.org/10.1177/0300985813476058 - Monaghan M.L. & Sheahan B.J. 1987. Liver biopsy in ragwort poisoning. Vet. Rec. 120(15):374. <http://dx.doi.org/10.1136/vr.120.15.374> <PMid:3590594>
» https://doi.org/10.1136/vr.120.15.374 - Navarro M., Dutra Quintela F. & Uzal F.A. 2016. Bacillary hemoglobinuria, p.265-274. In: Uzal F., Prescott J., Songer G. & Popoff M. (Eds), Clostridial Diseases of Animals. John Wiley and Sons, Ames. <http://dx.doi.org/10.1002/9781118728291.ch22>.
» https://doi.org/10.1002/9781118728291.ch22 - Odani J.S., Blanchard P.C., Adaska J.M., Moeller R.B. & Uzal F.A. 2009. Malignant edema in postpartum dairy cattle. J. Vet. Diagn. Invest. 21(6):920-924. <http://dx.doi.org/10.1177/104063870902100631> <PMid:19901305>
» https://doi.org/10.1177/104063870902100631 - Odendaal M.W. & Kriek N.P.J. 2004. Tetanus, p.1878-1884. In: Coetzer J.A.W. & Tustin R.C. (Eds), Infectious Diseases of Livestock. Vol.3. 3rd ed. Oxford Press, Cape Town.
- Peek S.F., Semrad S.D. & Perkins G.A. 2003. Clostridial myonecrosis in horses (37 cases 1985-2000). Equine Vet. J. 35(1):86-92. <http://dx.doi.org/10.2746/042516403775467513> <PMid:12553469>
» https://doi.org/10.2746/042516403775467513 - Popoff M.R. 2016. Tetanus, p.295-302. In: Uzal F., Prescott J., Songer G. & Popoff M. (Eds), Clostridial Diseases of Animals. John Wiley and Sons, Ames. <http://dx.doi.org/10.1002/9781118728291.ch25>.
» https://doi.org/10.1002/9781118728291.ch25 - Prescott J.F. 2016. Brief description of animal pathogenic clostridia, p.13-19. In: Uzal F., Prescott J., Songer G. & Popoff M. (Eds), Clostridial Diseases of Animals. John Wiley and Sons. Ames. <http://dx.doi.org/10.1002/9781118728291.ch3>.
» https://doi.org/10.1002/9781118728291.ch3 - Quevedo P.S., Ladeira S.R.L., Soares M.P., Marcolongo-Pereira C., Sallis E.S.V., Grecco F.B., Estima-Silva P. & Schild A.L. 2011. Tétano em bovinos no sul do Rio Grande do Sul: estudo de 24 surtos. Pesq. Vet. Bras. 31(12):1066-1070. <http://dx.doi.org/10.1590/S0100-736X2011001200005>
» https://doi.org/10.1590/S0100-736X2011001200005 - Raposo J.B. 2007. Tétano, p.425-432. In: Riet-Correa F., Schild A.L., Lemos R.A.A & Borges J.R.J. (Eds), Doenças de Ruminantes e Equídeos. Vol.1. 3rd ed. Pallotti, Santa Maria.
- Raymundo D.L., Pavarini S.P., Bezerra Junior P.S., Antoniassi N.A.B., Brecht B.S., Gomes M.J.P. & Driemeier D. 2010. Mionecrose aguda por Clostridium septicum em equinos. Pesq. Vet. Bras. 30(8):637-640. <http://dx.doi.org/10.1590/S0100-736X2010000800005>
» https://doi.org/10.1590/S0100-736X2010000800005 - Riet-Correa F. 2007a. Carbúnculo sintomático, p.264-288. In: Riet-Correa F., Schild A.L., Lemos R.A.A & Borges J.R.J. (Eds), Doenças de Ruminantes e Equídeos. Vol.1. 3rd ed. Pallotti, Santa Maria .
- Riet-Correa F. 2007b. Edema maligno, p.286-265. In: Riet-Correa F., Schild A.L., Lemos R.A.A. & Borges J.R.J. (Eds), Doenças de Ruminantes e Equídeos. Vol.1. 3rd ed. Pallotti, Santa Maria .
- Riet-Correa F., Tabosa L.M., Azevedo E.O., Medeiros R.M.T., Simões S.V.D., Dantas A.F., Alves C.J., Nobre V.M.T., Athayde A.C., Gomes A.A. & Lima E.F. 2003. Doenças dos ruminantes e equinos no semi-árido da Paraíba. Semi-Árido em Foco 1:2-86.
- Rood J.I. 2016. General Physiological and virulence properties of the pathogenic clostridia, p.7-12. In: Uzal F., Prescott J., Songer G. & Popoff M. (Eds), Clostridial Diseases of Animals. John Wiley and Sons. Ames. <http://dx.doi.org/10.1002/9781118728291.ch2>.
» https://doi.org/10.1002/9781118728291.ch2 - Salvador S.C. & Freire C.A. 1998. Ocorrência de tétano epizoótico em bovinos no Estado de Minas Gerais. Arqs Inst. Biológico, São Paulo, 65:95.
- Schild A.L. 2007. Hemoglobinúria bacilar, p.305-308. In: Riet-Correa F., Schild A.L., Lemos R.A.A & Borges J.R.J. (Eds), Doenças de Ruminantes e Equídeos. Vol.1. 3rd ed. Pallotti, Santa Maria .
- Silva R.O.S., Oliveira Junior C.A., Gonçalves L.A. & Lobato F.C.F. 2016a. Botulism in ruminants in Brazil. Ciência Rural 46(8):1411-1417. <http://dx.doi.org/10.1590/0103-8478cr20151486>
» https://doi.org/10.1590/0103-8478cr20151486 - Silva R.O.S., Uzal F.A., Oliveira Junior C.A. & Lobato F.C.F. 2016b. Gas gangrene (malignant edema), p.243-254. In: Uzal F., Prescott J., Songer G. & Popoff M. (Eds), Clostridial Diseases of Animals. John Wiley and Sons, Ames. <http://dx.doi.org/10.1002/9781118728291.ch20>.
» https://doi.org/10.1002/9781118728291.ch20 - Skarin H., Tevell Åberg A., Woudstra C., Hansen T., Löfström C., Koene M., Bano L., Hedeland M., Anniballi F., De Medici D. & Olsson Engvall E. 2013. The workshop on animal botulism in Europe. Biosecur Bioterror 11(Suppl.1):S183-S190. <http://dx.doi.org/10.1089/bsp.2012.0076> <PMid:23971805>
» https://doi.org/10.1089/bsp.2012.0076 - Soares M.C., Gaspar A.O., Brumatti R.C., Gomes D.C., Neves D.A., Alcântara L.O.B., Leal P.V. & Lemos R.A.A. 2018. Economic impact of an outbreak of botulism in a cattle feedlot. Pesq. Vet. Bras. 38(7):1365-1370. <http://dx.doi.org/10.1590/1678-5150-pvb-5643>
» https://doi.org/10.1590/1678-5150-pvb-5643 - Sojka J.E., Bowersock T.L., Parker J.E., Blevins W.G. & Irigoyen L. 1992. Clostridium chauvoei myositis infection in a neonatal calf. J. Vet. Diagn. Invest. 4(2):201-203. <http://dx.doi.org/10.1177/104063879200400219> <PMid:1616991>
» https://doi.org/10.1177/104063879200400219 - Songer J.G. 2010a. Histotoxic clostridia, p.203-209. In: Gyles C.L., Prescott J.F., Songer J.G. & Thoen C.O. (Eds), Pathogenesis of Bacterial Disease in Animals. 4th ed. Wiley Blackwell, Ames. <http://dx.doi.org/10.1002/9780470958209.ch12>.
» https://doi.org/10.1002/9780470958209.ch12 - Songer J.G. 2010b. Enteric clostridia, p.211-229. In: Gyles C.L., Prescott J.F., Songer J.G. & Thoen C.O. (Eds), Pathogenesis of Bacterial Disease in Animals. 4th ed. Wiley Blackwell, Ames. <http://dx.doi.org/10.1002/9780470958209.ch13>.
» https://doi.org/10.1002/9780470958209.ch13 - Tessele B., Brum J.S. & Barros C.S.L. 2013. Lesões parasitárias encontradas em bovinos abatidos para consumo humano. Pesq. Vet. Bras. 33(7):873-889. <http://dx.doi.org/10.1590/S0100-736X2013000700008>
» https://doi.org/10.1590/S0100-736X2013000700008 - Useh N.M., Ibrahim N.D.G., Nok A.J. & Esievo K.A.N. 2006. Relationship between outbreaks of blackleg of cattle and annual rainfall in Zaria, Nigeria. Vet. Rec. 158(3):100-101. <http://dx.doi.org/10.1136/vr.158.3.100> <PMid:16428667>
» https://doi.org/10.1136/vr.158.3.100 - Uzal F.A., Kelly W.R., Morris W.E. & Assis R.A. 2002. Effects of intravenous injection of Clostridium perfringens type D epsilon toxin in calves. J. Comp. Pathol. 126(1):71-75. <http://dx.doi.org/10.1053/jcpa.2001.0514> <PMid:11814324>
» https://doi.org/10.1053/jcpa.2001.0514 - Uzal F.A., Paramidani M., Assis R., Morris W. & Miyakawa M.F. 2003. Outbreak of clostridial myocarditis in calves. Vet. Rec. 152(5):134-136. <http://dx.doi.org/10.1136/vr.152.5.134> <PMid:12585599>
» https://doi.org/10.1136/vr.152.5.134 - Valette L. & Petermann H.G. 1988. Clostridium tetani, p.667-688. In: Blobel H. & Schliesser T. (Eds), Handbuch der bakteriellen Infektionen bei Tieren. Band II. Gustav Verlag Fischer, Sttutgart.
- Williams B.M. 1977. Clostridial myositis in cattle: bacteriology and gross pathology. Vet. Rec. 100(5):90-91. <http://dx.doi.org/10.1136/vr.100.5.90> <PMid:190758>
» https://doi.org/10.1136/vr.100.5.90
-
This work is part of the requirements for obtaining the doctoral degree by the first author.
Publication Dates
-
Publication in this collection
30 Sept 2019 -
Date of issue
July 2019
History
-
Received
15 Mar 2019 -
Accepted
22 Mar 2019