ABSTRACT:
Bovine digital dermatitis (BDD) is a polybacterial claw disease that is endemic to dairy cattle kept in loose house systems, and treponemas are the main bacteria implicated in this disease. The objective of this study was to report the occurrence of Treponema spp. in BDD from crossbred dairy cattle (Holstein x Zebu) kept in a pasture in the Brazilian Amazon biome. The diagnostic of BDD was performed by inspecting the distal extremities of cattle during milking in one or more visits comprising 15 farms. In total, it could be inspected 1,847 cows from August 2016 to July 2017, and 25 lesions of BDD were diagnosed. The feet were scored (System M: M0 = no lesion, M1 = ulcer stage <2cm, M2 = ulcer stage >2cm, M3 = healing stage, M4 = chronic stage, M4.1 = chronic stage with ulcer area). Twenty four biopsy samples were taken from feet with BDD and five biopsy samples from feet with no lesions. The histopathology of stained tissues was performed by hematoxylin and eosin and Warthin-Starry method. The samples were also tested by nested PCR for the three previously isolated BDD Treponema phylogroups (T. medium/T. vincentii-like, T. phagedenis-like and T. putidum/T. denticola-like). Spirochetes were observed in 54.2% (13/24) of the lesions, and in 91.7% (22/24) of the samples were detected the DNA of this spirochete belonging to the treponema phylogroups implicated in BDD. In 25% (6/24) of the lesions were detected all the phylogroups. Forty percent (40%, 2/5) of the M0 samples were also positive for the nested Polymerase Chain Reaction (nested-PCR), as 8.3% (2/24) of the lesions were negative in both techniques employed. Treponema putidum/T. denticola-like was the most detected bacterial in all the stages, and active lesions (M2 and M4.1) presented a greater proportion of T. medium/T. vincentii-like and T. phagedenis-like, but no statistical differences were observed (p>0.05). It could be concluded that BDD lesions in crossbred dairy cattle kept to pasture in the Amazon biome were classified as “polytreponemal” infections and the phylogroup T. putidum/T. denticola-like was the most frequent in the lesions.
INDEX TERMS:
Treponema spp.; bovine digital dermatitis; Amazon biome; Brazil; Mortellaro disease; Warthin-Starry; nested-PCR; cattle
RESUMO:
Dermatite digital bovina (DDB) é uma enfermidade polibacteriana dos dígitos endêmica em vacas leiteiras criadas em estábulos e as treponemas são as principais bactérias envolvidas. Este estudo teve como objetivo relatar a ocorrência de Treponema spp. em DDB em bovinos leiteiros mestiços (Holandês x Zebu) criados a pasto no bioma amazônico brasileiro. O diagnóstico da DDB foi realizado pela inspeção, em uma ou mais visitas, das extremidades distais das vacas durante a ordenha em 15 propriedades. No total, foram inspecionadas 1.847 vacas de agosto de 2016 a julho de 2017 e diagnosticou-se 25 lesões de DDB. As extremidades distais inspecionadas foram classificadas em escores (M0 = sem lesão, M1 = estágio ulcerado <2cm, M2 = estágio ulcerado >2cm, M3 = estágio em cicatrização, M4 = estágio crônico, M4.1 = estágio crônico com área ulcerada) e realizada 24 biópsias de dígitos com DDB e cinco biópsias de dígitos em estágio M0. Foram realizadas a histopatologia pelas colorações de hematoxilina e eosina e pelo método de Warthin-Starry, e a nested de reação em cadeia de polimerase (nested-PCR) para os três filogrupos de treponemas previamente isolados de DDB (Treponema medium/T. vincentii-like, T. phagedenis-like e T. putidum/T. denticola-like). Espiroquetas foram observadas em 54,2% (13/24) das lesões e em 91,7% (22/24) detectou-se o DNA de, pelo menos, um dos filogrupos de treponemas pesquisados. Em 25% (6/24) das lesões foram detectados o DNA dos três filogrupos. Em 40% (2/5) das amostras em estágio M0 também foram positivas na nested-PCR, assim como 8,3% (2/24) das lesões foram negativas em ambas as técnicas empregadas. T. putidum/T. denticola-like foi o filogrupo mais detectado em todos os estágios e lesões ativas (M2 e M4.1) apresentaram uma maior proporção para Treponema medium/T. vincentii-like e T. phagedenis-like, mas não se obteve diferença estatística na ocorrência dos filogrupos entre os estágios das lesões (P>0,05). Conclui-se que lesões de DDB em rebanhos leiteiros mestiços criados a pasto no bioma amazônico brasileiro são “politreponemais” e o filogrupo T. putidum/T. denticola-like é o mais frequente nas lesões.
TERMOS DE INDEXAÇÃO:
Treponema spp.; dermatite digital bovina; Amazônia; doença de Mortellaro; Warthin-Starry; nested-PCR; Brasil; bovinos
Introduction
Bovine digital dermatitis (BDD) is an infectious disease characterized by inflammation and ulceration of the skin of bovine digits, and also associated with different bacterial agents (Cheli & Mortellaro 1974Cheli R. & Mortellaro C. 1974. Digital dermatitis in cattle. Proceedings 8th International Meeting Diseases of Cattle, Milan, Italy. 213p., Santos et al. 2011Santos T.M.A., Pereira R.V., Caixeta L.S., Guard C.L. & Bicalho R.C. 2011. Microbial diversity in bovine papillomatous digital dermatitis in Holstein dairy cows from upstate New York. FEMS Microbiol. Ecol. 79(2):518-529. <http://dx.doi.org/10.1111/j.1574-6941.2011.01234.x> <PMid:22093037>
https://doi.org/10.1111/j.1574-6941.2011...
, Krull et al. 2014Krull A.C., Shearer J.K., Gorden P.J., Cooper V.L., Phillips G.J. & Plummer P.J. 2014. Deep sequencing analysis reveals temporal microbiota changes associated with development of bovine digital dermatitis. Infect. Immun. 82(8):3359-3373. <http://dx.doi.org/10.1128/IAI.02077-14> <PMid:24866801>
https://doi.org/10.1128/IAI.02077-14...
). In the BDD lesions, the spirochetes which are bacteria of the genus Mycoplasma, Fusobacterium, Porphyromonas, Bacteroides spp., Campylobacter spp. have been isolated, as well as the species of Guggenheimella bovis and Dichelobacter nodosus (Döpfer et al. 1997Döpfer D., Koopmans A., Meijer F.A., Szakáll I., Schukken Y.H., Klee W., Bosma R.B., Cornelisse J.L., Van Asten A.J.A.M. & ter Huurne A.A.H.M. 1997. Histological and bacteriological evaluation of digital dermatitis in cattle, with special reference to spirochaetes and Campylobacter faecalis. Vet. Rec. 140(24):620-623. <http://dx.doi.org/10.1136/vr.140.24.620> <PMid:9228692>
https://doi.org/10.1136/vr.140.24.620...
, Schlafer et al. 2008Schlafer S., Nordhoff M., Wyss C., Strub S., Hübner J., Gescher D.M., Petrich A., Göbel U.B. & Moter A. 2008. Involvement of Guggenheimella bovis in digital dermatitis lesions of dairy cows. Vet. Microbiol. 128(1/2):118-125. <http://dx.doi.org/10.1016/j.vetmic.2007.09.024> <PMid:18024006>
https://doi.org/10.1016/j.vetmic.2007.09...
, Rasmussen et al. 2012Rasmussen M., Capion N., Klitgaard K., Rogdo T., Fjeldass T., Boye M. & Jensen T.K. 2012. Bovine digital dermatitis: Possible pathogenic consortium consisting of Dichelobacter nodosus and multiple Treponema species. Vet. Microbiol. 160(1/2):151-161. <http://dx.doi.org/10.1016/j.vetmic.2012.05.018> <PMid:22698300>
https://doi.org/10.1016/j.vetmic.2012.05...
, Krull et al. 2014Krull A.C., Shearer J.K., Gorden P.J., Cooper V.L., Phillips G.J. & Plummer P.J. 2014. Deep sequencing analysis reveals temporal microbiota changes associated with development of bovine digital dermatitis. Infect. Immun. 82(8):3359-3373. <http://dx.doi.org/10.1128/IAI.02077-14> <PMid:24866801>
https://doi.org/10.1128/IAI.02077-14...
, Nielsen et al. 2016Nielsen M.W., Strube M.L., Isbrand A., Al-Medrasi W.D.H.M, Boye M., Jensen T.K. & Klitgaard K. 2016. Potencial bacterial core species associated with digital dermatitis in cattle herds identified by molecular profiling of interdigital skin samples. Vet. Microbiol. 186:139-149. <http://dx.doi.org/10.1016/j.vetmic.2016.03.003> <PMid:27016768>
https://doi.org/10.1016/j.vetmic.2016.03...
). Among these, spirochetes are the bacterial agents which are the most related to the disease, since they are detected in more significant proportions and found in deeper layers of the epidermis, these bacterial agents also have the ability to suppress the innate immune system and to induce the formation of lesions (Stamm et al. 2002Stamm L.V., Bergen H.L. & Walker R.L. 2002. Molecular typing of papillomatous digital dermatitis-associated Treponema isolates based on analysis of 16S-23S ribosomal DNA intergenic spacer regions. J. Clin. Microbiol. 40(9):3463-3469. <http://dx.doi.org/10.1128/jcm.40.9.3463-3469.2002> <PMid:12202594>
https://doi.org/10.1128/jcm.40.9.3463-34...
, Cruz et al. 2005Cruz C.E.F., Pescador C.A., Nakajima Y. & Driemeier D. 2005. Immunopathological investigations on bovine digital epidermitis. Vet. Rec. 157(26):834-840. <http://dx.doi.org/10.1136/vr.157.26.834> <PMid:16377788>
https://doi.org/10.1136/vr.157.26.834...
, Zuerner et al. 2007Zuerner R.L., Heidari M., Elliott M.K., Alt D.P. & Neill J.D. 2007. Papillomatous digital dermatitis spirochetes suppress the bovine macrophage innate immune response. Vet. Microbiol. 125(3/4):256-264. <http://dx.doi.org/10.1016/j.vetmic.2007.06.001> <PMid:17628359>
https://doi.org/10.1016/j.vetmic.2007.06...
, Klitgaard et al. 2008Klitgaard K., Boye M., Capion N. & Jensen T.K. 2008. Evidence of multiple Treponema phylotypes involved in bovine digital dermatitis as shown by 16S rRNA gene analysis and fluorescence in situ hybridization. J. Clin. Microbiol. 46(9):3012-3020. <http://dx.doi.org/10.1128/JCM.00670-08> <PMid:18562583>
https://doi.org/10.1128/JCM.00670-08...
, Nordhoff et al. 2008Nordhoff M., Moter A., Schrank K. & Wieler L.H. 2008. High prevalence of treponemes in bovine digital dermatitis - a molecular epidemiology. Vet. Microbiol. 131(3/4):293-300. <http://dx.doi.org/10.1016/j.vetmic.2008.04.019> <PMid:18524502>
https://doi.org/10.1016/j.vetmic.2008.04...
, Nielsen et al. 2016Nielsen M.W., Strube M.L., Isbrand A., Al-Medrasi W.D.H.M, Boye M., Jensen T.K. & Klitgaard K. 2016. Potencial bacterial core species associated with digital dermatitis in cattle herds identified by molecular profiling of interdigital skin samples. Vet. Microbiol. 186:139-149. <http://dx.doi.org/10.1016/j.vetmic.2016.03.003> <PMid:27016768>
https://doi.org/10.1016/j.vetmic.2016.03...
). According to molecular studies, Treponema is the most important genus of spirochetes isolated from BDD consisting of various strains, which characterizes a “polytreponemal” disease (Evans et al. 2008Evans N.J., Brown J.M., Demirkan I., Murray R.D., Vink D.W., Blowey R.W., Hart C.A. & Carter S.D. 2008. Three unique groups of spirochetes isolated from digital dermatitis lesions in UK cattle. Vet. Microbiol. 130(1/2):141-150. <http://dx.doi.org/10.1016/j.vetmic.2007.12.019> <PMid:18243592>
https://doi.org/10.1016/j.vetmic.2007.12...
, Klitgaard et al. 2008Klitgaard K., Boye M., Capion N. & Jensen T.K. 2008. Evidence of multiple Treponema phylotypes involved in bovine digital dermatitis as shown by 16S rRNA gene analysis and fluorescence in situ hybridization. J. Clin. Microbiol. 46(9):3012-3020. <http://dx.doi.org/10.1128/JCM.00670-08> <PMid:18562583>
https://doi.org/10.1128/JCM.00670-08...
, Krull et al. 2014Krull A.C., Shearer J.K., Gorden P.J., Cooper V.L., Phillips G.J. & Plummer P.J. 2014. Deep sequencing analysis reveals temporal microbiota changes associated with development of bovine digital dermatitis. Infect. Immun. 82(8):3359-3373. <http://dx.doi.org/10.1128/IAI.02077-14> <PMid:24866801>
https://doi.org/10.1128/IAI.02077-14...
, Nielsen et al. 2016Nielsen M.W., Strube M.L., Isbrand A., Al-Medrasi W.D.H.M, Boye M., Jensen T.K. & Klitgaard K. 2016. Potencial bacterial core species associated with digital dermatitis in cattle herds identified by molecular profiling of interdigital skin samples. Vet. Microbiol. 186:139-149. <http://dx.doi.org/10.1016/j.vetmic.2016.03.003> <PMid:27016768>
https://doi.org/10.1016/j.vetmic.2016.03...
).
The BDD is a common foot condition in dairy cows reared in a free-stall farming system in England, Germany, United States of America (USA), and Japan (Evans et al. 2008Evans N.J., Brown J.M., Demirkan I., Murray R.D., Vink D.W., Blowey R.W., Hart C.A. & Carter S.D. 2008. Three unique groups of spirochetes isolated from digital dermatitis lesions in UK cattle. Vet. Microbiol. 130(1/2):141-150. <http://dx.doi.org/10.1016/j.vetmic.2007.12.019> <PMid:18243592>
https://doi.org/10.1016/j.vetmic.2007.12...
, Klitgaard et al. 2008Klitgaard K., Boye M., Capion N. & Jensen T.K. 2008. Evidence of multiple Treponema phylotypes involved in bovine digital dermatitis as shown by 16S rRNA gene analysis and fluorescence in situ hybridization. J. Clin. Microbiol. 46(9):3012-3020. <http://dx.doi.org/10.1128/JCM.00670-08> <PMid:18562583>
https://doi.org/10.1128/JCM.00670-08...
, Nordhoff et al. 2008Nordhoff M., Moter A., Schrank K. & Wieler L.H. 2008. High prevalence of treponemes in bovine digital dermatitis - a molecular epidemiology. Vet. Microbiol. 131(3/4):293-300. <http://dx.doi.org/10.1016/j.vetmic.2008.04.019> <PMid:18524502>
https://doi.org/10.1016/j.vetmic.2008.04...
, Yano et al. 2010Yano T., Moe K.K., Yamazaki K., Ooka T., Hayashi T. & Misawa N. 2010. Identification of candidate pathogens of papillomatous digital dermatitis in dairy cattle from quantitative 16S rRNA clonal analysis. Vet. Microbiol. 143(2/4):352-262. <http://dx.doi.org/10.1016/j.vetmic.2009.12.009> <PMid:20036086>
https://doi.org/10.1016/j.vetmic.2009.12...
). In these countries, three different Treponema phylogroups are commonly identified in BDD lesions, such as: “T. medium/T. vincentti-like”, “T. phagedenis-like” and “T. pudidum/T. denticola-like” (Evans et al. 2008Evans N.J., Brown J.M., Demirkan I., Murray R.D., Vink D.W., Blowey R.W., Hart C.A. & Carter S.D. 2008. Three unique groups of spirochetes isolated from digital dermatitis lesions in UK cattle. Vet. Microbiol. 130(1/2):141-150. <http://dx.doi.org/10.1016/j.vetmic.2007.12.019> <PMid:18243592>
https://doi.org/10.1016/j.vetmic.2007.12...
, Yano et al. 2010Yano T., Moe K.K., Yamazaki K., Ooka T., Hayashi T. & Misawa N. 2010. Identification of candidate pathogens of papillomatous digital dermatitis in dairy cattle from quantitative 16S rRNA clonal analysis. Vet. Microbiol. 143(2/4):352-262. <http://dx.doi.org/10.1016/j.vetmic.2009.12.009> <PMid:20036086>
https://doi.org/10.1016/j.vetmic.2009.12...
, Döpfer et al. 2012Döpfer D., Ladell P., Anklam K., Mikheil D. & Dusick A. 2012. Growth curves and morphology of three Treponema subtypes isolated from digital dermatitis in cattle. Vet. J. 193(3):685-693. <http://dx.doi.org/10.1016/j.tvjl.2012.06.054> <PMid:22901455>
https://doi.org/10.1016/j.tvjl.2012.06.0...
, Marcatili et al. 2016Marcatili P., Nielsen M.W., Sicheritz-Pontén T., Jensen T.K., Schafer-Nielsen C., Boye M., Nielsen M. & Klitgaard K. 2016. A novel approach to probe host-pathogen interactions of bovine digital dermatitis, a model of a complex polymicrobial infection. BMC Genomics 17:987. <http://dx.doi.org/10.1186/s12864-016-3341-7> <PMid:27908274>
https://doi.org/10.1186/s12864-016-3341-...
). These bacterial phylogroups are also isolated from BDD in beef cattle (Sullivan et al. 2013Sullivan L.E., Carter S.D., Blowey R., Duncan J.S., Grove-White D. & Evans N.J. 2013. Digital dermatitis in beef cattle. Vet. Rec. 173(23):582-583. <http://dx.doi.org/10.1136/vr.101802>
https://doi.org/10.1136/vr.101802...
), sheep (Sullivan et al. 2015aSullivan L.E., Clegg S.R., Angell J.W., Newbrook K., Blowey R.W., Carter S.D., Bell J., Duncan J.S., Grove-White D.H., Murray R.D. & Evans N.J. 2015a. The high association of bovine digital dermatitis Treponema spp. with contagious ovine digital dermatitis lesions and the presence of Fusobacterium necrophorum and Dichelobacter nodosus. J. Clin. Microbiol. 53(5):1628-1638. <http://dx.doi.org/10.1128/JCM.00180-15> <PMid:25740778>
https://doi.org/10.1128/JCM.00180-15...
), goats (Sullivan et al. 2015bSullivan L.E., Evans N.J., Clegg S.R., Carter S.D., Horsfield J.E., Grove-White D. & Duncan J.S. 2015b. Digital dermatitis treponemes associated with a severe foot disease in dairy goats. Vet. Rec. 176(11):283-8. <http://dx.doi.org/10.1136/vr.102858> <PMid:25428906>
https://doi.org/10.1136/vr.102858...
), and in North American elk (Clegg et al. 2015Clegg S.R., Mansfield K.G., Newbrook K., Sullivan L.E., Blowey R.W., Carter S.D. & Evans N.J. 2015. Isolation of digital dermatitis treponemes from lesions in wild North American elk (Cervus elaphus) in Washington State, USA. J. Clin. Microbiol. 53(1):88-94. <http://dx.doi.org/10.1128/JCM.02276-14> <PMid:25355757>
https://doi.org/10.1128/JCM.02276-14...
).
In Brazil, BDD is a disease that occurs in cattle herds that may be raised under three different management systems (intensive, semi-intensive, or extensive practices) in their different regions, but with different rates of occurrence. Among foot lesions diagnosed in dairy cows, BDD comprised 38.9% of this disease in the state of Goiás (GO) (Silva et al. 2001Silva L.A.F., Silva L.M., Romani A.F., Rabelo R.E., Fioravanti M.C.S., Souza T.M. & Silva C.A. 2001. Características clínicas e epidemiológicas das enfermidades podais em vacas lactantes do município de Orizona-GO. Ciênc. Anim. Bras. 2(2):119-126.), 33% in the state of Minas Gerais (MG) (Moreira et al. 2018aMoreira T.F., Nicolino R.R., Andrade L.S., Facury Filho E.J. & Carvalho A.U. 2018a. Prevalence of lameness and hoof lesions in all year-round grazing cattle in Brazil. Trop. Anim. Health Prod. 50(8):1829-1834. <http://dx.doi.org/10.1007/s11250-018-1626-3> <PMid:29846882>
https://doi.org/10.1007/s11250-018-1626-...
), 29.9% in the state of Rio Grande do Sul (RS) (Cruz et al. 2001Cruz C., Driemeier D., Cerva C. & Corbellini L.G. 2001. Clinical and epidemiological aspects of bovine digital lesions in southern Brazil. Arq. Bras. Med. Vet. Zootec. 53(6):654-657. <http://dx.doi.org/10.1590/S0102-09352001000600006>
https://doi.org/10.1590/S0102-0935200100...
) and 0.92% in the state of Pará (PA) (Silveira et al. 2009Silveira J.A.C., Albernaz T.T., Oliveira C.M., Duarte M.D. & Barbosa J.D. 2009. Afecções podais em vacas da bacia leiteira de Rondon do Pará. Pesq. Vet. Bras. 29(11):905-909. <http://dx.doi.org/10.1590/S0100-736X2009001100007>
https://doi.org/10.1590/S0100-736X200900...
). However, studies related to bacterial agents in lesions are still limited. In dairy cows and beef cattle raised in the Midwest and South regions, the presence of spirochetes in stained tissues performed by silver has already been demonstrated (Cruz et al. 2005Cruz C.E.F., Pescador C.A., Nakajima Y. & Driemeier D. 2005. Immunopathological investigations on bovine digital epidermitis. Vet. Rec. 157(26):834-840. <http://dx.doi.org/10.1136/vr.157.26.834> <PMid:16377788>
https://doi.org/10.1136/vr.157.26.834...
, Castro et al. 2008Castro G.R., Brito L.A.B., Fioravanti M.C.S., Silva L.A.F., Araújo E.G., Orlando C.F.P., Franco L.G. & Moura M.I. 2008. Estudo anatomopatológico de lesões de dermatite digital em bovinos. Ciênc. Anim. Bras. 9(4):1159-1166.), as well as different species of the Treponema genus by using the fluorescent in situ hybridization (FISH) technique and by the nested-PCR (Nascimento et al. 2015Nascimento L.V., Mauerwerk M.T., Santos C.L., Barros Filho I.R., Birgel Júnior E.H., Sotomaior C.S., Madeira H.M.F. & Ollhoff R.D. 2015. Treponemes detected in digital dermatitis lesions in Brazilian dairy cattle and possible host reservoirs of infection. J. Clin. Microbiol. 53(6):1935-1937. <http://dx.doi.org/10.1128/JCM.03586-14> <PMid:25788552>
https://doi.org/10.1128/JCM.03586-14...
, Moreira et al. 2018bMoreira T.F., Facury Filho E.J., Carvalho A.U., Strube M.L., Nielsen M.W., Klitgaard K. & Jensen T.K. 2018b. Pathology and bacteria related to digital dermatitis in dairy cattle in all year round grazing system in Brazil. Plos One 13(3):e0193870. <http://dx.doi.org/10.1371/journal.pone.0193870> <PMid:29513739>
https://doi.org/10.1371/journal.pone.019...
).
In the Brazilian Amazon biome (northern region), the climatic conditions and territorial extension favor cattle-breeding on pasture land all year long, and it can be noted that the BDD is reported in dairy and beef cattle (Silveira et al. 2009Silveira J.A.C., Albernaz T.T., Oliveira C.M., Duarte M.D. & Barbosa J.D. 2009. Afecções podais em vacas da bacia leiteira de Rondon do Pará. Pesq. Vet. Bras. 29(11):905-909. <http://dx.doi.org/10.1590/S0100-736X2009001100007>
https://doi.org/10.1590/S0100-736X200900...
, Silveira et al. 2018Silveira J.A.S., Silva N.S., Albernaz T.T., Bomjardim H.A., Belo Reis A.S., Oliveira C.M.C., Duarte M.D. & Barbosa J.A. 2018. Estudo epidemiológico e clínico de afecções podais em bovinos de corte manejados extensivamente no sudeste do Pará. Pesq. Vet. Bras. 38(3):367-373. <http://dx.doi.org/10.1590/1678-5150-pvb-4411>
https://doi.org/10.1590/1678-5150-pvb-44...
). It is important to note, it seems that there are no studies related to the bacterial agents involved. This study aimed to demonstrate Treponema spp. in BDD lesions observed in histological fragments using the silver impregnation technique and complemented by nested-PCR in dairy cattle herds, raised on pasture in the Amazon biome.
Materials and Methods
Study region, diagnosis, lesion classification, and biopsies. A search for Treponema spp. through silver impregnation was performed and also complemented by nested-PCR in BDD lesions in crossbred dairy cattle (Holstein x Zebu) bred on pastures of Urochloa (Brachiaria) brizantha in southeastern Pará and western Maranhão, situated at the Amazon biome, from August 2016 to July 2017. 1,847 cattle from 15 rural properties were inspected in one or more visits, and 25 BDD lesions were diagnosed. The lesions were classified as follows: M1 = skin in an ulcer stage, diameter <2cm; M2 = skin in an ulcer stage >2cm; M3 = skin in a healing stage, covered by a crust; M4 = skin in a chronic stage, hyperkeratotic surface; and M4.1 = chronic stage skin with an ulcer area (Döpfer et al. 1997Döpfer D., Koopmans A., Meijer F.A., Szakáll I., Schukken Y.H., Klee W., Bosma R.B., Cornelisse J.L., Van Asten A.J.A.M. & ter Huurne A.A.H.M. 1997. Histological and bacteriological evaluation of digital dermatitis in cattle, with special reference to spirochaetes and Campylobacter faecalis. Vet. Rec. 140(24):620-623. <http://dx.doi.org/10.1136/vr.140.24.620> <PMid:9228692>
https://doi.org/10.1136/vr.140.24.620...
, Berry et al. 2012Berry S.L., Read D.H., Famula T.R., Mongini A. & Döpfer D. 2012. Long-term observations on the dynamics of bovine digital dermatitis lesions on a California dairy after topical treatment with lincomycin HCl. Vet. J. 193(3):654-658. <http://dx.doi.org/10.1016/j.tvjl.2012.06.048> <PMid:22892182>
https://doi.org/10.1016/j.tvjl.2012.06.0...
). Twenty-four biopsies of BDD and five digits were performed in stage M0. From the lesions and digits in stage M0 (digit without injury), two fragments were collected, approximately 0.5cm each, after anesthesia of the distal and by intravenous Bier block, with 20ml of 2% lidocaine. Regarding the BDD lesions, the first fragment, obtained with a scalpel blade and anatomical forceps, clean and sterile, was removed from the center of the lesions. In stage M4.1, the fragment was extracted from the ulcer area. These biopsies were stored in polyethylene tubes, previously identified, and kept at -20oC until laboratory procedures for molecular biology were performed. The second fragment, removed from the intersection of normal skin and the center of the lesion, was fixed in 10% buffered formaldehyde. In the digits of stage M0, both biopsies were performed at the caudal border of the pelvic limbs in the interdigital commissure, following the same procedures for collecting the lesions.
Histopathology. Samples fixed in formaldehyde were processed by the usual methods for histopathology, in the “Setor de Anatomia Patológica” of the “Universidade Federal Rural do Rio de Janeiro” (UFRRJ). These samples were soaked in paraffin, cut into a microtome at 5µm thickness, and stained with hematoxylin and eosin (HE) and by the Warthin-Starry method.
DNA extraction and nested-PCR. The biopsies were thawed at room temperature, and DNA extraction was performed following the protocol based on the use of phenol/chloroform as described by McIntosh et al. (2015)McIntosh D., Bezerra R.A., Luz H.R., Faccini J.L.H., Gaiotto F.A., Giné G.A.F. & Albuquerque G.R. 2015. Detection of Rickettsia bellii and Rickettsia amblyomma longirostre (Acari: Ixodidae) from Bahia state, Northeast Brazil. Braz. J. Microbiol. 46(3):879-883. <http://dx.doi.org/10.1590/S1517-838246320140623> <PMid:26413074>
https://doi.org/10.1590/S1517-8382463201...
.
The extracted DNA was submitted to nested-PCR using specific primers for the three treponema phylogroups “T. medium/T.vincentii-like”, “T. phagedenis-like” and “T. pudidum/T. denticola-like” according to the methodology described by Evans et al. (2008)Evans N.J., Brown J.M., Demirkan I., Murray R.D., Vink D.W., Blowey R.W., Hart C.A. & Carter S.D. 2008. Three unique groups of spirochetes isolated from digital dermatitis lesions in UK cattle. Vet. Microbiol. 130(1/2):141-150. <http://dx.doi.org/10.1016/j.vetmic.2007.12.019> <PMid:18243592>
https://doi.org/10.1016/j.vetmic.2007.12...
and (2009)Evans N.J., Brown J.M., Demirkan I., Murray R.D., Birtles R.J., Hart C.A. & Carter S.D. 2009. Treponema pedis sp. nov., a spirochaete isolated from bovine digital dermatitis lesions. Int. J. Syst. Evol. Microbiol. 59(Pt 5):987-991. <http://dx.doi.org/10.1099/ijs.0.002287-0> <PMid:19406779>
https://doi.org/10.1099/ijs.0.002287-0...
.
Statistical analysis. To evaluate a possible association between the phylogroups (T. medium/T. Vincentii-like, T. phagedenis-like and T. putidum/T. denticola-like) and the BDD stages (M0, M1, M2, M3, M4 and M4.1), the Fisher’s exact test was used, with a significance level (α) of 5%. All analyzes were performed after registration in spreadsheets (Microsoft Excel® 2010), and the data were analyzed using the statistical software SPSS 20.0 (IBM Corp. Released 2011, IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.). The frequencies of the variables presented descriptive analyzes.
Results
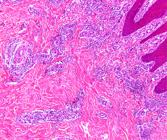
The BDD lesions were classified as 16.7% (4/24) in stage M1 (Fig.1), 37.5% (9/24) in M2, 12.5% (3/24 ) in M3, 12.5% (3/24) in M4 and 20.8% (5/24) in M4.1 (Fig.2) (Table 1). Histopathology revealed extensive ulceration (stages M1, M2, and M4.1) associated with ulcer clusters of bacterial colonies, acanthosis with hypergranulosis, and hyperkeratosis (sometimes referred to as parakeratotic, sometimes as orthokeratotic), which were accentuated in stages M3 and M4. Inflammatory cells had infiltrated the epidermis, the dermo-epidermal junction, and the perivascular region in the dermis (Fig.3 and 4). In the M0 stage, the skin presented its usual histological architecture with mild acanthosis, hypergranulosis, and a discrete perivascular infiltrate of inflammatory cells.
Bovine digital dermatitis. Spherical lesion, with a granular, moist surface and hypertrophied hair. Interdigital commissure of the plantar region of the left pelvic member of Bovine 9 (Table 1: stage M1, score by Döpfer et al. 1997Döpfer D., Koopmans A., Meijer F.A., Szakáll I., Schukken Y.H., Klee W., Bosma R.B., Cornelisse J.L., Van Asten A.J.A.M. & ter Huurne A.A.H.M. 1997. Histological and bacteriological evaluation of digital dermatitis in cattle, with special reference to spirochaetes and Campylobacter faecalis. Vet. Rec. 140(24):620-623. <http://dx.doi.org/10.1136/vr.140.24.620> <PMid:9228692>
https://doi.org/10.1136/vr.140.24.620... ). Amazon biome.
Bovine digital dermatitis. Ulcer lesion, irregularly shaped with a granular, reddish and moist surface, with raised and hyperkeratotic margins. Horny tissue with marked bead erosion. Plantar region of the left pelvic member of Bovine 28 (Table 1: stage M4.1, score by Döpfer et al. 1997Döpfer D., Koopmans A., Meijer F.A., Szakáll I., Schukken Y.H., Klee W., Bosma R.B., Cornelisse J.L., Van Asten A.J.A.M. & ter Huurne A.A.H.M. 1997. Histological and bacteriological evaluation of digital dermatitis in cattle, with special reference to spirochaetes and Campylobacter faecalis. Vet. Rec. 140(24):620-623. <http://dx.doi.org/10.1136/vr.140.24.620> <PMid:9228692>
https://doi.org/10.1136/vr.140.24.620... and Berry et al. 2012Berry S.L., Read D.H., Famula T.R., Mongini A. & Döpfer D. 2012. Long-term observations on the dynamics of bovine digital dermatitis lesions on a California dairy after topical treatment with lincomycin HCl. Vet. J. 193(3):654-658. <http://dx.doi.org/10.1016/j.tvjl.2012.06.048> <PMid:22892182>
https://doi.org/10.1016/j.tvjl.2012.06.0... ). Amazon biome.
Bovine digital dermatitis. Skin with extensive area of ulceration and exudation of the epidermis. Dermis with mild inflammatory infiltrate at the dermo-epidermal junction (DEJ). HE, obj.10x.
Bovine digital dermatitis. Skin with moderate inflammatory infiltrate, predominantly perivascular in the dermis and dermal-epidermal junction (DEJ). HE, obj.4x.
The silver impregnation revealed spirochetes in the epidermis superficial strata in 54.2% of the lesions (13/24) (Fig.5 and 6), and its absence in the M0 stage. Nested-PCR detected the genetic material of Treponema ssp. in 91.7% (22/24) of BDD injuries. The DNA of the phylogroup T. pudidum/T. denticola-like was the most frequently detected (83.3%, 20/24) in samples with lesions. In 25% (6/24) of the lesions, the three Treponema phylogroups surveyed were detected. In the M0 stage, Treponema genetic material was detected in 40% (2/5) of the samples, and 8.3% (2/24) of the BDD lesions were negative for spirochetes and Treponema spp. in both techniques employed, respectively (Table 1).
Bovine digital dermatitis. Spirochetes in the superficial dermis and around hair follicles. Warthin-Starry method, obj.100x.
Bovine digital dermatitis. Spirochetes in the spinous stratum of the epidermis. Warthin-Starry method, obj.100x.
Through the distribution of the genetic material between the stages of BDD, it could be observed that the T. pudidum/T. denticola-like phylogroup was the most frequently detected (p=0.048). In fact, it could be detected a higher proportion of positive samples for the T. medium/T. vincentii-like phylogroups and T. phagedenis-like in stages M2 and M4.1, however, there was no statistical difference (p=0.408 and p=0.279, respectively) in the occurrence of this phylogroup and the BDD stage (Fig.7).
Distribution of frequencies (%) of Treponema detected using the nested-PCR technique in biopsies of BDD and skin of the digit without injury (M0) according to the stage (scored by Döpfer et al. (1997)Döpfer D., Koopmans A., Meijer F.A., Szakáll I., Schukken Y.H., Klee W., Bosma R.B., Cornelisse J.L., Van Asten A.J.A.M. & ter Huurne A.A.H.M. 1997. Histological and bacteriological evaluation of digital dermatitis in cattle, with special reference to spirochaetes and Campylobacter faecalis. Vet. Rec. 140(24):620-623. <http://dx.doi.org/10.1136/vr.140.24.620> <PMid:9228692>
https://doi.org/10.1136/vr.140.24.620... and Berry et al. (2012)Berry S.L., Read D.H., Famula T.R., Mongini A. & Döpfer D. 2012. Long-term observations on the dynamics of bovine digital dermatitis lesions on a California dairy after topical treatment with lincomycin HCl. Vet. J. 193(3):654-658. <http://dx.doi.org/10.1016/j.tvjl.2012.06.048> <PMid:22892182>
https://doi.org/10.1016/j.tvjl.2012.06.0... of crossbred dairy cattle raised on pasture in the Amazon biome.
Discussion
These results suggested the presence of Treponema spp. in the etiology of BDD, as observed in dairy cows kept in pastures in the Brazilian Central region (Moreira et al. (2018b)Moreira T.F., Facury Filho E.J., Carvalho A.U., Strube M.L., Nielsen M.W., Klitgaard K. & Jensen T.K. 2018b. Pathology and bacteria related to digital dermatitis in dairy cattle in all year round grazing system in Brazil. Plos One 13(3):e0193870. <http://dx.doi.org/10.1371/journal.pone.0193870> <PMid:29513739>
https://doi.org/10.1371/journal.pone.019...
, in dairy cows housed in the southern region of Brazil (Nascimento et al. 2015Nascimento L.V., Mauerwerk M.T., Santos C.L., Barros Filho I.R., Birgel Júnior E.H., Sotomaior C.S., Madeira H.M.F. & Ollhoff R.D. 2015. Treponemes detected in digital dermatitis lesions in Brazilian dairy cattle and possible host reservoirs of infection. J. Clin. Microbiol. 53(6):1935-1937. <http://dx.doi.org/10.1128/JCM.03586-14> <PMid:25788552>
https://doi.org/10.1128/JCM.03586-14...
) and free-stalled dairy cows in the USA (Zinicola et al. 2015Zinicola M., Lima F., Lima S., Machado V., Gomez M., Döpfer D., Guard C. & Bicalho R. 2015. Altered microbiomes in bovine digital dermatitis lesions, and the gut as a pathogen reservoir. Plos One 10(3):e0120504. <http://dx.doi.org/10.1371/journal.pone.0120504> <PMid:25781328>
https://doi.org/3https://doi.org/10.1371...
), Germany (Nordhoff et al. 2008Nordhoff M., Moter A., Schrank K. & Wieler L.H. 2008. High prevalence of treponemes in bovine digital dermatitis - a molecular epidemiology. Vet. Microbiol. 131(3/4):293-300. <http://dx.doi.org/10.1016/j.vetmic.2008.04.019> <PMid:18524502>
https://doi.org/10.1016/j.vetmic.2008.04...
), Denmark (Klitgaard et al. 2008Klitgaard K., Boye M., Capion N. & Jensen T.K. 2008. Evidence of multiple Treponema phylotypes involved in bovine digital dermatitis as shown by 16S rRNA gene analysis and fluorescence in situ hybridization. J. Clin. Microbiol. 46(9):3012-3020. <http://dx.doi.org/10.1128/JCM.00670-08> <PMid:18562583>
https://doi.org/10.1128/JCM.00670-08...
), England (Evans et al. 2008Evans N.J., Brown J.M., Demirkan I., Murray R.D., Vink D.W., Blowey R.W., Hart C.A. & Carter S.D. 2008. Three unique groups of spirochetes isolated from digital dermatitis lesions in UK cattle. Vet. Microbiol. 130(1/2):141-150. <http://dx.doi.org/10.1016/j.vetmic.2007.12.019> <PMid:18243592>
https://doi.org/10.1016/j.vetmic.2007.12...
) and Japan (Yano et al. 2010Yano T., Moe K.K., Yamazaki K., Ooka T., Hayashi T. & Misawa N. 2010. Identification of candidate pathogens of papillomatous digital dermatitis in dairy cattle from quantitative 16S rRNA clonal analysis. Vet. Microbiol. 143(2/4):352-262. <http://dx.doi.org/10.1016/j.vetmic.2009.12.009> <PMid:20036086>
https://doi.org/10.1016/j.vetmic.2009.12...
).
By the methodology used, a high frequency of Treponema spp. was observed in the lesions of BDD, comprising 91.7%. However, there was lesser diversity of phylogroups between the lesions, consisting of 25%. In dairy cows intensively reared in southern Brazil, Nascimento et al. (2015)Nascimento L.V., Mauerwerk M.T., Santos C.L., Barros Filho I.R., Birgel Júnior E.H., Sotomaior C.S., Madeira H.M.F. & Ollhoff R.D. 2015. Treponemes detected in digital dermatitis lesions in Brazilian dairy cattle and possible host reservoirs of infection. J. Clin. Microbiol. 53(6):1935-1937. <http://dx.doi.org/10.1128/JCM.03586-14> <PMid:25788552>
https://doi.org/10.1128/JCM.03586-14...
detected Treponema spp. in 100% of the researched injuries, and 81.8% of the injuries showed all three phylogroups. This lower frequency, with a low variety of Treponema detected in the lesions, may be related to the lower environmental pressure to which these animals raised on pasture in the Amazon biome are subjected. On pasture, the animals are susceptible to low humidity (digit), especially in the non-rainy season, less contact with feces, and less contact between animals. In the etiopathogenesis, the main reservoirs of Treponema for healthy cattle suggested by Shibahara et al. (2002)Shibahara T., Ohya T., Ishii R., Ogihara Y., Maeda T., Ishikawa Y. & Kadota K. 2002. Concurrent spirochaetal infections of the feet and colon of cattle in Japan. Aust. Vet. J. 80(8):497-502. <http://dx.doi.org/10.1111/j.1751-0813.2002.tb12474.x> <PMid:12224620>
https://doi.org/10.1111/j.1751-0813.2002...
, Evans et al. (2012)Evans N.J., Timofte D., Isherwood D.R., Brown J.M., Williams J.M., Sherlock K., Lehane M.J., Murray R.D., Birtles R.J., Hart C.A. & Carter S.D. 2012. Host and environmental reservoirs of infection for bovine digital dermatitis treponemes. Vet. Microbiol. 156(1/2):102-109. <http://dx.doi.org/10.1016/j.vetmic.2011.09.029> <PMid:22019292>
https://doi.org/10.1016/j.vetmic.2011.09...
, Klitgaard et al. (2014)Klitgaard K., Nielsen M.W., Ingerslev H., Boye M. & Jensen T.K. 2014. Discovery of bovine digital dermatitis - associated Treponema spp. in the dairy herd environment by a targeted deep-sequencing approach. Appl. Environ. Microbiol. 80(14):4427-4432. <http://dx.doi.org/10.1128/AEM.00873-14> <PMid:24814794>
https://doi.org/10.1128/AEM.00873-14...
, Nascimento et al. (2015)Nascimento L.V., Mauerwerk M.T., Santos C.L., Barros Filho I.R., Birgel Júnior E.H., Sotomaior C.S., Madeira H.M.F. & Ollhoff R.D. 2015. Treponemes detected in digital dermatitis lesions in Brazilian dairy cattle and possible host reservoirs of infection. J. Clin. Microbiol. 53(6):1935-1937. <http://dx.doi.org/10.1128/JCM.03586-14> <PMid:25788552>
https://doi.org/10.1128/JCM.03586-14...
and Zinicola et al. (2015)Zinicola M., Lima F., Lima S., Machado V., Gomez M., Döpfer D., Guard C. & Bicalho R. 2015. Altered microbiomes in bovine digital dermatitis lesions, and the gut as a pathogen reservoir. Plos One 10(3):e0120504. <http://dx.doi.org/10.1371/journal.pone.0120504> <PMid:25781328>
https://doi.org/3https://doi.org/10.1371...
were the digestive tract and animals with BDD. A lower prevalence (72.9%) of Treponema in BDD lesions, using the same molecular technique, was also obtained by Moreira et al. (2018b)Moreira T.F., Facury Filho E.J., Carvalho A.U., Strube M.L., Nielsen M.W., Klitgaard K. & Jensen T.K. 2018b. Pathology and bacteria related to digital dermatitis in dairy cattle in all year round grazing system in Brazil. Plos One 13(3):e0193870. <http://dx.doi.org/10.1371/journal.pone.0193870> <PMid:29513739>
https://doi.org/10.1371/journal.pone.019...
in dairy cows grazing in Central region of Brazil. This lower prevalence pointed out to a breeding environment relationship influencing the frequency of Treponema in BDD lesions.
The histopathology of the lesions revealed extensive areas of ulceration and inflammatory changes in the epidermis and dermis. It was similar to the pathological changes in the BDD observed in dairy cows stabled by Döpfer et al. (1997)Döpfer D., Koopmans A., Meijer F.A., Szakáll I., Schukken Y.H., Klee W., Bosma R.B., Cornelisse J.L., Van Asten A.J.A.M. & ter Huurne A.A.H.M. 1997. Histological and bacteriological evaluation of digital dermatitis in cattle, with special reference to spirochaetes and Campylobacter faecalis. Vet. Rec. 140(24):620-623. <http://dx.doi.org/10.1136/vr.140.24.620> <PMid:9228692>
https://doi.org/10.1136/vr.140.24.620...
and in beef cattle by Sullivan et al. (2013)Sullivan L.E., Carter S.D., Blowey R., Duncan J.S., Grove-White D. & Evans N.J. 2013. Digital dermatitis in beef cattle. Vet. Rec. 173(23):582-583. <http://dx.doi.org/10.1136/vr.101802>
https://doi.org/10.1136/vr.101802...
. Bacterial colonies in the form of coconuts and bacilli were also observed, which suggests the presence of other bacterial agents, in addition to spirochetes, in the BDD lesions of crossbred dairy cows raised on pasture in the Amazon biome. In the BDD lesions, different phyla of bacteria were isolated and, therefore, this disease was characterized as polybacterial, according to Krull et al. (2014)Krull A.C., Shearer J.K., Gorden P.J., Cooper V.L., Phillips G.J. & Plummer P.J. 2014. Deep sequencing analysis reveals temporal microbiota changes associated with development of bovine digital dermatitis. Infect. Immun. 82(8):3359-3373. <http://dx.doi.org/10.1128/IAI.02077-14> <PMid:24866801>
https://doi.org/10.1128/IAI.02077-14...
, Klitgaard et al. (2014)Klitgaard K., Nielsen M.W., Ingerslev H., Boye M. & Jensen T.K. 2014. Discovery of bovine digital dermatitis - associated Treponema spp. in the dairy herd environment by a targeted deep-sequencing approach. Appl. Environ. Microbiol. 80(14):4427-4432. <http://dx.doi.org/10.1128/AEM.00873-14> <PMid:24814794>
https://doi.org/10.1128/AEM.00873-14...
and Zinicola et al. (2015)Zinicola M., Lima F., Lima S., Machado V., Gomez M., Döpfer D., Guard C. & Bicalho R. 2015. Altered microbiomes in bovine digital dermatitis lesions, and the gut as a pathogen reservoir. Plos One 10(3):e0120504. <http://dx.doi.org/10.1371/journal.pone.0120504> <PMid:25781328>
https://doi.org/3https://doi.org/10.1371...
. However, spirochetes are the most prevalent bacterial agent in BDD lesions and are commonly found in deep strata of the epidermis, which points to a close relationship with the pathogenesis of this disease (Klitgaard et al. 2014Klitgaard K., Nielsen M.W., Ingerslev H., Boye M. & Jensen T.K. 2014. Discovery of bovine digital dermatitis - associated Treponema spp. in the dairy herd environment by a targeted deep-sequencing approach. Appl. Environ. Microbiol. 80(14):4427-4432. <http://dx.doi.org/10.1128/AEM.00873-14> <PMid:24814794>
https://doi.org/10.1128/AEM.00873-14...
, Zinicola et al. 2015Zinicola M., Lima F., Lima S., Machado V., Gomez M., Döpfer D., Guard C. & Bicalho R. 2015. Altered microbiomes in bovine digital dermatitis lesions, and the gut as a pathogen reservoir. Plos One 10(3):e0120504. <http://dx.doi.org/10.1371/journal.pone.0120504> <PMid:25781328>
https://doi.org/3https://doi.org/10.1371...
, Moreira et al. 2018bMoreira T.F., Facury Filho E.J., Carvalho A.U., Strube M.L., Nielsen M.W., Klitgaard K. & Jensen T.K. 2018b. Pathology and bacteria related to digital dermatitis in dairy cattle in all year round grazing system in Brazil. Plos One 13(3):e0193870. <http://dx.doi.org/10.1371/journal.pone.0193870> <PMid:29513739>
https://doi.org/10.1371/journal.pone.019...
).
According to the frequency distribution of the surveyed phylogroups among the BDD scores, and to the M system, a high frequency of the phylogroup T. pudidum/T. denticola-like was observed in all stages, which suggests that this phylogroup is dominant in BDD lesions in the Amazon biome, as observed by Yano et al. (2010)Yano T., Moe K.K., Yamazaki K., Ooka T., Hayashi T. & Misawa N. 2010. Identification of candidate pathogens of papillomatous digital dermatitis in dairy cattle from quantitative 16S rRNA clonal analysis. Vet. Microbiol. 143(2/4):352-262. <http://dx.doi.org/10.1016/j.vetmic.2009.12.009> <PMid:20036086>
https://doi.org/10.1016/j.vetmic.2009.12...
in dairy cows housed in Japan. A higher proportion of the T. medium/T. vincentii-like phylogroups were also obtained and T. phagedenis-like in active lesions (stages M2 and M4.1) in relation to healthy skin (stage M0) and non-active lesions (stages M3 and M4), which indicated a change in the population of Treponema regarding the stage of the injury. However, no statistical association was found between the Treponema phylogroup and the stage of BDD lesions (p>0.05). A marked difference in the microbiota between active (M1, M2, and M4.1) and non-active (M3 and M4) lesions were observed by Krull et al. (2014)Krull A.C., Shearer J.K., Gorden P.J., Cooper V.L., Phillips G.J. & Plummer P.J. 2014. Deep sequencing analysis reveals temporal microbiota changes associated with development of bovine digital dermatitis. Infect. Immun. 82(8):3359-3373. <http://dx.doi.org/10.1128/IAI.02077-14> <PMid:24866801>
https://doi.org/10.1128/IAI.02077-14...
and Zinicola et al. (2015)Zinicola M., Lima F., Lima S., Machado V., Gomez M., Döpfer D., Guard C. & Bicalho R. 2015. Altered microbiomes in bovine digital dermatitis lesions, and the gut as a pathogen reservoir. Plos One 10(3):e0120504. <http://dx.doi.org/10.1371/journal.pone.0120504> <PMid:25781328>
https://doi.org/3https://doi.org/10.1371...
. Treponema denticola, T. medium, T. maltophilum, T. paraluiscuniculi, T. phagedenis, T. putidum, and T. vincentii were detected more frequently in active lesions in the study by Zinicola et al. (2015)Zinicola M., Lima F., Lima S., Machado V., Gomez M., Döpfer D., Guard C. & Bicalho R. 2015. Altered microbiomes in bovine digital dermatitis lesions, and the gut as a pathogen reservoir. Plos One 10(3):e0120504. <http://dx.doi.org/10.1371/journal.pone.0120504> <PMid:25781328>
https://doi.org/3https://doi.org/10.1371...
.
Moter et al. (1998)Moter A., Leist G., Rudolph R., Schrank K., Choi B., Wagner M. & Göbel U.B. 1998. Fluorescence in situ hybridization shows spatial distribution of as yet uncultured treponemes in biopsies from digital dermatitis lesions. Microbiology 144(Pt 9):2459-2467. <http://dx.doi.org/10.1099/00221287-144-9-2459> <PMid:9782493>
https://doi.org/10.1099/00221287-144-9-2...
observed that T. denticola was distributed between cellular debris and the superficial layers of the spinous stratum of ulcerative BDD lesions. Therefore, it suggested this agent as a secondary one in the pathogenesis of the disease. Nordhoff et al. (2008)Nordhoff M., Moter A., Schrank K. & Wieler L.H. 2008. High prevalence of treponemes in bovine digital dermatitis - a molecular epidemiology. Vet. Microbiol. 131(3/4):293-300. <http://dx.doi.org/10.1016/j.vetmic.2008.04.019> <PMid:18524502>
https://doi.org/10.1016/j.vetmic.2008.04...
observed that Treponema of the T. medium/T. vincentii-like phylogroups and T. phagedenis-like were located between the interface of healthy tissue with injured one. They inferred a close relationship between these phylogroups with the etiology of BDD. Probably, humoral and cellular immune responses triggered by Treponema in BDD, as described by Trott et al. (2003)Trott D.J., Moeller M.R., Zuerner R.L., Goff J.P., Waters W.R., Alt D.P., Walker R.L. & Wannemuehler M.J. 2003. Characterization of Treponema phagedenis-like spirochetes isolated from papillomatous digital dermatitis lesions in dairy cattle. J. Clin. Microbiol. 41(6):2522-2529. <http://dx.doi.org/10.1128/jcm.41.6.2522-2529.2003> <PMid:12791876>
https://doi.org/10.1128/jcm.41.6.2522-25...
, can change the bacterial population present in BDD and characterize the stage of the lesion. Additional studies, involving a larger number of biopsies, is necessary to assess whether there is a significant change in the Treponema population according to the lesion stage in the BDD of crossbred dairy cows raised on pasture in the Amazon biome.
Bacterial research involving molecular techniques of PCR and FISH pointed out that bacteria of the genus Treponema spp. are the main dominant agents in the BDD lesions, as observed by Moreira et al. (2018bMoreira T.F., Facury Filho E.J., Carvalho A.U., Strube M.L., Nielsen M.W., Klitgaard K. & Jensen T.K. 2018b. Pathology and bacteria related to digital dermatitis in dairy cattle in all year round grazing system in Brazil. Plos One 13(3):e0193870. <http://dx.doi.org/10.1371/journal.pone.0193870> <PMid:29513739>
https://doi.org/10.1371/journal.pone.019...
), Nordhoff et al. (2008)Nordhoff M., Moter A., Schrank K. & Wieler L.H. 2008. High prevalence of treponemes in bovine digital dermatitis - a molecular epidemiology. Vet. Microbiol. 131(3/4):293-300. <http://dx.doi.org/10.1016/j.vetmic.2008.04.019> <PMid:18524502>
https://doi.org/10.1016/j.vetmic.2008.04...
and Zinicola et al. (2015)Zinicola M., Lima F., Lima S., Machado V., Gomez M., Döpfer D., Guard C. & Bicalho R. 2015. Altered microbiomes in bovine digital dermatitis lesions, and the gut as a pathogen reservoir. Plos One 10(3):e0120504. <http://dx.doi.org/10.1371/journal.pone.0120504> <PMid:25781328>
https://doi.org/3https://doi.org/10.1371...
. However, two samples from the present study, in stages M1 and M3, were negative for these both techniques employed. Negative results by FISH were also obtained in a sample by Moreira et al. (2018b)Moreira T.F., Facury Filho E.J., Carvalho A.U., Strube M.L., Nielsen M.W., Klitgaard K. & Jensen T.K. 2018b. Pathology and bacteria related to digital dermatitis in dairy cattle in all year round grazing system in Brazil. Plos One 13(3):e0193870. <http://dx.doi.org/10.1371/journal.pone.0193870> <PMid:29513739>
https://doi.org/10.1371/journal.pone.019...
. These authors correlated this result with the final stage of wound healing. These results indicated that BDD lesions in the early stages (M1), in addition to lesions in the final stage of healing (M3), may lack the presence of Treponema in the tissues. Also, they may have a low concentration of these agents, in which the techniques employed were not capable of detecting it, or may be distributed in a non-homogeneous manner in which only the biopsies did not contain the genetic material. In two samples of skin in stage M0 (without lesion), the phylogroups T. phagedenis-like and T. putidum/T.denticola-like were detected, but no spirochetes were observed in the silver impregnation. Rasmussen et al. (2012)Rasmussen M., Capion N., Klitgaard K., Rogdo T., Fjeldass T., Boye M. & Jensen T.K. 2012. Bovine digital dermatitis: Possible pathogenic consortium consisting of Dichelobacter nodosus and multiple Treponema species. Vet. Microbiol. 160(1/2):151-161. <http://dx.doi.org/10.1016/j.vetmic.2012.05.018> <PMid:22698300>
https://doi.org/10.1016/j.vetmic.2012.05...
, Knappe-Poindecker et al. (2013)Knappe-Poindecker M., Gilhuus M., Jensen T.K., Klitgaard K., Larssen R.B. & Fjeldaas T. 2013. Interdigital dermatitis, heel horn erosion, and digital dermatitis in 14 Norwegian dairy herds. J. Dairy Sci. 96(12):7617-7629. <http://dx.doi.org/10.3168/jds.2013-6717> <PMid:24140335>
https://doi.org/10.3168/jds.2013-6717...
, and Moreira et al. (2018b)Moreira T.F., Facury Filho E.J., Carvalho A.U., Strube M.L., Nielsen M.W., Klitgaard K. & Jensen T.K. 2018b. Pathology and bacteria related to digital dermatitis in dairy cattle in all year round grazing system in Brazil. Plos One 13(3):e0193870. <http://dx.doi.org/10.1371/journal.pone.0193870> <PMid:29513739>
https://doi.org/10.1371/journal.pone.019...
also obtained positive samples for Treponema in PCR in apparently healthy tissues. However, they are not frequently observed in BDD lesions, according to Yano et al. (2010)Yano T., Moe K.K., Yamazaki K., Ooka T., Hayashi T. & Misawa N. 2010. Identification of candidate pathogens of papillomatous digital dermatitis in dairy cattle from quantitative 16S rRNA clonal analysis. Vet. Microbiol. 143(2/4):352-262. <http://dx.doi.org/10.1016/j.vetmic.2009.12.009> <PMid:20036086>
https://doi.org/10.1016/j.vetmic.2009.12...
and Zinicola et al. (2015)Zinicola M., Lima F., Lima S., Machado V., Gomez M., Döpfer D., Guard C. & Bicalho R. 2015. Altered microbiomes in bovine digital dermatitis lesions, and the gut as a pathogen reservoir. Plos One 10(3):e0120504. <http://dx.doi.org/10.1371/journal.pone.0120504> <PMid:25781328>
https://doi.org/3https://doi.org/10.1371...
.
Conclusion
The bovine digital dermatitis (BDD) in crossbred dairy cattle herds raised in a pasture in the Amazon biome was characterized as a “polytreponemal” lesion with more significant frequency from the phylogroup T. pudidum/T. denticola-like in all stages.
Acknowledgments
The authors would like to thank the “Pró-Reitoria de Pesquisa e Pós-Graduação” of the “Universidade Federal do Pará” (Edital 01/2020 - PROPESP/PAPQ) and to the “Conselho Nacional de Desenvolvimento Científico e Tecnológico” (CNPq) (process number 432100/2016-4), for the financial support; and also, to the “Coordenação de Nível de Aperfeiçoamento de Pessoal de Nível Superior” (CAPES) for the exchange scholarship for doctoral researcher.
References
- Berry S.L., Read D.H., Famula T.R., Mongini A. & Döpfer D. 2012. Long-term observations on the dynamics of bovine digital dermatitis lesions on a California dairy after topical treatment with lincomycin HCl. Vet. J. 193(3):654-658. <http://dx.doi.org/10.1016/j.tvjl.2012.06.048> <PMid:22892182>
» https://doi.org/10.1016/j.tvjl.2012.06.048 - Castro G.R., Brito L.A.B., Fioravanti M.C.S., Silva L.A.F., Araújo E.G., Orlando C.F.P., Franco L.G. & Moura M.I. 2008. Estudo anatomopatológico de lesões de dermatite digital em bovinos. Ciênc. Anim. Bras. 9(4):1159-1166.
- Cheli R. & Mortellaro C. 1974. Digital dermatitis in cattle. Proceedings 8th International Meeting Diseases of Cattle, Milan, Italy. 213p.
- Clegg S.R., Mansfield K.G., Newbrook K., Sullivan L.E., Blowey R.W., Carter S.D. & Evans N.J. 2015. Isolation of digital dermatitis treponemes from lesions in wild North American elk (Cervus elaphus) in Washington State, USA. J. Clin. Microbiol. 53(1):88-94. <http://dx.doi.org/10.1128/JCM.02276-14> <PMid:25355757>
» https://doi.org/10.1128/JCM.02276-14 - Cruz C., Driemeier D., Cerva C. & Corbellini L.G. 2001. Clinical and epidemiological aspects of bovine digital lesions in southern Brazil. Arq. Bras. Med. Vet. Zootec. 53(6):654-657. <http://dx.doi.org/10.1590/S0102-09352001000600006>
» https://doi.org/10.1590/S0102-09352001000600006 - Cruz C.E.F., Pescador C.A., Nakajima Y. & Driemeier D. 2005. Immunopathological investigations on bovine digital epidermitis. Vet. Rec. 157(26):834-840. <http://dx.doi.org/10.1136/vr.157.26.834> <PMid:16377788>
» https://doi.org/10.1136/vr.157.26.834 - Döpfer D., Koopmans A., Meijer F.A., Szakáll I., Schukken Y.H., Klee W., Bosma R.B., Cornelisse J.L., Van Asten A.J.A.M. & ter Huurne A.A.H.M. 1997. Histological and bacteriological evaluation of digital dermatitis in cattle, with special reference to spirochaetes and Campylobacter faecalis Vet. Rec. 140(24):620-623. <http://dx.doi.org/10.1136/vr.140.24.620> <PMid:9228692>
» https://doi.org/10.1136/vr.140.24.620 - Döpfer D., Ladell P., Anklam K., Mikheil D. & Dusick A. 2012. Growth curves and morphology of three Treponema subtypes isolated from digital dermatitis in cattle. Vet. J. 193(3):685-693. <http://dx.doi.org/10.1016/j.tvjl.2012.06.054> <PMid:22901455>
» https://doi.org/10.1016/j.tvjl.2012.06.054 - Evans N.J., Brown J.M., Demirkan I., Murray R.D., Birtles R.J., Hart C.A. & Carter S.D. 2009. Treponema pedis sp. nov., a spirochaete isolated from bovine digital dermatitis lesions. Int. J. Syst. Evol. Microbiol. 59(Pt 5):987-991. <http://dx.doi.org/10.1099/ijs.0.002287-0> <PMid:19406779>
» https://doi.org/10.1099/ijs.0.002287-0 - Evans N.J., Brown J.M., Demirkan I., Murray R.D., Vink D.W., Blowey R.W., Hart C.A. & Carter S.D. 2008. Three unique groups of spirochetes isolated from digital dermatitis lesions in UK cattle. Vet. Microbiol. 130(1/2):141-150. <http://dx.doi.org/10.1016/j.vetmic.2007.12.019> <PMid:18243592>
» https://doi.org/10.1016/j.vetmic.2007.12.019 - Evans N.J., Timofte D., Isherwood D.R., Brown J.M., Williams J.M., Sherlock K., Lehane M.J., Murray R.D., Birtles R.J., Hart C.A. & Carter S.D. 2012. Host and environmental reservoirs of infection for bovine digital dermatitis treponemes. Vet. Microbiol. 156(1/2):102-109. <http://dx.doi.org/10.1016/j.vetmic.2011.09.029> <PMid:22019292>
» https://doi.org/10.1016/j.vetmic.2011.09.029 - Klitgaard K., Boye M., Capion N. & Jensen T.K. 2008. Evidence of multiple Treponema phylotypes involved in bovine digital dermatitis as shown by 16S rRNA gene analysis and fluorescence in situ hybridization. J. Clin. Microbiol. 46(9):3012-3020. <http://dx.doi.org/10.1128/JCM.00670-08> <PMid:18562583>
» https://doi.org/10.1128/JCM.00670-08 - Klitgaard K., Nielsen M.W., Ingerslev H., Boye M. & Jensen T.K. 2014. Discovery of bovine digital dermatitis - associated Treponema spp. in the dairy herd environment by a targeted deep-sequencing approach. Appl. Environ. Microbiol. 80(14):4427-4432. <http://dx.doi.org/10.1128/AEM.00873-14> <PMid:24814794>
» https://doi.org/10.1128/AEM.00873-14 - Knappe-Poindecker M., Gilhuus M., Jensen T.K., Klitgaard K., Larssen R.B. & Fjeldaas T. 2013. Interdigital dermatitis, heel horn erosion, and digital dermatitis in 14 Norwegian dairy herds. J. Dairy Sci. 96(12):7617-7629. <http://dx.doi.org/10.3168/jds.2013-6717> <PMid:24140335>
» https://doi.org/10.3168/jds.2013-6717 - Krull A.C., Shearer J.K., Gorden P.J., Cooper V.L., Phillips G.J. & Plummer P.J. 2014. Deep sequencing analysis reveals temporal microbiota changes associated with development of bovine digital dermatitis. Infect. Immun. 82(8):3359-3373. <http://dx.doi.org/10.1128/IAI.02077-14> <PMid:24866801>
» https://doi.org/10.1128/IAI.02077-14 - Marcatili P., Nielsen M.W., Sicheritz-Pontén T., Jensen T.K., Schafer-Nielsen C., Boye M., Nielsen M. & Klitgaard K. 2016. A novel approach to probe host-pathogen interactions of bovine digital dermatitis, a model of a complex polymicrobial infection. BMC Genomics 17:987. <http://dx.doi.org/10.1186/s12864-016-3341-7> <PMid:27908274>
» https://doi.org/10.1186/s12864-016-3341-7 - McIntosh D., Bezerra R.A., Luz H.R., Faccini J.L.H., Gaiotto F.A., Giné G.A.F. & Albuquerque G.R. 2015. Detection of Rickettsia bellii and Rickettsia amblyomma longirostre (Acari: Ixodidae) from Bahia state, Northeast Brazil. Braz. J. Microbiol. 46(3):879-883. <http://dx.doi.org/10.1590/S1517-838246320140623> <PMid:26413074>
» https://doi.org/10.1590/S1517-838246320140623 - Moreira T.F., Nicolino R.R., Andrade L.S., Facury Filho E.J. & Carvalho A.U. 2018a. Prevalence of lameness and hoof lesions in all year-round grazing cattle in Brazil. Trop. Anim. Health Prod. 50(8):1829-1834. <http://dx.doi.org/10.1007/s11250-018-1626-3> <PMid:29846882>
» https://doi.org/10.1007/s11250-018-1626-3 - Moreira T.F., Facury Filho E.J., Carvalho A.U., Strube M.L., Nielsen M.W., Klitgaard K. & Jensen T.K. 2018b. Pathology and bacteria related to digital dermatitis in dairy cattle in all year round grazing system in Brazil. Plos One 13(3):e0193870. <http://dx.doi.org/10.1371/journal.pone.0193870> <PMid:29513739>
» https://doi.org/10.1371/journal.pone.0193870 - Moter A., Leist G., Rudolph R., Schrank K., Choi B., Wagner M. & Göbel U.B. 1998. Fluorescence in situ hybridization shows spatial distribution of as yet uncultured treponemes in biopsies from digital dermatitis lesions. Microbiology 144(Pt 9):2459-2467. <http://dx.doi.org/10.1099/00221287-144-9-2459> <PMid:9782493>
» https://doi.org/10.1099/00221287-144-9-2459 - Nascimento L.V., Mauerwerk M.T., Santos C.L., Barros Filho I.R., Birgel Júnior E.H., Sotomaior C.S., Madeira H.M.F. & Ollhoff R.D. 2015. Treponemes detected in digital dermatitis lesions in Brazilian dairy cattle and possible host reservoirs of infection. J. Clin. Microbiol. 53(6):1935-1937. <http://dx.doi.org/10.1128/JCM.03586-14> <PMid:25788552>
» https://doi.org/10.1128/JCM.03586-14 - Nielsen M.W., Strube M.L., Isbrand A., Al-Medrasi W.D.H.M, Boye M., Jensen T.K. & Klitgaard K. 2016. Potencial bacterial core species associated with digital dermatitis in cattle herds identified by molecular profiling of interdigital skin samples. Vet. Microbiol. 186:139-149. <http://dx.doi.org/10.1016/j.vetmic.2016.03.003> <PMid:27016768>
» https://doi.org/10.1016/j.vetmic.2016.03.003 - Nordhoff M., Moter A., Schrank K. & Wieler L.H. 2008. High prevalence of treponemes in bovine digital dermatitis - a molecular epidemiology. Vet. Microbiol. 131(3/4):293-300. <http://dx.doi.org/10.1016/j.vetmic.2008.04.019> <PMid:18524502>
» https://doi.org/10.1016/j.vetmic.2008.04.019 - Rasmussen M., Capion N., Klitgaard K., Rogdo T., Fjeldass T., Boye M. & Jensen T.K. 2012. Bovine digital dermatitis: Possible pathogenic consortium consisting of Dichelobacter nodosus and multiple Treponema species. Vet. Microbiol. 160(1/2):151-161. <http://dx.doi.org/10.1016/j.vetmic.2012.05.018> <PMid:22698300>
» https://doi.org/10.1016/j.vetmic.2012.05.018 - Santos T.M.A., Pereira R.V., Caixeta L.S., Guard C.L. & Bicalho R.C. 2011. Microbial diversity in bovine papillomatous digital dermatitis in Holstein dairy cows from upstate New York. FEMS Microbiol. Ecol. 79(2):518-529. <http://dx.doi.org/10.1111/j.1574-6941.2011.01234.x> <PMid:22093037>
» https://doi.org/10.1111/j.1574-6941.2011.01234.x - Schlafer S., Nordhoff M., Wyss C., Strub S., Hübner J., Gescher D.M., Petrich A., Göbel U.B. & Moter A. 2008. Involvement of Guggenheimella bovis in digital dermatitis lesions of dairy cows. Vet. Microbiol. 128(1/2):118-125. <http://dx.doi.org/10.1016/j.vetmic.2007.09.024> <PMid:18024006>
» https://doi.org/10.1016/j.vetmic.2007.09.024 - Shibahara T., Ohya T., Ishii R., Ogihara Y., Maeda T., Ishikawa Y. & Kadota K. 2002. Concurrent spirochaetal infections of the feet and colon of cattle in Japan. Aust. Vet. J. 80(8):497-502. <http://dx.doi.org/10.1111/j.1751-0813.2002.tb12474.x> <PMid:12224620>
» https://doi.org/10.1111/j.1751-0813.2002.tb12474.x - Silva L.A.F., Silva L.M., Romani A.F., Rabelo R.E., Fioravanti M.C.S., Souza T.M. & Silva C.A. 2001. Características clínicas e epidemiológicas das enfermidades podais em vacas lactantes do município de Orizona-GO. Ciênc. Anim. Bras. 2(2):119-126.
- Silveira J.A.C., Albernaz T.T., Oliveira C.M., Duarte M.D. & Barbosa J.D. 2009. Afecções podais em vacas da bacia leiteira de Rondon do Pará. Pesq. Vet. Bras. 29(11):905-909. <http://dx.doi.org/10.1590/S0100-736X2009001100007>
» https://doi.org/10.1590/S0100-736X2009001100007 - Silveira J.A.S., Silva N.S., Albernaz T.T., Bomjardim H.A., Belo Reis A.S., Oliveira C.M.C., Duarte M.D. & Barbosa J.A. 2018. Estudo epidemiológico e clínico de afecções podais em bovinos de corte manejados extensivamente no sudeste do Pará. Pesq. Vet. Bras. 38(3):367-373. <http://dx.doi.org/10.1590/1678-5150-pvb-4411>
» https://doi.org/10.1590/1678-5150-pvb-4411 - Stamm L.V., Bergen H.L. & Walker R.L. 2002. Molecular typing of papillomatous digital dermatitis-associated Treponema isolates based on analysis of 16S-23S ribosomal DNA intergenic spacer regions. J. Clin. Microbiol. 40(9):3463-3469. <http://dx.doi.org/10.1128/jcm.40.9.3463-3469.2002> <PMid:12202594>
» https://doi.org/10.1128/jcm.40.9.3463-3469.2002 - Sullivan L.E., Carter S.D., Blowey R., Duncan J.S., Grove-White D. & Evans N.J. 2013. Digital dermatitis in beef cattle. Vet. Rec. 173(23):582-583. <http://dx.doi.org/10.1136/vr.101802>
» https://doi.org/10.1136/vr.101802 - Sullivan L.E., Clegg S.R., Angell J.W., Newbrook K., Blowey R.W., Carter S.D., Bell J., Duncan J.S., Grove-White D.H., Murray R.D. & Evans N.J. 2015a. The high association of bovine digital dermatitis Treponema spp. with contagious ovine digital dermatitis lesions and the presence of Fusobacterium necrophorum and Dichelobacter nodosus J. Clin. Microbiol. 53(5):1628-1638. <http://dx.doi.org/10.1128/JCM.00180-15> <PMid:25740778>
» https://doi.org/10.1128/JCM.00180-15 - Sullivan L.E., Evans N.J., Clegg S.R., Carter S.D., Horsfield J.E., Grove-White D. & Duncan J.S. 2015b. Digital dermatitis treponemes associated with a severe foot disease in dairy goats. Vet. Rec. 176(11):283-8. <http://dx.doi.org/10.1136/vr.102858> <PMid:25428906>
» https://doi.org/10.1136/vr.102858 - Trott D.J., Moeller M.R., Zuerner R.L., Goff J.P., Waters W.R., Alt D.P., Walker R.L. & Wannemuehler M.J. 2003. Characterization of Treponema phagedenis-like spirochetes isolated from papillomatous digital dermatitis lesions in dairy cattle. J. Clin. Microbiol. 41(6):2522-2529. <http://dx.doi.org/10.1128/jcm.41.6.2522-2529.2003> <PMid:12791876>
» https://doi.org/10.1128/jcm.41.6.2522-2529.2003 - Yano T., Moe K.K., Yamazaki K., Ooka T., Hayashi T. & Misawa N. 2010. Identification of candidate pathogens of papillomatous digital dermatitis in dairy cattle from quantitative 16S rRNA clonal analysis. Vet. Microbiol. 143(2/4):352-262. <http://dx.doi.org/10.1016/j.vetmic.2009.12.009> <PMid:20036086>
» https://doi.org/10.1016/j.vetmic.2009.12.009 - Zinicola M., Lima F., Lima S., Machado V., Gomez M., Döpfer D., Guard C. & Bicalho R. 2015. Altered microbiomes in bovine digital dermatitis lesions, and the gut as a pathogen reservoir. Plos One 10(3):e0120504. <http://dx.doi.org/10.1371/journal.pone.0120504> <PMid:25781328>
» https://doi.org/3» https://doi.org/10.1371/journal.pone.0120504 - Zuerner R.L., Heidari M., Elliott M.K., Alt D.P. & Neill J.D. 2007. Papillomatous digital dermatitis spirochetes suppress the bovine macrophage innate immune response. Vet. Microbiol. 125(3/4):256-264. <http://dx.doi.org/10.1016/j.vetmic.2007.06.001> <PMid:17628359>
» https://doi.org/10.1016/j.vetmic.2007.06.001
Publication Dates
-
Publication in this collection
10 Aug 2020 -
Date of issue
June 2020
History
-
Received
11 July 2019 -
Accepted
13 Aug 2019