ABSTRACT
Ametryn is one of the most widely used herbicides in the sugarcane culture. Little is known about the interactions between this herbicide and the attributes of soils in the sugarcane region of northeastern Brazil. This knowledge, before recommending herbicide, will minimize the negative effects on the environment, particularly on water resources, and will ensure weed control efficacy. In this work, ametryn leaching potential was estimated through bioassays and chromatography, in five soils from the sugarcane region in northeastern Brazil: Quartzarenic Neosol (Entisol); Red Argisol (Ultisol); Ferrihumiluvic Spodosol (Spodosols); Red-Yellow Acrisol (Oxisol) and Haplic Cambisol (Inceptisols). To achieve this, columns were prepared with samples of the respective soils. On top of these columns ametryn was applied and, 12 hours later, a 60 mm rainfall was simulated. After water draining (72 hours after herbicide application), the columns were longitudinally opened to withdraw samples of each soil, every 5 cm. On some of these samples, ametryn quantification was performed by high-performance liquid chromatography and, on the others, biological assays were performed to confirm the results. Ametryn mobility was influenced by the physical-chemical characteristics of soils, mainly by organic matter content, texture and cation exchange capacity (CEC). However, this cannot be considered for Ferrihumiluvic Spodosol, whose cementing characteristics restrict the infiltration of water and organic compounds. Increased leaching ametryn occurred in Quartzarenic Neosol (Entisol), with higher herbicide concentration in the 5 to 10 cm depth layer, in relation to the 0 to 5 cm surface layer, indicating possible agronomic efficiency loss and higher risk of groundwater contamination.
Keywords:
herbicide; liquid chromatography; bioassay; mobility
RESUMO
O ametryn é um dos herbicidas mais utilizados na cultura da cana-de-açúcar, e pouco se conhece sobre as interações desse herbicida com os atributos dos solos da região canavieira do Nordeste brasileiro. Esse conhecimento antes da recomendação do herbicida permite minimizar os efeitos negativos ao ambiente - em especial, aos recursos hídricos - e garantir a eficiência no controle de plantas daninhas. Neste trabalho foi estimado o potencial de lixiviação do ametryn, por meio de bioensaios e cromatografia, em cinco solos da região canavieira do Nordeste brasileiro: Neossolo Quartzarênico, Argissolo Vermelho, Espodossolo Ferri-humilúvico, Latossolo Vermelho-Amarelo e Cambissolo Háplico. Para isso, foram preparadas colunas com amostras dos respectivos solos. No topo dessas colunas foi aplicado o ametryn e, 12 horas depois, foi simulada uma precipitação pluviométrica de 60 mm. Após a drenagem da água (72 horas após aplicação do herbicida), as colunas foram abertas longitudinalmente, para retirada de amostras de cada solo a cada 5 cm de profundidade. Numa parte dessas amostras fez-se a quantificação do ametryn por cromatografia líquida de alta eficiência e, na outra, foram realizados ensaios biológicos para confirmar esses resultados. A mobilidade do ametryn foi influenciada pelas características físico-químicas dos solos, principalmente pelo teor de matéria orgânica, textura e capacidade de troca catiônica (CTC). Entretanto, isso não pode ser considerado para o Espodossolo Ferri-humilúvico, cujas características cimentantes restringem a infiltração de água e compostos orgânicos. A maior lixiviação do ametryn ocorreu no Neossolo Quartzarênico, com maior concentração do herbicida na camada de 5 a 10 cm de profundidade, em relação à camada superficial de 0 a 5 cm, indicando possibilidade de perda da eficiência agronômica e maior risco de contaminação de águas subterrâneas.
Palavras-chave:
herbicida; cromatografia líquida; bioensaio; mobilidade
INTRODUCTION
Among the most used herbicides in Brazilian sugarcane cultures, ametryn stands out. It belongs to the triazine chemical group; it is recommended for pre- and post-initial emergence use, to control mono- and dicotyledonous weeds from different cultures. It was observed that in the sugarcane region of northeastern Brazil, as well as in other regions of the country, this herbicide was applied on a large scale, on different soil types, without the basic knowledge of its interaction with them. The incorrect use of this tool, especially for what concerns the applied dose, leads to the contamination of soil and surface water in sugarcane cultivated regions (Queiroz and Lanças, 1997; Monquero et al., 2008; Jacomini et al., 2009).
One of the ways to minimize the negative effect of using herbicides is knowing its interaction with soil, in particular its leaching potential, since it is a fundamental process for the superficial incorporation of most herbicides, affecting seeds or germinating plants. However, when excessive, it can take them to the deepest soil layers, limiting their action and also being able to promote water table contamination (Monquero et al., 2014).
In these terms, studies have been carried out to better understand the behavior of this herbicide on Brazilian soils (Andrade et al., 2010; Freitas et al., 2012; Silva et al., 2012). These works proved that the dynamic of ametryn depends on the physical and chemical characteristics of soil, with emphasis on organic matter, pH and texture. However, little is known about ametryn behavior on the soils of the northeastern region, whose chemical and mineralogical characteristics are different from other regions in Brazil.
Studies about the dynamic of herbicides on soil are, normally, performed in laboratory conditions that require the use of expensive equipments to analyze compounds, such as liquid or gas chromatographs. Yet, due to the expenses involved in these analyses, the use of bioassay techniques is an alternative to determine sorption capacity, persistence and herbicide mobility on soils. Freitas et al. (2012) noted that the bioassay method with cucumber as indicator plant was more effective in confirming ametryn leaching, compared to the chromatographic technique. Nonetheless, chromatography presents the advantage of quantifying the herbicide on the soil, whereas with the bioassay it is only possible to detect it.
Based on the above considerations, this work evaluated ametryn leaching in five soil types from the sugarcane region in northeastern Brazil, through bioassays with result validation, using high efficiency liquid chromatography.
MATERIAL AND METHODS
Five soil types classified according to the Brazilian System of Soil Classification - SiBCS (Embrapa, 2013) were evaluated; they were collected in the 0-30 cm depth layer, in areas with no herbicide application history, in the sugarcane region of northeastern Brazil: Quartzarenic Neosol (RQ) - (Entisol), in the municipality of Pedro Velho, Rio Grande do Norte state; Red Argisol (PV) - (Ultisol), in the municipality of Carpina - Pernambuco state; Ferrihumiluvic Spodosol (ESK) - (Spodosols), in the municipality of Carpina - Pernambuco state; Red-Yellow Acrisol (LVA) - (Oxisol), in the municipality of Maceió - Alagoas state; and Haplic Cambisol (CX) - (Inceptisols), in the municipality of Quixeré - Ceará state.
Soil samples were air-dried, pounded to break up clods and passed in a 4 mm mesh sieve. Limestone application was performed on soils by base saturation method in order to adjust pH; they were incubated during 30 days. A subsample was extracted from each sample, in order to perform chemical and physical analyses (Tables 1 and 2), according to the methodology described by Embrapa (1997).
Physical attributes of the 0 - 30 cm layer of soils from different sugarcane regions in northeastern Brazil
The test was performed in subdivided plot scheme, distributed in completely randomized design with four repetitions. Plots were composed by five soils, and subplots were made of 10 layers with 5 cm interval depths (0-5, 5-10, 10-15, 15-20, 20-25, 25-30, 30-35, 35-40, 40-45 and 45-50 cm) in PVC columns with 10 cm diameter x 50 cm length and removable side cover, according to Andrade et al. (2010) and Freitas et al. (2012).
Soils were placed in previously prepared columns that were covered with paraffin on the inside, to prevent lateral water draining. All columns were marked and sectioned every 5 cm. After being filled with soils, they were saturated with water for 48 hours and subsequently left in the vertical position for 72 hours, in order to drain water excess, according to Andrade et al. (2010). After that, herbicides were applied on the columns' upper part, in the recommended maximum dose for weed control (4,0 kg ha-1 ametryn i.a.), using a precision spray equipped with two XR 110.02 nozzles, spaced 0.5 m apart and kept under a 2.5 bar pressure, applying the equivalent of 150 L mixture.
Twelve hours after herbicide application, with the columns still in vertical position, rain simulation was performed, applying a 60 mm single blade, in a three-hour interval, for RQ, CX, PV and LVA. However, 10 hours were necessary for ESK, due to the lower water infiltration speed.
After rain simulation, the columns were kept in vertical position for 72 hours, so that water could drain into soil. Subsequently, they were placed in the horizontal position, and were laterally opened and sectioned every 5 cm with a PVC blade, according to Andrade et al. (2010) and Freitas et al. (2012).
In the center of every section, about 10 g of soil sample were collected; they were air-dried, pounded to break up clods, and stored in freezers at a temperature of approximately -20 oC, for future extraction and herbicide quantification by chromatographic analysis. Subsequently, five cucumber (Cucumis sativus) seeds were planted per each column segment, as a bioindicator of ametryn presence. During test performance, soil moisture in the columns was kept through daily irrigations, maintaining the soil close to field capacity.
On day 14 after emergence (DAE) of the bioindicator species, the assessments of intoxication index and dry mass accumulation in the aerial part of plants were performed. In the intoxication index evaluation of indicator plants, grades from 0 (no intoxication) to 100 (plant death) were assigned. For dry mass determination, all plants were cut near the soil surface and were placed in greenhouse with forced air circulation (70 ? 2 oC) until reaching constant mass.
To quantify ametryn concentration in the different depths of the columns, herbicide was extracted from soil samples, using the solid liquid extraction technique with low-temperature partitioning, proposed by Vieira et al. (2007) and Goulart et al. (2008).
The process consisted in using 2.0 g dry soil, previously homogenized and divided in four parts, in 30.0 mL capacity glass jars with screw caps and then adding 12.0 mL extraction mixture, composed of 4.0 mL water, 6.5 mL acetonitrile and 1.5 mL ethyl acetate. These jars were submitted to vertical agitation for 30 minutes. Subsequently, the samples were left for 12 hours ? 30 minutes in a freezer at approximately -20 oC. After this period, filtering of non-frozen fraction, organic extract and herbicide was performed, with a 10.0 mL volumetric flask. Fractions containing soil and frozen water were discarded. After reaching room temperature, filtered solutions were transferred to a 10.0 mL capacity round-bottom flask, for solvent evaporation in rotary evaporator, at a 50 ? 1 oC temperature. After evaporation, the round-bottom flask was washed with three 0.50 mL aliquots of acetonitrile; the final extract was filtered once again in a 0.45 μm membrane and stored in 1.5 mL capacity vials, for future analysis by high efficiency liquid chromatography.
Ametryn determination was made using a high performance liquid chromatography system (HPLC), Varian Pro Star 325 model, with UV-Vis detector and column (Varian Microsorb 100-3 C18, 100 mm x 4.6 mmd.i.).
The chromatographic conditions for the analysis were mobile phase: water and acetonitrile in 55:45 (v/v) proportion respectively, acidified with 0.02% phosphoric acid (H3PO4), respectively; 1,0 mL min-1 flow; 20 µL injection volume; 30 oC column temperature; and 214 nm wavelength.
The herbicide stock solution was prepared with the 98.3% purity standard, in a 1,000 μg mL-1 concentration, in acetonitrile, and the working solutions were prepared with it. Quantification was performed through the comparison of the areas obtained in the chromatograms by external calibration method.
To interpret the results referring to the biological test (intoxication and dry mass of the indicator plant aerial part) and to concentrate ametryn by HPLC, the averages obtained in each depth with the respective standard deviations were plotted in a bar chart.
RESULTS AND DISCUSSION
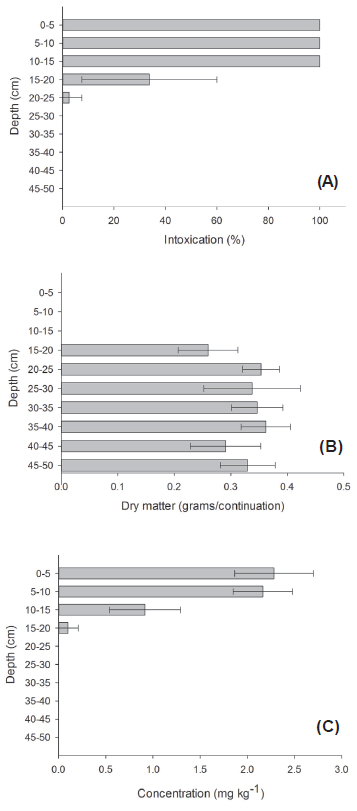
Cucumber plants died when planted in Quartzarenic Neosol (RQ), in 0 to 5, 5 to 10 and 10 to 15 cm depths; intense intoxication symptoms were observed up to 20 cm (Figure 1A), resulting in lower plant growth, with lower dry mass accumulation (Figure 1B). In plants that were cultivated in samples collected in the 20 to 25 cm and 25 to 30 cm depth segments, intoxication was detected, but only in moderate scale, without damaging dry mass accumulation (Figure 1B).
Intoxication percentage (A), dry matter mass (B) and ametryn concentration obtained by chromatography (C) in Quartzarenic Neosol (RQ) in the different column depths, after ametryn application and 60 mm rain simulation.
In this soil, ametryn was detected by high efficiency liquid chromatography (HPLC) up to 20 cm depth; however, there were higher concentrations in the 5 to 10 cm layer, indicating that the applied 60 mm rain were enough to considerably reduce the herbicide concentration in the 0 to 5 cm layer of soil. Hence, for RQ, an increase in rain intensity could cause higher herbicide leaching in the soil profile, damaging its efficacy in controlling weeds and increasing the contamination risk of groundwater.
The herbicide high mobility was favored by the chemical and physical characteristics of RQ (Tables 1 and 2). Sandy texture (93% sand), high pH (6.7), low organic matter content (0.5 dag kg-1) and low ionic exchange capacity (CEC) (2.2 cmolc dm-3) contributed to ametryn mobility on the soil profile. Andrade et al. (2010) verified higher ametryn mobility in soils with low organic matter content. According to Liu et al. (2010), in soils with low content of organic matter and clay, herbicide percolation may occur with higher intensity, facilitating its availability in soil solution, making it likely to be taken to the water table. Silva et al. (2012) verified that the increase in soil pH may decrease the sorption capacity of ametryn, and result in higher leaching of this molecule in soil profile, because when soil pH is close or under the herbicide pKa, this may protone and receive positive charge and be absorbed by the plant as cation. However, when pH becomes higher than the herbicide pKa (which in ametryn's case is 4.1), the quantity of ametryn in its molecular form increases, reducing sorption capacity (Silva et al., 2012).
The herbicide moderate mobility on the soil profile may be beneficial, so that herbicides applied in pre-emergence may have their effect on seeds or seedlings during germination or emergence (Monquero et al., 2014). However, when in excess, it may cause effectiveness decrease, due to the compound percolation in the soil profile, as well as the possibility of water table contamination (Monquero et al., 2014; Braga et al., 2016Andrade S.R.B. et al. Lixiviação do ametryn em Argissolo Vermelho-Amarelo e Latossolo Vermelho-Amarelo, com diferentes valores de pH. Planta Daninha, 2010:28:655-63.).
For Red Argisol (PV), a similar behavior was observed between the intoxication percentage of plants (Figure 2A) and the concentration of ametryn (Figure 2C); there were higher intoxication indices that resulted in the plant death on the soil surface layer, up to 5 cm, and subsequent decreases in deeper layers. The herbicide presence was noted up to 15 cm depth, even if in lower intensity. This moderate mobility is related to high clay content (Table 2) associated to higher CEC in relation to RQ (Table 1); they help sorption and, consequently, hinder herbicide leaching in the soil profile (Silva et al., 2012). The sorption of basic herbicides to soils increases with CEC increment (Oliveira Jr et al., 1999), which is associated to the clay fraction of soil and to the content of organic matter.
Intoxication percentage (A), dry matter mass (B) and ametryn concentration obtained by chromatography (C) in Red Argisol (PV) in the different column depths, after ametryn application and 60 mm rain simulation.
The results observed in this work support the ones obtained by Passos et al. (2011), who, working with the leaching of clomazone + hexazinone and ametryn herbicides on the soil, also verified ametryn mobility only up to the 10-15 cm layer in a Distroferric Argisol.
In spite of the symptoms visualized up to 15 cm in PV, the accumulation of cucumber dry matter was reduced only to 10 cm (Figure 2), indicating that it is not a very precise variable to detect herbicide presence, since the plant may present symptoms of herbicide intoxication without affecting its growth rate (Velini et al., 2008). In addition, herbicide sub doses may positively influence height growth and mass accumulation of plant dry mass, and they may turn this variable into a not very precise one (Silva et al., 2009).
In Ferrhumiluvic Spodossol (ESK), a similar behavior was verified between plant intoxication percentage (Figure 3A) and ametryn concentration (Figure 3C); plant death only up to 5 cm and moderate intoxication symptoms in the 5 to 10 cm depth layer were found, indicating lower herbicide concentration, as shown in Figure 3C.
Intoxication percentage (A), dry matter mass (B) and ametryn concentration obtained by chromatography (C) in Ferrihumiluvic Spodosol (ESK) in the different column depths, after ametryn application and 60 mm rain simulation.
This low ametryn mobility in ESK, in spite of 72% sand in its composition (Table 2), low organic matter content and low CEC (Table 1), was probably influenced by water infiltration difficulty in this soil, as observed during rain simulation; this may have hinder herbicide movement in its profile, acting as a filter, given that to stimulate 60 mm rain on this soil, interval applications were necessary for 10 hours, whereas for the other soils rain simulation was carried out in three hours. Spodosols are mineral, hydromorphic soils, and most of them present cemented horizons that restrict water infiltration and, as a consequence, also stop or hinder organic compounds to be leached out of the system, thus contributing to the podzolization process (Oliveira et al., 2010).
In Red-Yellow Acrisol (LVA), ametryn intoxication resulted in plants death up to 15 cm depth inside the column, with intoxication symptoms up to 25 cm depth (Figure 4A), whereas herbicide concentration was detected only up to 20 cm (Figure 4C). Probably, high herbicide mobility is related to the sandy texture of this soil (63% sand) and to pH (6.0), which facilitate ametryn movement in the soil matrix. According to Prata et al. (2003), the composition, size and distribution of soil particles and their respective porosity influence the descendant movement of herbicides.
Intoxication percentage (A), dry matter mass (B) and ametryn concentration obtained by chromatography (C) in Red-Yellow Acrisol (LVA) in the different column depths, after ametryn application and 60 mm rain simulation.
Ametryn was detected up to 15 cm depth in Haplic Cambisol (CX), but with higher intoxication indices and a reduction in the accumulation of dry matter in the indicator plans, in the superficial 10 cm. Chromatographic analysis also detected herbicide up to 15 cm depth (Figure 5C), complementing the use of biological test.
Intoxication percentage (A), dry matter mass (B) and ametryn concentration obtained by chromatography (C) in Haplic Cambisol (CX) in the different column depths, after ametryn application and 60 mm rain simulation.
This ametryn intermediate mobility must be related to contents of organic matter (1.56 dag kg-1) and clay (37%) in this soil, which result in higher CEC compared to other soils (Tables 1 and 2), helping herbicide retention due to wider specific surface associated to the presence of herbicide adsorption spots (Rojas et al., 2015). Freitas et al. (2012), working with ametryn in Cambisol from the very same region, verified leaching of this herbicide up to 20 cm depth. Higher mobility in the soil profile observed by these authors is due to lower contents of organic matter (1.2%) and cl (17%), compared to this work.
In this work, it was verified that the bioassay method, through intoxication assessment in the indicator plant, presented similar results to the ones obtained in HPLC, being also more sensitive to detect ametryn presence in soils with lower sorption capacity (RQ and LVA). However, HPLC allows to quantify herbicide, which is interesting, mainly when its concentration may cause the death of the indicator plant, as occurred in the first RQ 15 cm (Figure 1A), and it is not possible to deduce the quantity of present herbicide; HPLC allowed to detect higher ametryn concentration in the 5-10 cm depth, in relation to the superficial layer from 0 to 5 cm (Figure 1B); it indicated the possibility of agronomic efficiency loss and the potential for groundwater contamination.
Ametryn mobility was influenced by the physical-chemical characteristics of soils, mainly CEC, organic matter content and texture. However, this cannot be considered for Ferrihumiluvic Spodosol, whose cementing characteristics restrict the infiltration of water and organic compounds. Ametryn high leaching potential in Quartzarenic Neosol indicates that the use of this herbicide on this soil must be avoided, due to the possibility of agronomic efficiency loss and to the potential for groundwater contamination.
ACKNOWLEDGMENTS
To the CNPq (National Council for Scientific and Technological Development), and to the CAPES (Brazilian Federal Agency for Support and Evaluation of Graduate Education), for the financial support and the granted scholarships.
REFERENCES
- Andrade S.R.B. et al. Lixiviação do ametryn em Argissolo Vermelho-Amarelo e Latossolo Vermelho-Amarelo, com diferentes valores de pH. Planta Daninha, 2010:28:655-63.
- Braga D.F. et al. Leaching of sulfentrazone in soils from the sugarcane region in the Northeast region of Brazil. Planta Daninha. 2016;34:161-9.
- Empresa Brasileira de Pesquisa Agropecuária - Embrapa. Manual de métodos de análise de solo. 2ª. ed. Rio de Janeiro: 1997. 212p.
- Empresa Brasileira de Pesquisa Agropecuária - Embrapa. Sistema brasileiro de classificação de solos. 3ª. ed. Brasília: 2013. 353p.
- Freitas F.C.L. et al. Mobilidade do ametryn em solos da região semiárida do Rio Grande do Norte. Planta Daninha. 2012;30:641-8.
- Goulart S.M. et al. Low-temperature clean-up method for the determination of pyrethroids in milk using gas chromatography with electron capture detection. Talanta. 2008;75:1320-3.
- Jacomini A.E. et al. Determination of ametryn in river water, river sediment and bivalve mussels by liquid chromatography-tandem mass spectrometry. J Braz Chem Soc. 2009;20:107-16.
- Liu Y. et al. Adsorption and desorption behavior of herbicide diuron on various Chinese cultivated soils. J Hazardous Mat. 2010;178:462-8.
- Monquero P.A. et al. Manejo de Merremia aegyptia com misturas de herbicidas utilizando diferentes lâminas de água na presença ou ausência de palha de cana-de-açúcar. Rev Bras Herb. 2014;13:88-96.
- Monquero P.A. et al. Lixiviação de clomazone + ametryn, diuron + hexazinone e isoxaflutole em dois tipos de solo. Planta Daninha. 2008;26:685-91.
- Oliveira A.P. et al. Spodosol spedogenesis under Barreiras Formation and Sandbank environments in the south of Bahia. Rev Bras Cienc Solo. 2010;34:847-60.
- Oliveira Jr R.S. et al. Spatial variability of imazethapyr sorption in soil. Weed Sci. 1999;47:243-8.
- Passos A.T.M. et al. Lixiviação no solo de herbicidas em razão da percolação de água. Científica. 2011;39:85-93.
- Prata F. et al. Glyphosate sorption and desorption in soils with different phosphorous levels. Sci Agric. 2003;60:175-80.
- Queiroz M.E.C., Lanças F.M. HRGC study of sorption and dessorption of Atrazine, Ametryn and Metolachlor on Brazilian soils. J Braz Chem Soc. 1997;8:1-6.
- Rojas R. et al. Adsorption study of low-cost and locally available organic substance sand a soil to remove pesticides from aqueous solutions. J Hydrol. 2015;520:461-72.
- Silva M.A. et al. Efeito osmótico de gliphosate no desenvolvimento inicial de cana-de - açúcar. Bragantia. 2009;68:973-8.
- Silva L.O.C. et al. Sorção e dessorção do ametryn em solos brasileiros. Planta Daninha. 2012;30:633-40.
- Velini E.D. et al. Glyphosate applied at low doses can stimulate plant growth. Pest Manage Sci. 2008;4:489-96.
- Vieira H.P. et al. Otimização e validação da técnica de extração líquido com partição em baixa temperatura (ELL-PBT) para piretróides em água e análise por CG. Quím. Nova. 2007;30:535-40.
-
1
Recebido para publicação em 21.3.2016 e aprovado em 9.5.2016.
Publication Dates
-
Publication in this collection
Oct-Dec 2016
History
-
Received
21 Mar 2016 -
Accepted
09 May 2016