ABSTRACT:
The most consumed cheese in Brazil, Minas Frescal cheese (MFC) is highly susceptible to microbial contamination and clandestine production and commercialization can pose a risk to consumer health. The storage of this fresh product under refrigeration, although more appropriate, may favor the growth of spoilage psychrotrophic bacteria. The objective of this study was to quantify and compare Pseudomonas spp. and other psychrotrophic bacteria in inspected and non-inspected MFC samples, evaluate their lipolytic and proteolytic activities and their metalloprotease production potentials. Twenty MFC samples were evaluated: 10 inspected and 10 non-inspected. Counts of psychrotrophic bacteria and Pseudomonas spp., evaluation of the proteolytic and lipolytic potential of the isolates, and identification of potential producers of alkaline metalloprotease (AprX) were assessed. The mean total psychrotrophic counts were 1.07 (±2.18) × 109CFU/g in the inspected samples and 4.5 (±5.86) × 108CFU/g in the non-inspected, with no significant difference (p=0.37). The average score of Pseudomonas spp. was 6.86 (±18.6) × 105 and 2.08 (±3.65) × 106 CFU/g for the inspected and non-inspected MFC samples, respectively, with no significant difference (p=0.1). Pseudomonas spp. represented 0.06% and 0.004% of psychrotrophic bacteria found in inspected and non-inspected MFC samples, respectively. Collectively, 694 psychrotrophic strains and 47Pseudomonas spp. were isolated, of which 59.9% and 68.1% were simultaneously proteolytic and lipolytic, respectively. Of the 470 psychrotrophs isolated from inspected and 224 from non-inspected cheese samples, 5.74% and 2.23% contained aprX, respectively, while 100 and 86.96% of the Pseudomonas spp. isolates in inspected and non-inspected cheese samples contained the gene. The production potential of AprX did not, however, determine the proteolytic activity on plates of these isolates under the conditions evaluated in this study. Of total, 65.63% of the psychrotrophs that contained aprX gene were confirmed as Pseudomonas spp., using genus-specific PCR. Phylogenetic analysis of the 16S rRNA gene of the other psychrotrophs that were potential producers of AprX identified them as Serratia spp. (n=7), Raoultella ornithinolytica (n=1), and Acinetobacter schindleri (n=1) in the inspected samples and Psychrobacter sanguinis (n=1) and Leuconostoc mesenteroides (n=1) in the non-inspected samples. The production conditions of Brazilian MFC of these samples, while meeting the legal determinations, are not sufficient to control Pseudomonas and other spoilage-related psychrotrophs. Thus, stricter hygienic measures are required during the formal production of this type of cheese.
INDEX TERMS:
Pseudomonas spp.; psychrotrophic; microorganisms; Minas Frescal cheese; cheese; AprX production; alkaline metalloprotease; proteolysis; lipolysis
RESUMO:
O mais consumido no Brasil, o queijo Minas Frescal (QMF) é altamente suscetível à contaminação microbiana e a produção e comercialização clandestina podem representar um risco para a saúde do consumidor. O armazenamento deste produto fresco sob refrigeração, embora mais apropriado, pode favorecer a multiplicação de bactérias psicrotróficas deteriorantes. O objetivo deste estudo foi quantificar e comparar Pseudomonas spp. e outras bactérias psicrotróficas em amostras de QMF inspecionadas e não inspecionadas, avaliar o potencial lipolítico, proteolítico e de produção de metaloprotease alcalina. Vinte amostras de QMF foram avaliadas: 10 inspecionadas e 10 não inspecionadas. Foram avaliadas as contagens de bactérias psicrotróficas e Pseudomonas spp., o potencial proteolítico e lipolítico dos isolados e a identificação de potenciais produtores de metaloprotease alcalina (AprX). A média total das contagens de bactérias psicrotróficas foi de 1,07 (±2,18) × 109UFC/g nas amostras inspecionadas e 4,5 (±5,86) × 108UFC/g nas não inspecionadas, sem diferença significativa (p=0,37). A média de Pseudomonasspp. foi de 6,86 (±18,6) × 105 e 2,08 (±3,65) × 106UFC/g para as amostras QMF inspecionadas e não-inspecionadas, respectivamente, sem diferença significativa (p=0,1). Pseudomonas spp. representaram 0,06% e 0,004% de bactérias psicrotróficas encontradas em amostras QMF inspecionadas e não-inspecionadas, respectivamente. Das amostras inspecionadas e não inspecionadas, foram isoladas 694 colônias psicrotróficas e 47 Pseudomonasspp., dos quais 59,9% e 68,1% foram simultaneamente proteolíticos e lipolíticos, respectivamente. Dos 470 isolados de psicrotróficos das amostras de queijo inspecionados e dos 224 isolados das não inspecionadas, 5,74% e 2,23% continham o gene aprX, respectivamente, enquanto 100 e 86,96% das Pseudomonasspp. isoladas em amostras de queijo inspecionadas e não inspecionadas continham o potencial de expressão de AprX. Esse potencial, no entanto, não determinou a atividade proteolítica em placas desses isolados nas condições avaliadas neste estudo. Do total, 65,63% dos psicrotróficos que continham o gene aprX foram confirmados como Pseudomonasspp., utilizando PCR gênero-específico. A análise filogenética do gene 16S rRNA dos outros psicrotróficos que foram produtores potenciais de AprX os identificou como Serratia spp. (n=7), Raoultella ornithinolytica (n=1) e Acinetobacter schindleri (n=1) nas amostras inspecionadas e Psychrobacter sanguinis (n=1) e Leuconostoc mesenteroides (n=1) nas amostras não inspecionadas. As condições de produção do QMF dessas amostras, atendendo às determinações legais, não são suficientes para controlar a Pseudomonas e outros psicrotróficos relacionados à deterioração. Assim, medidas higiênicas mais rígidas são necessárias durante a produção formal deste tipo de queijo.
TERMOS DE INDEXAÇÃO:
Pseudomonas spp.; micro-organismos; psicrotróficos; queijo; Minas Frescal; produção de AprX; metaloprotease alcalina; proteólise; lipólise
Introduction
By definition, Brazilian Minas Frescal cheese (MFC) is a fresh product obtained by the enzymatic coagulation of milk with rennet and/or other suitable coagulating enzymes, with or without supplementation with specific lactic bacteria, powdered milk, cream, milk solids, sodium chloride, and calcium chloride (Brasil 1997Brasil 1997. Regulamento Técnico Para Fixação de Identidade e Qualidade do Queijo Minas Frescal. Portaria nº 352, de 04 de setembro de 1997, Diário Oficial da União, Ministério da Agricultura, Pecuária e Abastecimento, Brasília, DF. ). It is classified as a semi-fat (25 to 44.9% fat) cheese with very high moisture content (not less than 55.0%) (Brasil 2004Brasil 2004. Regulamento Técnico para Fixação de Identidade e Qualidade do Queijo Minas Frescal. Instrução Normativa nº 4, de 1 de março de 2004, Diário Oficial da União,.). The production of this type of cheese in Brazil is carried out formally by dairies that follow the legal stipulations of the “Ministério da Agricultura, Pecuária e Abastecimento” (Brasil 1997Brasil 1997. Regulamento Técnico Para Fixação de Identidade e Qualidade do Queijo Minas Frescal. Portaria nº 352, de 04 de setembro de 1997, Diário Oficial da União, Ministério da Agricultura, Pecuária e Abastecimento, Brasília, DF. ) and mainly small milk producers, with the objective of adding value and increases the shelf life of milk through the preparation of derivatives.
The MFC is quite susceptible to microbial contamination when prepared using raw milk, or during or after processing (Carvalho et al. 2007Carvalho J.D.G., Viotto W.H. & Kuaye A.Y. 2007. The quality of Minas Frescal cheese produced by different technological processes. Food Control 18(3):262-267. <http://dx.doi.org/10.1016/j.foodcont.2005.10.005>
https://doi.org/10.1016/j.foodcont.2005....
). Moreover, it can be a source of food pathogens (Campos et al. 2017Campos A.C.L.P., Puño-Sarmiento J.J., Medeiros L.P., Gazal L.E.S., Maluta R.P., Navarro A., Kobayashi R.K.T., Fagan E.P. & Nakazato G. 2017. Virulence genes and antimicrobial resistance in Escherichia coli from cheese made from unpasteurized milk in Brazil. Foodborne Pathog Dis. 15(2):94-110. <http://dx.doi.org/10.1089/fpd.2017.2345> <PMid:29215297>
https://doi.org/10.1089/fpd.2017.2345...
).
In addition to pathogenic microorganisms, proteolytic and/or lipolytic microorganisms, mainly psychrotrophs, may interfere with the quality of the MFC, since as it is a fresh product, refrigeration is necessary for its storage. When present in the raw material, psychrotrophic bacteria reduce the industrial yield, flavor, and aroma, being able to render the cheese improper for consumption (Murphy et al. 2016Murphy S.C., Martin N.H., Barbano D.M. & Wiedmann M. 2016. Influence of raw milk quality on processed dairy products: How do raw milk quality test results relate to product quality and yield? J. Dairy Sci. 99(12):10128-10149. <http://dx.doi.org/10.3168/jds.2016-11172> <PMid:27665134>
https://doi.org/10.3168/jds.2016-11172...
).
As the degradation of proteins results in non-acid metabolites, protease activity confers a bitter taste and putrid smell to the cheese. Undesirable effects are also influenced by the reduction of the integrity of milk proteins, deficient coagulation, and greater loss of casein fragments in the serum, requiring 20-30 percent more milk per kilo of cheese (Samaržija et al. 2012Samaržija D., Zamberlin Š. & Pogačić T. 2012. Psychrotrophic bacteria and milk and dairy products quality. Mljekarstvo. Dairy 62(2):77-95.).
Alkaline metalloprotease (AprX), which is heat-resistant, is considered the main microbial protease, and is encoded by aprX. This gene is found in various proteolytic bacteria, such as Pseudomonas spp. and Serratia spp. (Dufour et al. 2008Dufour D., Nicodeme M., Perrin C., Driou A., Brusseaux E., Humbert G., Gaillard J.L. & Dary A. 2008. Molecular typing of industrial strains of Pseudomonas spp. isolated from milk and genetical and biochemical characterization of an extracellular protease produced by one of them. Int. J. Food Microbiol. 125(2):188-196. <http://dx.doi.org/10.1016/j.ijfoodmicro.2008.04.004> <PMid:18511140>
https://doi.org/10.1016/j.ijfoodmicro.20...
, Marchand et al. 2009Marchand S., Vandriesche G., Coorevits A., Coudijzer K., De Jonghe V., Dewettinck K., De Vos P., Devreese B., Heyndrickx M. & De Block J. 2009. Heterogeneity of heat-resistant proteases from milk Pseudomonas species. Int. J. Food Microbiol. 133(1/2):68-77. <http://dx.doi.org/10.1016/j.ijfoodmicro.2009.04.027> <PMid:19481283>
https://doi.org/10.1016/j.ijfoodmicro.20...
, Bagliniere et al. 2013Bagliniere F., Mateos A., Tanguy G., Jardin J., Briard-Bion V., Rousseau F., Robert B., Beaucher E., Gaillard J.L., Amiel C., Humbert G., Dary A. & Gaucheron F. 2013. Proteolysis of ultra-high temperature- treated casein micelles by AprX enzyme from Pseudomonas fluorescens induces their destabilisation. Int. Dairy J. 31(2):55-61. <http://dx.doi.org/10.1016/j.idairyj.2013.02.011>
https://doi.org/10.1016/j.idairyj.2013.0...
). This enzyme is of great significance to dairy industry, because it leads to the deterioration of casein, which causes significant alterations of the physical and chemical quality and organoleptic properties of raw milk and its derivatives (Dufour et al. 2008Dufour D., Nicodeme M., Perrin C., Driou A., Brusseaux E., Humbert G., Gaillard J.L. & Dary A. 2008. Molecular typing of industrial strains of Pseudomonas spp. isolated from milk and genetical and biochemical characterization of an extracellular protease produced by one of them. Int. J. Food Microbiol. 125(2):188-196. <http://dx.doi.org/10.1016/j.ijfoodmicro.2008.04.004> <PMid:18511140>
https://doi.org/10.1016/j.ijfoodmicro.20...
).
Milk fat is equally compromised by microbial activity. Microbial lipases elevate heat resistance and promote a rancid flavor and aroma in dairy products. Microbial lipases and proteases remain active even after the elimination of the vegetative forms of micro-organisms by pasteurization (Oliveira et al. 2015Oliveira G.B., Favarin L., Luchese R.H. & McIntosh D. 2015. Psychrotrophic bacteria in milk: how much do we really know? Braz. J. Microbiol. 46(2):313-321. <http://dx.doi.org/10.1590/S1517-838246220130963> <PMid:26273245>
https://doi.org/10.1590/S1517-8382462201...
, Murphy et al. 2016Murphy S.C., Martin N.H., Barbano D.M. & Wiedmann M. 2016. Influence of raw milk quality on processed dairy products: How do raw milk quality test results relate to product quality and yield? J. Dairy Sci. 99(12):10128-10149. <http://dx.doi.org/10.3168/jds.2016-11172> <PMid:27665134>
https://doi.org/10.3168/jds.2016-11172...
), as in case of Minas cheese.
Several studies have demonstrated the predominance of the genus Pseudomonas among dairy psychrotrophs (Ozturkoglu-Budak et al. 2016Ozturkoglu-Budak S., Wiebenga A., Bron P.A. & de Vries R.P. 2016. Protease and lipase activities of fungal and bacterial strains derived from an artisanal raw ewe’s milk cheese. Int. J. Food Microbiol. 237:17-27. <http://dx.doi.org/10.1016/j.ijfoodmicro.2016.08.007> <PMid:27541978>
https://doi.org/10.1016/j.ijfoodmicro.20...
, Vithanage et al. 2016Vithanage N.R., Dissanayake M., Bolge G., Palombo E.A., Yeager T.R. & Datta N. 2016. Biodiversity of culturable psychrotrophic microbiota in raw milk attributable to refrigeration conditions, seasonality and their spoilage potential. Int. Dairy J. 57:80-90. <http://dx.doi.org/10.1016/j.idairyj.2016.02.042>
https://doi.org/10.1016/j.idairyj.2016.0...
, Xin et al. 2017Xin L., Meng Z., Zhang L., Cui Y., Han X. & Yi H. 2017. The diversity and proteolytic properties of psychrotrophic bacteria in raw cows´ milk from North China. Int. Dairy J. 66:34-41. <http://dx.doi.org/10.1016/j.idairyj.2016.10.014>
https://doi.org/10.1016/j.idairyj.2016.1...
). Several species of Pseudomonas are responsible for the deterioration of other refrigerated foodstuffs (Samaržija et al. 2012Samaržija D., Zamberlin Š. & Pogačić T. 2012. Psychrotrophic bacteria and milk and dairy products quality. Mljekarstvo. Dairy 62(2):77-95., Oliveira et al. 2015Oliveira G.B., Favarin L., Luchese R.H. & McIntosh D. 2015. Psychrotrophic bacteria in milk: how much do we really know? Braz. J. Microbiol. 46(2):313-321. <http://dx.doi.org/10.1590/S1517-838246220130963> <PMid:26273245>
https://doi.org/10.1590/S1517-8382462201...
), since they have the capacity to produce proteolytic and lipolytic enzymes at different temperatures, which reinforces their importance as spoilage agents of the dairy product chain (Scatamburlo et al. 2015Scatamburlo T.M., Yamazi A.K., Cavicchioli V.Q., Pieri F.A. & Nero L.A. 2015. Spoilage potential of Pseudomonas species isolated from goat milk. J. Dairy Sci. 98(2):759-764. <http://dx.doi.org/10.3168/jds.2014-8747> <PMid:25497792>
https://doi.org/10.3168/jds.2014-8747...
, Ribeiro Júnior et al. 2018Ribeiro Júnior J.C., Oliveira A.M., Silva F.G., Tamanini R., Oliveira A.L.M. & Beloti V. 2018. The main spoilage related psychrotrophic bacteria in refrigerated raw milk. J. Dairy Sci. 101(1):75-83. <http://dx.doi.org/10.3168/jds.2017-13069> <PMid:29102138>
https://doi.org/10.3168/jds.2017-13069...
). Furthermore, because they have a high capacity to form biofilms, allowing them to proliferate in a wide variety of environments (Murphy et al. 2016Murphy S.C., Martin N.H., Barbano D.M. & Wiedmann M. 2016. Influence of raw milk quality on processed dairy products: How do raw milk quality test results relate to product quality and yield? J. Dairy Sci. 99(12):10128-10149. <http://dx.doi.org/10.3168/jds.2016-11172> <PMid:27665134>
https://doi.org/10.3168/jds.2016-11172...
).
Taking into account the technical problems that spoilage microorganisms cause in dairy products, the objective of this study was to quantify and compare Pseudomonas spp. and other psychrotrophic bacteria in inspected and non-inspected MFC samples, evaluate their lipolytic and proteolytic activities and their metalloprotease production potentials by identification of aprX gene.
Materials and Methods
Sampling and preparation of cheese. Twenty samples of MFC marketed in the municipality of Londrina/PR, from May to June 2017, were evaluated. Ten samples of different brands were collected from supermarkets and were recorded by the Brazilian state or federal inspection system and were thus regarded as formal, inspected. These inspected samples had from 11 to 41 days of manufacture, with 22 days on average.
The remaining 10 MFC samples were collected from different fairs in the municipality and were marketed in a clandestine manner, and were therefore considered non-inspected. It was not possible to determine the period between the manufacture date and the analysis of these non-inspected samples, since the sellers were not necessarily the producers.
The MFC samples evaluated did not present any type of alteration of coloration (white, slightly yellowish), texture (soft) or smell (slightly acidic). They had varying amounts of white or yellowish serum, characteristic of MFC.
The samples were transported under refrigeration to the Laboratory of Inspection of Products of Animal Origin, which is a part of the “Instituto Nacional de Ciência e Tecnologia para a Cadeia Produtiva do Leite” (INCT-Cadeia do Leite) of the “Universidade Estadual de Londrina” (UEL), Paraná, Brazil, where they were immediately processed.
The external surfaces of packaging were sanitized with 70% alcohol. For the psychrotrophic bacterial count, a 25-g aliquot obtained aseptically from different fragments of the cheese sample was homogenized with 225mL of 0.1% peptone saline in Stomacher blender for 180 seconds, obtaining a 10-1 dilution. From this dilution, serial decimal dilutions were performed with the same diluent.
For estimating the count of Pseudomonas spp., another 25-g aliquot of each sample was diluted in 225 mL buffered peptone water (Oxoid®, England) and homogenized in a Stomacher blender, according to ISO 11.059 (ISO 2009ISO 2009. ISO 11.059, Milk and Milk Products: method for the enumeration of Pseudomonas spp. ISO/TS 11059:2009 (IDF/RM 225:2009), International Organization for Standardization, Geneva, Switzerland.) recommendations.
Counting of microorganisms. For estimating the psychrotrophic bacterial count, 0.1mL of the dilutions were grown in duplicate on the surface of Plate Count Agar (PCA) (Acumedia, Baltimore, USA) plates and incubated at 7°C for 10 days.
The count of Pseudomonas spp. was performed according to ISO 11059 (2009). A tenth of mL of the dilutions was spread on the surface of penicillin pimaricin agar (PPA) plates, prepared with Pseudomonas agar base (Oxoid) supplemented with 100,000IU/L of penicillin G potassium (Sigma Aldrich®, United States), and 0.01g/L of piramicin (Coalhopar F-E-B Biotecnologia®, Brazil). The plates were incubated at 25°C for 48h. All colonies were subjected to tests for oxidase and glucose fermentation. Only the oxidase positive, non-glucose fermentative colonies were taken into account for the counts of Pseudomonas spp.
The counts were compared by the t-test using the Statistica v. 6.0 software (StatSoft, OK, USA).
Proteolytic and lipolytic potential of Pseudomonas spp. and others psychrotrophic bacteria. The colonies of Pseudomonas spp. and psychrotrophic bacteria were inoculated onto milk agar plates (Acumedia) supplemented with a solution of 10% reconstituted milk powder (Molico®, Nestlé, São Paulo, Brazil), and in tributyrin agar (HiMedia, Mumbai, India) supplemented with 1% tributyrin (HiMedia) to assess the proteolytic and lipolytic activity, respectively, according to the procedure stated by Hantsis-Zacharov & Halpern (2007)Hantsis-Zacharov E. & Halpern M. 2007. Culturable psychrotrophic bacterial communities in raw milk and their proteolytic and lipolytic traits. Appl. Environ. Microbiol. 73(22):7162-7168. <http://dx.doi.org/10.1128/AEM.00866-07> <PMid:17890340>
https://doi.org/10.1128/AEM.00866-07...
. The plates were incubated under the same conditions recommended for the bacterial counts.
DNA extraction. The psychrotrophic colonies that showed spoiling potential on plates were grown in brain heart broth (Merck®, Germany) and incubated at 35oC for 48h, under the same incubation conditions as that for the colonies of Pseudomonas spp. grown in tryptone soy broth (Oxoid). An aliquot of 1mL of each broth was used to extract DNA by the simple boiling method, according to the study by Ribeiro Júnior et al. (2016Ribeiro Júnior J.C., Tamanini R., Soares B.F., Oliveira A.M., Silva F.G., Silva F.F., Augusto N.A. & Beloti V. 2016. Efficiency of boiling and four other methods for genomic DNA extraction of deteriorating spore-forming bacteria from milk. Semina, Ciênc. Agrarárias 37:3069-3078.).
Molecular confirmation of Pseudomonas spp. The isolates of Pseudomonas spp. obtained in the counts and the other psychrotrophs were subjected to PCR amplification of a specific region in the 16S rRNA gene of the genus Pseudomonas, according to the amplification protocol described by Spilker et al. (2004)Spilker T., Coenye T., Vandamme P. & Lipuma J.J. 2004. PCR-based assay for differentiation of Pseudomonas aeruginosa from other Pseudomonas species recovered from cystic fibrosis patients. J. Clin. Microbiol. 42(5):2074-2079. <http://dx.doi.org/10.1128/JCM.42.5.2074-2079.2004> <PMid:15131172>
https://doi.org/10.1128/JCM.42.5.2074-20...
, using the primers F-GS-PA (GACGGGTGAGTAATGCCTA) and PA-GS-R (CACTGGTGTTCCTTCCTATA). PCR reactions were performed using 50ng of template DNA, 10nM of each dNTP, 1× buffer, 75mmol/L of MgCl2, 20pmol/L of each primer, and 2.5U of Platinum Taq DNA polymerase (Invitrogen, CA, USA), to yield a final reaction volume of 50μL, recommended by Ribeiro Júnior et al. (2016)Ribeiro Júnior J.C., Tamanini R., Soares B.F., Oliveira A.M., Silva F.G., Silva F.F., Augusto N.A. & Beloti V. 2016. Efficiency of boiling and four other methods for genomic DNA extraction of deteriorating spore-forming bacteria from milk. Semina, Ciênc. Agrarárias 37:3069-3078.. Samples which displayed 618-bp amplicons were considered as Pseudomonas spp.
Detection of aprX. PCR of aprX gene (AprX enzyme) was performed using the primers apr I (TAYGGBTTCAAYTCCAAYAC) and apr II (VGCGATSGAMACRTTRCC) and amplification conditions described by Bach et al. (2001Bach H.J., Hartmann A., Schloter M. & Munch J.C. 2001. PCR primers and functional probes for amplification and detection of bacterial genes for extracellular peptidases in single strains and in soil. J. Microbiol. Methods 44(2):173-182. <http://dx.doi.org/10.1016/S0167-7012(00)00239-6> <PMid:11165346>
https://doi.org/10.1016/S0167-7012(00)00...
). The reaction conditions were the same as those cited above (Ribeiro Júnior et al. 2016Ribeiro Júnior J.C., Tamanini R., Soares B.F., Oliveira A.M., Silva F.G., Silva F.F., Augusto N.A. & Beloti V. 2016. Efficiency of boiling and four other methods for genomic DNA extraction of deteriorating spore-forming bacteria from milk. Semina, Ciênc. Agrarárias 37:3069-3078.), with 194-bp amplicons being considered positive.
Amplification and sequencing of the 16S rRNA gene. The isolates that were positive for aprX and were not confirmed as Pseudomonas spp. by genus-specific reaction were subjected to the partial amplification of the 16S rRNA gene using the primers 27f (5′-GAGTTTGATCMTGGCTCAG-3′) and 1492r (5′-GGYTACCTTGTTACGACTT-3′) (Osborne et al. 2005Osborne C.A., Galic M., Sangwan P. & Janssen P.H. 2005. PCRgenerated artefact from 16S rRNA gene-specific primers. FEMS Microbiol. Lett. 248(2):183-187. <http://dx.doi.org/10.1016/j.femsle.2005.05.043> <PMid:15961258>
https://doi.org/10.1016/j.femsle.2005.05...
). The amplification conditions were as follows: 1 cycle of initial denaturation at 94°C for 5min; 35 cycles at 94°C for 1min, annealing at 58°C for 1min, and primer extension at 72°C for 1min; and a final extension cycle at 72°C for 10min.
The PCR product from the 16S rRNA gene was then purified (PureLinkTM Genomic DNA Purification Kit, Invitrogen) and quantified (Qubit® dsDNA HS Assay Kit, Invitrogen). DNA sequencing was performed by the Sanger method (ABI 3500 Genetic Analyzer, Applied Biosystems, Foster City, USA) in both directions. A representative sequence of each species found was selected for deposit in GenBank.
Phylogenetic analysis for species identification. The quality of the 16S rRNA sequences was evaluated by BioEdit v. 7.2.5 software (Hall 1999Hall T.A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41:95-98.) and the consensual sequences were generated by CAP 3 (Huang & Madan 1999Huang X. & Madan A. 1999. CAP3: a DNA sequence assembly program. Genome Res. 9(9):868-877. <http://dx.doi.org/10.1101/gr.9.9.868> <PMid:10508846>
https://doi.org/10.1101/gr.9.9.868...
). Preliminary identification at the genus level was performed by the BLAST tool of the National Center for Biotechnology Information (NCBI). Once the genera were identified, the sequences were individually aligned by Clustal W with the representative type sequences of all species of the genus available in the Ribosomal Database Project (RDP)4
4
Ribosomal Database Project (RDP), Center for Microbial Ecology, Michigan State University, Michigan, USA. Available at <https://rdp.cme.msu.edu/hierarchy>
; the identification of the species was based on the genetic identity matrix calculated by the Tamura-Nei model in the MEGA software v. 7.0 (Kumar et al. 2016Kumar S., Stecher G. & Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33(7):1870-1874. <http://dx.doi.org/10.1093/molbev/msw054> <PMid:27004904>
https://doi.org/10.1093/molbev/msw054...
). The phylogenetic trees were elaborated in the same software, using the Neighbor Joining method, Tamura-Nei model, and bootstrap support for 1000 replicates.
Results
The psychrotroph counts ranged from 3.5×107-6.85 × 109 UFC/g in the inspected cheese samples, with a mean of 1.07 (±2.18) × 109UFC/g. In the non-inspected cheese samples, psychrotroph counts ranged between 2.6 × 107-1.65 × 109UFC/g, with a mean of 4.5 (±5.86) × 108CFU/g. No significant difference was observed between the psychrotroph counts in the inspected and non-inspected cheese samples (p=0.37).
The mean of Pseudomonas spp. counts was 6.86 (±18.6) × 105 and 2.08 (±3.65) × 106CFU/g for formally and informally marketed cheese, respectively. There was no significant difference (p=0.1) between the counts of Pseudomonas spp. among the inspected and non-inspected cheeses, although the average of counts of Pseudomonas spp. in inspected cheese samples was only 32.9% of that in the non-inspected cheese samples.
From the plates used for the psychrotroph counts, 694 colonies were isolated: 470 from inspected cheese and 224 from non-inspected cheese. With regards to the isolation of Pseudomonas spp. confirmed by biochemical tests (oxidase positive and glucose negative), 47 isolates were identified, of which 24 were from inspected cheeses and 23 were from non-inspected cheese samples. The spoilage potential of these isolates is described in Table 1.
Spoilage potential of isolates of psychrotrophic bacteria, and the genus Pseudomonas isolated from 20 samples of inspected (10) and non-inspected (10) cheeses marketed in the municipality of Londrina, Paraná, from May to June 2017
Of these total number of isolates, 85.9 and 91.5% of psychrotrophic bacteria and Pseudomonas isolated from the total number of samples of MFC, respectively, showed some type of spoilage potential. Moreover, there was a predominance of proteolytic and lipolytic potential simultaneously in nearly 60% of psychrotrophic and 70% of Pseudomonas spp. (Table 1)
Of the 47 isolates confirmed as Pseudomonas spp. by biochemical tests, 46 (97.9%) were confirmed by genus-specific PCR.
To verify the potential metalloprotease production capacity, a search of aprX was conducted individually in all isolates of this study, i.e. 694 psychrotrophic and 46 Pseudomonas spp. isolates proven by genus-specific PCR, totaling 740 reactions, as detailed in Table 2.
Production potential of metalloprotease (aprX) of psychrotrophic bacteria and of the Pseudomonas spp., and the spoilage potential of these isolates from inspected and non-inspected Minas Frescal cheese on agar plates
The 32 psychrotrophic isolates that displayed positive results in the assessment of aprX (Table 2) were also subjected to genus-specific PCR for Pseudomonas spp. Of these, 21 (65.63%) were confirmed, and the other 11 isolates were submitted to partial sequencing of the 16S rRNA for identification and phylogenetic analysis.
The identification of these 11 isolates that contained aprX were not identified as Pseudomonas is described in Table 3. Five genera were identified: Serratia spp. (n=7), Raoultella ornithinolytica (n=1), Acinetobacter schindleri (n=1), Psychrobacter sanguinis (n=1) and Leuconostoc mesenteroides (n=1). The species of the seven strains of Serratia were not identified because high percentages of similarity in the identity matrix (greater than 99%) were observed within several species of the 17 representative sequences of the genus available in RDP. The possible species indicated by a sequence representative of all isolates in the present study can be observed in Figure 1.
Phylogenetic tree of species of the genus Serratia (GenBank accession number) prepared using the alignment of 668bp of the 16S rRNA gene, Neighbor Joining method, Tamura-Nei model, and bootstrap support for 1000 replicates. The strain marked with a diamond symbol is representative of seven species of this genus isolated in this study. The bar indicates the percentage of nucleotide substitution.
Discussion
The highest mean score of psychrotrophic in the inspected cheeses compared to non-inspected cheeses can be explained by the greater control in storage, mandatory refrigeration (Brasil 1997Brasil 1997. Regulamento Técnico Para Fixação de Identidade e Qualidade do Queijo Minas Frescal. Portaria nº 352, de 04 de setembro de 1997, Diário Oficial da União, Ministério da Agricultura, Pecuária e Abastecimento, Brasília, DF. ), and strict observation in all inspected samples evaluated. Sangaletti et al. (2009)Sangaletti N., Porto E., Brazaca S.G.C., Yagasaki C.A., Dalla Dea R.C. & Silva M.V. 2009. Shelf-life of Minas Frescal cheese. Ciênc. Tecnol. Aliment. 29(2):262-269. <http://dx.doi.org/10.1590/S0101-20612009000200004>
https://doi.org/10.1590/S0101-2061200900...
found that the psychrotrophic counts increase in MFC with the refrigeration storage period, increasing from 1.4×103CFU/g on the first day after manufacturing to 4.5×1011CFU/g after 30 days under these conditions. The lack of control or even the absence of refrigeration until the moment of commercialization of non-inspected cheeses can lead to higher counts of mesophilic microorganisms, but result in lower counts of psychrophic bacteria.
As the formally marketed cheese samples already had, on average, 22 days of manufacture, and therefore, were kept under refrigeration, it is possible that the psychrotrophic counts would be lower if the samples were evaluated with a smaller storage period. In addition, the higher psychrotrophic counts in inspected cheese samples can be due to the inoculation of lactic acid bacteria (LAB) cultures in pasteurized milk intended for the production of cheese in dairy products. Several LAB cultures are also psychrotrophic and may demonstrate proteolytic and/or lipolytic activity (Ribeiro Júnior et al. 2018Ribeiro Júnior J.C., Oliveira A.M., Silva F.G., Tamanini R., Oliveira A.L.M. & Beloti V. 2018. The main spoilage related psychrotrophic bacteria in refrigerated raw milk. J. Dairy Sci. 101(1):75-83. <http://dx.doi.org/10.3168/jds.2017-13069> <PMid:29102138>
https://doi.org/10.3168/jds.2017-13069...
).
Pseudomonas is considered the main genus among psychrotrophic microorganisms in milk and dairy products (Ozturkoglu-Budak et al. 2016Ozturkoglu-Budak S., Wiebenga A., Bron P.A. & de Vries R.P. 2016. Protease and lipase activities of fungal and bacterial strains derived from an artisanal raw ewe’s milk cheese. Int. J. Food Microbiol. 237:17-27. <http://dx.doi.org/10.1016/j.ijfoodmicro.2016.08.007> <PMid:27541978>
https://doi.org/10.1016/j.ijfoodmicro.20...
, Vithanage et al. 2016Vithanage N.R., Dissanayake M., Bolge G., Palombo E.A., Yeager T.R. & Datta N. 2016. Biodiversity of culturable psychrotrophic microbiota in raw milk attributable to refrigeration conditions, seasonality and their spoilage potential. Int. Dairy J. 57:80-90. <http://dx.doi.org/10.1016/j.idairyj.2016.02.042>
https://doi.org/10.1016/j.idairyj.2016.0...
, Xin et al. 2017Xin L., Meng Z., Zhang L., Cui Y., Han X. & Yi H. 2017. The diversity and proteolytic properties of psychrotrophic bacteria in raw cows´ milk from North China. Int. Dairy J. 66:34-41. <http://dx.doi.org/10.1016/j.idairyj.2016.10.014>
https://doi.org/10.1016/j.idairyj.2016.1...
) and contamination can occur by the hands of milkers, and from the surface of the cows’ udders and milking equipment, and from poorly sanitized refrigerated tanks (Vidal et al. 2017Vidal A.M.C., Saran Netto A., Vaz A.C.N., Capodifóglio E., Gonçalves A.C.S., Rossi G.A.M., Figueiredo A.S. & Ruiz V.L.A. 2017. Pseudomonas spp.: contamination sources in bulk tanks of dairy farms. Pesq. Vet. Bras. 37(9):941-948. <http://dx.doi.org/10.1590/s0100-736x2017000900008>
https://doi.org/10.1590/s0100-736x201700...
). From the average counts in the samples evaluated in the present study, however, it is possible to affirm that Pseudomonas spp. represent only 0,06% of the psychrotrophs (6.86×105 of 1.07×109UFC/g) of the inspected Frescal Minas cheese samples and 0.46% (2.08×106 of 4.5×108CFU/g) of the non-inspected ones evaluated in the present study. Other psychrotrophs than non-Pseudomonas can be more important for the Brazilian MFC quality and shelf-life, as reported by Ribeiro Júnior et al. (2018)Ribeiro Júnior J.C., Oliveira A.M., Silva F.G., Tamanini R., Oliveira A.L.M. & Beloti V. 2018. The main spoilage related psychrotrophic bacteria in refrigerated raw milk. J. Dairy Sci. 101(1):75-83. <http://dx.doi.org/10.3168/jds.2017-13069> <PMid:29102138>
https://doi.org/10.3168/jds.2017-13069...
in Brazilian raw milk.
Microorganisms of the Pseudomonas genus in raw milk are eliminated by pasteurization (Dogan & Boor 2003Dogan B. & Boor K.J. 2003. Genetic diversity and spoilage potentials among Pseudomonas spp. isolated from fluid milk products and dairy processing plants. Appl. Environ. Microbiol. 69(1):130-138. <http://dx.doi.org/10.1128/AEM.69.1.130-138.2003> <PMid:12513987>
https://doi.org/10.1128/AEM.69.1.130-138...
). Their isolation from inspected cheese samples can be due to recontamination after milk pasteurization, at any stage of production.
It is known that the low efficiency of cleaning and sanitizing of utensils (Gruetzmacher & Bradley 1999Gruetzmacher T.J. & Bradley Junior R.L. 1999. Identification and control of processing variables that affect the quality and safety of fluid milk. J. Food Protect. 62(6):625-631. <http://dx.doi.org/10.4315/0362-028X-62.6.625> <PMid:10382651>
https://doi.org/10.4315/0362-028X-62.6.6...
), the capacity of biofilm formation on equipment, temperature, storage time, (Hammad 2015Hammad A.M. 2015. Spoilage potential of Pseudomonas spp. isolated from domiati cheese. Assiut Vet. Med. J. 61(147):18-23.) and the poor quality of water (Cousin & Bramley 1981Cousin M.A. & Bramley A.J. 1981. The microbiology of raw milk, p.119-163. In: Robinson R.K. (Ed.), Dairy Microbiology of Milk. Applied Science Publishers, London., Fagundes et al. 2006Fagundes C.M., Fischer V., Silva W.P., Carbonera N. & Araújo M.R. 2006. Presence of Pseudomonas spp. in milking phases with different hygienic handling procedures and in refrigerated milk. Ciência Rural 36(2):568-572. <http://dx.doi.org/10.1590/S0103-84782006000200032>
https://doi.org/10.1590/S0103-8478200600...
) may influence the contamination of processed cheese samples by Pseudomonasspp. in industries. Therefore, additional cleaning and sanitizing measures for equipment should be implemented, which will uphold the quality of pasteurized milk, thus ensuring a better quality of the final product. The presence of Pseudomonas spp. in non-inspected cheeses can be due to possible manufacturing with raw milk or environmental contamination at any point in the processing or marketing of the cheese.
A study by Sangaletti et al. (2009)Sangaletti N., Porto E., Brazaca S.G.C., Yagasaki C.A., Dalla Dea R.C. & Silva M.V. 2009. Shelf-life of Minas Frescal cheese. Ciênc. Tecnol. Aliment. 29(2):262-269. <http://dx.doi.org/10.1590/S0101-20612009000200004>
https://doi.org/10.1590/S0101-2061200900...
demonstrated that 96.38% of psychrotrophic bacteria isolated from MFC showed lipolytic potential, and 78.17% showed proteolytic potential, which is as high as the value in the present study (Table 1).
Dogan & Boor (2003)Dogan B. & Boor K.J. 2003. Genetic diversity and spoilage potentials among Pseudomonas spp. isolated from fluid milk products and dairy processing plants. Appl. Environ. Microbiol. 69(1):130-138. <http://dx.doi.org/10.1128/AEM.69.1.130-138.2003> <PMid:12513987>
https://doi.org/10.1128/AEM.69.1.130-138...
isolated 338 strains of Pseudomonas spp. from raw milk and found that 51% were producers of proteases and 67% were producers of lipase, suggesting that the lipolytic and proteolytic activities varied among the different species of Pseudomonas spp. In these study the Pseudomonas are identify only at genus level. In the study by Hammad (2015)Hammad A.M. 2015. Spoilage potential of Pseudomonas spp. isolated from domiati cheese. Assiut Vet. Med. J. 61(147):18-23., of 80 Domiati cheese samples collected, 70 were positive for Pseudomonas spp. Of the 80 isolates confirmed as Pseudomonas, 97.5% and 87.5% were potentially proteolytic and lipolytic, respectively. P. fluorescens was the most common species isolated.
The results of AprX production by Pseudomonas spp. in this study were higher than that in the study of Hammad (2015)Hammad A.M. 2015. Spoilage potential of Pseudomonas spp. isolated from domiati cheese. Assiut Vet. Med. J. 61(147):18-23., who reported that 33 (41.25%) isolates of Pseudomonas spp. from cheeses were aprX positive.
It can be verified that not all the isolates that exhibit aprX express it constantly, since isolates that showed the aprX gene did not present proteolytic activity in plaques (Table 2). This intermittence in the expression of aprX may be conditioned by other factors, such as the availability of other substrates in the medium, as was also observed in another study (Ribeiro Júnior et al. 2018Ribeiro Júnior J.C., Oliveira A.M., Silva F.G., Tamanini R., Oliveira A.L.M. & Beloti V. 2018. The main spoilage related psychrotrophic bacteria in refrigerated raw milk. J. Dairy Sci. 101(1):75-83. <http://dx.doi.org/10.3168/jds.2017-13069> <PMid:29102138>
https://doi.org/10.3168/jds.2017-13069...
).
Serratia spp. are known psychrotrophs with a potential expression of AprX (Ribeiro Júnior et al. 2018Ribeiro Júnior J.C., Oliveira A.M., Silva F.G., Tamanini R., Oliveira A.L.M. & Beloti V. 2018. The main spoilage related psychrotrophic bacteria in refrigerated raw milk. J. Dairy Sci. 101(1):75-83. <http://dx.doi.org/10.3168/jds.2017-13069> <PMid:29102138>
https://doi.org/10.3168/jds.2017-13069...
). The present study identified all the isolates of this genus in samples of formally produced MFC (Table 3). This bacterium, as well as all other gram-negative bacteria, is not thermoduric bacteria. Thus, it is possible to affirm that the contamination of cheeses by these micro-organisms occurred after pasteurization, i.e., during processing.
Genetic Identification and spoilage activity of psychrotrophic bacteria with expression potential of alkaline metalloprotease (aprX) not confirmed as Pseudomonas spp., isolated from inspected and non-inspected Minas Frescal cheese samples
Despite displaying an expression potential of AprX, Acinetobacter schindleri, Psychrobacter sanguinis, and Leuconostoc mesenteroides showed no proteolytic activity on the plates, but only a lipolytic activity (Table 3). Thus, like strains of Pseudomonas spp., the expression of aprX may be related to other unknown factors.
The genus Raoultella was separated from the genus Klebsiella in 2001 (Drancourt et al. 2001Drancourt M., Bollet C., Carta A. & Rousselier P. 2001. Phylogenetic analyses of Klebsiella species delineate Klebsiella and Raoultella gen. nov., with description of Raoultella ornithinolytica comb. nov., Raoultella terrigena comb. nov. and Raoultella planticola comb. nov. Int. J. Syst. Evol. Microbiol. 51(Pt 3):925-932. <http://dx.doi.org/10.1099/00207713-51-3-925> <PMid:11411716>
https://doi.org/10.1099/00207713-51-3-92...
). This is a known human pathogen (Seng et al. 2016Seng P., Boushab B.M., Romain F., Gouriet F., Bruder N., Martin C., Paganelli F., Bernit E., Treut Y.P.L., Thomas P., Papazian L., Raoult D. & Stein A. 2016. Emerging role of Raoultella ornithinolytica in human infections: a series of cases and review of the literature. Int. J. Infect. Dis. 45:65-71. <http://dx.doi.org/10.1016/j.ijid.2016.02.014> <PMid:26921549>
https://doi.org/10.1016/j.ijid.2016.02.0...
) and presents only four type sequences available in the RDP. In Figure 2, it is possible to observe that the isolate of the present study was grouped with the species Raoultella ornithinolytica and was also 100% compatible with this species in the identification by the identity matrix (Table 2). No previous reports of the production potential of AprX were found, but there is a description of a psychotrophic proteolytic agent of milk in Slovakia (Pukančíková et al. 2016Pukančíková L., Lipničanová S., Kačániová M., Chmelová D. & Ondrejovič M. 2016. Natural microflora of raw cow milk and their enzymatic spoilage potential. Nova Biotechnologica Chimica 15(2):142-155. <http://dx.doi.org/10.1515/nbec-2016-0015>).
Phylogenetic tree of type sequences of species of the genus Raoultella (GenBank accession number) prepared using the alignment of 384bp of the 16S rRNA gene, Neighbor Joining method, Tamura-Nei model, and bootstrap support for 1000 replicates. The strain marked with a diamond symbol was isolated in this study. The bar indicates the percentage of nucleotide substitution.
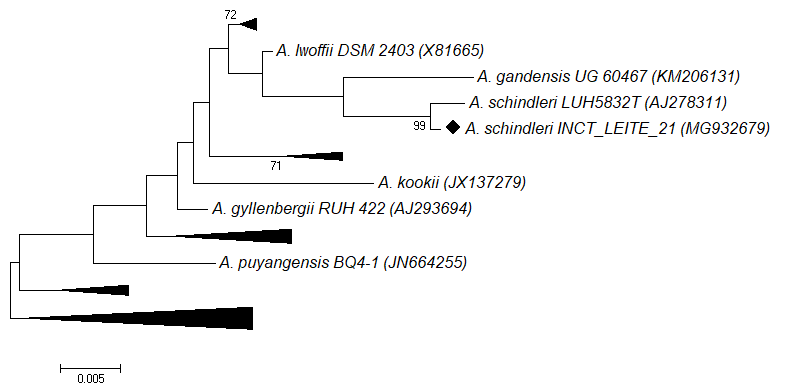
The genus Acinetobacter is a known component of spoilage-related microbiota of milk (Von Neubeck et al. 2015Von Neubeck M., Baur C., Krewinkel M., Stoeckel M., Kranz B., Stressler T., Fischer L., Hinrichs J., Scherer S. & Wenning M. 2015. Biodiversity of refrigerated raw milk microbiota and their enzymatic spoilage potential. Int. J. Food Microbiol. 211:57-65. <http://dx.doi.org/10.1016/j.ijfoodmicro.2015.07.001> <PMid:26173200>
https://doi.org/10.1016/j.ijfoodmicro.20...
). These bacteria are psychrotrophs (Vithanage et al. 2016Vithanage N.R., Dissanayake M., Bolge G., Palombo E.A., Yeager T.R. & Datta N. 2016. Biodiversity of culturable psychrotrophic microbiota in raw milk attributable to refrigeration conditions, seasonality and their spoilage potential. Int. Dairy J. 57:80-90. <http://dx.doi.org/10.1016/j.idairyj.2016.02.042>
https://doi.org/10.1016/j.idairyj.2016.0...
, Xin et al. 2017Xin L., Meng Z., Zhang L., Cui Y., Han X. & Yi H. 2017. The diversity and proteolytic properties of psychrotrophic bacteria in raw cows´ milk from North China. Int. Dairy J. 66:34-41. <http://dx.doi.org/10.1016/j.idairyj.2016.10.014>
https://doi.org/10.1016/j.idairyj.2016.1...
) and considered as emerging pathogens associated with human infections (Turton et al. 2010Turton J.F., Shah J., Ozongwu C. & Pike R. 2010. Incidence of Acinetobacter species other than A. baumannii among clinical isolates of Acinetobacter: evidence for emerging species. J. Clin. Microbiol. 48(4):1445-1449. <http://dx.doi.org/10.1128/JCM.02467-09> <PMid:20181894>
https://doi.org/10.1128/JCM.02467-09...
). The large percentage of identity matrix similarity (Table 3) observed in the strain isolated in this study with the species A. schindleri strain type LUH5232T (accession number AJ278311) can also be found in the phylogenetic proximity of the strains in Figure 3. No previous reports on the potential of AprX expression in Acinetobacter species or the isolation of A. schindleri species from cheeses were found.
Phylogenetic tree of type sequences of species of the genus Acinetobacter (GenBank accession number) prepared using the alignment of 541bp of the 16S rRNA gene, Neighbor Joining method, Tamura-Nei model, and bootstrap support for 1000 replicates. The strain marked with a diamond symbol was isolated in this study. The bar indicates the percentage of nucleotide substitution.
P. sanguinis isolated in this study is reported to be an uncommon human pathogen (Le Guern et al. 2014Le Guern R., Wallet F., Vega E., Courcol R.J. & Loïez C. 2014. Psychrobacter sanguinis: an unusual bacteria for a nosocomial meningitis. J. Clin. Microbiol. 52(9):3475-3477. <http://dx.doi.org/10.1128/JCM.01197-14> <PMid:24989605>
https://doi.org/10.1128/JCM.01197-14...
) with a preference for cold (Rodrigues et al. 2009Rodrigues D.F., Jesus E.C., Ayala-Del-Río H.L., Pellizari V.H., Gilichinsky D., Sepulveda-Torres L. & Tiedje J.M. 2009. Biogeography of two cold-adapted genera: Psychrobacter and Exiguobacterium. ISME J. 3(6):658-665. <http://dx.doi.org/10.1038/ismej.2009.25> <PMid:19322243>
https://doi.org/10.1038/ismej.2009.25...
) and aquatic environments (Wirth et al. 2012Wirth S.E., Ayala-Del-Río H.L., Cole J.A., Kohlerschmidt D.J., Musser K.A., Sepúlveda-Torres L.C., Thompson L.M. & Wolfgang W.J. 2012. Psychrobacter sanguinis sp. nov., recovered from four clinical specimens over a 4-year period. Int. J. Syst. Evol. Microbiol. 62(Pt 1):49-54. <http://dx.doi.org/10.1099/ijs.0.029058-0> <PMid:21317274>
https://doi.org/10.1099/ijs.0.029058-0...
). No previous reports of the isolation of this species in milk or cheeses, or their spoilage potential, were found. Delbès et al. (2007)Delbès C., Ali-Mandjee L. & Montel M.C. 2007. Monitoring bacterial communities in raw milk and cheese by culture-dependent and -independent 16s rrna gene-based analyses. Appl. Environ. Microbiol. 73(6):1882-1891. <http://dx.doi.org/10.1128/AEM.01716-06> <PMid:17259356>
https://doi.org/10.1128/AEM.01716-06...
found only the species P. faecalis in raw milk and cheeses in France. In Figure 4, one can observe the phylogenetic proximity of the strain isolated by the present work with the P. sanguinis type strain, with a similarity calculated at 99.8%.
Phylogenetic tree of sequences of type species of the genus Psychrobacter accession (GenBank accession number) prepared using the alignment of 468bp of the 16S rRNA gene, Neighbor Joining method, Tamura-Nei model, and bootstrap support for 1000 replicates. The CEPA marked with diamond symbol was isolated in this study. The bar indicates the percentage of nucleotide substitution.
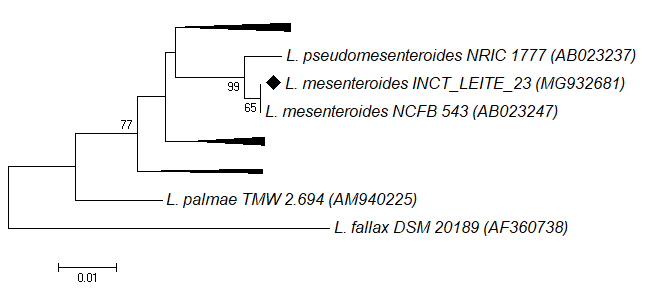
Bacteria of the genus Leuconostoc are described as lactic acid bacteria (LAB) (Kleppen et al. 2012Kleppen H.P., Nes I.F. & Holo H. 2012. Characterization of a Leuconostoc bacteriophage infecting flavor producers of cheese starter cultures. Appl. Environ. Microbiol. 78(18):6769-6772. <http://dx.doi.org/10.1128/AEM.00562-12> <PMid:22798359>
https://doi.org/10.1128/AEM.00562-12...
). Figure 5 indicates that the isolate from this study presents 100% of phylogenetic proximity with the type species L. mesenteroides (AB023247). This isolate was obtained from a sample of non-inspected cheese. Therefore, the possibility that this strain originates from some LAB culture used as a fermenting agent in cheese production is discarded, or rather, it is a component of the autochthonous milk microbiota. The expression potential of AprX by this strain is also relevant, since this enzyme is related to the Gammaproteobacteria class, according to Table 3.
Phylogenetic tree of sequences of type species of the genus Leuconostoc (GenBank accession number) prepared using the alignment of 323bp of the 16S rRNA gene, Neighbor Joining method, Tamura-Nei model, and bootstrap support for 1000 replicates. The strain marked with a diamond symbol was isolated in this study. The bar indicates the percentage of nucleotide substitution.
A study by Ribeiro Júnior et al. (2018)Ribeiro Júnior J.C., Oliveira A.M., Silva F.G., Tamanini R., Oliveira A.L.M. & Beloti V. 2018. The main spoilage related psychrotrophic bacteria in refrigerated raw milk. J. Dairy Sci. 101(1):75-83. <http://dx.doi.org/10.3168/jds.2017-13069> <PMid:29102138>
https://doi.org/10.3168/jds.2017-13069...
also demonstrated that other psychrotrophic microorganisms from milk, besides Pseudomonas spp., may present a potential expression of AprX, such as Serratia ureilytica, Enterobacter kobei and Yersinia enterocolitica, all belonging to the Gammaproteobacteria class, in addition to R. ornithinolytica, A. schindleri and P. sanguinis identified in this study (Table 3). However, the isolation of L. mesenteroides (Bacilli) reveals that the dispersion of aprX can go beyond the Gammaproteobacteria class, broadening the spectrum of spoilage-related microorganisms of milk and dairy products.
Conclusions
Pseudomonas spp. and other potential spoilage-related psychrotrophs can be isolated in equivalent quantities, both in formally produced and commercialized and non-inspected MFC in Brazil.
The industrial production processes are not sufficient to control the contamination of MFC by these microorganisms, since the cheese possibly manufactured from raw milk present lower counts of spoilage-related psychrotrophs than that industrially processed with pasteurized milk. Or, it is possible that the lack of refrigeration of the informal cheese was determinant of the low count of psychrotrophs in relation to the formal MFC.
Pseudomonas and other psychrotrophs isolated from MFC are, in their majority, simultaneously proteolytic and lipolytic, and may deteriorate the quality of the cheeses. Furthermore, microorganisms not yet described as cheese-spoilage bacteria may be emerging targets for quality control, and the factors that influence the expression and the dispersion of aprX gene between genera of psychrotrophs need to be elucidated.
The production of MFC with pasteurized milk should be safeguarded to avoid the risk to consumer health. In addition, industrial hygiene practices should be followed to ensure cheese production with less contamination by spoilage microorganisms.
Acknowledgements
This study was supported by the following Brazilian institutes: National Council of Scientific and Technological Development (CNPq, grant number 305062/2015-8, Brasília, Brazil), Brazilian Federal Agency for Support and Evaluation of Graduate Education (CAPES, process 88887.145705/2017-00, Brasília, Brazil), Financing of Studies and Projects (FINEP, Brasília, Brazil), and the Araucaria Foundation (FAP-PR, Curitiba, Paraná, Brazil). The authors are grateful for the collaboration of the researcher Elis Lorenzetti for support in molecular biology.
References
- Bach H.J., Hartmann A., Schloter M. & Munch J.C. 2001. PCR primers and functional probes for amplification and detection of bacterial genes for extracellular peptidases in single strains and in soil. J. Microbiol. Methods 44(2):173-182. <http://dx.doi.org/10.1016/S0167-7012(00)00239-6> <PMid:11165346>
» https://doi.org/10.1016/S0167-7012(00)00239-6 - Bagliniere F., Mateos A., Tanguy G., Jardin J., Briard-Bion V., Rousseau F., Robert B., Beaucher E., Gaillard J.L., Amiel C., Humbert G., Dary A. & Gaucheron F. 2013. Proteolysis of ultra-high temperature- treated casein micelles by AprX enzyme from Pseudomonas fluorescens induces their destabilisation. Int. Dairy J. 31(2):55-61. <http://dx.doi.org/10.1016/j.idairyj.2013.02.011>
» https://doi.org/10.1016/j.idairyj.2013.02.011 - Brasil 1997. Regulamento Técnico Para Fixação de Identidade e Qualidade do Queijo Minas Frescal. Portaria nº 352, de 04 de setembro de 1997, Diário Oficial da União, Ministério da Agricultura, Pecuária e Abastecimento, Brasília, DF.
- Brasil 2004. Regulamento Técnico para Fixação de Identidade e Qualidade do Queijo Minas Frescal. Instrução Normativa nº 4, de 1 de março de 2004, Diário Oficial da União,.
- Campos A.C.L.P., Puño-Sarmiento J.J., Medeiros L.P., Gazal L.E.S., Maluta R.P., Navarro A., Kobayashi R.K.T., Fagan E.P. & Nakazato G. 2017. Virulence genes and antimicrobial resistance in Escherichia coli from cheese made from unpasteurized milk in Brazil. Foodborne Pathog Dis. 15(2):94-110. <http://dx.doi.org/10.1089/fpd.2017.2345> <PMid:29215297>
» https://doi.org/10.1089/fpd.2017.2345 - Carvalho J.D.G., Viotto W.H. & Kuaye A.Y. 2007. The quality of Minas Frescal cheese produced by different technological processes. Food Control 18(3):262-267. <http://dx.doi.org/10.1016/j.foodcont.2005.10.005>
» https://doi.org/10.1016/j.foodcont.2005.10.005 - Cousin M.A. & Bramley A.J. 1981. The microbiology of raw milk, p.119-163. In: Robinson R.K. (Ed.), Dairy Microbiology of Milk. Applied Science Publishers, London.
- Delbès C., Ali-Mandjee L. & Montel M.C. 2007. Monitoring bacterial communities in raw milk and cheese by culture-dependent and -independent 16s rrna gene-based analyses. Appl. Environ. Microbiol. 73(6):1882-1891. <http://dx.doi.org/10.1128/AEM.01716-06> <PMid:17259356>
» https://doi.org/10.1128/AEM.01716-06 - Dogan B. & Boor K.J. 2003. Genetic diversity and spoilage potentials among Pseudomonas spp. isolated from fluid milk products and dairy processing plants. Appl. Environ. Microbiol. 69(1):130-138. <http://dx.doi.org/10.1128/AEM.69.1.130-138.2003> <PMid:12513987>
» https://doi.org/10.1128/AEM.69.1.130-138.2003 - Drancourt M., Bollet C., Carta A. & Rousselier P. 2001. Phylogenetic analyses of Klebsiella species delineate Klebsiella and Raoultella gen. nov., with description of Raoultella ornithinolytica comb. nov., Raoultella terrigena comb. nov. and Raoultella planticola comb. nov. Int. J. Syst. Evol. Microbiol. 51(Pt 3):925-932. <http://dx.doi.org/10.1099/00207713-51-3-925> <PMid:11411716>
» https://doi.org/10.1099/00207713-51-3-925 - Dufour D., Nicodeme M., Perrin C., Driou A., Brusseaux E., Humbert G., Gaillard J.L. & Dary A. 2008. Molecular typing of industrial strains of Pseudomonas spp. isolated from milk and genetical and biochemical characterization of an extracellular protease produced by one of them. Int. J. Food Microbiol. 125(2):188-196. <http://dx.doi.org/10.1016/j.ijfoodmicro.2008.04.004> <PMid:18511140>
» https://doi.org/10.1016/j.ijfoodmicro.2008.04.004 - Fagundes C.M., Fischer V., Silva W.P., Carbonera N. & Araújo M.R. 2006. Presence of Pseudomonas spp. in milking phases with different hygienic handling procedures and in refrigerated milk. Ciência Rural 36(2):568-572. <http://dx.doi.org/10.1590/S0103-84782006000200032>
» https://doi.org/10.1590/S0103-84782006000200032 - Gruetzmacher T.J. & Bradley Junior R.L. 1999. Identification and control of processing variables that affect the quality and safety of fluid milk. J. Food Protect. 62(6):625-631. <http://dx.doi.org/10.4315/0362-028X-62.6.625> <PMid:10382651>
» https://doi.org/10.4315/0362-028X-62.6.625 - Hall T.A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41:95-98.
- Hammad A.M. 2015. Spoilage potential of Pseudomonas spp. isolated from domiati cheese. Assiut Vet. Med. J. 61(147):18-23.
- Hantsis-Zacharov E. & Halpern M. 2007. Culturable psychrotrophic bacterial communities in raw milk and their proteolytic and lipolytic traits. Appl. Environ. Microbiol. 73(22):7162-7168. <http://dx.doi.org/10.1128/AEM.00866-07> <PMid:17890340>
» https://doi.org/10.1128/AEM.00866-07 - Huang X. & Madan A. 1999. CAP3: a DNA sequence assembly program. Genome Res. 9(9):868-877. <http://dx.doi.org/10.1101/gr.9.9.868> <PMid:10508846>
» https://doi.org/10.1101/gr.9.9.868 - ISO 2009. ISO 11.059, Milk and Milk Products: method for the enumeration of Pseudomonas spp. ISO/TS 11059:2009 (IDF/RM 225:2009), International Organization for Standardization, Geneva, Switzerland.
- Kleppen H.P., Nes I.F. & Holo H. 2012. Characterization of a Leuconostoc bacteriophage infecting flavor producers of cheese starter cultures. Appl. Environ. Microbiol. 78(18):6769-6772. <http://dx.doi.org/10.1128/AEM.00562-12> <PMid:22798359>
» https://doi.org/10.1128/AEM.00562-12 - Kumar S., Stecher G. & Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33(7):1870-1874. <http://dx.doi.org/10.1093/molbev/msw054> <PMid:27004904>
» https://doi.org/10.1093/molbev/msw054 - Le Guern R., Wallet F., Vega E., Courcol R.J. & Loïez C. 2014. Psychrobacter sanguinis: an unusual bacteria for a nosocomial meningitis. J. Clin. Microbiol. 52(9):3475-3477. <http://dx.doi.org/10.1128/JCM.01197-14> <PMid:24989605>
» https://doi.org/10.1128/JCM.01197-14 - Marchand S., Vandriesche G., Coorevits A., Coudijzer K., De Jonghe V., Dewettinck K., De Vos P., Devreese B., Heyndrickx M. & De Block J. 2009. Heterogeneity of heat-resistant proteases from milk Pseudomonas species. Int. J. Food Microbiol. 133(1/2):68-77. <http://dx.doi.org/10.1016/j.ijfoodmicro.2009.04.027> <PMid:19481283>
» https://doi.org/10.1016/j.ijfoodmicro.2009.04.027 - Murphy S.C., Martin N.H., Barbano D.M. & Wiedmann M. 2016. Influence of raw milk quality on processed dairy products: How do raw milk quality test results relate to product quality and yield? J. Dairy Sci. 99(12):10128-10149. <http://dx.doi.org/10.3168/jds.2016-11172> <PMid:27665134>
» https://doi.org/10.3168/jds.2016-11172 - Oliveira G.B., Favarin L., Luchese R.H. & McIntosh D. 2015. Psychrotrophic bacteria in milk: how much do we really know? Braz. J. Microbiol. 46(2):313-321. <http://dx.doi.org/10.1590/S1517-838246220130963> <PMid:26273245>
» https://doi.org/10.1590/S1517-838246220130963 - Osborne C.A., Galic M., Sangwan P. & Janssen P.H. 2005. PCRgenerated artefact from 16S rRNA gene-specific primers. FEMS Microbiol. Lett. 248(2):183-187. <http://dx.doi.org/10.1016/j.femsle.2005.05.043> <PMid:15961258>
» https://doi.org/10.1016/j.femsle.2005.05.043 - Ozturkoglu-Budak S., Wiebenga A., Bron P.A. & de Vries R.P. 2016. Protease and lipase activities of fungal and bacterial strains derived from an artisanal raw ewe’s milk cheese. Int. J. Food Microbiol. 237:17-27. <http://dx.doi.org/10.1016/j.ijfoodmicro.2016.08.007> <PMid:27541978>
» https://doi.org/10.1016/j.ijfoodmicro.2016.08.007 - Pukančíková L., Lipničanová S., Kačániová M., Chmelová D. & Ondrejovič M. 2016. Natural microflora of raw cow milk and their enzymatic spoilage potential. Nova Biotechnologica Chimica 15(2):142-155. <http://dx.doi.org/10.1515/nbec-2016-0015>
- Ribeiro Júnior J.C., Tamanini R., Soares B.F., Oliveira A.M., Silva F.G., Silva F.F., Augusto N.A. & Beloti V. 2016. Efficiency of boiling and four other methods for genomic DNA extraction of deteriorating spore-forming bacteria from milk. Semina, Ciênc. Agrarárias 37:3069-3078.
- Ribeiro Júnior J.C., Oliveira A.M., Silva F.G., Tamanini R., Oliveira A.L.M. & Beloti V. 2018. The main spoilage related psychrotrophic bacteria in refrigerated raw milk. J. Dairy Sci. 101(1):75-83. <http://dx.doi.org/10.3168/jds.2017-13069> <PMid:29102138>
» https://doi.org/10.3168/jds.2017-13069 - Rodrigues D.F., Jesus E.C., Ayala-Del-Río H.L., Pellizari V.H., Gilichinsky D., Sepulveda-Torres L. & Tiedje J.M. 2009. Biogeography of two cold-adapted genera: Psychrobacter and Exiguobacterium ISME J. 3(6):658-665. <http://dx.doi.org/10.1038/ismej.2009.25> <PMid:19322243>
» https://doi.org/10.1038/ismej.2009.25 - Samaržija D., Zamberlin Š. & Pogačić T. 2012. Psychrotrophic bacteria and milk and dairy products quality. Mljekarstvo. Dairy 62(2):77-95.
- Sangaletti N., Porto E., Brazaca S.G.C., Yagasaki C.A., Dalla Dea R.C. & Silva M.V. 2009. Shelf-life of Minas Frescal cheese. Ciênc. Tecnol. Aliment. 29(2):262-269. <http://dx.doi.org/10.1590/S0101-20612009000200004>
» https://doi.org/10.1590/S0101-20612009000200004 - Scatamburlo T.M., Yamazi A.K., Cavicchioli V.Q., Pieri F.A. & Nero L.A. 2015. Spoilage potential of Pseudomonas species isolated from goat milk. J. Dairy Sci. 98(2):759-764. <http://dx.doi.org/10.3168/jds.2014-8747> <PMid:25497792>
» https://doi.org/10.3168/jds.2014-8747 - Seng P., Boushab B.M., Romain F., Gouriet F., Bruder N., Martin C., Paganelli F., Bernit E., Treut Y.P.L., Thomas P., Papazian L., Raoult D. & Stein A. 2016. Emerging role of Raoultella ornithinolytica in human infections: a series of cases and review of the literature. Int. J. Infect. Dis. 45:65-71. <http://dx.doi.org/10.1016/j.ijid.2016.02.014> <PMid:26921549>
» https://doi.org/10.1016/j.ijid.2016.02.014 - Spilker T., Coenye T., Vandamme P. & Lipuma J.J. 2004. PCR-based assay for differentiation of Pseudomonas aeruginosa from other Pseudomonas species recovered from cystic fibrosis patients. J. Clin. Microbiol. 42(5):2074-2079. <http://dx.doi.org/10.1128/JCM.42.5.2074-2079.2004> <PMid:15131172>
» https://doi.org/10.1128/JCM.42.5.2074-2079.2004 - Turton J.F., Shah J., Ozongwu C. & Pike R. 2010. Incidence of Acinetobacter species other than A. baumannii among clinical isolates of Acinetobacter: evidence for emerging species. J. Clin. Microbiol. 48(4):1445-1449. <http://dx.doi.org/10.1128/JCM.02467-09> <PMid:20181894>
» https://doi.org/10.1128/JCM.02467-09 - Vidal A.M.C., Saran Netto A., Vaz A.C.N., Capodifóglio E., Gonçalves A.C.S., Rossi G.A.M., Figueiredo A.S. & Ruiz V.L.A. 2017. Pseudomonas spp.: contamination sources in bulk tanks of dairy farms. Pesq. Vet. Bras. 37(9):941-948. <http://dx.doi.org/10.1590/s0100-736x2017000900008>
» https://doi.org/10.1590/s0100-736x2017000900008 - Vithanage N.R., Dissanayake M., Bolge G., Palombo E.A., Yeager T.R. & Datta N. 2016. Biodiversity of culturable psychrotrophic microbiota in raw milk attributable to refrigeration conditions, seasonality and their spoilage potential. Int. Dairy J. 57:80-90. <http://dx.doi.org/10.1016/j.idairyj.2016.02.042>
» https://doi.org/10.1016/j.idairyj.2016.02.042 - Von Neubeck M., Baur C., Krewinkel M., Stoeckel M., Kranz B., Stressler T., Fischer L., Hinrichs J., Scherer S. & Wenning M. 2015. Biodiversity of refrigerated raw milk microbiota and their enzymatic spoilage potential. Int. J. Food Microbiol. 211:57-65. <http://dx.doi.org/10.1016/j.ijfoodmicro.2015.07.001> <PMid:26173200>
» https://doi.org/10.1016/j.ijfoodmicro.2015.07.001 - Wirth S.E., Ayala-Del-Río H.L., Cole J.A., Kohlerschmidt D.J., Musser K.A., Sepúlveda-Torres L.C., Thompson L.M. & Wolfgang W.J. 2012. Psychrobacter sanguinis sp. nov., recovered from four clinical specimens over a 4-year period. Int. J. Syst. Evol. Microbiol. 62(Pt 1):49-54. <http://dx.doi.org/10.1099/ijs.0.029058-0> <PMid:21317274>
» https://doi.org/10.1099/ijs.0.029058-0 - Xin L., Meng Z., Zhang L., Cui Y., Han X. & Yi H. 2017. The diversity and proteolytic properties of psychrotrophic bacteria in raw cows´ milk from North China. Int. Dairy J. 66:34-41. <http://dx.doi.org/10.1016/j.idairyj.2016.10.014>
» https://doi.org/10.1016/j.idairyj.2016.10.014
-
4
Ribosomal Database Project (RDP), Center for Microbial Ecology, Michigan State University, Michigan, USA. Available at <https://rdp.cme.msu.edu/hierarchy>
Publication Dates
-
Publication in this collection
02 Dec 2019 -
Date of issue
Oct 2019
History
-
Received
18 Feb 2019 -
Accepted
01 Apr 2019