ABSTRACT:
Eleven cases of renal cystadenoma/cystadenocarcinoma-nodular dermatofibrosis syndrome (RCND) are described in German Shepherd dogs diagnosed from January 1994 to January 2018 at the Veterinary Pathology Laboratory of the “Universidade Federal de Santa Maria” (LPV-UFSM). The study sample was composed of eight male and three female dogs at a ratio of 2.67:1. Age ranged from six to 12 years (mean=8.7 years). The main clinical signs reported in descending order of frequency were multiple cutaneous nodules (nodular dermatofibrosis), dyspnea, anorexia, weight loss, recurrent hematuria, vomiting, and polydipsia. Results demonstrated that it is not always easy to clinically recognize this syndrome, but its peculiar anatomical-pathological characteristics allow safe diagnosis. Histologically, it was possible to detect all phases (cysts, papillary intratubular hyperplasia, and cystadenomas or cystadenocarcinomas) of a possible pathological continuum of the renal lesions. Uterine leiomyomas were observed in only one of the cases. Through histochemical techniques, it was possible to identify the presence of type I collagen in both cutaneous and renal lesions and consider its possible involvement in the pathogenesis of renal cystadenocarcinoma. Immunohistochemistry (IHC) showed partially satisfactory results in the staining of epithelial cells of renal cysts and neoplasms for pan-cytokeratin.
INDEX TERMS:
Clinics; epidemiology; anatomic-pathology; histochemistry; immunohistochemistry; renal cystadenocarcinoma; nodular dermatofibrosis; syndrome; German Shepherd dogs; dog diseases; pathology
RESUMO:
São descritos 11 casos da síndrome cistadenoma/cistadenocarcinoma-dermatofibrose nodular (CR-DN) em cães Pastor Alemão, diagnosticados entre janeiro de 1994 e janeiro de 2018 no Laboratório de Patologia Veterinária da Universidade Federal de Santa Maria (LPV-UFSM). Os cães afetados foram oito machos e três fêmeas, estabelecendo-se uma relação de 2,67:1. A idade variou de seis a 12 anos, sendo a média de idade de 8,7 anos. Os principais sinais clínicos relatados foram, em ordem decrescente de frequência, múltiplos nódulos cutâneos (dermatofibrose nodular), dispneia, anorexia, emagrecimento, hematúria recorrente, vômito e polidipsia. Este estudo permitiu estabelecer que o reconhecimento clínico da síndrome nem sempre é fácil, porém suas características anátomo-patológicas peculiares permitem um diagnóstico com segurança. Histologicamente, foi possível detectar todas as fases (cistos, hiperplasia intratubular papilífera, cistadenomas ou cistadenocarcinomas) de um possível continuum patológico das lesões renais. Leiomiomas uterinos foram observados somente em um caso. Através das técnicas histoquímicas foi possível estabelecer que o colágeno tipo I está presente em ambas as lesões, cutâneas e renais, e cogitar seu possível envolvimento na patogênese dos cistadenocarcinomas renais. A técnica de IHQ mostrou resultados parcialmente satisfatórios na imunomarcação das células epiteliais dos cistos e dos neoplasmas renais para pancitoceratina.
TERMOS DE INDEXAÇÃO:
Clínica; epidemiologia; anátomo-patologia; histoquímica; imuno-histoquímica; síndrome; cistadenocarcinoma; dermatofibrose nodular; Pastor Alemão; doenças de cães; patologia
Introduction
Renal cystadenocarcinoma and nodular dermatofibrosis (RCND) is a rare syndrome characterized by bilateral and multifocal renal cystadenoma/cystadenocarcinoma, nodular dermatofibrosis, and uterine leiomyoma that has been reported mainly in German Shepherd dogs (Lium & Moe 1985Lium B. & Moe L. 1985. Hereditary multifocal renal cystadenocarcinomas and nodular dermatofibrosis in the German Shepherd dog: macroscopic and histopathologic changes. Vet. Pathol. 22(5):447-455. <http://dx.doi.org/10.1177/030098588502200503> <PMid:4049673>
https://doi.org/10.1177/0300985885022005...
, Moe & Lium 1997Moe L. & Lium B. 1997. Hereditary multifocal renal cystadenocarcinomas and nodular dermatofibrosis in 51 German Shepherd dogs. J. Small Anim. Pract. 38(11):498-505. <http://dx.doi.org/10.1111/j.1748-5827.1997.tb03306.x> <PMid:9403809>
https://doi.org/10.1111/j.1748-5827.1997...
). This syndrome is caused by mutations in the folliculin gene (FLCN, previously BHD) gene, which is located in chromosome 5 and is a dominantly inherited disease (Lingaas et al. 2003Lingaas F., Comstock K.E., Kirkness E.F., Sorensen A., Aarskaug T., Hitte C., Nickerson M.L., Moe L., Schmidt L.S., Thomas R., Breen M., Galibert F., Zbar B. & Ostrander E.A. 2003. A mutation in the canine BHD gene is associated with hereditary multifocal renal cystadenocarcinoma and nodular dermatofibrosis in the German Shepherd Dog. Hum. Mol. Genet. 12(23):3043-3053. <http://dx.doi.org/10.1093/hmg/ddg336> <PMid:14532326>
https://doi.org/10.1093/hmg/ddg336...
, Bonsdorff et al. 2009Bønsdorff T.B., Jansen J.H., Thomassen R.F. & Lingaas F. 2009. Loss of heterozygosity at the FLCN locus in early renal cystic lesions in dogs with renal cystadenocarcinoma and nodular dermatofibrosis. Mamm. Genome 20(5):315-320. <http://dx.doi.org/10.1007/s00335-009-9183-8> <PMid:19387735>
https://doi.org/10.1007/s00335-009-9183-...
, Pressler et al. 2009Pressler B.M., Williams L.E., Ramos-Vara J.A. & Anderson K. 2009. Sequencing of the Von Hippel-Lindau gene in canine renal carcinoma. J. Vet. Intern. Med. 23(3):592-597. <http://dx.doi.org/10.1111/j.1939-1676.2009.0310.x> <PMid:19422471>
https://doi.org/10.1111/j.1939-1676.2009...
). Inactivation of this tumor suppressor gene is one of the critical steps in this disease (Bonsdorff et al. 2009Bønsdorff T.B., Jansen J.H., Thomassen R.F. & Lingaas F. 2009. Loss of heterozygosity at the FLCN locus in early renal cystic lesions in dogs with renal cystadenocarcinoma and nodular dermatofibrosis. Mamm. Genome 20(5):315-320. <http://dx.doi.org/10.1007/s00335-009-9183-8> <PMid:19387735>
https://doi.org/10.1007/s00335-009-9183-...
). This syndrome has also been sporadically reported in Boxer, crossbred (White et al. 1998White S.D., Rosychuk R.A.W., Schultheiss P. & Scott K.V. 1998. Nodular dermatofibrosis and cystic renal disease in three mixed breed dogs and a Boxer dog. Vet. Dermatol. 9(2):119-126. <http://dx.doi.org/10.1046/j.1365-3164.1998.00100.x>
https://doi.org/10.1046/j.1365-3164.1998...
), and Golden Retriever dogs (Marks et al. 1993Marks S.L., Farman C.A. & Peaston A. 1993. Nodular dermatofibrosis and renal cystadenomas in a Golden Retriever. Vet. Dermatol. 4(3):133-137. <http://dx.doi.org/10.1111/j.1365-3164.1993.tb00206.x>
https://doi.org/10.1111/j.1365-3164.1993...
).
Clinical signs vary greatly among dogs depending on age and stage of the disease at examination (Moe & Lium 1997Moe L. & Lium B. 1997. Hereditary multifocal renal cystadenocarcinomas and nodular dermatofibrosis in 51 German Shepherd dogs. J. Small Anim. Pract. 38(11):498-505. <http://dx.doi.org/10.1111/j.1748-5827.1997.tb03306.x> <PMid:9403809>
https://doi.org/10.1111/j.1748-5827.1997...
). They usually include numerous firm cutaneous and subcutaneous nodules, abdominal distension, greatly enlarged kidneys (on palpation), anorexia, fatigue, progressive weight loss, polydipsia, vomiting, and constipation or diarrhea (Lium & Moe 1985Lium B. & Moe L. 1985. Hereditary multifocal renal cystadenocarcinomas and nodular dermatofibrosis in the German Shepherd dog: macroscopic and histopathologic changes. Vet. Pathol. 22(5):447-455. <http://dx.doi.org/10.1177/030098588502200503> <PMid:4049673>
https://doi.org/10.1177/0300985885022005...
).
Gross lesions observed at necropsy are multiple firm and spherical skin and subcutaneous nodules. They may be distributed throughout the body, but with marked preference for limbs, head and back (Suter et al. 1983Suter M., Lott-Stolz G. & Wild P. 1983. Generalized nodular dermatofibrosis in six Alsatians. Vet. Pathol. 20(5):632-634. <http://dx.doi.org/10.1177/030098588302000515> <PMid:6636470>
https://doi.org/10.1177/0300985883020005...
, Lium & Moe 1985Lium B. & Moe L. 1985. Hereditary multifocal renal cystadenocarcinomas and nodular dermatofibrosis in the German Shepherd dog: macroscopic and histopathologic changes. Vet. Pathol. 22(5):447-455. <http://dx.doi.org/10.1177/030098588502200503> <PMid:4049673>
https://doi.org/10.1177/0300985885022005...
, Moe & Lium 1997Moe L. & Lium B. 1997. Hereditary multifocal renal cystadenocarcinomas and nodular dermatofibrosis in 51 German Shepherd dogs. J. Small Anim. Pract. 38(11):498-505. <http://dx.doi.org/10.1111/j.1748-5827.1997.tb03306.x> <PMid:9403809>
https://doi.org/10.1111/j.1748-5827.1997...
). In the kidneys, lesions are bilateral, multiple and cystic, containing a gelatinous, limpid or reddish brown fluid, often with areas of necrosis. The cysts can rupture and release their contents into the peritoneal cavity. (Lium & Moe 1985Lium B. & Moe L. 1985. Hereditary multifocal renal cystadenocarcinomas and nodular dermatofibrosis in the German Shepherd dog: macroscopic and histopathologic changes. Vet. Pathol. 22(5):447-455. <http://dx.doi.org/10.1177/030098588502200503> <PMid:4049673>
https://doi.org/10.1177/0300985885022005...
, Meuten & Meuten 2017Meuten J.D. & Meuten T.L.K. 2017. Tumors of the urinary system, p.632-688. In: Meuten D.J. (Ed), Tumors in Domestic Animals. 5th ed. Wiley-Blackwell, Ames.). Affected females also tend to present several uterine leiomyomas (Cianciolo & Mohr 2016Cianciolo R.E. & Mohr F.C. 2016. Urinary system, p.376-464. In: Maxie M.G., Jubb K.V.F., Kennedy P.C. & Palmer N.C. (Ed), Pathology of Domestic Animals. Vol.1. 6th ed. Elsevier, St Louis .). Histologically, the cutaneous lesion is denominated nodular dermatofibrosis and, cystadenoma or cystadenocarcinoma can be observed in the kidneys (Lium & Moe 1985Lium B. & Moe L. 1985. Hereditary multifocal renal cystadenocarcinomas and nodular dermatofibrosis in the German Shepherd dog: macroscopic and histopathologic changes. Vet. Pathol. 22(5):447-455. <http://dx.doi.org/10.1177/030098588502200503> <PMid:4049673>
https://doi.org/10.1177/0300985885022005...
).
In general, most textbooks of veterinary medicine (Cianciolo & Mohr 2016Cianciolo R.E. & Mohr F.C. 2016. Urinary system, p.376-464. In: Maxie M.G., Jubb K.V.F., Kennedy P.C. & Palmer N.C. (Ed), Pathology of Domestic Animals. Vol.1. 6th ed. Elsevier, St Louis ., Breshears & Confer 2017Breshears M.A. & Confer A.W. 2017. O Sistema urinário, p.617-681. In: Zachary J.F. (Ed), Pathology Basis of Veterinary Disease. 6th ed. Elsevier, St Louis. <http://dx.doi.org/10.1016/B978-0-323-35775-3.00011-4>.
https://doi.org/10.1016/B978-0-323-35775...
, Serakides & Silva 2017Serakides R. & Silva J.F. 2017. Sistema urinário, p.267-309. In: Santos R.L. & Alessi A.C. (Ed.), Patologia Veterinária. 2ª ed. Guanabara Koogan, Rio de Janeiro.) contain little, generic information on RCND, and this syndrome is mostly described in the international literature, with little information on its prevalence and presentation characteristics found in the national literature (Langohr et al. 2002Langohr I.M., Irigoyen L.F., Salles M.W.S., Kommers G.D. & Barros C.S.L. 2002. Cistadenocarcinoma renal e dermatofibrose nodular em cães Pastor Alemão: 4 casos. Ciência Rural 32(4):621-626. <http://dx.doi.org/10.1590/S0103-84782002000400012>
https://doi.org/10.1590/S0103-8478200200...
, Inkelmann et al. 2012Inkelmann M.A., Kommers G.D., Trost M.E., Barros C.S.L., Fighera R.A., Irigoyen L.F. & Silveira I.P. 2012. Lesões do Sistema urinário em 1.063 cães. Pesq. Vet. Bras. 32(8):761-771. <http://dx.doi.org/10.1590/S0100-736X2012000800015>
https://doi.org/S0100-736X2012000800015...
), which is important for the diagnosis and knowledge of the occurrence of this disease in the country.
The main objectives of this retrospective study were to determine the prevalence of RC-ND in the necropsies routine of the LPV-UFSM and to characterize the clinical-epidemiological, anatomical-pathological, histochemical and immunohistochemical aspects of this syndrome in German Shepherd dogs in the central region of Rio Grande do Sul state, Brazil.
Materials and Methods
Protocols of dog necropsies conducted from January 1994 to January 2018 at the Laboratory of Veterinary Pathology of the “Universidade Federal de Santa Maria” (LPV-UFSM) were reviewed in search of cases of cutaneous and renal lesions compatible with those described for renal cystadenoma/cystadenocarcinoma-nodular dermatofibrosis syndrome (RC-ND) in German Shepherd dogs. The total number of German Shepherd dogs necropsied during this period was also calculated. Cases 1 to 4 and 1 to 6 of RC-ND syndrome included in this survey were part of the studies by Langohr et al. (2002)Langohr I.M., Irigoyen L.F., Salles M.W.S., Kommers G.D. & Barros C.S.L. 2002. Cistadenocarcinoma renal e dermatofibrose nodular em cães Pastor Alemão: 4 casos. Ciência Rural 32(4):621-626. <http://dx.doi.org/10.1590/S0103-84782002000400012>
https://doi.org/10.1590/S0103-8478200200...
and Inkelmann et al. (2012)Inkelmann M.A., Kommers G.D., Trost M.E., Barros C.S.L., Fighera R.A., Irigoyen L.F. & Silveira I.P. 2012. Lesões do Sistema urinário em 1.063 cães. Pesq. Vet. Bras. 32(8):761-771. <http://dx.doi.org/10.1590/S0100-736X2012000800015>
https://doi.org/S0100-736X2012000800015...
respectively.
From these necropsy protocols, information on the gender and age of the dogs was obtained. As for age, dogs were divided into three categories, as previously reported (Fighera et al. 2008Fighera R.A., Souza T.M., Silva M.C., Brum J.S., Graça D.L., Kommers G.D., Irigoyen L.F. & Barros C.S.L. 2008. Causas de morte e razões para eutanásia de cães. Pesq. Vet. Bras. 28(4):223-230. <http://dx.doi.org/10.1590/S0100-736X2008000400005>
https://doi.org/10.1590/S0100-736X200800...
), namely, puppies (<1 year), adults (≥1 to ≤9 years), and elderly (>10 years).
Clinical signs, outcome (spontaneous death or euthanasia), macroscopic findings (pattern of dermatofibrosis lesions, including number, size, distribution and presence of ulceration in cutaneous nodules; size, location, distribution and characteristics of renal lesions and presence of metastases), and microscopic findings were also determined. Other related injuries, such as uterine and extra-renal lesions of uremia, were also recorded.
As for gross lesions, the data were computed from the descriptions present in the medical reports, complemented by observation of the archives case photographs. Regarding histopathological evaluation, the aspects described in the necropsy reports were considered and, depending on the availability of paraffin-embedded tissues on the LPV-UFSM archives, new histological sections (stained with hematoxylin-eosin, HE) were evaluated.
Renal and cutaneous lesions, depending on the availability of paraffin-embedded tissues, were evaluated using the histochemical techniques of Masson’s Trichrome (MT, Masson’s Trichrome Histokit, EasyPath, EP-11-20013) and Picro-Sirius Red (PR, Picro-Sirius-Hematoxylin Histokit, EasyPath, EP-11-20011) for better collagen evidencing and typification, respectively. Using TM, the collagen areas stain in blue, whereas using PR under polarized light, type 1 collagen is observed as dense, yellow, orange or red fibers and type 3 collagen as fine, green fibers (Whittaker et al. 1994Whittaker P., Kloner R.A., Boughner D.R. & Pickering J.G. 1994. Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized light. Basic Res. Cardiol. 89(5):397-410. <http://dx.doi.org/10.1007/BF00788278> <PMid:7535519>
https://doi.org/10.1007/BF00788278...
).
Renal lesions were also assessed by immunohistochemistry (IHC) using bovine polyclonal anti-pancytokeratin antibody (Dako Cytomation, code Z-0622) produced in rabbit (1:2,000). Silanized slides with 3μm histological sections were used. After dewaxing and rehydration of the tissues, antigenic recovery with TRIS-EDTA at pH 9.0 was performed, followed by endogenous peroxidase blocking with 3% hydrogen peroxide. Blocking of non-specific reactions was performed with protein blocker (EasyPath kit, EP-12-20504). Incubation with the primary antibody occurred in oven for 60 min at 37°C in a humid chamber. Easy Link One polymer (EasyPath kit, EP-12-20504) was used as secondary antibody, incubated in oven at 25°C. 3-3’-diaminobenzidine tetrachloride (DAB; EasyPath kit, EP-12-20504) was used as substrate-chromogen. Sections were counterstained with Harris hematoxylin, dehydrated, and mounted using synthetic mounting medium (Entellan, Merck) and coverslips. Bovine alimentary tract squamous cell carcinoma was used as positive control. The same sections to be tested, with replacement of primary antibody with antibody diluent, were used as negative control.
Results
Epidemiological and clinical aspects
A total of 6,231 dogs (2,958 males and 3,271 females) were necropsied during the study period. Of these, 379 were German Shepherd - 208 males (54.89%) and 171 females (45.11%). Of these, 11 (2.9%) German Shepherd dogs were diagnosed with RCND compatible lesions. The cases were chronologically distributed: two cases in 1994, 1996, and 2012; one case in 2005, 2010, 2011, 2015, and 2018. Epidemiological and clinical aspects are detailed in Table 1. Affected dogs were eight males (80%) and three females (30%), establishing a ratio of 2.67:1. Of the three bitches, two were unspayed. The age of the affected dogs ranged from 6 to 12 years. In one case, the age was not reported. Of the 10 dogs whose age was known, 7 (70%) were classified as adults and three (30%) as elderly. Overall, mean age was 8.7 years and median was 8.5 years.
The main clinical signs described in the medical reports in descending order of frequency were multiple cutaneous nodules (4/11, 36.36%), dyspnea (3/11, 27.27%), anorexia (2/11, 18.18%), progressive weight loss (2/11, 18.18%), recurrent hematuria (2/11, 18.18%), and vomiting (2/11, 18.18%). Seven dogs (7/11, 63.63%) died spontaneously and four (4/11, 36.36%) were euthanized.
Macroscopic changes
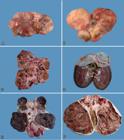
Necropsy findings usually showed enlarged kidneys and with irregular contour due to presence of firm or floating cysts and/or neoplastic nodular tissue that protruded on the capsular surface (Fig.1A,B). Table 2 shows these aspects in detail for each dog. The kidneys of the eleven dogs were bilaterally affected. In 10 cases (Dogs 1-9 and 11), cysts and neoplasms were observed in both kidneys. In one case (Dog 10), the left kidney had cysts and neoplasm, whereas the right kidney showed only small cysts.
Renal cystadenocarcinoma. (A) Kidney of Dog 9 is increased in size, with parenchyma distorted by small subcapsular cystic masses. (B) Kidney of Dog 9 increased markedly in size with parenchyma distorted by subcapsular cystic masses of various sizes. The cysts show thick walls. (C) Dog 9. At cut, multiple cysts containing neoplastic and sometimes necrotic formations, are observed. There is little renal parenchyma remaining. (D) Dog 10. At cut, there is a cyst of approximately 3 cm in diameter located in the corticomedullary region of the cranial pole with fibrous wall and adjacent smaller cystic formations. (E) Dog 8. At cut, the kidney shows multiple cystic formations with dark-red, porous and extremely friable content, and some firm, whitish solid portions. (F) Dog 8. At cut, the kidney shows multiple cystic formations with brownish, porous and extremely friable content, and some firm, whitish solid portions. A thick, white, fibrous capsule is observed.
Cut surface of the kidneys revealed cysts of a few millimeters in diameter (usually filled with clear fluid) (Fig.1C) to large cystic cavities (up to 15cm in diameter and containing clear, reddish brown and dark brown liquids, or clots) (Fig.1D), which replaced much of the renal parenchyma, affecting the cortical (mainly), medullary and pelvic (less often) regions. In most cases, the cystic cavities were also filled by scarce or abundant, dark red or dark brown, porous, extremely friable (necrotic aspect) neoplastic tissue (Fig.1E,F). Rarely (Dogs 4, 8, and 11), solid, whitish neoplastic masses were also observed. Cystic cavity capsules were generally fibrous and firm (Fig.1F). In three cases (Dogs 4, 7, and 8), there was rupture of cysts, with extravasation of contents and/or hemorrhage into the abdominal cavity (hemoperitoneum). Peritonitis was also described in Dog 8.
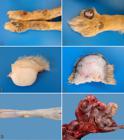
Skin lesions were observed at necropsy in 11 dogs. The gross characteristics of the cutaneous lesions in each case are detailed in Table 3. In nine cases, the lesions were multiple and numerous, affecting more than a single body area. A few nodules were found in one case (Dog 10) and a single nodule was observed in only one situation (Dog 1). Nodular lesions were distributed in descending order of frequency as follows: hind limbs (9/11, 81.81%) (Fig.2A), forelimbs (8/11, 72.72%), head (3/11, 27.27%) (Fig.2B), and trunk (2/11, 18.18%).
(A) Nodular dermatofibrosis in Dog 9. Skin, on the cranial face of the pelvic limbs there are multiple nodules, very close to each other, sometimes coalescing and forming larger masses, occasionally ulcerated. (B) Nodular dermatofibrosis in Dog 9. Multiple nodules can be observed in the cranial face of the anterior limb, with the largest nodule showing areas with ulceration and depigmentation. (C) Nodular dermatofibrosis in Dog 11. Skin, at cut, a white, firm nodule that extends from the superficial dermis to the deep dermis can be observed. (D) Nodular dermatofibrosis in Dog 9. Skin, at cut, the surface shows a firm, whitish nodule that is strictly located in the subcutaneous tissue. (E) Uterine leiomyoma in Dog 8. Multiple, smooth, whitish nodules can be observed in the serosa layer of the uterus. (F) Intestine of Dog 10. There is a mass consisting of pleated intestine, surrounded by a fibrous capsule, which corresponds to the omentum with metastasis of cystadenocarcinoma.
These lesions were observed as papules (<1cm) or nodules (>1cm). In several cases, nodular lesions were very close to each other, sometimes coalescing and forming larger masses/plaques. The lesions were alopecic or not, sometimes ulcerated (four cases). The epidermal surface was blackened in several cases. At cut, it was observed that the nodular lesions were located in the dermis (Fig.2C) or in the subcutaneous tissue (Fig.2D), and were firm (fibrous) and white in most cases. Occasionally, soft lesions were described.
In Dog 8, there were two protruded nodules in the serosa layer of the uterus (Fig.2E), 0.3 and 0.6 cm in diameter. They were smooth and yellowish. At cut, they were not very firm and homogeneous. Additional findings associated with RCND were firm-elastic, whitish, neoplastic nodules (interpreted as metastatic) in the liver and spleen (Dog 4). In Dog 10, near the stomach (pylorus), there was a fibrous mass in the omentum, which had been transformed into a fibrous capsule that enveloped the entire intestine, causing its pleating and shrinking of the intestinal loops (Fig.2F). Small nodules were also observed in the costal pleura (and pleural effusion). The lungs showed retracted areas in the pleura and the liver had rounded edges (as a result of sharp retraction of the capsule). Extra-renal lesions of uremia were described in Dog 1 (pneumopathy) and Dog 2 (gastropathy, pneumopathy, and parathyroid hyperplasia). In the urinary bladder, urine color was described as chocolate-red (Dog 1) or dark red (Dogs 8 and 9).
Microscopic changes
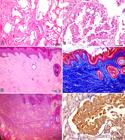
Renal histological changes were multifocal, affecting the cortical and/or medullary regions, and comprised discrete epithelial cysts (Fig.3A), areas of papillary intratubular hyperplasia (Fig.3A), cystadenomas and/or cystadenocarcinomas (Fig.3B). The presence of these lesions in each case is detailed in Table 4. In the entire study sample, there were multiple small cysts composed of a thin wall and internally lined with a layer of epithelial cells, similar to the normal tubular epithelium, but generally elongated and flat (interpreted as caused by fluid - not stained - distension of the wall). Six cases presented some cystic formations internally lined with a high cuboidal epithelium, at times with two to three layers of cells, or forming discrete papillary projections (papillary intratubular hyperplasia). In three cases, in the wall of some cystic structures, moderate to marked papillary proliferation of well-differentiated neoplastic epithelial cells was observed, with abundant and eosinophilic cytoplasm. These cells showed single, central nuclei with a single nucleolus (cystadenomas). In general, cystadenocarcinomas were characterized as being part of a larger cystic structure that showed a thick, fibrous wall and was internally lined with neoplastic cuboidal or polyhedral epithelial cells arranged in an irregular papillary pattern. These cells presented abundant cytoplasm and round or oval, vesiculous nuclei with dispersed chromatin. Some cells were binucleated and others had a large cytoplasmic vacuole pushing the nucleus to the periphery (signet-ring cell morphology) (Fig.3B). Mitotic figures were rare. Occasionally, cystic structures filled with neoplastic cells, similar to those described for the papillary areas but arranged in a more solid pattern, were observed. The following features were frequently observed in the cystadenocarcinomas: areas of intense peritumoral (contiguous with the wall of the cystic cavities) and intratumoral (often as connective septa) fibrosis, marked multifocal necrosis sometimes containing cholesterol clefts, areas of focally extensive hemorrhage, mild multifocal mineralization, macrophage infiltrate containing golden-brown granular pigment (hemosiderin) and irregular deposits of golden-yellow crystalloid material (hematoidin).
(A) Kidney. Focally extensive area with formation of cysts characterized by wall with a single layer of cells. There are two areas with onset of hyperplasia within the cysts. HE, bar=50μm. (B) Kidney. Proliferation of neoplastic epithelial cells forming papillary projections. There are binucleated neoplastic cells and a signet-ring cell (vacuolated cytoplasm), characteristic of renal cystadenocarcinoma. HE, bar=20μm. (C) Skin. Abundant presence of collagen fibers in the superficial and deep dermis (not shown in the microphotograph) and reduction of skin appendages. Irregular hyperplasia with moderate orthokeratotic hyperkeratosis is observed in the epidermis. HE, bar=100μm. (D) Skin. There is proliferation of collagen fibers in the superficial and deep dermis (not shown in the microphotograph) stained in blue by MT. Irregular hyperplasia of the epidermis is observed with moderate orthokeratotic hyperkeratosis and keratosis of the remaining hair follicles. MT, bar=100μm. (E) Skin. Same section of Figure 3 D, showing thick orange collagen fibers in the dermis, characteristic of type I collagen. PR, bar=100μm. (F) Kidney. Positive immunostaining for pancytokeratin of the epithelial cells of a cystadenocarcinoma. IHC HRP-polymer, bar=20μm.
Histological aspects of renal lesions in 11 German Shepherd dogs with renal cystadenoma/cystadenocarcinoma-nodular dermatofibrosis syndrome (RCND)
Metastases of the cystadenocarcinomas were observed in the liver and spleen (Dog 4) and in multiple organs and tissues in Dog 10. In the latter, the fibrous mass in the omentum and the fibrous capsule into which it was transformed (which involved the entire intestine) consisted of abundant fibrous connective tissue (interpreted as a desmoplastic cirrhous reaction), well vascularized, and interspersed with moderately pleomorphic, metastatic epithelial cells, including signet-ring cells. Metastases were also observed in the esophageal serosa, costal pleura, parietal pleura, and liver capsule.
Cutaneous nodules were composed of accumulation of mature collagen, generally arranged in thick fibers and interspersed with a few fibrocytes (Fig.3C). In the dermal nodules, this collagen redundancy was observed in the skin adnexa, which were morphologically normal, or in the presence of dilated hair follicles with keratosis. In the dermis, the limits of these nodules were barely perceptible among the normal adjacent collagen. However, in the subcutaneous tissue, the collagenous nodules were well delimited among the adipose tissue. The cutaneous and subcutaneous nodules were interpreted as nodular dermatofibrosis. The epidermis was acanthotic and with orthokeratotic hyperkeratosis or ulcerated in some nodules. Mononuclear inflammatory infiltrate was occasionally observed.
In the uterus wall of Dog 8, there were well-delimited and non-encapsulated areas of neoplastic proliferation of spindle cells, which were organized in dense bundles; they were well differentiated and similar to smooth muscle cells. The cytoplasm was elongated, abundant, and eosinophilic. The nucleus was elongated and composed of moderately aggregated chromatin. The nucleoli were unique and barely perceptible. The neoplasms were interpreted as leiomyomas.
Histochemistry and immunohistochemistry (IHC)
MT staining provided clearer identification of areas with greater deposition of collagen fibers, mainly those surrounding and interspersing renal cystadenocarcinomas (Dogs 1, 4, 6-8, 10, 11), and those with mild fibrosis surrounding mainly cystadenomas (Dogs 3 and 5). Sequential sections of these cases were PR stained and observed under polarized light. It was observed that areas with fibrosis, regardless of the degree of severity, were composed of type 1 collagen. Likewise, in the areas of nodular dermatofibrosis, collagen fibers were intensely stained in blue under MT staining (Fig.3D) and type 1 collagen was identified under PR staining (Fig.3E).
IHC using the anti-pancytokeratin antibody applied on sections of the kidney showed irregular or absent immunostaining of the epithelial cells of the cysts and neoplasms. The most intensely immunostained cells were observed in areas with cystadenocarcinoma in a more solid pattern in Dog 10 (Fig.3F), as well as in metastasis in the omentum in this case.
Discussion
The age of the 11 affected dogs and their mean age were very similar to those observed in another study conducted with 43 German Shepherd dogs, which is considered as the first detailed pathological description of this hereditary syndrome (Lium & Moe 1985Lium B. & Moe L. 1985. Hereditary multifocal renal cystadenocarcinomas and nodular dermatofibrosis in the German Shepherd dog: macroscopic and histopathologic changes. Vet. Pathol. 22(5):447-455. <http://dx.doi.org/10.1177/030098588502200503> <PMid:4049673>
https://doi.org/10.1177/0300985885022005...
). A male:female ratio of 2.67:1 was established for the 11 dogs of this study. Most reports suggest a predominance of males, in a ratio of 2:1 (Meuten & Meuten 2017Meuten J.D. & Meuten T.L.K. 2017. Tumors of the urinary system, p.632-688. In: Meuten D.J. (Ed), Tumors in Domestic Animals. 5th ed. Wiley-Blackwell, Ames.). It is worth mentioning that of the 379 German Shepherd dogs necropsied during the study period, 54.89% were male and 45.11% were female.
Clinical recognition of RCND syndrome is not always easy. Based on clinical reports, only two cases had both the observation of urinary tract disease and cutaneous lesions compatible with the syndrome (Lium & Moe 1985Lium B. & Moe L. 1985. Hereditary multifocal renal cystadenocarcinomas and nodular dermatofibrosis in the German Shepherd dog: macroscopic and histopathologic changes. Vet. Pathol. 22(5):447-455. <http://dx.doi.org/10.1177/030098588502200503> <PMid:4049673>
https://doi.org/10.1177/0300985885022005...
). Six cases had signs indicative of urinary tract disease alone (or its consequences) and two dogs showed cutaneous nodules (perhaps renal disease had not yet manifested in these cases). Nonspecific clinical signs, confused with other diseases, may be factors that make clinical suspicion of the RCND syndrome difficult, and the diagnosis is often established only at necropsy and histopathological examination.
Eight dogs had some signs indicative of urinary tract disease (hematuria, urinary retention, azotemia and/or masses in the renal region), but others showed only secondary changes to chronic kidney disease (progressive weight loss, dyspnea, anorexia, vomiting, polydipsia or anemia). Similar clinical signs were observed in a study conducted with 51 dogs with this syndrome, where polydipsia and hematuria (macroscopic) were described in 25% of the cases. Severe clinical signs, including depression, fever and anorexia, were observed in 22% of the dogs. Two-thirds of these animals had peritonitis (sterile) caused by rupture of renal cysts. Pain and dyspnea were due to peritonitis (resulting from cyst rupture) and/or metastases (Moe & Lium 1997Moe L. & Lium B. 1997. Hereditary multifocal renal cystadenocarcinomas and nodular dermatofibrosis in 51 German Shepherd dogs. J. Small Anim. Pract. 38(11):498-505. <http://dx.doi.org/10.1111/j.1748-5827.1997.tb03306.x> <PMid:9403809>
https://doi.org/10.1111/j.1748-5827.1997...
).
In only four cases there was description of cutaneous nodules in clinical reports, and in Dog 3, recurrent cutaneous nodules were the reason for the consultation, although they were detected at the necropsy of the 11 dogs studied. Skin lesions were the main reason for owners to take their dog to the clinic in 37% of the cases in a study conducted with 51 dogs with RCND syndrome (Moe & Lium 1997Moe L. & Lium B. 1997. Hereditary multifocal renal cystadenocarcinomas and nodular dermatofibrosis in 51 German Shepherd dogs. J. Small Anim. Pract. 38(11):498-505. <http://dx.doi.org/10.1111/j.1748-5827.1997.tb03306.x> <PMid:9403809>
https://doi.org/10.1111/j.1748-5827.1997...
). In the present study, it can be inferred from the description of necropsy that, in some dogs, perhaps because they are small or multiple, but very small in the middle of the long coat, the nodules were not detected or clinically mentioned. Skin lesions were more frequent in the limbs, head and trunk, in descending order of frequency, in this survey. In another study (51 dogs), the most frequent sites of the lesions were limbs and head, and the authors emphasized that the nodules were often difficult to observe (Moe & Lium 1997Moe L. & Lium B. 1997. Hereditary multifocal renal cystadenocarcinomas and nodular dermatofibrosis in 51 German Shepherd dogs. J. Small Anim. Pract. 38(11):498-505. <http://dx.doi.org/10.1111/j.1748-5827.1997.tb03306.x> <PMid:9403809>
https://doi.org/10.1111/j.1748-5827.1997...
).
Regarding renal changes at necropsy, the main characteristics were bilateral lesions, presence of multiple cysts of different sizes, and of neoplastic masses contained in cystic cavities, usually necrotic, destructive, and with hemorrhage or, occasionally, as solid masses. Neoplastic cysts and masses often increased kidney size and completely altered its shape. The same characteristics have been described in other studies on this syndrome (Lium & Moe 1985Lium B. & Moe L. 1985. Hereditary multifocal renal cystadenocarcinomas and nodular dermatofibrosis in the German Shepherd dog: macroscopic and histopathologic changes. Vet. Pathol. 22(5):447-455. <http://dx.doi.org/10.1177/030098588502200503> <PMid:4049673>
https://doi.org/10.1177/0300985885022005...
, Moe & Lium 1997Moe L. & Lium B. 1997. Hereditary multifocal renal cystadenocarcinomas and nodular dermatofibrosis in 51 German Shepherd dogs. J. Small Anim. Pract. 38(11):498-505. <http://dx.doi.org/10.1111/j.1748-5827.1997.tb03306.x> <PMid:9403809>
https://doi.org/10.1111/j.1748-5827.1997...
) and were considered as indicative of primary multicenter origin (Lium & Moe 1985Lium B. & Moe L. 1985. Hereditary multifocal renal cystadenocarcinomas and nodular dermatofibrosis in the German Shepherd dog: macroscopic and histopathologic changes. Vet. Pathol. 22(5):447-455. <http://dx.doi.org/10.1177/030098588502200503> <PMid:4049673>
https://doi.org/10.1177/0300985885022005...
). The neoplastic lesions observed in the RCND syndrome differ from the sporadic forms of renal carcinomas, which are usually unilateral and single, with occasional metastases to the contralateral kidney (Lucke & Kelly 1976Lucke V.M. & Kelly D.F. 1976. Renal carcinoma in the dog. Vet. Pathol. 13(4):264-276. <http://dx.doi.org/10.1177/030098587601300403> <PMid:969167>
https://doi.org/10.1177/0300985876013004...
, Baskin & De Paoli 1977Baskin G.B. & De Paoli A. 1977. Primary renal neoplasms of the dog. Vet. Pathol. 14(6):591-605. <http://dx.doi.org/10.1177/030098587701400606> <PMid:201076>
https://doi.org/10.1177/0300985877014006...
, Lium & Moe 1985Lium B. & Moe L. 1985. Hereditary multifocal renal cystadenocarcinomas and nodular dermatofibrosis in the German Shepherd dog: macroscopic and histopathologic changes. Vet. Pathol. 22(5):447-455. <http://dx.doi.org/10.1177/030098588502200503> <PMid:4049673>
https://doi.org/10.1177/0300985885022005...
, Meuten & Meuten 2017Meuten J.D. & Meuten T.L.K. 2017. Tumors of the urinary system, p.632-688. In: Meuten D.J. (Ed), Tumors in Domestic Animals. 5th ed. Wiley-Blackwell, Ames.).
The simultaneous presence of cysts and the cystic nature of the neoplasms of this syndrome are different from other renal tumors described in dogs (Meuten & Meuten 2017Meuten J.D. & Meuten T.L.K. 2017. Tumors of the urinary system, p.632-688. In: Meuten D.J. (Ed), Tumors in Domestic Animals. 5th ed. Wiley-Blackwell, Ames.). Renal cysts increase in size with age, and they also seem to present a tendency to malignant transformation of renal lesions with advanced age in dogs predisposed to this syndrome (Lium & Moe 1985Lium B. & Moe L. 1985. Hereditary multifocal renal cystadenocarcinomas and nodular dermatofibrosis in the German Shepherd dog: macroscopic and histopathologic changes. Vet. Pathol. 22(5):447-455. <http://dx.doi.org/10.1177/030098588502200503> <PMid:4049673>
https://doi.org/10.1177/0300985885022005...
). Cysts may be the initial lesion of this disease, and they seem to progress through stages of hyperplasia, adenomas, and adenocarcinomas (Meuten & Meuten 2017Meuten J.D. & Meuten T.L.K. 2017. Tumors of the urinary system, p.632-688. In: Meuten D.J. (Ed), Tumors in Domestic Animals. 5th ed. Wiley-Blackwell, Ames.). In several cases, the presence of small cysts and the intermediate stages (hyperplasia and/or cystadenoma) of this pathological continuum until cystadenocarcinoma were observed. In only two cases the most advanced lesion was described as cystadenoma, without morphological evidence of intratumoral malignancy. Progression of the pathological continuum (from cysts to cystadenocarcinoma) would be influenced by genetic factors, with cists of purebred German Shepherd from certain strains prone to malignant transformation more rapidly (White et al. 1998White S.D., Rosychuk R.A.W., Schultheiss P. & Scott K.V. 1998. Nodular dermatofibrosis and cystic renal disease in three mixed breed dogs and a Boxer dog. Vet. Dermatol. 9(2):119-126. <http://dx.doi.org/10.1046/j.1365-3164.1998.00100.x>
https://doi.org/10.1046/j.1365-3164.1998...
).
According to the histological classification of mesenchymal tumors of the skin and soft tissues in domestic animals of the World Health Organization (Hendrick et al. 1998Hendrick M.J., Mahaffey E.A., Moore F.M., Vos J.H. & Walder E.J. 1998. Histological Classification of Mesenchymal Tumors of Skin and Soft Tissues of Domestic Animals. Vol.2. 2nd ed. Armed Forces Institute of Pathology, Washington, p.1-64.), nodular dermatofibrosis has been considered as part of a rare syndrome, described mainly in German Shepherd dogs (but may occasionally affect other breeds), where multiple fibrous nodules are observed in the dermis and subcutaneous tissue. According to this classification, these lesions are histologically differentiated from collagenous hamartomas because they are not limited to the superficial dermis and have normal or hyperplastic cutaneous adnexa within the redundant collagen. They are also differentiated from fibromas, which are benign neoplasms of mature fibrocytes producing abundant collagen, in which the fibers are arranged in interlocking fascicles or, less frequently, in swirls. All dogs in this study had predominantly multiple cutaneous nodules in the limbs, as well as in the head or trunk. This distribution has also been observed in studies conducted with larger samples on German Shepherd dogs (Lium & Moe 1985Lium B. & Moe L. 1985. Hereditary multifocal renal cystadenocarcinomas and nodular dermatofibrosis in the German Shepherd dog: macroscopic and histopathologic changes. Vet. Pathol. 22(5):447-455. <http://dx.doi.org/10.1177/030098588502200503> <PMid:4049673>
https://doi.org/10.1177/0300985885022005...
, Moe & Lium 1997Moe L. & Lium B. 1997. Hereditary multifocal renal cystadenocarcinomas and nodular dermatofibrosis in 51 German Shepherd dogs. J. Small Anim. Pract. 38(11):498-505. <http://dx.doi.org/10.1111/j.1748-5827.1997.tb03306.x> <PMid:9403809>
https://doi.org/10.1111/j.1748-5827.1997...
) and with dogs of other breeds (Marks et al. 1993Marks S.L., Farman C.A. & Peaston A. 1993. Nodular dermatofibrosis and renal cystadenomas in a Golden Retriever. Vet. Dermatol. 4(3):133-137. <http://dx.doi.org/10.1111/j.1365-3164.1993.tb00206.x>
https://doi.org/10.1111/j.1365-3164.1993...
, White et al. 1998White S.D., Rosychuk R.A.W., Schultheiss P. & Scott K.V. 1998. Nodular dermatofibrosis and cystic renal disease in three mixed breed dogs and a Boxer dog. Vet. Dermatol. 9(2):119-126. <http://dx.doi.org/10.1046/j.1365-3164.1998.00100.x>
https://doi.org/10.1046/j.1365-3164.1998...
, Meuten & Meuten 2017Meuten J.D. & Meuten T.L.K. 2017. Tumors of the urinary system, p.632-688. In: Meuten D.J. (Ed), Tumors in Domestic Animals. 5th ed. Wiley-Blackwell, Ames., Serakides & Silva 2017Serakides R. & Silva J.F. 2017. Sistema urinário, p.267-309. In: Santos R.L. & Alessi A.C. (Ed.), Patologia Veterinária. 2ª ed. Guanabara Koogan, Rio de Janeiro.).
Collagenous lesion in the skin and desmoplastic fibrous reaction in renal neoplasms are noteworthy in RCND. In the present study, it was possible to observe that, in both lesions, collagen was dense and showed characteristics indicative of type I staining. This type of collagen is considered mature (Junqueira et al. 1979Junqueira L.C.U., Bignolas G. & Brentani R.R. 1979. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 11(4):447-455. <http://dx.doi.org/10.1007/BF01002772> <PMid:91593>
https://doi.org/10.1007/BF01002772...
), and its presence in tumoral desmoplastic tumoral reactions has been studied in other types of carcinomas, including esophageal squamous cell carcinomas (SCC) in cattle (Faccin et al. 2017Faccin T.C., Masuda E.K., Piazer J.V.M., Melo S.M.P. & Kommers G.D. 2017. Annular stenotic oesopageal squamous cell carcinoma in cattle exposed naturally to bracken fern (Pteridium arachnoideum). J. Comp. Pathol. 157(2/3):174-180. <http://dx.doi.org/10.1016/j.jcpa.2017.07.008> <PMid:28942300>
https://doi.org/10.1016/j.jcpa.2017.07.0...
) and pancreatic ductal adenocarcinomas in humans (Armstrong et al. 2004Armstrong T., Packham G., Murphy L.B., Bateman A.C., Conti J.A., Fine D.R., Johnson C.D., Benyon C. & Iredale J.P. 2004. Type I collagen promotes the malignant phenotype of pancreatic ductal adenocarcinoma. Clin. Cancer Res. 10(21):7427-7437. <http://dx.doi.org/10.1158/1078-0432.CCR-03-0825> <PMid:15534120>
https://doi.org/10.1158/1078-0432.CCR-03...
, Shields et al. 2011Shields M.A., Dangi-Garimella S., Krantz S.B., Bentrem D.J. & Munshi H.G. 2011. Pancreatic cancer cells respond to type I collagen by inducing snail expression to promote membrane type 1 matrix metalloproteinase-dependent collagen invasion. J. Biol. Chem. 286(12):10495-10504.). The role attributed to collagen in the desmoplastic reaction has been somewhat contradictory, ranging from acting as a barrier to tumor invasion to an association of its greater expression with a worse prognosis and increased metastatization in human neoplasias (Shields et al. 2011Shields M.A., Dangi-Garimella S., Krantz S.B., Bentrem D.J. & Munshi H.G. 2011. Pancreatic cancer cells respond to type I collagen by inducing snail expression to promote membrane type 1 matrix metalloproteinase-dependent collagen invasion. J. Biol. Chem. 286(12):10495-10504.). Type I collagen has been associated with proliferation of neoplastic cells in human pancreatic cancer, and was also observed that desmoplastic reaction, through collagen, promoted a malignant phenotype of the neoplastic cells, resulting in a worse prognosis for the host (Armstrong et al. 2004Armstrong T., Packham G., Murphy L.B., Bateman A.C., Conti J.A., Fine D.R., Johnson C.D., Benyon C. & Iredale J.P. 2004. Type I collagen promotes the malignant phenotype of pancreatic ductal adenocarcinoma. Clin. Cancer Res. 10(21):7427-7437. <http://dx.doi.org/10.1158/1078-0432.CCR-03-0825> <PMid:15534120>
https://doi.org/10.1158/1078-0432.CCR-03...
). The observation of markedly collagenous desmoplastic reaction (caused by type I collagen) in renal cystadenocarcinomas contrasted with the mild fibrous reaction in the cystadenomas of this study, suggesting the need for further studies addressing the existence or not of a prognostic role of this intratumoral reaction in RCND.
Several theories for the pathogenesis of renal and cutaneous lesions in RCND syndrome have been postulated. White et al. (1998)White S.D., Rosychuk R.A.W., Schultheiss P. & Scott K.V. 1998. Nodular dermatofibrosis and cystic renal disease in three mixed breed dogs and a Boxer dog. Vet. Dermatol. 9(2):119-126. <http://dx.doi.org/10.1046/j.1365-3164.1998.00100.x>
https://doi.org/10.1046/j.1365-3164.1998...
, when studying this syndrome in dogs of other breeds, summarized these theories as follows: one hypothesis is that dermatofibrosis is a paraneoplastic syndrome secondary to renal neoplasia; the other theory postulates that they are two different diseases that arise independently and are united by a common hereditary mechanism. Considering the fibrosis observed in the histological evaluation of the kidneys in the cases studied by these authors, they proposed a theory of concomitant onset of fibrosis in the skin and kidney; renal fibrosis would cause obstruction to the tubule flow, with consequent expansion and eventual formation of cysts in the renal tubules. In the cases herein studied, fibrosis seemed to be associated mainly with neoplasms, and may not have been associated with the genesis of cysts through obstructive mechanisms.
Only one of the three bitches in this survey presented uterine leiomyomas. These have also been frequently described in bitches with RCND (Lium & Moe 1985Lium B. & Moe L. 1985. Hereditary multifocal renal cystadenocarcinomas and nodular dermatofibrosis in the German Shepherd dog: macroscopic and histopathologic changes. Vet. Pathol. 22(5):447-455. <http://dx.doi.org/10.1177/030098588502200503> <PMid:4049673>
https://doi.org/10.1177/0300985885022005...
, Moe & Lium 1997Moe L. & Lium B. 1997. Hereditary multifocal renal cystadenocarcinomas and nodular dermatofibrosis in 51 German Shepherd dogs. J. Small Anim. Pract. 38(11):498-505. <http://dx.doi.org/10.1111/j.1748-5827.1997.tb03306.x> <PMid:9403809>
https://doi.org/10.1111/j.1748-5827.1997...
); however, a pathogenetic mechanism for this concomitant neoplasm has not yet been proposed.
It is worth noting that in seven cases, almost all of the renal parenchyma was replaced by cystic and neoplastic cystic and necrotic masses; however, only two dogs showed extra-renal lesions of uremia at necropsy; a larger number of dogs with renal insufficiency and, consequently, uremic lesions would be expected. In a study conducted with 43 German Shepherd dogs with RCND, uremia was only observed in two cases (Lium & Moe 1985Lium B. & Moe L. 1985. Hereditary multifocal renal cystadenocarcinomas and nodular dermatofibrosis in the German Shepherd dog: macroscopic and histopathologic changes. Vet. Pathol. 22(5):447-455. <http://dx.doi.org/10.1177/030098588502200503> <PMid:4049673>
https://doi.org/10.1177/0300985885022005...
). The absence of extra-renal lesions of uremia in a larger number of cases is an intriguing finding, considering the marked renal morphological changes observed in the vast majority of the kidneys analyzed in this study.
The literature surveyed showed that a specific immunohistochemical panel has not yet been established for the epithelial cells that comprise the cysts and renal epithelial neoplasms of this syndrome. For renal carcinomas of sporadic occurrence (excluding RCND), it is known that double IHC staining for cytokeratin and vimentin, uromodulin, Pax8, napsin A, and neprilysin confirms the tumor originates from kidney cells (Meuten & Meuten 2017Meuten J.D. & Meuten T.L.K. 2017. Tumors of the urinary system, p.632-688. In: Meuten D.J. (Ed), Tumors in Domestic Animals. 5th ed. Wiley-Blackwell, Ames.). In the present study, pancytokeratin immunostaining was not constant in all cases. An IHC study conducted with young dogs with early RCND lesions demonstrated strong staining for cytokeratin (broad spectrum) in the epithelium that lined the renal cysts in the dogs assessed, indicating that the epithelium had a distal tubular or collecting ducts origin, because the normal distal epithelial tubule cells presented intense staining for cytokeratin, whereas normal proximal tubular cells showed weak or no staining (Moe et al. 2000Moe L., Gamlem H., Jonasdottir T.J. & Lingaas F. 2000. Renal microcystic tubular lesions in two 1-year-old dogs an early sign of hereditary renal cystadenocarcinoma? J. Comp. Pathol. 123(2/3):218-221. <http://dx.doi.org/10.1053/jcpa.2000.0408> <PMid:11032680>
https://doi.org/10.1053/jcpa.2000.0408...
).
Conclusions
This study enabled identification of the prevalence and main clinical-epidemiological and morphological characteristics of renal cystadenoma/cystadenocarcinoma-nodular dermatofibrosis syndrome (RCND).
It also demonstrated that it is not always easy to clinically recognize this syndrome, but its peculiar characteristics (renal neoplasms and nodular dermatofibrosis) observed in the gross and microscopic evaluations allow safe morphological diagnosis. It was possible to detect all phases of a possible pathological continuum of the renal lesions.
Through histochemical techniques, it was possible to identify the presence of type I collagen in both cutaneous and renal lesions and consider its possible involvement in the pathogenesis of renal cystadenocarcinomas.
Immunohistochemistry (IHC) showed partially satisfactory results in the staining of epithelial cells of renal cysts and neoplasms for pancytokeratin.
Acknowledgements
The authors are grateful to the Coordination for the Improvement of Higher Education Personnel (CAPES) for the financial support provided to this study. R.P.M. Thompson has a graduate studies scholarship from the National Council for Scientific and Technological Development (CNPq). E.C. Lamego has an undergraduate research scholarship from the Research Support Foundation of the State of Rio Grande do Sul (FAPERGS). S.M.P. Melo has an undergraduate research scholarship from CNPq. G.D. Kommers holds a grant for productivity in research from CNPq.
References
- Armstrong T., Packham G., Murphy L.B., Bateman A.C., Conti J.A., Fine D.R., Johnson C.D., Benyon C. & Iredale J.P. 2004. Type I collagen promotes the malignant phenotype of pancreatic ductal adenocarcinoma. Clin. Cancer Res. 10(21):7427-7437. <http://dx.doi.org/10.1158/1078-0432.CCR-03-0825> <PMid:15534120>
» https://doi.org/10.1158/1078-0432.CCR-03-0825 - Baskin G.B. & De Paoli A. 1977. Primary renal neoplasms of the dog. Vet. Pathol. 14(6):591-605. <http://dx.doi.org/10.1177/030098587701400606> <PMid:201076>
» https://doi.org/10.1177/030098587701400606 - Bønsdorff T.B., Jansen J.H., Thomassen R.F. & Lingaas F. 2009. Loss of heterozygosity at the FLCN locus in early renal cystic lesions in dogs with renal cystadenocarcinoma and nodular dermatofibrosis. Mamm. Genome 20(5):315-320. <http://dx.doi.org/10.1007/s00335-009-9183-8> <PMid:19387735>
» https://doi.org/10.1007/s00335-009-9183-8 - Breshears M.A. & Confer A.W. 2017. O Sistema urinário, p.617-681. In: Zachary J.F. (Ed), Pathology Basis of Veterinary Disease. 6th ed. Elsevier, St Louis. <http://dx.doi.org/10.1016/B978-0-323-35775-3.00011-4>.
» https://doi.org/10.1016/B978-0-323-35775-3.00011-4 - Cianciolo R.E. & Mohr F.C. 2016. Urinary system, p.376-464. In: Maxie M.G., Jubb K.V.F., Kennedy P.C. & Palmer N.C. (Ed), Pathology of Domestic Animals. Vol.1. 6th ed. Elsevier, St Louis .
- Faccin T.C., Masuda E.K., Piazer J.V.M., Melo S.M.P. & Kommers G.D. 2017. Annular stenotic oesopageal squamous cell carcinoma in cattle exposed naturally to bracken fern (Pteridium arachnoideum). J. Comp. Pathol. 157(2/3):174-180. <http://dx.doi.org/10.1016/j.jcpa.2017.07.008> <PMid:28942300>
» https://doi.org/10.1016/j.jcpa.2017.07.008 - Fighera R.A., Souza T.M., Silva M.C., Brum J.S., Graça D.L., Kommers G.D., Irigoyen L.F. & Barros C.S.L. 2008. Causas de morte e razões para eutanásia de cães. Pesq. Vet. Bras. 28(4):223-230. <http://dx.doi.org/10.1590/S0100-736X2008000400005>
» https://doi.org/10.1590/S0100-736X2008000400005 - Hendrick M.J., Mahaffey E.A., Moore F.M., Vos J.H. & Walder E.J. 1998. Histological Classification of Mesenchymal Tumors of Skin and Soft Tissues of Domestic Animals. Vol.2. 2nd ed. Armed Forces Institute of Pathology, Washington, p.1-64.
- Inkelmann M.A., Kommers G.D., Trost M.E., Barros C.S.L., Fighera R.A., Irigoyen L.F. & Silveira I.P. 2012. Lesões do Sistema urinário em 1.063 cães. Pesq. Vet. Bras. 32(8):761-771. <http://dx.doi.org/10.1590/S0100-736X2012000800015>
» https://doi.org/S0100-736X2012000800015 - Junqueira L.C.U., Bignolas G. & Brentani R.R. 1979. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 11(4):447-455. <http://dx.doi.org/10.1007/BF01002772> <PMid:91593>
» https://doi.org/10.1007/BF01002772 - Langohr I.M., Irigoyen L.F., Salles M.W.S., Kommers G.D. & Barros C.S.L. 2002. Cistadenocarcinoma renal e dermatofibrose nodular em cães Pastor Alemão: 4 casos. Ciência Rural 32(4):621-626. <http://dx.doi.org/10.1590/S0103-84782002000400012>
» https://doi.org/10.1590/S0103-84782002000400012 - Lingaas F., Comstock K.E., Kirkness E.F., Sorensen A., Aarskaug T., Hitte C., Nickerson M.L., Moe L., Schmidt L.S., Thomas R., Breen M., Galibert F., Zbar B. & Ostrander E.A. 2003. A mutation in the canine BHD gene is associated with hereditary multifocal renal cystadenocarcinoma and nodular dermatofibrosis in the German Shepherd Dog. Hum. Mol. Genet. 12(23):3043-3053. <http://dx.doi.org/10.1093/hmg/ddg336> <PMid:14532326>
» https://doi.org/10.1093/hmg/ddg336 - Lium B. & Moe L. 1985. Hereditary multifocal renal cystadenocarcinomas and nodular dermatofibrosis in the German Shepherd dog: macroscopic and histopathologic changes. Vet. Pathol. 22(5):447-455. <http://dx.doi.org/10.1177/030098588502200503> <PMid:4049673>
» https://doi.org/10.1177/030098588502200503 - Lucke V.M. & Kelly D.F. 1976. Renal carcinoma in the dog. Vet. Pathol. 13(4):264-276. <http://dx.doi.org/10.1177/030098587601300403> <PMid:969167>
» https://doi.org/10.1177/030098587601300403 - Marks S.L., Farman C.A. & Peaston A. 1993. Nodular dermatofibrosis and renal cystadenomas in a Golden Retriever. Vet. Dermatol. 4(3):133-137. <http://dx.doi.org/10.1111/j.1365-3164.1993.tb00206.x>
» https://doi.org/10.1111/j.1365-3164.1993.tb00206.x - Meuten J.D. & Meuten T.L.K. 2017. Tumors of the urinary system, p.632-688. In: Meuten D.J. (Ed), Tumors in Domestic Animals. 5th ed. Wiley-Blackwell, Ames.
- Moe L. & Lium B. 1997. Hereditary multifocal renal cystadenocarcinomas and nodular dermatofibrosis in 51 German Shepherd dogs. J. Small Anim. Pract. 38(11):498-505. <http://dx.doi.org/10.1111/j.1748-5827.1997.tb03306.x> <PMid:9403809>
» https://doi.org/10.1111/j.1748-5827.1997.tb03306.x - Moe L., Gamlem H., Jonasdottir T.J. & Lingaas F. 2000. Renal microcystic tubular lesions in two 1-year-old dogs an early sign of hereditary renal cystadenocarcinoma? J. Comp. Pathol. 123(2/3):218-221. <http://dx.doi.org/10.1053/jcpa.2000.0408> <PMid:11032680>
» https://doi.org/10.1053/jcpa.2000.0408 - Pressler B.M., Williams L.E., Ramos-Vara J.A. & Anderson K. 2009. Sequencing of the Von Hippel-Lindau gene in canine renal carcinoma. J. Vet. Intern. Med. 23(3):592-597. <http://dx.doi.org/10.1111/j.1939-1676.2009.0310.x> <PMid:19422471>
» https://doi.org/10.1111/j.1939-1676.2009.0310.x - Serakides R. & Silva J.F. 2017. Sistema urinário, p.267-309. In: Santos R.L. & Alessi A.C. (Ed.), Patologia Veterinária. 2ª ed. Guanabara Koogan, Rio de Janeiro.
- Shields M.A., Dangi-Garimella S., Krantz S.B., Bentrem D.J. & Munshi H.G. 2011. Pancreatic cancer cells respond to type I collagen by inducing snail expression to promote membrane type 1 matrix metalloproteinase-dependent collagen invasion. J. Biol. Chem. 286(12):10495-10504.
- Suter M., Lott-Stolz G. & Wild P. 1983. Generalized nodular dermatofibrosis in six Alsatians. Vet. Pathol. 20(5):632-634. <http://dx.doi.org/10.1177/030098588302000515> <PMid:6636470>
» https://doi.org/10.1177/030098588302000515 - White S.D., Rosychuk R.A.W., Schultheiss P. & Scott K.V. 1998. Nodular dermatofibrosis and cystic renal disease in three mixed breed dogs and a Boxer dog. Vet. Dermatol. 9(2):119-126. <http://dx.doi.org/10.1046/j.1365-3164.1998.00100.x>
» https://doi.org/10.1046/j.1365-3164.1998.00100.x - Whittaker P., Kloner R.A., Boughner D.R. & Pickering J.G. 1994. Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized light. Basic Res. Cardiol. 89(5):397-410. <http://dx.doi.org/10.1007/BF00788278> <PMid:7535519>
» https://doi.org/10.1007/BF00788278
-
Part of Master’s Thesis of the first author.
Publication Dates
-
Publication in this collection
30 Sept 2019 -
Date of issue
July 2019
History
-
Received
20 Mar 2019 -
Accepted
01 Apr 2019