ABSTRACT:
Primary hepatobiliary neoplasms (PHN) are uncommon in cats, and originate in hepatocytes, intra- and extrahepatic bile ducts, mesenchymal cells, and cells of neuroendocrine origin. The aim of this study was to determine the frequency of PHN in cats diagnosed in the metropolitan region of Porto Alegre (RS), Brazil, for a period of 17 years, determining their epidemiological, anatomopathological and immunohistochemical aspects. Necropsy reports of 2.090 cats were analyzed, 125 were diagnosed with primary hepatobiliary diseases, of which 15 were cases of PHN, representing 12% of the specific hepatobiliary conditions and 0.7% of the necropsies. All PHN were malignant, of which 93.3% had epithelial origin and 6.7% presented mesenchymal origin. Cholangiocarcinoma was the most commonly diagnosed neoplasm, followed by hepatocellular carcinoma and hemangiosarcoma. In general, cats with no defined breed were the most affected. Concerning sex, 60% were females and 40% males. Age ranged from five to 18 years, with a mean age of 10.5 years (median of ten years). Grossly, cholangiocarcinoma and hemangiosarcoma were multinodular and hepatocellular carcinoma was massive. Microscopically, cholangiocarcinomas were arranged in acini and ducts, whereas hepatocellular carcinomas were arranged in solid sheets or trabeculae. On immunohistochemistry, cholangiocarcinomas, hepatocellular carcinomas, and hemangiosarcomas were positive for the antibodies CK 7, Hep Par-1, and vimentin and von Willebrand factor, respectively.
INDEX TERMS:
Felines; pathology; immunohistochemistry; hepatobiliary carcinoma; neoplasm; cats; cholangiocarcinoma; hepatic hemangiosarcoma
RESUMO:
Neoplasias hepatobiliares primárias (NHP) são incomuns em gatos e se originam de hepatócitos, células dos ductos biliares intra e extra-hepáticos, células mesenquimais e ainda células de origem neuroendócrina. O objetivo do trabalho foi determinar a frequência das NHP em gatos diagnosticados na Região Metropolitana de Porto Alegre, no período de 17 anos, abordando seus aspectos epidemiológicos, anatomopatológicos e imuno-histoquímicos (IHQ). Foram analisados os laudos de necropsia de 2.090 gatos sendo que 125 foram diagnosticados com doenças hepatobiliares primárias, destes 15 foram casos de NHP, representando 12% das condições hepatobiliares específicas e 0,7% do total de necropsias. Todos os diagnósticos de NHP eram malignos, destes 93,3% apresentaram origem epitelial e 6,7% mesenquimal. Colangiocarcinoma foi a neoplasia mais diagnosticada, seguido do carcinoma hepatocelular e hemangiossarcoma. De uma maneira geral, os gatos sem raça definida foram os mais acometidos. Em relação ao sexo 60% eram fêmeas e 40% machos. A idade variou de cinco a 18 anos, com a idade média de 10,5 anos (mediana de 10 anos). Macroscopicamente o colangiocarcinoma e hemangiossarcoma eram multinodulares, e o carcinoma hepatocelular, maciço. À histologia, houve predomínio do arranjo acinar e ductal nos colangiocarcinomas e sólido, no carcinoma hepatocelular. Na IHQ os colangiocarcinomas foram reativos para CK 7, carcinoma hepatocelular para Hep Par-1 e hemangiossarcoma para vimentina e fator de von Willebrand.
TERMOS DE INDEXAÇÃO:
Felinos; patologia; imuno-histoquímica; carcinoma hepatobiliar; colangiocarcinoma; hemangiossarcoma hepático
Introduction
Primary hepatobiliary neoplasms (PHN) are uncommon in cats (Cullen & Stalker 2016Cullen J.M. & Stalker M.J. 2016. Liver and biliary system, p.307-308. In: Maxie M.G. (Ed.), Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol.2. 6th ed. Elsevier, St Louis. <http://dx.doi.org/10.1016/B978-0-7020-5318-4.00008-5>.
https://doi.org/10.1016/B978-0-7020-5318...
), and originate in hepatocytes, intra- and extrahepatic bile duct cells, mesenchymal cells, and cells of neuroendocrine origin (Head et al. 2003Head K.W., Cullen J.M., Dubielzig R.R., Else R.W., Misdorp W., Patnaik A.K., Tateyama S. & Van der Gaag I. 2003. Histological Classification of Tumors of the Alimentary System of Domestic Animals. Vol.5. 2nd ed. Armed Forces Institute of Pathology, Washington. 257p.). They usually affect older cats, with no predisposition for breed or sex (Cullen 2017Cullen J.M. 2017. Tumors of the liver and gallbladder, p.602-631. In: Meuten D.J. (Ed), Tumors in Domestic Animals. 5th ed. John Wiley & Sons, Iowa.). Anorexia, lethargy, weight loss, hepatomegaly identified on abdominal palpation, and jaundice may be present, but clinical signs are often nonspecific, making clinical diagnosis difficult (Rutgers 1998Rutgers C. 1998. Feline liver disease. In Practice 20(1):16-25. <http://dx.doi.org/10.1136/inpract.20.1.16>
https://doi.org/10.1136/inpract.20.1.16...
, Barros 2016Barros C.S.L. 2016. Fígado, vias biliares e pâncreas exócrino, p.222-265. In: Santos R.L. & Alessi A.C. (Eds), Patologia Veterinária. 2ª ed. Roca, Rio de Janeiro.). The use of immunohistochemistry assists with the diagnosis of PHN (Patnaik 1992Patnaik A.K. 1992. A morphological and immunocytochemical study of hepatic neoplasms in cats. Vet. Pathol. 29(5):405-415. <http://dx.doi.org/10.1177/030098589202900506> <PMid:1413408>
https://doi.org/10.1177/0300985892029005...
), and treatment and prognosis are determined by gross pattern and histological features, with indication for surgical resection in cases of neoplasms confined to only one hepatic lobe (Stonehewer 2006Stonehewer J. 2006. Fígado e pâncreas, p.358-372. In: Chandler E.A. & Gaskell R.M. (Eds), Clínica e Terapêutica em Felinos. 3ª ed. Roca, São Paulo.). The present study aimed to determine the frequency of PHN in cats diagnosed in the metropolitan region of Porto Alegre (RS), Brazil, for 17 years, addressing their epidemiological and pathological aspects.
Materials and Methods
PHN cases in the necropsy reports of cats archived at the Department of Veterinary Pathology of the “Universidade Federal do Rio Grande do Sul”, from January 1999 to December 2016, were reviewed and selected. Data described in necropsy requests, such as breed, sex, age and gross lesions, were reviewed and compiled. All cases analyzed were from the metropolitan region of Porto Alegre. Of the selected cases, the archived paraffin-embedded blocks were searched for the preparation of 3 µm-thick sections on histological slides for subsequent staining by the hematoxylin and eosin technique and visualization by optical microscopy.
The gross pattern and histological classification of the present study followed the criteria established by World Health Organization (WHO) (Head et al. 2003Head K.W., Cullen J.M., Dubielzig R.R., Else R.W., Misdorp W., Patnaik A.K., Tateyama S. & Van der Gaag I. 2003. Histological Classification of Tumors of the Alimentary System of Domestic Animals. Vol.5. 2nd ed. Armed Forces Institute of Pathology, Washington. 257p.). For evaluation of the degree of fibrosis and quantification of mucin expression, histological sections were subjected to special Masson’s trichrome (MT) and periodic acid-Schiff (PAS) stains. The special staining techniques followed the protocols described in the records of the Armed Forces Institute of Pathology (Gaffney 1992Gaffney E. 1992. Carbohydrates, p.151. In: Prophet E.B., Mills B., Arrington J.B. & Sobin L.H (Eds), Laboratory Methods in Histotechnology. Armed Forces Institute of Pathology, American Registry of Pathology, Washington., McElroy 1992McElroy D.A. 1992. Connective tissue, p.132. In: Prophet E.B., Mills B., Arrington J.B. & Sobin L.H (Eds), Laboratory Methods in Histotechnology. Armed Forces Institute of Pathology, American Registry of Pathology, Washington.).
Neoplasm sections were submitted to immunohistochemistry (IHC) for biliary epithelium cells (cytokeratin 7 - CK 7), hepatocytes (Hepatocyte Paraffin 1 - Hep Par-1), mesenchymal cells (vimentin), and vascular endothelium (von Willebrand factor). The primary antibodies and protocols used are specified in Table 1.
Results
Necropsy reports of 2.090 cats were analyzed, and 125 were diagnosed with primary hepatobiliary diseases, of which 15 were cases of PHN, representing 12% of the specific hepatobiliary conditions and 0.7% of the necropsies. All PHN (100%, 15/15) were malignant; of these, 93.3% (14/15) had epithelial origin and 6.7% (1/15) presented mesenchymal origin. Cholangiocarcinoma was identified in 66.6% (10/15), hepatocellular carcinoma in 26.7% (4/15), and hemangiosarcoma in 6.7% (1/15) of the cats.
In general, cats with no defined breed (NDB) were the most affected, representing 80% (12/15) of the cases, and the Siamese breed represented the remaining 20% (3/15) of the cases. Regarding sex, 60% (9/15) were females and 40% (6/15) males. Age ranged from five to 18 years, with a mean age of 10.5 years (median of ten years).
Cholangiocarcinoma
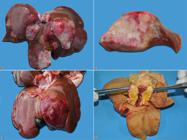
Cholangiocarcinoma was diagnosed in 66.6% (10/15) of the cases. It affected NDB cats in 80% (8/10) and Siamese cats in 20% (2/10). As for sex, 50% (5/10) were females and 50% (5/10) males. Age ranged from five to 13 years, with a mean age of 10.3 years (median of 11 years). In 80% (8/10) of the cases, cholangiocarcinoma originated in intrahepatic bile ducts and in the remaining 20% (2/10) from extrahepatic ducts (13.3%, 2/15). Grossly, the multinodular pattern was identified in 60% (6/10), and was characterized by multifocal to coalescent nodules, firm on palpation, and whitish with red areas (Fig.1A,B), and sometimes presented central depression (umbilicated aspect). The massive pattern was observed in 20% (2/10) of the cases, with the right lateral lobe affected in one case (50%, 1/2) and the left medial lobe affected one other case (50%, 1/2). These were firm on palpation and red interspersed with whitish areas (Fig.1C). The extrahepatic cases involved the cystic duct and were characterized by whitish to yellowish masses, firm on palpation, and with biliary flow obstruction (Fig.1D). Extrahepatic gross findings were mainly characterized by poor body condition and free slightly reddish serous fluid in the abdominal cavity (ascites), identified in 50% (5/10) of cats with cholangiocarcinoma. In 40% (4/10) of the cases, nodules with gross appearance similar to those of the liver were identified in various organs, such as regional (hepatic) lymph nodes (75%, 3/4), peritoneum, diaphragm, intestinal and gastric serosa, lungs (50%, 2/4 each), and kidneys (25%, 1/4). Yellow oral and conjunctival mucosa, skin and subcutaneous tissue (jaundice) and yellow slightly diminished liver with lobular pattern accentuation were observed in cats with extrahepatic cholangiocarcinomas.
Gross aspects of cholangiocarcinoma in cats. (A) Multinodular pattern. Multifocal to coalescent nodules of different sizes, whitish interspersed with red areas. (B) Cholangiocarcinoma cutting surface of Figure 1 A. (C) Massive pattern. Extensive focal mass located in the left medial lobe, red with lighter areas and central depression (umbilicated aspect). (D) Extrahepatic cholangiocarcinoma. Yellowish mass located in the cystic duct.
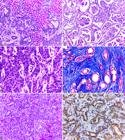
Histologically, 60% (6/10) of the cases were characterized by replacement of the hepatic parenchyma by proliferation of non-delimited and non-encapsulated epithelial cells, with formation of acini and/or irregular ducts (Fig.2A). In 30% (3/10) of the cases, the solid pattern was predominant. One case (10%, 1/10) was identified as biliary cystadenocarcinoma, characterized by formation of numerous cysts, with papillary projections to their lumen (Fig.2B). Cells were cuboid to rounded, with relatively undefined cytoplasmic borders, moderate and eosinophilic cytoplasm, round nucleus with chromatin, which ranged from dense to finely stippled, and with one to two conspicuous nucleoli (Fig.2C). Cellular pleomorphism was moderate in 50% (5/10), marked in 30% (3/10), and mild in 20% (2/10) of the cases. The mitotic index per high power field (HPF, 400x) was discrete (1/HPF) in 60% (6/10) of the cases, and moderate (2/HPF) in the remaining 40% (4/10) of the cases. Fibrous connective tissue proliferation, visualized mainly using MT staining, was marked in 50% (5/10) (scirrhous pattern) (Fig.2D), moderate in 30% (3/10), and mild in 20% (2/10) of the cases. Mucin expression was quantified as moderate in 40% (4/10), discrete in 20% (2/10), and marked in 10% (1/10) of the cases. This histological change was not identified in the remaining 30% (3/10) of the cases. Mucin was characterized by amorphous, slightly basophilic material within the acinar and ductal structures, which was intensely stained pink by PAS (Fig.2E). Intratumoral necrosis and hemorrhage were observed in 70% (7/10) and 60% (6/10) of the cases, respectively. Tumor invasion in lymphatic and/or blood vessels was identified in 40% (4/10) of the cases, and extrahepatic metastases were found mainly in hepatic lymph nodes (4/10), spleen (3/10), lungs (3/10), stomach and intestinal serosa (2/10), peritoneum (1/10), diaphragm (1/10), and kidneys (1/10).
Histological and immunohistochemical aspects of cholangiocarcinoma in cats. (A) Replacement of the hepatic parenchyma by neoplastic proliferation of epithelial cells, with formation of acini and irregular ducts, supported by moderate connective stroma. HE, obj.10x. (B) Biliary cystadenocarcinoma. Neoplastic proliferation of epithelial cells with formation of cystic structures, sometimes with papillary projections to the lumen. HE, obj.10x. (C) Enlargement of Figure 2 A showing neoplastic epithelial cells with moderate pleomorphism. HE, obj.40x. (D) Proliferation of duct-forming epithelial cells (red), interspersed with marked proliferation of fibrous connective tissue (blue). MT, obj.20x. (E) Proliferation of epithelial cells arranged in ductal and acinar pattern, with amorphous slightly basophilic material within these structures (mucin). HE, obj.20x. Inset: evidence of mucin intensely pink stained by PAS. PAS, obj.40x. (F) Marked anti-CK 7 staining in the cytoplasm of neoplastic epithelial cells, sometimes more intense near the plasma membranes. IHC, DAB, obj.40x.
Immunohistochemistry (IHC) showed that intracytoplasmic and membrane staining for CK 7 was marked in 50% (5/10) (Fig.2F), moderate in 40% (4/10), and discrete in 10% (1/10) of the cases. No cases of cholangiocarcinoma showed IHC positivity for Hep Par-1.
Hepatocellular carcinoma
Hepatocellular carcinoma represented 26.7% (4/15) of the diagnoses. All affected cats were NDB (100%, 4/4). Females corresponded to 75% (3/4) and males to 25% (1/4) of the cases. Age ranged from 10 to 18 years, with a mean age of 12.5 years (median of 11 years).
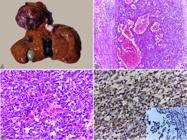
The massive gross pattern was identified in all cats (100%, 4/4), characterized by large masses, predominantly located in the quadrate lobe with extension to the right medial and lateral lobes (75%, 3/4) (Fig.3A). The left medial lobe was also affected in one case (25%, 1/4). The masses were friable, whitish to yellowish, with irregular surface and red multifocal areas. Extrahepatic gross findings were characterized by poor body condition (75%, 3/4), ascites, jaundice in mucosa and subcutaneous tissue, and large amounts of free blood and clots in the abdominal cavity (hemoperitoneum) (25%, 1/4 each).
Anatomopathological and immunohistochemical aspects of hepatocellular carcinoma in cats. (A) Gross pattern. Large whitish mass with multifocal red and yellowish areas located in the quadrate lobe extending to the right medial and lateral lobes. (B) Histological aspect. Neoplastic proliferation of hepatocytes arranged in a solid pattern. HE, obj.10x. (C) Enlargement of Figure 3 B showing neoplastic hepatocytes with moderate pleomorphism. HE, obj.40x. (D) Intense anti-Hep Par-1 staining in the cytoplasm of neoplastic hepatocytes. IHC, DAB, obj.40x.
Histologically, there was disorganization of the organ architecture caused by proliferation of neoplastic hepatocytes with solid pattern (75%, 3/4), which were characterized by dense mantle cells (Fig.3B), sometimes with formation of trabeculae of variable thickness (25%, 1/4). Cells were polygonal, with relatively distinct cytoplasmic borders, abundant and eosinophilic cytoplasm, round nucleus, with finely stippled chromatin and one to two conspicuous nucleoli (Fig.3C). Marked intracytoplasmic vacuolization was identified in 50% (2/4) of the cases. Cell pleomorphism ranged from moderate to severe (50%, 2/4 each), and binucleated cells were identified in 50% (2/4) of the cases. The mitotic index HPF (400x) was discrete (1/HPF) in all cases (100%, 4/4). Fibrous connective tissue proliferation was classified as mild, and no mucin expression was identified in all cases (100%, 4/4). Intratumoral necrosis and hemorrhage were visualized in 100% (4/4) and 75% (3/4) of the cases, respectively. No tumor invasion in the blood and/or lymphatic vessels or extrahepatic metastases was identified.
All cases (100%, 4/4) showed marked intracytoplasmic, sometimes granular, staining for Hep Par-1 (Fig.3D). No cases of hepatocellular carcinoma presented IHC positivity for CK 7.
Hemangiosarcoma
Only one animal was diagnosed with primary liver hemangiosarcoma, accounting for 6.7% (1/15) of the cases. It was a five-year-old male NDB cat.
At necropsy, the liver showed multiple dark red nodules (Fig.4A) and soft on palpation. On the cutting surface, numerous cystic areas containing blood, interspersed with whitish firm areas were observed. The cat presented good body condition and pale mucous membranes. In abdominal cavity, a large amount of free blood and clots was observed.
Anatomopathological and immunohistochemical aspects of hepatic hemangiosarcoma. (A) Gross pattern characterized by multifocal dark red nodules of varying sizes. (B) Histological aspects. Neoplastic proliferation of spindle cells arranged in a solid pattern, sometimes with formation of vascular structures of different sizes and filled with red blood cells. HE, obj.10x. (C) Enlargement of Figure 4 B showing endothelial neoplastic cells. HE, obj.40x. (D) Intracytoplasmic accentuated multifocal staining for vimentin. Inset: intracytoplasmic staining for von Willebrand factor. IHC, DAB, obj.40x.
Microscopic analysis was characterized by non-delimited and non-encapsulated endothelial cells proliferation, arranged in a solid pattern, often forming vascular structures, and sustained in a discrete conjunctival stroma (Fig.4B). The cells were spindle-shaped, with indistinct cytoplasmic borders, discrete and eosinophilic cytoplasm, oval to elongated nucleus, stippled chromatin, and evident single nucleoli (Fig.4C). Cellular pleomorphism was moderate, and the mitotic index HPF (400x) was discrete (1/HPF). An extensive area of necrosis and intratumoral hemorrhage was observed. No tumor invasion in vessels or extrahepatic metastases was identified.
IHC showed marked multifocal intracytoplasmic staining for vimentin and moderate for von Willebrand factor (Fig.4D).
Discussion
Frequency of PHN in the present study was 0.7% of the necropsies in cats, and when analyzed only the category of hepatobiliary diseases, the frequency was 12%. In similar studies, PHN frequency ranged from 0.7 to 2.3% of the necropsies (Schmidt & Langham 1967Schmidt R.E. & Langham R.F. 1967. A survey of feline neoplasms. J. Am. Vet. Med. Assoc. 151(559):1325-1328., Engle & Brodey 1969Engle G.C. & Brodey R.S. 1969. A retrospective study of 395 feline neoplasms. J. Am. Anim. Hosp. Assoc. 5(2):21-31., Patnaik et al. 1975Patnaik A.K., Liu S.K., Hurvitz A.I. & McClelland A.J. 1975. Nonhematopoietic neoplasms in cats. J. Nat. Cancer Inst. 54(4):855-860. <PMid:1055268>, Martins 2016Martins T.M. 2016. Causas de morte e razões para eutanásia de gatos na Região Central do Rio Grande do Sul. Doctoral Dissertation in Pathology and Clinical Pathology, Universidade Federal de Santa Maria, Santa Maria, RS.). In the analyses of biopsy samples, these neoplasms corresponded to 9.7 to 11.3% (Gagne et al. 1996Gagne J.M., Weiss J. & Armstrong P.J. 1996. Histopathologic evaluation of feline inflammatory liver disease. Vet. Pathol. 33(5):521-526. <http://dx.doi.org/10.1177/030098589603300506> <PMid:8885178>
https://doi.org/10.1177/0300985896033005...
, Hirose et al. 2014Hirose N., Uchida K., Kanemoto H., Ohno K., Chambers J.K. & Nakayama H. 2014. A retrospective histopathological survey on canine and feline liver diseases at the University of Tokyo between 2006 and 2012. J. Vet. Med. Sci. 76(7):1015-1020. <http://dx.doi.org/10.1292/jvms.14-0083> <PMid:24717415>
https://doi.org/10.1292/jvms.14-0083...
). Researchers have reported that PHN corresponded to 1.0 to 5.7% of all cat neoplasms (Hammer & Sikkema 1995Hammer A.S. & Sikkema D.A. 1995. Hepatic neoplasia in the dog and cat. Vet. Clin. N. Am., Small Anim. Pract. 25(2):419-435. <http://dx.doi.org/10.1016/S0195-5616(95)50035-X> <PMid:7785172>
https://doi.org/10.1016/S0195-5616(95)50...
, Rutgers 1998Rutgers C. 1998. Feline liver disease. In Practice 20(1):16-25. <http://dx.doi.org/10.1136/inpract.20.1.16>
https://doi.org/10.1136/inpract.20.1.16...
, Andrade et al. 2012Andrade R.L.F.S., Oliveira D.M., Dantas A.F.M., Souza A.P., Nóbrega Neto P.I. & Riet-Correa F. 2012. Tumores de cães e gatos diagnosticados no semiárido da Paraíba. Pesq. Vet. Bras. 32(10):1037-1040. <http://dx.doi.org/10.1590/S0100-736X2012001000016>
https://doi.org/10.1590/S0100-736X201200...
, Van Sprundel et al. 2014Van Sprundel R.G.H.M., Van den Ingh T.S.G.A.M., Guscetti F., Kershaw O., Van Wolferen M.E., Rothuizen J. & Spee B. 2014. Classification of primary hepatic tumours in the cat. Vet. J. 202(2):255-266. <http://dx.doi.org/10.1016/j.tvjl.2014.07.002> <PMid:25439443>
https://doi.org/10.1016/j.tvjl.2014.07.0...
). NDB cats were the most affected in this study; however, no breed predisposition to PHN has been described (Balkman 2009Balkman C. 2009. Hepatobiliary neoplasia in dogs and cats. Vet. Clin. N. Am., Small Anim. Pract. 39(3):617-625. <http://dx.doi.org/10.1016/j.cvsm.2009.01.001> <PMid:19524795>
https://doi.org/10.1016/j.cvsm.2009.01.0...
). The larger number of NDB cats is probably due to the fact that they are the most assisted in the metropolitan region of Porto Alegre and, consequently, referred to the Department of Veterinary Pathology. There was no apparent sex predisposition, as previously described (Lawrence et al. 1994Lawrence H.J., Erb H.N. & Harvey H.J. 1994. Nonlymphomatous hepatobiliary masses in cats: 41 cases (1972-1991). Vet. Surg. 23(5):365-368. <http://dx.doi.org/10.1111/j.1532-950X.1994.tb00496.x> <PMid:7839594>
https://doi.org/10.1111/j.1532-950X.1994...
, Van Sprundel et al. 2014Van Sprundel R.G.H.M., Van den Ingh T.S.G.A.M., Guscetti F., Kershaw O., Van Wolferen M.E., Rothuizen J. & Spee B. 2014. Classification of primary hepatic tumours in the cat. Vet. J. 202(2):255-266. <http://dx.doi.org/10.1016/j.tvjl.2014.07.002> <PMid:25439443>
https://doi.org/10.1016/j.tvjl.2014.07.0...
). Elderly cats were the most affected, corroborating the literature (Patnaik 1992Patnaik A.K. 1992. A morphological and immunocytochemical study of hepatic neoplasms in cats. Vet. Pathol. 29(5):405-415. <http://dx.doi.org/10.1177/030098589202900506> <PMid:1413408>
https://doi.org/10.1177/0300985892029005...
, Post & Patnaik 1992Post G. & Patnaik A.K. 1992. Nonhematopoietic hepatic neoplasms in cats: 21 cases (1983-1988). J. Am. Vet. Med. Assoc. 201(7):1080-1082. <PMid:1330999>, Lawrence et al. 1994Lawrence H.J., Erb H.N. & Harvey H.J. 1994. Nonlymphomatous hepatobiliary masses in cats: 41 cases (1972-1991). Vet. Surg. 23(5):365-368. <http://dx.doi.org/10.1111/j.1532-950X.1994.tb00496.x> <PMid:7839594>
https://doi.org/10.1111/j.1532-950X.1994...
, Andrade et al. 2012Andrade R.L.F.S., Oliveira D.M., Dantas A.F.M., Souza A.P., Nóbrega Neto P.I. & Riet-Correa F. 2012. Tumores de cães e gatos diagnosticados no semiárido da Paraíba. Pesq. Vet. Bras. 32(10):1037-1040. <http://dx.doi.org/10.1590/S0100-736X2012001000016>
https://doi.org/10.1590/S0100-736X201200...
, Cullen 2017Cullen J.M. 2017. Tumors of the liver and gallbladder, p.602-631. In: Meuten D.J. (Ed), Tumors in Domestic Animals. 5th ed. John Wiley & Sons, Iowa.), and this may be associated with their longevity (O’Neill et al. 2015O’Neill D.G., Church D.B., Mcgreevy P.D., Thomson P.C. & Brodbelt D.C. 2015. Longevity and mortality of cats attending primary care veterinary practices in England. J. Feline Med. Surg. 17(2):125-133. <http://dx.doi.org/10.1177/1098612X14536176> <PMid:24925771>
https://doi.org/10.1177/1098612X14536176...
).
In the present study, all PHN were malignant, as described by Patnaik et al. (1975)Patnaik A.K., Liu S.K., Hurvitz A.I. & McClelland A.J. 1975. Nonhematopoietic neoplasms in cats. J. Nat. Cancer Inst. 54(4):855-860. <PMid:1055268>. However, numerous researchers have reported that the benign form is most commonly found (Post & Patnaik 1992Post G. & Patnaik A.K. 1992. Nonhematopoietic hepatic neoplasms in cats: 21 cases (1983-1988). J. Am. Vet. Med. Assoc. 201(7):1080-1082. <PMid:1330999>, Lawrence et al. 1994Lawrence H.J., Erb H.N. & Harvey H.J. 1994. Nonlymphomatous hepatobiliary masses in cats: 41 cases (1972-1991). Vet. Surg. 23(5):365-368. <http://dx.doi.org/10.1111/j.1532-950X.1994.tb00496.x> <PMid:7839594>
https://doi.org/10.1111/j.1532-950X.1994...
). Cholangiocarcinoma was the most commonly diagnosed neoplasm, followed by hepatocellular carcinoma, corresponding to 66.6 and 26.7% of the cases, respectively. In cats, neoplasms originating in bile duct cells occur more frequently than those originating in hepatocytes (Van Sprundel et al. 2014Van Sprundel R.G.H.M., Van den Ingh T.S.G.A.M., Guscetti F., Kershaw O., Van Wolferen M.E., Rothuizen J. & Spee B. 2014. Classification of primary hepatic tumours in the cat. Vet. J. 202(2):255-266. <http://dx.doi.org/10.1016/j.tvjl.2014.07.002> <PMid:25439443>
https://doi.org/10.1016/j.tvjl.2014.07.0...
, Otte et al. 2017Otte C.M., Penning L.C. & Rothuizen J. 2017. Feline biliary tree and gallbladder disease: aetiology, diagnosis and treatment. J. Feline Med. Surg. 19(5):514-528. <http://dx.doi.org/10.1177/1098612X17706465> <PMid:28438089>
https://doi.org/10.1177/1098612X17706465...
). Several studies have reported cholangiocarcinoma as the main PHN in cats (Engle & Brodey 1969Engle G.C. & Brodey R.S. 1969. A retrospective study of 395 feline neoplasms. J. Am. Anim. Hosp. Assoc. 5(2):21-31., Patnaik 1992Patnaik A.K. 1992. A morphological and immunocytochemical study of hepatic neoplasms in cats. Vet. Pathol. 29(5):405-415. <http://dx.doi.org/10.1177/030098589202900506> <PMid:1413408>
https://doi.org/10.1177/0300985892029005...
, Post & Patnaik 1992Post G. & Patnaik A.K. 1992. Nonhematopoietic hepatic neoplasms in cats: 21 cases (1983-1988). J. Am. Vet. Med. Assoc. 201(7):1080-1082. <PMid:1330999>, Andrade et al. 2012Andrade R.L.F.S., Oliveira D.M., Dantas A.F.M., Souza A.P., Nóbrega Neto P.I. & Riet-Correa F. 2012. Tumores de cães e gatos diagnosticados no semiárido da Paraíba. Pesq. Vet. Bras. 32(10):1037-1040. <http://dx.doi.org/10.1590/S0100-736X2012001000016>
https://doi.org/10.1590/S0100-736X201200...
, Martins 2016Martins T.M. 2016. Causas de morte e razões para eutanásia de gatos na Região Central do Rio Grande do Sul. Doctoral Dissertation in Pathology and Clinical Pathology, Universidade Federal de Santa Maria, Santa Maria, RS., Cullen 2017Cullen J.M. 2017. Tumors of the liver and gallbladder, p.602-631. In: Meuten D.J. (Ed), Tumors in Domestic Animals. 5th ed. John Wiley & Sons, Iowa.). However, some researchers have described hepatocellular carcinoma (Patnaik et al. 1975Patnaik A.K., Liu S.K., Hurvitz A.I. & McClelland A.J. 1975. Nonhematopoietic neoplasms in cats. J. Nat. Cancer Inst. 54(4):855-860. <PMid:1055268>) and biliary adenoma (Post & Patnaik 1992Post G. & Patnaik A.K. 1992. Nonhematopoietic hepatic neoplasms in cats: 21 cases (1983-1988). J. Am. Vet. Med. Assoc. 201(7):1080-1082. <PMid:1330999>, Lawrence et al. 1994Lawrence H.J., Erb H.N. & Harvey H.J. 1994. Nonlymphomatous hepatobiliary masses in cats: 41 cases (1972-1991). Vet. Surg. 23(5):365-368. <http://dx.doi.org/10.1111/j.1532-950X.1994.tb00496.x> <PMid:7839594>
https://doi.org/10.1111/j.1532-950X.1994...
) as the most common. Cholangiocarcinomas may develop from intra- or extrahepatic bile ducts (Crawford & Liu 2010Crawford J.M. & Liu C. 2010. Fígado e trato biliar, p.979-1012. In: Kumar V., Abbas A.K., Fausto N. & Aster J.C. (Eds), Robbins e Cotran, Bases Patológicas das Doenças. Elsevier, Rio de Janeiro.), with intrahepatic ducts as the most frequent (Patnaik 1992Patnaik A.K. 1992. A morphological and immunocytochemical study of hepatic neoplasms in cats. Vet. Pathol. 29(5):405-415. <http://dx.doi.org/10.1177/030098589202900506> <PMid:1413408>
https://doi.org/10.1177/0300985892029005...
, Cullen 2009Cullen J.M. 2009. Summary of the World small animal veterinary association standardization committee guide to classification of liver disease in dogs and cats. Vet. Clin. N. Am., Small Anim. Pract. 39(3):395-418. <http://dx.doi.org/10.1016/j.cvsm.2009.02.003> <PMid:19524786>
https://doi.org/10.1016/j.cvsm.2009.02.0...
, 2017Cullen J.M. 2017. Tumors of the liver and gallbladder, p.602-631. In: Meuten D.J. (Ed), Tumors in Domestic Animals. 5th ed. John Wiley & Sons, Iowa.). In the present study, approximately 13% of the cases originated in extrahepatic ducts, corroborating the literature (Patnaik et al. 1975Patnaik A.K., Liu S.K., Hurvitz A.I. & McClelland A.J. 1975. Nonhematopoietic neoplasms in cats. J. Nat. Cancer Inst. 54(4):855-860. <PMid:1055268>, Patnaik 1992Patnaik A.K. 1992. A morphological and immunocytochemical study of hepatic neoplasms in cats. Vet. Pathol. 29(5):405-415. <http://dx.doi.org/10.1177/030098589202900506> <PMid:1413408>
https://doi.org/10.1177/0300985892029005...
). Extrahepatic bile duct and gallbladder neoplasms are rare in cats (Feldman et al. 1976Feldman B.F., Strafuss A.C. & Gabbert N. 1976. Bile duct carcinoma in the cat: 3 case reports. Feline Pract. 6:33-39., Cullen 2017Cullen J.M. 2017. Tumors of the liver and gallbladder, p.602-631. In: Meuten D.J. (Ed), Tumors in Domestic Animals. 5th ed. John Wiley & Sons, Iowa.), but researchers have found a similar frequency between intra- and extrahepatic tumors (Lawrence et al. 1994Lawrence H.J., Erb H.N. & Harvey H.J. 1994. Nonlymphomatous hepatobiliary masses in cats: 41 cases (1972-1991). Vet. Surg. 23(5):365-368. <http://dx.doi.org/10.1111/j.1532-950X.1994.tb00496.x> <PMid:7839594>
https://doi.org/10.1111/j.1532-950X.1994...
).
In this study, approximately 6.0% of the cases were of mesenchymal origin. Primary hepatobiliary sarcomas are rare in cats (Balkman 2009Balkman C. 2009. Hepatobiliary neoplasia in dogs and cats. Vet. Clin. N. Am., Small Anim. Pract. 39(3):617-625. <http://dx.doi.org/10.1016/j.cvsm.2009.01.001> <PMid:19524795>
https://doi.org/10.1016/j.cvsm.2009.01.0...
). Primary liver hemangiosarcoma varies widely in frequency (Scavelli et al. 1985Scavelli T.D., Patnaik A.K., Mehlhaff C.J. & Hayes A.A. 1985. Hemangiosarcoma in the cat: retrospective evaluation of 31 surgical cases. J. Am. Vet. Med. Assoc. 187(8):817-819. <PMid:4055500>, Patnaik 1992Patnaik A.K. 1992. A morphological and immunocytochemical study of hepatic neoplasms in cats. Vet. Pathol. 29(5):405-415. <http://dx.doi.org/10.1177/030098589202900506> <PMid:1413408>
https://doi.org/10.1177/0300985892029005...
, Post & Patnaik 1992Post G. & Patnaik A.K. 1992. Nonhematopoietic hepatic neoplasms in cats: 21 cases (1983-1988). J. Am. Vet. Med. Assoc. 201(7):1080-1082. <PMid:1330999>). This variability can result from two factors: the low incidence of this neoplasm and the difficulty in establishing the primary site when more than one organ is involved (Barros 2016Barros C.S.L. 2016. Fígado, vias biliares e pâncreas exócrino, p.222-265. In: Santos R.L. & Alessi A.C. (Eds), Patologia Veterinária. 2ª ed. Roca, Rio de Janeiro., Cullen 2017Cullen J.M. 2017. Tumors of the liver and gallbladder, p.602-631. In: Meuten D.J. (Ed), Tumors in Domestic Animals. 5th ed. John Wiley & Sons, Iowa.). Researchers have reported that hemangiosarcoma is the second most common malignant neoplasm in cats (Post & Patnaik 1992Post G. & Patnaik A.K. 1992. Nonhematopoietic hepatic neoplasms in cats: 21 cases (1983-1988). J. Am. Vet. Med. Assoc. 201(7):1080-1082. <PMid:1330999>).
Cholangiocarcinoma and hepatocellular carcinoma present different gross appearance (Head et al. 2003Head K.W., Cullen J.M., Dubielzig R.R., Else R.W., Misdorp W., Patnaik A.K., Tateyama S. & Van der Gaag I. 2003. Histological Classification of Tumors of the Alimentary System of Domestic Animals. Vol.5. 2nd ed. Armed Forces Institute of Pathology, Washington. 257p., Cullen & Stalker 2016Cullen J.M. & Stalker M.J. 2016. Liver and biliary system, p.307-308. In: Maxie M.G. (Ed.), Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol.2. 6th ed. Elsevier, St Louis. <http://dx.doi.org/10.1016/B978-0-7020-5318-4.00008-5>.
https://doi.org/10.1016/B978-0-7020-5318...
). In the present study, the multinodular pattern was identified in 60% of cholangiocarcinomas, corroborating the literature, which describes this as the most common gross presentation in dogs and cats (Patnaik 1992Patnaik A.K. 1992. A morphological and immunocytochemical study of hepatic neoplasms in cats. Vet. Pathol. 29(5):405-415. <http://dx.doi.org/10.1177/030098589202900506> <PMid:1413408>
https://doi.org/10.1177/0300985892029005...
, Cullen & Stalker 2016Cullen J.M. & Stalker M.J. 2016. Liver and biliary system, p.307-308. In: Maxie M.G. (Ed.), Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol.2. 6th ed. Elsevier, St Louis. <http://dx.doi.org/10.1016/B978-0-7020-5318-4.00008-5>.
https://doi.org/10.1016/B978-0-7020-5318...
). In a survey with dogs, 83% of the cases were multinodular (Flores et al. 2013Flores M.M., Bianchi R.M., Kommers G.D., Irigoyen L.F., Barros C.L. & Fighera R.A. 2013. Prevalência e achados epidemiológicos, anatomopatológicos e imuno-histoquímicos dos tumores hepáticos malignos primários de cães da Região Central do Rio Grande do Sul (1965-2012). Pesq. Vet. Bras. 33(4):497-511. <http://dx.doi.org/10.1590/S0100-736X2013000400014>
https://doi.org/10.1590/S0100-736X201300...
). In the present study, 20% of cholangiocarcinomas were classified as of massive pattern, and were characterized by large masses that obliterated the entire hepatic lobe (Cullen 2017Cullen J.M. 2017. Tumors of the liver and gallbladder, p.602-631. In: Meuten D.J. (Ed), Tumors in Domestic Animals. 5th ed. John Wiley & Sons, Iowa.). In dogs, this presentation has been identified in approximately 17% of bile duct neoplasms (Flores et al. 2013Flores M.M., Bianchi R.M., Kommers G.D., Irigoyen L.F., Barros C.L. & Fighera R.A. 2013. Prevalência e achados epidemiológicos, anatomopatológicos e imuno-histoquímicos dos tumores hepáticos malignos primários de cães da Região Central do Rio Grande do Sul (1965-2012). Pesq. Vet. Bras. 33(4):497-511. <http://dx.doi.org/10.1590/S0100-736X2013000400014>
https://doi.org/10.1590/S0100-736X201300...
). It is not yet clear whether multiple nodules result from intrahepatic metastases or primary lesions in different foci (Barros 2016Barros C.S.L. 2016. Fígado, vias biliares e pâncreas exócrino, p.222-265. In: Santos R.L. & Alessi A.C. (Eds), Patologia Veterinária. 2ª ed. Roca, Rio de Janeiro., Cullen & Stalker 2016Cullen J.M. & Stalker M.J. 2016. Liver and biliary system, p.307-308. In: Maxie M.G. (Ed.), Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol.2. 6th ed. Elsevier, St Louis. <http://dx.doi.org/10.1016/B978-0-7020-5318-4.00008-5>.
https://doi.org/10.1016/B978-0-7020-5318...
). Firm consistency and whitish color are common gross features (Head et al. 2003Head K.W., Cullen J.M., Dubielzig R.R., Else R.W., Misdorp W., Patnaik A.K., Tateyama S. & Van der Gaag I. 2003. Histological Classification of Tumors of the Alimentary System of Domestic Animals. Vol.5. 2nd ed. Armed Forces Institute of Pathology, Washington. 257p., Barros 2016Barros C.S.L. 2016. Fígado, vias biliares e pâncreas exócrino, p.222-265. In: Santos R.L. & Alessi A.C. (Eds), Patologia Veterinária. 2ª ed. Roca, Rio de Janeiro., Cullen 2017Cullen J.M. 2017. Tumors of the liver and gallbladder, p.602-631. In: Meuten D.J. (Ed), Tumors in Domestic Animals. 5th ed. John Wiley & Sons, Iowa.), and are attributed to the large amount of fibrous stroma present in cholangiocarcinomas (Head et al. 2003Head K.W., Cullen J.M., Dubielzig R.R., Else R.W., Misdorp W., Patnaik A.K., Tateyama S. & Van der Gaag I. 2003. Histological Classification of Tumors of the Alimentary System of Domestic Animals. Vol.5. 2nd ed. Armed Forces Institute of Pathology, Washington. 257p., Cullen & Stalker 2016Cullen J.M. & Stalker M.J. 2016. Liver and biliary system, p.307-308. In: Maxie M.G. (Ed.), Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol.2. 6th ed. Elsevier, St Louis. <http://dx.doi.org/10.1016/B978-0-7020-5318-4.00008-5>.
https://doi.org/10.1016/B978-0-7020-5318...
). The umbilicated aspect is probably attributed to intratumor necrosis (Head et al. 2003Head K.W., Cullen J.M., Dubielzig R.R., Else R.W., Misdorp W., Patnaik A.K., Tateyama S. & Van der Gaag I. 2003. Histological Classification of Tumors of the Alimentary System of Domestic Animals. Vol.5. 2nd ed. Armed Forces Institute of Pathology, Washington. 257p., Cullen & Stalker 2016Cullen J.M. & Stalker M.J. 2016. Liver and biliary system, p.307-308. In: Maxie M.G. (Ed.), Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol.2. 6th ed. Elsevier, St Louis. <http://dx.doi.org/10.1016/B978-0-7020-5318-4.00008-5>.
https://doi.org/10.1016/B978-0-7020-5318...
, Cullen 2017Cullen J.M. 2017. Tumors of the liver and gallbladder, p.602-631. In: Meuten D.J. (Ed), Tumors in Domestic Animals. 5th ed. John Wiley & Sons, Iowa.). Occasionally multiple cystic areas are observed, and when there is predominance of this presentation, the neoplasm is named biliary cystadenocarcinoma (Head et al. 2003Head K.W., Cullen J.M., Dubielzig R.R., Else R.W., Misdorp W., Patnaik A.K., Tateyama S. & Van der Gaag I. 2003. Histological Classification of Tumors of the Alimentary System of Domestic Animals. Vol.5. 2nd ed. Armed Forces Institute of Pathology, Washington. 257p., Cullen 2017Cullen J.M. 2017. Tumors of the liver and gallbladder, p.602-631. In: Meuten D.J. (Ed), Tumors in Domestic Animals. 5th ed. John Wiley & Sons, Iowa.), as observed in a cat of the present study. Hepatocellular carcinomas exhibited massive gross pattern. This presentation is the most commonly found, and is characterized by large masses involving a single hepatic lobe or extending to adjacent lobes (Barros 2016Barros C.S.L. 2016. Fígado, vias biliares e pâncreas exócrino, p.222-265. In: Santos R.L. & Alessi A.C. (Eds), Patologia Veterinária. 2ª ed. Roca, Rio de Janeiro., Cullen 2017Cullen J.M. 2017. Tumors of the liver and gallbladder, p.602-631. In: Meuten D.J. (Ed), Tumors in Domestic Animals. 5th ed. John Wiley & Sons, Iowa.). This neoplasm varies in size and gross appearance, and may be present in massive, nodular or diffuse patterns (Cullen & Stalker 2016Cullen J.M. & Stalker M.J. 2016. Liver and biliary system, p.307-308. In: Maxie M.G. (Ed.), Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol.2. 6th ed. Elsevier, St Louis. <http://dx.doi.org/10.1016/B978-0-7020-5318-4.00008-5>.
https://doi.org/10.1016/B978-0-7020-5318...
). In the present study, quadrate, right medial and lateral lobes were the most affected. According to Patnaik (1992)Patnaik A.K. 1992. A morphological and immunocytochemical study of hepatic neoplasms in cats. Vet. Pathol. 29(5):405-415. <http://dx.doi.org/10.1177/030098589202900506> <PMid:1413408>
https://doi.org/10.1177/0300985892029005...
, there is no predilection for hepatic lobe, but the left side is involved in more than two-thirds of hepatocellular carcinomas in dogs (Liptak et al. 2004Liptak J.M., Dernell W.S., Monnet E., Powers B.E., Bachand A.M., Kenney J.G. & Withrow S.J. 2004. Massive hepatocellular carcinoma in dogs: 48 cases (1992-2002). J. Am. Vet. Med. Assoc. 225(8):1225-1230. <http://dx.doi.org/10.2460/javma.2004.225.1225> <PMid:15521445>
https://doi.org/10.2460/javma.2004.225.1...
). Color and consistency vary according to the degrees of hemorrhage and necrosis of the neoplasm and vacuolization of neoplastic cells (Barros 2016Barros C.S.L. 2016. Fígado, vias biliares e pâncreas exócrino, p.222-265. In: Santos R.L. & Alessi A.C. (Eds), Patologia Veterinária. 2ª ed. Roca, Rio de Janeiro., Cullen 2017Cullen J.M. 2017. Tumors of the liver and gallbladder, p.602-631. In: Meuten D.J. (Ed), Tumors in Domestic Animals. 5th ed. John Wiley & Sons, Iowa.).
Extrahepatic gross findings were mainly characterized by poor body condition, ascites, and jaundice. These changes are frequent in cases of liver disease (Barros 2016Barros C.S.L. 2016. Fígado, vias biliares e pâncreas exócrino, p.222-265. In: Santos R.L. & Alessi A.C. (Eds), Patologia Veterinária. 2ª ed. Roca, Rio de Janeiro.). It has been suggested that ascites occurs as a result of portal hypertension owing to compression caused by neoplasms. Jaundice resulted from bile flow obstruction with consequent intra- and extrahepatic cholestasis (Charles et al. 2006Charles J.A., Cullen J.M., Van den Ingh T.S.G.A.M., Winkle T.V. & Desmet V.J. 2006. Morphological classification of neoplastic disorders of the canine and feline liver, p.117-123. In: Rothuizen J. (Ed), WSAVA Standards for Clinical and Histological Diagnosis of Canine and Feline Liver Diseases. Elsevier, Edinburgh.). In the literature, approximately 20% of the cases presented jaundice (Lawrence et al. 1994Lawrence H.J., Erb H.N. & Harvey H.J. 1994. Nonlymphomatous hepatobiliary masses in cats: 41 cases (1972-1991). Vet. Surg. 23(5):365-368. <http://dx.doi.org/10.1111/j.1532-950X.1994.tb00496.x> <PMid:7839594>
https://doi.org/10.1111/j.1532-950X.1994...
). Hemoperitoneum as a result of rupture of neoplasms was observed in two cats: one with hepatocellular carcinoma and one with hemangiosarcoma. Generally, when the neoplasm is friable, there is rupture with consequent hemoperitoneum, anemia, and sudden death (Barros 2016Barros C.S.L. 2016. Fígado, vias biliares e pâncreas exócrino, p.222-265. In: Santos R.L. & Alessi A.C. (Eds), Patologia Veterinária. 2ª ed. Roca, Rio de Janeiro., Cullen & Stalker 2016Cullen J.M. & Stalker M.J. 2016. Liver and biliary system, p.307-308. In: Maxie M.G. (Ed.), Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol.2. 6th ed. Elsevier, St Louis. <http://dx.doi.org/10.1016/B978-0-7020-5318-4.00008-5>.
https://doi.org/10.1016/B978-0-7020-5318...
, Cullen 2017Cullen J.M. 2017. Tumors of the liver and gallbladder, p.602-631. In: Meuten D.J. (Ed), Tumors in Domestic Animals. 5th ed. John Wiley & Sons, Iowa.). Hemoperitoneum, ascites, and jaundice have been frequently reported in dogs with PHN (Flores et al. 2013Flores M.M., Bianchi R.M., Kommers G.D., Irigoyen L.F., Barros C.L. & Fighera R.A. 2013. Prevalência e achados epidemiológicos, anatomopatológicos e imuno-histoquímicos dos tumores hepáticos malignos primários de cães da Região Central do Rio Grande do Sul (1965-2012). Pesq. Vet. Bras. 33(4):497-511. <http://dx.doi.org/10.1590/S0100-736X2013000400014>
https://doi.org/10.1590/S0100-736X201300...
).
The histological characteristics of PHN vary according to the degree of differentiation (Head et al. 2003Head K.W., Cullen J.M., Dubielzig R.R., Else R.W., Misdorp W., Patnaik A.K., Tateyama S. & Van der Gaag I. 2003. Histological Classification of Tumors of the Alimentary System of Domestic Animals. Vol.5. 2nd ed. Armed Forces Institute of Pathology, Washington. 257p., Cullen 2017Cullen J.M. 2017. Tumors of the liver and gallbladder, p.602-631. In: Meuten D.J. (Ed), Tumors in Domestic Animals. 5th ed. John Wiley & Sons, Iowa.). Well-differentiated cholangiocarcinomas are composed of cells that resemble the normal biliary epithelium and present a tubular or acinar arrangement (Barros 2016Barros C.S.L. 2016. Fígado, vias biliares e pâncreas exócrino, p.222-265. In: Santos R.L. & Alessi A.C. (Eds), Patologia Veterinária. 2ª ed. Roca, Rio de Janeiro.). As they become undifferentiated, the solid pattern is more prevalent (Head et al. 2003Head K.W., Cullen J.M., Dubielzig R.R., Else R.W., Misdorp W., Patnaik A.K., Tateyama S. & Van der Gaag I. 2003. Histological Classification of Tumors of the Alimentary System of Domestic Animals. Vol.5. 2nd ed. Armed Forces Institute of Pathology, Washington. 257p., Cullen 2017Cullen J.M. 2017. Tumors of the liver and gallbladder, p.602-631. In: Meuten D.J. (Ed), Tumors in Domestic Animals. 5th ed. John Wiley & Sons, Iowa.). Biliary cystadenocarcinoma is a variation histologically characterized by numerous cysts of varying sizes, which often contain mucin and present papillary projections to the lumen (Cullen & Stalker 2016Cullen J.M. & Stalker M.J. 2016. Liver and biliary system, p.307-308. In: Maxie M.G. (Ed.), Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol.2. 6th ed. Elsevier, St Louis. <http://dx.doi.org/10.1016/B978-0-7020-5318-4.00008-5>.
https://doi.org/10.1016/B978-0-7020-5318...
). Although the acinar/ductal arrangement was the most frequently found in this study, well-differentiated cholangiocarcinomas were not identified, since their cellular pleomorphism ranged from moderate to severe. Hepatocellular carcinoma usually has three microscopic patterns: trabecular, adenoid and solid (Head et al. 2003Head K.W., Cullen J.M., Dubielzig R.R., Else R.W., Misdorp W., Patnaik A.K., Tateyama S. & Van der Gaag I. 2003. Histological Classification of Tumors of the Alimentary System of Domestic Animals. Vol.5. 2nd ed. Armed Forces Institute of Pathology, Washington. 257p., Barros 2016Barros C.S.L. 2016. Fígado, vias biliares e pâncreas exócrino, p.222-265. In: Santos R.L. & Alessi A.C. (Eds), Patologia Veterinária. 2ª ed. Roca, Rio de Janeiro.). According to Cullen (2017)Cullen J.M. 2017. Tumors of the liver and gallbladder, p.602-631. In: Meuten D.J. (Ed), Tumors in Domestic Animals. 5th ed. John Wiley & Sons, Iowa., the solid pattern is poorly differentiated and characterized by pleomorphic cells (Crawford & Liu 2010Crawford J.M. & Liu C. 2010. Fígado e trato biliar, p.979-1012. In: Kumar V., Abbas A.K., Fausto N. & Aster J.C. (Eds), Robbins e Cotran, Bases Patológicas das Doenças. Elsevier, Rio de Janeiro., Flores et al. 2013Flores M.M., Bianchi R.M., Kommers G.D., Irigoyen L.F., Barros C.L. & Fighera R.A. 2013. Prevalência e achados epidemiológicos, anatomopatológicos e imuno-histoquímicos dos tumores hepáticos malignos primários de cães da Região Central do Rio Grande do Sul (1965-2012). Pesq. Vet. Bras. 33(4):497-511. <http://dx.doi.org/10.1590/S0100-736X2013000400014>
https://doi.org/10.1590/S0100-736X201300...
). Vacuolization in the cytoplasm of neoplastic hepatocytes is a frequent finding, and is associated with glycogen or lipid deposition (Cullen 2017Cullen J.M. 2017. Tumors of the liver and gallbladder, p.602-631. In: Meuten D.J. (Ed), Tumors in Domestic Animals. 5th ed. John Wiley & Sons, Iowa.). Hemangiosarcomas are composed of neoplastic endothelial cells, often with formation of vascular spaces, but the solid arrangement may be found in some situations (Head et al. 2003Head K.W., Cullen J.M., Dubielzig R.R., Else R.W., Misdorp W., Patnaik A.K., Tateyama S. & Van der Gaag I. 2003. Histological Classification of Tumors of the Alimentary System of Domestic Animals. Vol.5. 2nd ed. Armed Forces Institute of Pathology, Washington. 257p., Barros 2016Barros C.S.L. 2016. Fígado, vias biliares e pâncreas exócrino, p.222-265. In: Santos R.L. & Alessi A.C. (Eds), Patologia Veterinária. 2ª ed. Roca, Rio de Janeiro.). Hemorrhage, necrosis and thrombus formation are frequent (Cullen 2017Cullen J.M. 2017. Tumors of the liver and gallbladder, p.602-631. In: Meuten D.J. (Ed), Tumors in Domestic Animals. 5th ed. John Wiley & Sons, Iowa.), and these findings are associated with the gross presentation, characterized by soft dark red nodules. The mitotic index was discrete in most PHN cases; however, researchers have reported that the mitotic index is more pronounced in cholangiocarcinoma than hepatocellular carcinoma (Head et al. 2003Head K.W., Cullen J.M., Dubielzig R.R., Else R.W., Misdorp W., Patnaik A.K., Tateyama S. & Van der Gaag I. 2003. Histological Classification of Tumors of the Alimentary System of Domestic Animals. Vol.5. 2nd ed. Armed Forces Institute of Pathology, Washington. 257p.), and can be used to differentiate between them (Barros 2016Barros C.S.L. 2016. Fígado, vias biliares e pâncreas exócrino, p.222-265. In: Santos R.L. & Alessi A.C. (Eds), Patologia Veterinária. 2ª ed. Roca, Rio de Janeiro.).
Fibrous connective tissue and mucin are histological features frequent in cholangiocarcinomas and uncommon in hepatocellular carcinomas (Patnaik 1992Patnaik A.K. 1992. A morphological and immunocytochemical study of hepatic neoplasms in cats. Vet. Pathol. 29(5):405-415. <http://dx.doi.org/10.1177/030098589202900506> <PMid:1413408>
https://doi.org/10.1177/0300985892029005...
, Head et al. 2003Head K.W., Cullen J.M., Dubielzig R.R., Else R.W., Misdorp W., Patnaik A.K., Tateyama S. & Van der Gaag I. 2003. Histological Classification of Tumors of the Alimentary System of Domestic Animals. Vol.5. 2nd ed. Armed Forces Institute of Pathology, Washington. 257p., Barros 2016Barros C.S.L. 2016. Fígado, vias biliares e pâncreas exócrino, p.222-265. In: Santos R.L. & Alessi A.C. (Eds), Patologia Veterinária. 2ª ed. Roca, Rio de Janeiro., Cullen & Stalker 2016Cullen J.M. & Stalker M.J. 2016. Liver and biliary system, p.307-308. In: Maxie M.G. (Ed.), Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol.2. 6th ed. Elsevier, St Louis. <http://dx.doi.org/10.1016/B978-0-7020-5318-4.00008-5>.
https://doi.org/10.1016/B978-0-7020-5318...
, Cullen 2017Cullen J.M. 2017. Tumors of the liver and gallbladder, p.602-631. In: Meuten D.J. (Ed), Tumors in Domestic Animals. 5th ed. John Wiley & Sons, Iowa.). Cholangiocarcinomas may vary in the amount of fibrous connective tissue proliferation, and researchers have reported a scirrhous pattern when there is marked fibrosis (Head et al. 2003Head K.W., Cullen J.M., Dubielzig R.R., Else R.W., Misdorp W., Patnaik A.K., Tateyama S. & Van der Gaag I. 2003. Histological Classification of Tumors of the Alimentary System of Domestic Animals. Vol.5. 2nd ed. Armed Forces Institute of Pathology, Washington. 257p.). As observed in the present study, researchers have reported that mucin stains strongly with PAS (Barros 2016Barros C.S.L. 2016. Fígado, vias biliares e pâncreas exócrino, p.222-265. In: Santos R.L. & Alessi A.C. (Eds), Patologia Veterinária. 2ª ed. Roca, Rio de Janeiro., Cullen 2017Cullen J.M. 2017. Tumors of the liver and gallbladder, p.602-631. In: Meuten D.J. (Ed), Tumors in Domestic Animals. 5th ed. John Wiley & Sons, Iowa.). Multiple foci of necrosis and hemorrhage are common in cholangiocarcinomas and hepatocellular carcinomas (Patnaik 1992Patnaik A.K. 1992. A morphological and immunocytochemical study of hepatic neoplasms in cats. Vet. Pathol. 29(5):405-415. <http://dx.doi.org/10.1177/030098589202900506> <PMid:1413408>
https://doi.org/10.1177/0300985892029005...
, Cullen 2017Cullen J.M. 2017. Tumors of the liver and gallbladder, p.602-631. In: Meuten D.J. (Ed), Tumors in Domestic Animals. 5th ed. John Wiley & Sons, Iowa.). In the present study, these characteristics were visualized in most of the PHN.
Vascular invasion and extrahepatic metastases were identified in 40% of cholangiocarcinomas. In a similar study, most cholangiocarcinomas showed invasion in lymphatic and/or blood vessels. In dogs, vascular invasion was identified in 64% of these neoplasms (Flores et al. 2013Flores M.M., Bianchi R.M., Kommers G.D., Irigoyen L.F., Barros C.L. & Fighera R.A. 2013. Prevalência e achados epidemiológicos, anatomopatológicos e imuno-histoquímicos dos tumores hepáticos malignos primários de cães da Região Central do Rio Grande do Sul (1965-2012). Pesq. Vet. Bras. 33(4):497-511. <http://dx.doi.org/10.1590/S0100-736X2013000400014>
https://doi.org/10.1590/S0100-736X201300...
). In hepatocellular carcinomas, invasion in vessels is not a common feature (Flores et al. 2013Flores M.M., Bianchi R.M., Kommers G.D., Irigoyen L.F., Barros C.L. & Fighera R.A. 2013. Prevalência e achados epidemiológicos, anatomopatológicos e imuno-histoquímicos dos tumores hepáticos malignos primários de cães da Região Central do Rio Grande do Sul (1965-2012). Pesq. Vet. Bras. 33(4):497-511. <http://dx.doi.org/10.1590/S0100-736X2013000400014>
https://doi.org/10.1590/S0100-736X201300...
, Cullen 2017Cullen J.M. 2017. Tumors of the liver and gallbladder, p.602-631. In: Meuten D.J. (Ed), Tumors in Domestic Animals. 5th ed. John Wiley & Sons, Iowa.), and this finding corroborates those of the present study. Several authors have described that cholangiocarcinoma is the most commonly found metastatic PHN (Patnaik 1992Patnaik A.K. 1992. A morphological and immunocytochemical study of hepatic neoplasms in cats. Vet. Pathol. 29(5):405-415. <http://dx.doi.org/10.1177/030098589202900506> <PMid:1413408>
https://doi.org/10.1177/0300985892029005...
, Lawrence et al. 1994Lawrence H.J., Erb H.N. & Harvey H.J. 1994. Nonlymphomatous hepatobiliary masses in cats: 41 cases (1972-1991). Vet. Surg. 23(5):365-368. <http://dx.doi.org/10.1111/j.1532-950X.1994.tb00496.x> <PMid:7839594>
https://doi.org/10.1111/j.1532-950X.1994...
, Head et al. 2003Head K.W., Cullen J.M., Dubielzig R.R., Else R.W., Misdorp W., Patnaik A.K., Tateyama S. & Van der Gaag I. 2003. Histological Classification of Tumors of the Alimentary System of Domestic Animals. Vol.5. 2nd ed. Armed Forces Institute of Pathology, Washington. 257p., Cullen 2017Cullen J.M. 2017. Tumors of the liver and gallbladder, p.602-631. In: Meuten D.J. (Ed), Tumors in Domestic Animals. 5th ed. John Wiley & Sons, Iowa.), and that hepatocellular carcinoma metastasis is uncommon in all animal species (Cullen 2009Cullen J.M. 2009. Summary of the World small animal veterinary association standardization committee guide to classification of liver disease in dogs and cats. Vet. Clin. N. Am., Small Anim. Pract. 39(3):395-418. <http://dx.doi.org/10.1016/j.cvsm.2009.02.003> <PMid:19524786>
https://doi.org/10.1016/j.cvsm.2009.02.0...
, Cullen & Stalker 2016Cullen J.M. & Stalker M.J. 2016. Liver and biliary system, p.307-308. In: Maxie M.G. (Ed.), Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol.2. 6th ed. Elsevier, St Louis. <http://dx.doi.org/10.1016/B978-0-7020-5318-4.00008-5>.
https://doi.org/10.1016/B978-0-7020-5318...
). Regional lymph nodes are the major sites of metastatic PHN (Cullen & Stalker 2016Cullen J.M. & Stalker M.J. 2016. Liver and biliary system, p.307-308. In: Maxie M.G. (Ed.), Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol.2. 6th ed. Elsevier, St Louis. <http://dx.doi.org/10.1016/B978-0-7020-5318-4.00008-5>.
https://doi.org/10.1016/B978-0-7020-5318...
). Especially in cats, cholangiocarcinomas can invade the Glisson capsule, with implantation of neoplastic cells in the peritoneum and serosa of various organs of the abdominal cavity (Cullen 2017Cullen J.M. 2017. Tumors of the liver and gallbladder, p.602-631. In: Meuten D.J. (Ed), Tumors in Domestic Animals. 5th ed. John Wiley & Sons, Iowa.). In a similar research, the frequency of metastatic cholangiocarcinomas ranged from 67 to 80% of the cases (Patnaik 1992Patnaik A.K. 1992. A morphological and immunocytochemical study of hepatic neoplasms in cats. Vet. Pathol. 29(5):405-415. <http://dx.doi.org/10.1177/030098589202900506> <PMid:1413408>
https://doi.org/10.1177/0300985892029005...
, Lawrence et al. 1994Lawrence H.J., Erb H.N. & Harvey H.J. 1994. Nonlymphomatous hepatobiliary masses in cats: 41 cases (1972-1991). Vet. Surg. 23(5):365-368. <http://dx.doi.org/10.1111/j.1532-950X.1994.tb00496.x> <PMid:7839594>
https://doi.org/10.1111/j.1532-950X.1994...
). In a study with dogs, metastases were observed in approximately 78% of the cases, with lungs, lymph nodes, and abdominal cavity (omentum, mesentery and parietal peritoneum) as the most frequently affected organs (Flores et al. 2013Flores M.M., Bianchi R.M., Kommers G.D., Irigoyen L.F., Barros C.L. & Fighera R.A. 2013. Prevalência e achados epidemiológicos, anatomopatológicos e imuno-histoquímicos dos tumores hepáticos malignos primários de cães da Região Central do Rio Grande do Sul (1965-2012). Pesq. Vet. Bras. 33(4):497-511. <http://dx.doi.org/10.1590/S0100-736X2013000400014>
https://doi.org/10.1590/S0100-736X201300...
), corroborating the findings of the present study.
All cholangiocarcinomas expressed CK 7, whereas hepatocellular carcinomas expressed Hep Par-1 in immunohistochemical analysis. In humans, the Hep Par-1 and CK 7 IHC assessments are used to differentiate PHN and in cases of metastatic carcinomas (Lau et al. 2002Lau S.K., Prakash S., Geller S.A. & Alsabeh R. 2002. Comparative immunohistochemical profile of hepatocellular carcinoma, cholangiocarcinoma, and metastatic adenocarcinoma. Hum. Pathol. 33(12):1175-1181. <http://dx.doi.org/10.1053/hupa.2002.130104> <PMid:12514785>
https://doi.org/10.1053/hupa.2002.130104...
). Several studies in dogs have reported that CK 7 immunostaining in ductal epithelial cells has good sensitivity and specificity (Ramos-Vara et al. 2001Ramos-Vara J.A., Miller M.A. & Johnson G.C. 2001. Immunohistochemical characterization of canine hyperplastic hepatic lesions and hepatocellular and biliary neoplasms with monoclonal antibody hepatocyte paraffin 1 and a monoclonal antibody to cytokeratin 7. Vet. Pathol. 38(6):636-643. <http://dx.doi.org/10.1354/vp.38-6-636> <PMid:11732796>
https://doi.org/10.1354/vp.38-6-636...
, Flores et al. 2013Flores M.M., Bianchi R.M., Kommers G.D., Irigoyen L.F., Barros C.L. & Fighera R.A. 2013. Prevalência e achados epidemiológicos, anatomopatológicos e imuno-histoquímicos dos tumores hepáticos malignos primários de cães da Região Central do Rio Grande do Sul (1965-2012). Pesq. Vet. Bras. 33(4):497-511. <http://dx.doi.org/10.1590/S0100-736X2013000400014>
https://doi.org/10.1590/S0100-736X201300...
). Cholangiocarcinoma does not show immunoreaction for Hep Par-1 (Shimonishi et al. 2000Shimonishi T., Miyazaki K. & Nakanuma Y. 2000. Cytokeratin profile relates to histological subtypes and intrahepatic location of intrahepatic cholangiocarcinoma and primary sites of metastatic adenocarcinoma of liver. Histopathology 37(1):55-63. <http://dx.doi.org/10.1046/j.1365-2559.2000.00932.x> <PMid:10931219>
https://doi.org/10.1046/j.1365-2559.2000...
, Lau et al. 2002Lau S.K., Prakash S., Geller S.A. & Alsabeh R. 2002. Comparative immunohistochemical profile of hepatocellular carcinoma, cholangiocarcinoma, and metastatic adenocarcinoma. Hum. Pathol. 33(12):1175-1181. <http://dx.doi.org/10.1053/hupa.2002.130104> <PMid:12514785>
https://doi.org/10.1053/hupa.2002.130104...
), as observed in the present study. Hep Par-1 is a highly specific and sensitive marker of normal, hyperplastic and/or neoplastic hepatocytes. Therefore, the Hep Par-1 associated with the CK 7 IHC techniques establish the diagnosis of PHN (Ramos-Vara et al. 2001Ramos-Vara J.A., Miller M.A. & Johnson G.C. 2001. Immunohistochemical characterization of canine hyperplastic hepatic lesions and hepatocellular and biliary neoplasms with monoclonal antibody hepatocyte paraffin 1 and a monoclonal antibody to cytokeratin 7. Vet. Pathol. 38(6):636-643. <http://dx.doi.org/10.1354/vp.38-6-636> <PMid:11732796>
https://doi.org/10.1354/vp.38-6-636...
). Hemangiosarcoma showed immunostaining for vimentin and von Willebrand factor, which are mesenchymal and endothelial cell-specific antibodies, respectively (Mello & Alves 1999Mello E.S. & Alves V.A.F. 1999. Glossário dos principais marcadores imuno-histoquímicos, p.266-270. In: Alves V.A.F., Bacchi C.E. & Vassallo J. (Eds), Manual de Imuno-histoquímica. Sociedade Brasileira de Patologia, São Paulo., Bertazzolo et al. 2005Bertazzolo W., Dell’Orco M., Bonfanti U., Ghisleni G., Caniatti M., Masserdotti C., Antoniazzi E., Crippa L. & Roccabianca P. 2005. Canine angiosarcoma: cytologic, histologic, and immunohistochemical correlations. Vet. Clin. Pathol. 34(1):28-34. <http://dx.doi.org/10.1111/j.1939-165X.2005.tb00005.x> <PMid:15732014>
https://doi.org/10.1111/j.1939-165X.2005...
).
In humans, hepatocellular carcinoma is the major PHN, because there are several associated etiological factors, such as viral infection, chronic alcoholism, nonalcoholic steatohepatitis and food contaminants as aflatoxins (Crawford & Liu 2010Crawford J.M. & Liu C. 2010. Fígado e trato biliar, p.979-1012. In: Kumar V., Abbas A.K., Fausto N. & Aster J.C. (Eds), Robbins e Cotran, Bases Patológicas das Doenças. Elsevier, Rio de Janeiro.). In cats, the etiology of PHN is unknown (Lawrence et al. 1994Lawrence H.J., Erb H.N. & Harvey H.J. 1994. Nonlymphomatous hepatobiliary masses in cats: 41 cases (1972-1991). Vet. Surg. 23(5):365-368. <http://dx.doi.org/10.1111/j.1532-950X.1994.tb00496.x> <PMid:7839594>
https://doi.org/10.1111/j.1532-950X.1994...
), but Platynosomum fastosum infection has been described in the literature as a predisposing factor for the development of cholangiocarcinoma (Santos et al. 1981Santos J.A., Lopes M.A.F., Schott A.C., Santos A.E.S., Porfírio L.C. & Passos L. 1981. Colangiocarcinomas em gatos com parasitismo de ductos biliares por Platynossomum fastosum. Pesq. Vet. Bras. 1:31-36., Andrade et al. 2012Andrade R.L.F.S., Oliveira D.M., Dantas A.F.M., Souza A.P., Nóbrega Neto P.I. & Riet-Correa F. 2012. Tumores de cães e gatos diagnosticados no semiárido da Paraíba. Pesq. Vet. Bras. 32(10):1037-1040. <http://dx.doi.org/10.1590/S0100-736X2012001000016>
https://doi.org/10.1590/S0100-736X201200...
). In the present study, no hepatobiliary trematodes were identified, because occurrence of Platynosomum sp. in the metropolitan region of Porto Alegre is infrequent (Michaelsen et al. 2012Michaelsen R., Silveira E., Marques S.M.T., Pimentel M.C. & Costa F.V.A. 2012. Platynosomum concinnum (Trematoda: Dicrocoeliidae) em gato doméstico da cidade de Porto Alegre, Rio Grande do Sul, Brasil. Vet. Foco 10(1):53-60.).
Conclusions
All primary hepatobiliary neoplasms (PHN) presented characteristics of malignancy and affected mainly elderly cats.
Cholangiocarcinoma was the most commonly diagnosed neoplasm, followed by hepatocellular carcinoma and hemangiosarcoma. Most cases of cholangiocarcinoma originated in intrahepatic bile ducts.
Grossly, cholangiocarcinoma and hemangiosarcoma presented predominance of the multinodular pattern, whereas hepatocellular carcinoma showed predominance of the massive pattern.
Extrahepatic gross findings were mainly characterized by poor body condition, ascites, and jaundice.
Histologically, there was predominance of acinar/ductal arrangement in cholangiocarcinomas and solid arrangement in hepatocellular carcinoma.
PHN showed moderate to severe cellular pleomorphism and mild mitotic index.
Proliferation of fibrous connective tissue and presence of mucin, identified by MT and PAS staining, respectively, were common histological findings in cholangiocarcinoma.
The use of hepatocyte and bile duct epithelial cells specific antibodies, such as Hep Par-1 and CK 7 assisted with the diagnosis of PHN.
References
- Andrade R.L.F.S., Oliveira D.M., Dantas A.F.M., Souza A.P., Nóbrega Neto P.I. & Riet-Correa F. 2012. Tumores de cães e gatos diagnosticados no semiárido da Paraíba. Pesq. Vet. Bras. 32(10):1037-1040. <http://dx.doi.org/10.1590/S0100-736X2012001000016>
» https://doi.org/10.1590/S0100-736X2012001000016 - Balkman C. 2009. Hepatobiliary neoplasia in dogs and cats. Vet. Clin. N. Am., Small Anim. Pract. 39(3):617-625. <http://dx.doi.org/10.1016/j.cvsm.2009.01.001> <PMid:19524795>
» https://doi.org/10.1016/j.cvsm.2009.01.001 - Barros C.S.L. 2016. Fígado, vias biliares e pâncreas exócrino, p.222-265. In: Santos R.L. & Alessi A.C. (Eds), Patologia Veterinária. 2ª ed. Roca, Rio de Janeiro.
- Bertazzolo W., Dell’Orco M., Bonfanti U., Ghisleni G., Caniatti M., Masserdotti C., Antoniazzi E., Crippa L. & Roccabianca P. 2005. Canine angiosarcoma: cytologic, histologic, and immunohistochemical correlations. Vet. Clin. Pathol. 34(1):28-34. <http://dx.doi.org/10.1111/j.1939-165X.2005.tb00005.x> <PMid:15732014>
» https://doi.org/10.1111/j.1939-165X.2005.tb00005.x - Charles J.A., Cullen J.M., Van den Ingh T.S.G.A.M., Winkle T.V. & Desmet V.J. 2006. Morphological classification of neoplastic disorders of the canine and feline liver, p.117-123. In: Rothuizen J. (Ed), WSAVA Standards for Clinical and Histological Diagnosis of Canine and Feline Liver Diseases. Elsevier, Edinburgh.
- Crawford J.M. & Liu C. 2010. Fígado e trato biliar, p.979-1012. In: Kumar V., Abbas A.K., Fausto N. & Aster J.C. (Eds), Robbins e Cotran, Bases Patológicas das Doenças. Elsevier, Rio de Janeiro.
- Cullen J.M. 2009. Summary of the World small animal veterinary association standardization committee guide to classification of liver disease in dogs and cats. Vet. Clin. N. Am., Small Anim. Pract. 39(3):395-418. <http://dx.doi.org/10.1016/j.cvsm.2009.02.003> <PMid:19524786>
» https://doi.org/10.1016/j.cvsm.2009.02.003 - Cullen J.M. 2017. Tumors of the liver and gallbladder, p.602-631. In: Meuten D.J. (Ed), Tumors in Domestic Animals. 5th ed. John Wiley & Sons, Iowa.
- Cullen J.M. & Stalker M.J. 2016. Liver and biliary system, p.307-308. In: Maxie M.G. (Ed.), Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol.2. 6th ed. Elsevier, St Louis. <http://dx.doi.org/10.1016/B978-0-7020-5318-4.00008-5>.
» https://doi.org/10.1016/B978-0-7020-5318-4.00008-5 - Engle G.C. & Brodey R.S. 1969. A retrospective study of 395 feline neoplasms. J. Am. Anim. Hosp. Assoc. 5(2):21-31.
- Feldman B.F., Strafuss A.C. & Gabbert N. 1976. Bile duct carcinoma in the cat: 3 case reports. Feline Pract. 6:33-39.
- Flores M.M., Bianchi R.M., Kommers G.D., Irigoyen L.F., Barros C.L. & Fighera R.A. 2013. Prevalência e achados epidemiológicos, anatomopatológicos e imuno-histoquímicos dos tumores hepáticos malignos primários de cães da Região Central do Rio Grande do Sul (1965-2012). Pesq. Vet. Bras. 33(4):497-511. <http://dx.doi.org/10.1590/S0100-736X2013000400014>
» https://doi.org/10.1590/S0100-736X2013000400014 - Gaffney E. 1992. Carbohydrates, p.151. In: Prophet E.B., Mills B., Arrington J.B. & Sobin L.H (Eds), Laboratory Methods in Histotechnology. Armed Forces Institute of Pathology, American Registry of Pathology, Washington.
- Gagne J.M., Weiss J. & Armstrong P.J. 1996. Histopathologic evaluation of feline inflammatory liver disease. Vet. Pathol. 33(5):521-526. <http://dx.doi.org/10.1177/030098589603300506> <PMid:8885178>
» https://doi.org/10.1177/030098589603300506 - Hammer A.S. & Sikkema D.A. 1995. Hepatic neoplasia in the dog and cat. Vet. Clin. N. Am., Small Anim. Pract. 25(2):419-435. <http://dx.doi.org/10.1016/S0195-5616(95)50035-X> <PMid:7785172>
» https://doi.org/10.1016/S0195-5616(95)50035-X - Head K.W., Cullen J.M., Dubielzig R.R., Else R.W., Misdorp W., Patnaik A.K., Tateyama S. & Van der Gaag I. 2003. Histological Classification of Tumors of the Alimentary System of Domestic Animals. Vol.5. 2nd ed. Armed Forces Institute of Pathology, Washington. 257p.
- Hirose N., Uchida K., Kanemoto H., Ohno K., Chambers J.K. & Nakayama H. 2014. A retrospective histopathological survey on canine and feline liver diseases at the University of Tokyo between 2006 and 2012. J. Vet. Med. Sci. 76(7):1015-1020. <http://dx.doi.org/10.1292/jvms.14-0083> <PMid:24717415>
» https://doi.org/10.1292/jvms.14-0083 - Lau S.K., Prakash S., Geller S.A. & Alsabeh R. 2002. Comparative immunohistochemical profile of hepatocellular carcinoma, cholangiocarcinoma, and metastatic adenocarcinoma. Hum. Pathol. 33(12):1175-1181. <http://dx.doi.org/10.1053/hupa.2002.130104> <PMid:12514785>
» https://doi.org/10.1053/hupa.2002.130104 - Lawrence H.J., Erb H.N. & Harvey H.J. 1994. Nonlymphomatous hepatobiliary masses in cats: 41 cases (1972-1991). Vet. Surg. 23(5):365-368. <http://dx.doi.org/10.1111/j.1532-950X.1994.tb00496.x> <PMid:7839594>
» https://doi.org/10.1111/j.1532-950X.1994.tb00496.x - Liptak J.M., Dernell W.S., Monnet E., Powers B.E., Bachand A.M., Kenney J.G. & Withrow S.J. 2004. Massive hepatocellular carcinoma in dogs: 48 cases (1992-2002). J. Am. Vet. Med. Assoc. 225(8):1225-1230. <http://dx.doi.org/10.2460/javma.2004.225.1225> <PMid:15521445>
» https://doi.org/10.2460/javma.2004.225.1225 - Martins T.M. 2016. Causas de morte e razões para eutanásia de gatos na Região Central do Rio Grande do Sul. Doctoral Dissertation in Pathology and Clinical Pathology, Universidade Federal de Santa Maria, Santa Maria, RS.
- McElroy D.A. 1992. Connective tissue, p.132. In: Prophet E.B., Mills B., Arrington J.B. & Sobin L.H (Eds), Laboratory Methods in Histotechnology. Armed Forces Institute of Pathology, American Registry of Pathology, Washington.
- Mello E.S. & Alves V.A.F. 1999. Glossário dos principais marcadores imuno-histoquímicos, p.266-270. In: Alves V.A.F., Bacchi C.E. & Vassallo J. (Eds), Manual de Imuno-histoquímica. Sociedade Brasileira de Patologia, São Paulo.
- Michaelsen R., Silveira E., Marques S.M.T., Pimentel M.C. & Costa F.V.A. 2012. Platynosomum concinnum (Trematoda: Dicrocoeliidae) em gato doméstico da cidade de Porto Alegre, Rio Grande do Sul, Brasil. Vet. Foco 10(1):53-60.
- O’Neill D.G., Church D.B., Mcgreevy P.D., Thomson P.C. & Brodbelt D.C. 2015. Longevity and mortality of cats attending primary care veterinary practices in England. J. Feline Med. Surg. 17(2):125-133. <http://dx.doi.org/10.1177/1098612X14536176> <PMid:24925771>
» https://doi.org/10.1177/1098612X14536176 - Otte C.M., Penning L.C. & Rothuizen J. 2017. Feline biliary tree and gallbladder disease: aetiology, diagnosis and treatment. J. Feline Med. Surg. 19(5):514-528. <http://dx.doi.org/10.1177/1098612X17706465> <PMid:28438089>
» https://doi.org/10.1177/1098612X17706465 - Patnaik A.K. 1992. A morphological and immunocytochemical study of hepatic neoplasms in cats. Vet. Pathol. 29(5):405-415. <http://dx.doi.org/10.1177/030098589202900506> <PMid:1413408>
» https://doi.org/10.1177/030098589202900506 - Patnaik A.K., Liu S.K., Hurvitz A.I. & McClelland A.J. 1975. Nonhematopoietic neoplasms in cats. J. Nat. Cancer Inst. 54(4):855-860. <PMid:1055268>
- Post G. & Patnaik A.K. 1992. Nonhematopoietic hepatic neoplasms in cats: 21 cases (1983-1988). J. Am. Vet. Med. Assoc. 201(7):1080-1082. <PMid:1330999>
- Ramos-Vara J.A., Miller M.A. & Johnson G.C. 2001. Immunohistochemical characterization of canine hyperplastic hepatic lesions and hepatocellular and biliary neoplasms with monoclonal antibody hepatocyte paraffin 1 and a monoclonal antibody to cytokeratin 7. Vet. Pathol. 38(6):636-643. <http://dx.doi.org/10.1354/vp.38-6-636> <PMid:11732796>
» https://doi.org/10.1354/vp.38-6-636 - Rutgers C. 1998. Feline liver disease. In Practice 20(1):16-25. <http://dx.doi.org/10.1136/inpract.20.1.16>
» https://doi.org/10.1136/inpract.20.1.16 - Santos J.A., Lopes M.A.F., Schott A.C., Santos A.E.S., Porfírio L.C. & Passos L. 1981. Colangiocarcinomas em gatos com parasitismo de ductos biliares por Platynossomum fastosum Pesq. Vet. Bras. 1:31-36.
- Scavelli T.D., Patnaik A.K., Mehlhaff C.J. & Hayes A.A. 1985. Hemangiosarcoma in the cat: retrospective evaluation of 31 surgical cases. J. Am. Vet. Med. Assoc. 187(8):817-819. <PMid:4055500>
- Schmidt R.E. & Langham R.F. 1967. A survey of feline neoplasms. J. Am. Vet. Med. Assoc. 151(559):1325-1328.
- Shimonishi T., Miyazaki K. & Nakanuma Y. 2000. Cytokeratin profile relates to histological subtypes and intrahepatic location of intrahepatic cholangiocarcinoma and primary sites of metastatic adenocarcinoma of liver. Histopathology 37(1):55-63. <http://dx.doi.org/10.1046/j.1365-2559.2000.00932.x> <PMid:10931219>
» https://doi.org/10.1046/j.1365-2559.2000.00932.x - Stonehewer J. 2006. Fígado e pâncreas, p.358-372. In: Chandler E.A. & Gaskell R.M. (Eds), Clínica e Terapêutica em Felinos. 3ª ed. Roca, São Paulo.
- Van Sprundel R.G.H.M., Van den Ingh T.S.G.A.M., Guscetti F., Kershaw O., Van Wolferen M.E., Rothuizen J. & Spee B. 2014. Classification of primary hepatic tumours in the cat. Vet. J. 202(2):255-266. <http://dx.doi.org/10.1016/j.tvjl.2014.07.002> <PMid:25439443>
» https://doi.org/10.1016/j.tvjl.2014.07.002
Publication Dates
-
Publication in this collection
03 Apr 2020 -
Date of issue
Jan 2020
History
-
Received
12 Nov 2018 -
Accepted
16 Feb 2019