ABSTRACT:
An efficient protocol for in vitro flowering was successfully established for Impatiens balsamina cv Dwarf Bush, an important medicinal plant, through tissue culture techniques. Shoot, stem and petiole explants obtained from 4 week-old aseptic seedlings cultured on MS medium supplemented with different concentrations of plant growth regulator (PGR) were used for in vitro flower induction. Gibberellic acid (GA3), benzylaminopurine (BAP) and kinetin (Kin) treatment singly applied in MS media (pH 5.8), could all stimulate flowering at 23-26 oC with photoperiod of 16 hours light and 8 hours dark. It was observed that shoot explants were more responsive than stem explants in floral formation. Regeneration was achieved via direct organogenesis. For shoot explants, the treatment that induced the highest rate of in vitro flowering (7.30 ± 0.16 flowers per plantlet) was 1.0 mg L-1 GA3. Ultrastructural and histological analysis of in vivo and in vitro flowers were done to discover any somaclonal variation. This research described a simple protocol for rapid in vitro flowering that will be very beneficial for further breeding, cytological and molecular biology research.
Keywords:
in vitro regeneration; ultrastructural; histology; gibberellic acid; flowering response
RESUMO:
Um protocolo eficiente para a floração in vitro foi estabelecido com sucesso para Impatiens balsamina cv. Dwarf Bush, uma planta medicinal importante, através de técnicas de cultura de tecidos. Foram utilizados explantes com broto, talo e pecíolo, extraídos de mudas assépticas com quatro semanas de vida, cultivadas em meio MS, suplementadas com diferentes concentrações de reguladores de crescimento de plantas (RCP), para indução de floração in vitro. O tratamento com ácido giberélico (GA3), benzilaminopurina (BAP) e cinetina (Kin), aplicado isoladamente em meio MS (pH 5,8), conseguiu estimular a floração a 23-26 oC, com fotoperíodo de 16 horas com luz e 8 horas sem. Observou-se que os explantes de broto eram mais responsivos que os explantes de talo na formação floral. A regeneração foi obtida via organogênese direta. Para explantes de broto, o tratamento que induziu a maior taxa de floração in vitro (7,30 ± 0,16 flores por plântula) foi o de 1,0 mg L-1 GA3. Análises ultraestruturais e histológicas de flores in vivo e in vitro foram realizadas para detectar qualquer variação somaclonal. Esta pesquisa descreveu um protocolo simples para a rápida floração in vitro, que será muito benéfico para futuras pesquisas em genética, citologia e biologia molecular.
Palavras-chave:
regeneração in vitro; ultraestrutural; histologia; ácido giberélico; resposta da floração
INTRODUCTION
Impatiens balsamina is an important medicinal plant that belongs to the family Balsaminaceae. The flower of I. balsamina is very attractive for research, because its flowering process requires short day conditions, and flower reversion can be obtained in an expected way after conversion to long day conditions (Pouteau et al., 1998Pouteau S., Tooke F., Battey N. Quantitative control of inflorescence formation in Impatiens balsamina. Plant Physiol . 1998;118:1191-201.). The flowers have been exploited to cool burning skin or cool fever (Taha et al., 2009Taha A. et al. In vitro regeneration of Garden Balsam, Impatiens balsamina using cotyledons derived from seedlings. Biotechnology. 2009;8:44-52.). In vitro flowering acts as an essential tool in examining flower induction, initiation, and the floral developmental process through plant growth regulators, such as cytokinins, gibberellins, and auxins (Ziv and Noar, 2006Ziv M., Naor V. Flowering of geophytes in vitro. Prop Ornam Plants. 2006;6:3-16.). Furthermore, in vitro flowering provides an ideal experimental system, preferable to in vivo grown plants, for studying the biological mechanism of flowering (Zhang, 2007Zhang T. Studies on in vitro Flowering and Fruiting of Perilla frutescens. Agric Sci China. 2007;6:33-7.).
In vitro flowering can also reduce external factors that affect the flowering process by allowing researchers to control environmental factors and use exogenous plant growth regulators (Zhang et al., 2008Zhang Y.W. et al. Within-season adjustment of sex expression in females and hermaphrodites of the clonal gynodioecious herb Glechoma longituba (Lamiaceae). Ecol Res. 2008;23:873-81.). In vitro flower formation can provide a model system for studying flower induction and development, allowing a means for conducting microbreeding, and a source of biochemicals and pharmaceuticals (Tisserat et al., 1990Tisserat B. et al. In vitro flowering from Citrus limon lateral buds. J Plant Physiol. 1990;136:56-60.). In many plants, in vitro flowering was normally achieved by the application of exogenous hormones to the culture medium (Taha, 1997Taha R.M. In vitro Flowering of Murraya paniculata (Jack) Linn. Asia Pacific J Molec Biol Biotechnol. 1997;5:68-71.; Jana and Sekhawat, 2011Jana S., Sekhawat G.S. Plant growth regulators, adenine sulfate and carbohydrates regulate organogenesis and in vitro flowering of Anethum graveolens. Acta Physiol Plant. 2011;33:305-11.). In tissue culture, in vitro flowering serves as an important tool for many reasons. One of the most important is that it shortens the life cycle of plants for breeding programs. Another is that it aims to include studying flower induction and initiation, and floral development.
Explants that have been used for regeneration in Impatiens include cotyledons of immature ovules of I. platypetala (Kyungkul, 1993Kyungkul H. In vitro shoot regeneration from cotyledons of immature ovules of Impatiens platypetala Lindl. In vitro Cell Dev Biol Plant. 1993;30:108-12.) and shoot tips from Impatiens hybrids (Kyungkul and Stephens, 1987Kyungkul H., Stephens L.C. Growth regulators affect in vitro propagation two interspecific Impatiens hybrids. Sci Hortic. 1987;32:307-13.). Impatiens most commonly propagate by seed, but this method has many limitations, such as low rate of germination, especially in Malaysia. Breeding cycle can be shortened to generate better quality of plant varieties for crop improvement to meet market demand. In addition, the product has potential for commercial scientific handicraft, futuristic gifs, and for miniature garden. This present study highlights the effort to develop an effective system for inducing in vitro flowering for one of Malaysia’s native balsams, I. balsamina. In vitro flowering for I. balsamina was developed using shoot, petiole, and stem explants derived from aseptic seedlings. Ultrastructural and histological studies were also conducted to compare variations, if any, which might occurr as a result of in vitro flowering.
MATERIALS AND METHODS
In vitro flowering of I. balsamina cv. Dwarf Bush
The seeds of I. balsamina were collected from the garden at the Institute of Biological Sciences, University of Malaya, and washed carefully under running tap water for 30 min. The seeds were then washed with Dettol detergent and two drops of Tween 20 for 5 min., followed by rinsing three times with distilled water. Seeds were surface sterilized with different concentrations (70%, 50% and 30%) of sodium hypochlorite solution for 5 min. each and washed thoroughly with distilled water. Finally, the seeds were treated with 70% ethanol for 1 min. and rinsed with sterile distilled water, once again in a laminar airflow cabinet. Seeds were cultured aseptically on basal medium (Murashige and Skoog 1962Murashige T., Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962;15:473-97.) supplemented with 3% (w/v) sucrose and solidified with 0.25% (w/v) gelrite. The germination of seeds in tissue culture tend to grow very fast compared of being sowed in traditional method.
The pH of the medium was adjusted to 5.8 using NaOH and HCl. The media were autoclaved at 121 oC for 20 min after adjusting the pH. Seeds were germinated aseptically. Young stem, shoot, and petiole obtained from aseptic seedlings were used as source of explants. Stem, shoot, and petiole explants were excised from 4 week-old aseptic seedlings into 5 x 5 mm, then cultured onto the experimental media supplemented with different plant growth regulators for inducing in vitro flowering. The hormones used were gibberellic acid (GA3), kinetin, and benzylaminopurine (BAP) at various concentrations and the cultures were maintained at temperature of 23-26 oC with 16 h of light and 60 µmol m2 s-1 of photosynthetic photon flux density, and 8 h of dark. Thirty replicates were used for each treatment. The number of flowers per explant, number of adventitious shoots, and plant height were measured weekly for 8 weeks.
Histological and ultrastructural analysis
In vitro and in vivo flowers were used and both samples were sliced and fixed in 3% (w/v) paraformaldehyde and 2% (v/v) glutaraldehyde in 0.2 M phosphate buffer at pH 7.2 for 24 h at room temperature. Samples were dehydrated in a graded ethanol series (30-100%). Later, the samples were infiltrated and embedded in basic resin (Technovit 7100) for one-week retention time and cut into cross section. Embedded samples were sectioned into 3.5 μm thick segments with a microtome (RM 2135 Leica, Germany). Sections were double stained with periodic acid shchiff (PAS) and naphthol blue black. The samples were then viewed under an Axioskop Zeiss (Germany) microscope attached to an AxioCamMRc video camera and were then analyzed using the AxioVision 4.7 software. Simultaneously, ultrastructural examination of in vitro and in vivo flowers were carried out and compared. Ultrastructural features were viewed by Field-Emission Scanning Electron Microscopy (FESEM), QuantaTM 450FEG. FESEM was used to check for any abnormalities and differences between in vivo and in vitro flower buds of I. balsamina.
Statistical analysis
Data analysis was conducted through analysis of variance (ANOVA) for completely randomized design. Duncan’s multiple range test (DMRT) and 5% significant level was used to compare means.
RESULTS AND DISCUSSION
In vitro flowering of I. balsamina cv. Dwarf Bush
Figure 1A shows I. balsamina as a control plant. Stem, shoot, and petiole explants from 4-week-old aseptic seedlings were used to induce in vitro flowering (Figure 1B). In vitro ûowering was successfully induced from all explants after 8 weeks of culture (Figure 1C). The in vitro flower buds began to bloom on week 4 of culture. The control consisted of all similar types of explants cultured on MS media without PGRs. The application of exogenous PGRs has significantly reduced the time of flowering. It seems that a period of 8 weeks in culture was appropriate for flowering in this present study. Table 1 shows the highest rate of in vitro flowering, which was induced from shoot explants (7.30 ± 0.16 flowers per plantlet) cultured on MS medium supplemented with 1.0 mg L-1 GA3. Meanwhile, the highest number of shoots formed (12.83 ± 0.10) and the tallest plantlets (10.80 ± 0.10 cm) were also observed in this treatment.
(A) Four-month-old in vivo flowering of Impatiens balsamina (B) Aseptic seedlings (C) In vitro flowering of Impatiens balsamina cultured on MS supplemented with 1.0 mg L-1 GA3 after 8 weeks. (D) In vitro flowering after 3 months.
Table 2 shows the effects of different concentrations of PGRs on stem explants. However, the rate of in vitro flowering was less for stem explants than for shoot explants. The highest rate of in vitro flowering was 4.30 ± 0.15 flowers per plantlet. Meanwhile, the highest number of shoots per explant (8.60 ± 0.15) and the highest height of plantlets (8.70 ± 0.17 cm) were observed in the 0.5 mg L-1 kinetin treatment group. Table 3 shows the effects of different concentrations of PGRs on petiole explants. The petiole explants started to induce shoot after 2 weeks of culture. As with shoot explants, the 1.0 mg L-1 GA3 treatment also resulted in the highest number of in vitro flowers (3.20 ± 0.17), while 0.5 mg L-1 kinetin induced the highest number of shoots per explant (6.57 ± 0.30) and the highest height of plantlets (8.47 ± 0.16 cm), as noted with stem explants. Therefore, GA3 was found to be the most effective PGR for inducing flowering in vitro. The number of shoots and plant height were related to the rate of in vitro flowering. This study has also found that shoot explants are more responsive compared to stem and petiole explants.
Histological and ultrastructural analysis
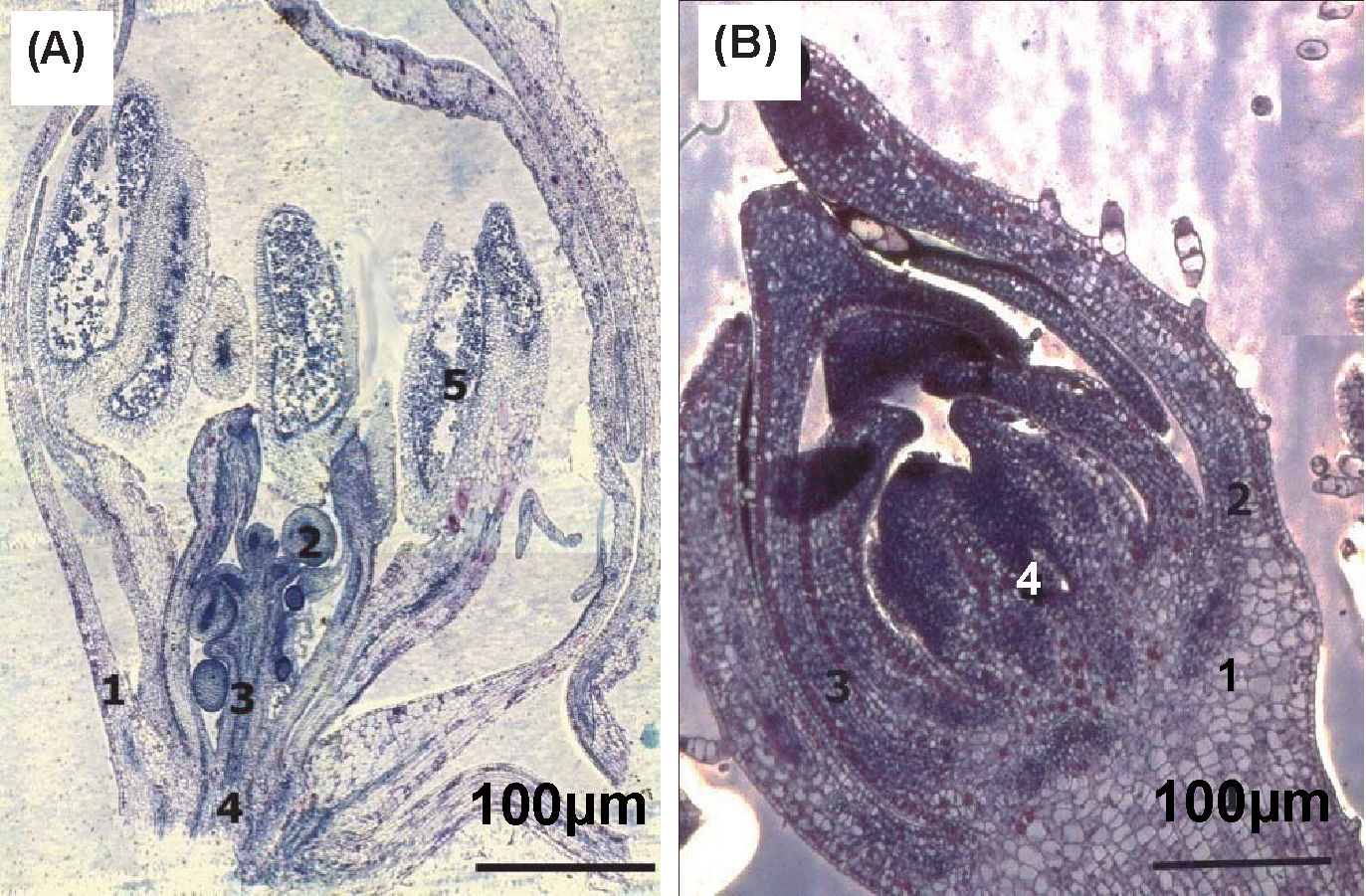
Longitudinal sections were prepared from in vivo and in vitro flowers. In vitro flowers of I. balsamina were collected from 2-month-old plantlets. Meanwhile, in vivo flowers were collected from 3 month-old intact plants. Histological analysis of in vivo carpels showed 5 different tissues, namely, abaxial surface, ovule, funiculus, placenta, and stamen (Figure 2A). The ovules attached to a thickened region of the ovary wall termed as the placenta. Each ovule is connected at its base to the placenta via the funiculus. Histological analysis showed that sexual reproduction was already fully developed in in vivo flowers compared to in vitro flowers. Meanwhile, histological analysis of in vitro flowers showed that there are 4 different tissues, namely, capitulum, sepal, petal, and ovary (Figure 2B). The abaxial surface was thick compared to in vivo flowers. Cell structures were not fully developed, perhaps due to the age of in vitro explants, which was just 8 weeks. Nonetheless, morphologically, the flowers were the same. In the early development of most flowers the primordia arises in centripetal sequence, so that the perianth is initiated first and the gynoecium last. Field Emission Scanning Electron Microscopic (FESEM) examinations were performed on in vitro and in vivo flower buds of 2 week-old I. balsamina. FESEM of in vitro I. balsamina. flower buds of, treated with 1.0 mg L-1 GA3, showed structures more clearly compared to in vivo flower buds (Figure 3A, B). This substantiates the premise that tissue culture promoted faster growth of the plantlets, allowing them to reach maturity at least about 2-3 weeks earlier. Outer surface of in vivo flower bud also showed more trichomes compared to outer surface of in vitro flower bud (Figure 3C, D). This may be due to the controlled environment with less trichomes found in in vitro plantlet.
Longitudinal sections of flower of Impatiens balsamina (A) In vivo flower showing the presence of (1) abaxial surface (2) ovule (3) funiculus (4) placenta (5) stamen (B) in vitro flower showing the presence of (1) capitulum (2) sepal (3) petal (4) ovary.
Field Emission Scanning Electron Micrographs (FESEM) of 2-week-old in vivo and in vitro Impatiens balsamina flower bud (A) In vivo flower bud (B) In vitro flower bud from plantlet cultured in MS medium with 1.0 mg L-1 GA3 (C) outer surface of in vivo flower bud (D) outer surface of in vitro flower bud.
In vitro flowering has great importance for selective hybridization, especially in the case of using pollen from rare stocks, and may be the first step towards the possibility of recombining genetic material via in vitro fertilization (Murthy et al., 2012Murthy K.S.R. et al. In vitro flowering- A review. J Agric Technol. 2012;8:1517-36.). In vitro flowering is presumably the most obscure of all the in vitro plant developmental processes. GA3 comprise hundreds of compounds, some of which regulate different aspects of plant growth and development, including seed germination, stem elongation, leaf expansion, and flower and seed development. They may act alone or in association with other hormones (Weiss and Ori, 2007Weiss D., Ori N. Mechanisms of crosstalk between gibberellin and other hormones. Plant Physiol. 2007;144:1240-6.). Early in vitro flowering of micropropagated plantlets can shorten their breeding cycle to generate better quality of plant varieties to meet market demand (Haque and Ghosh, 2013Haque S.M., Ghosh B. In vitro completion of sexual life cycle: production of R1 plants of Ipomoea quamoclit L. Propag Ornam Plants. 2013;13:19-24.). In this study, we have induced in vitro flowering and promoted adventitious shoot formation of I. balsamina GA3 was found to promote earlier in vitro flowering in I. balsamina GA3 has previously been reported to promote flowering in long day ornamental plants, such as Zantedeschia (Kozlowska et al., 2007Kozlowska M. et al. Changes in carbohydrate contents of Zantedeschia leaves under gibberellins-stimulated flowering. Acta Physiol Plant . 2007;29:27-32.) and a facultative long day plant, Brunonia (Wahyuni et al., 2011Wahyuni S. et al. Plant growth regulators and flowering of Brunonia and Calandrinia sp. Sci Hortic . 2011;128:141-5.).
The most effective plant growth regulator for inducing in vitro flowering in our study was GA3, followed by BAP, and kinetin. This result agrees with reports on the application of GA3 to Henckelia humboldtianus, which also encouraged earlier flowering (Sumanasiri et al., 2013Sumanasiri H. et al. Effect of gibberellic acid on growth and flowering of Henkelia humboldtianus Gardner (Ceylon Rock Primrose). Sci Hortic . 2013;159:29-32.). The effect on some species is greater than that on others. The effectiveness of GA3 in inducing in vitro flowering was also observed in many other plant species such as Phalaenopsis hybrida (Su et al., 2001Su W.R., et al. Changes in gibberellins levels in the flowering shoot of Phalaenopsis hybrida under high temperature conditions when flower development is blocked. Plant Physiol Biochemistry. 2001;39:45-50.). GA3 are a group of key hormones regulating many aspects of plant growth and development (Yamaguchi, 2008Yamaguchi S. Gibberellin metabolism and its regulation. Ann Rev Plant Biol. 2008;59:225-51.).
The most effective concentration of GA3 was 1.0 mg L-1, which strongly affected the maximum production of in vitro flowering in I. balsamina. Meanwhile, BAP was also found to promote in vitro flowering, as observed in Perilla frutescens (Zhang, 2007Zhang T. Studies on in vitro Flowering and Fruiting of Perilla frutescens. Agric Sci China. 2007;6:33-7.) and Boerhaavia diffusa (Sudarshana et al., 2008Sudarshana M.S. et al. In vitro flowering, somatic embryogenesis and regeneration in Boerhaavia diffusa Linn- A medicinal plant. Global J Biotechnol Biochem. 2008;3:83-6.). This is similar to our results, in which BAP also induced in vitro flowering. Moreover, kinetin applied singly also induced in vitro flowering of I. balsamina as well. Saha and Ghosh (2014Saha S.P., Ghosh B. Micropropagation and in vitro flowering of Luffa acutangula (L.) Roxb-an important vegetable crop. Inter J Bio-Res Stress Manag. 2014;5:12-21.) reported that kinetin could induce in vitro flowering in Luffa acutangula. Miles and Hagen Jr (1968Miles C.D., Hagen Jr C.W. The differentiation of pigmentation in flower. Parts IV. flavonoid elaborating enzymes from petals of Impatiens balsamina. Plant Physiol. 1968;43:1347-54.) reported that there are flavones in flower petals of I. balsamina.
Silva et al. (2014Silva T.J.A.D. et al. Genetic control of flower development, color and senescence of Dendrobium orchids. Sci Hortic . 2014;175:74-86.) reported that level of plant growth regulators (PGRs), genotype, and culture conditions are strongly associated with the initiation and development of floral organs from buds or callus tissue of Dendrobium. This may be due to the level of flavones in the flower petals that are affected by plant growth regulators. There are enormous physical and chemical aspects that affect the in vitro flowering mechanism. The in vitro flowering processes are under the influence of signals, including variations in endogenous levels (Campos and Kerbauy, 2004Campos K.O., Kerbauy G.B. Thermoperiodic effect on flowering and endogenous hormonal status in Dendrobium (Orchidaceae). J Plant Physiol. 2004;161:1385-7.). However, only few studies have addressed the effect of PGRs in in vitro flowering. Therefore, the observations reported hereby could render opportunities for further studies on the molecular physiology of flowering, particularly to understand how exogenous hormones regulate flower color in I. balsamina under controlled in vitro conditions.
The in vivo and in vitro morphology of the I. balsamina flowers were similar although histology analysis revealed that cell structures in in vitro flowers were not fully developed, possibly due to the environmental cultural conditions. In vitro flowering exhibited miscellaneous pollination biology. Numerous species of the genus Impatiens have diverse pollination biology (Schoen et al., 1994Schoen D.J. et al. The ecology and genetics of fitness in forest plants. Quantitative genetics of fitness components in Impatiens pallida (Balsaminacea). Am J Bot . 1994;81:232-9.). The carpels in Impatiens enclose the ovules and provide the style and stigma, as has been reported for some other species (Sattler and Lacroix, 1988Sattler R., Lacroix C. Development and evolution of basal cauline placentation- Basella rubra. Am J Bot. 1988;75:918-27.). The carpel column connections in Impatiens provide evidence to support the theory of how the primordial carpel fused to form the placenta in angiosperm evolution (Muller et al., 2001Muller B.M. et al. The MADS-box gene DEFH28 from Antirrhinum is involved in the regulation of floral meristem identity and fruit development. Plant J. 2001;28:169-79.). The Impatiens placenta is consequently best described as a near-cylindrical terminal extension of the floral apex, whose base is linked to the adjacent carpels (Chiurugwi et al., 2007Chiurugwi T. et al. Floral meristem interdeterminacy depends on flower position and is facilitated by acarpellate gynoecium development in Impatiens balsamina. New Phytol. 2007;173:79-90.). Field Emission Scanning Electron Microscopic (FESEM) examination was performed on 2-week old in vitro and in vivo flower buds of I. balsamina. FESEM of in vitro flower buds of I. balsamina treated with 1.0 mg L-1 GA3 showed internal structures more clearly compared to in vivo flower buds. Ovule structure could be clearly seen on in vitro flower buds. This proved that tissue culture promoted faster growth of plantlet.
The in vitro flowering process is very useful for large-scale production of plants. Moreover, it also can be used to study the physiological, biochemical, and molecular basis of the complex process of flowering. Effective protocols must be developed for mass propagation to conserve germplasm and to get uniform plants of a selected genotype. It is also helpful for I. balsamina, since it is very important to increase in vitro flowering frequency for genetic transformation studies, breeding programs, and even for scientific decorations or handicrafts.
ACKNOWLEDGEMENTS
This work has been financially supported by the Institute of Research Management and Monitoring (IPPP) of the University of Malaya (PG044/2013A). The authors would like to thank the University of Malaya for making their facilities available and providing financial support for the successful development of this research.
REFERENCES
- Campos K.O., Kerbauy G.B. Thermoperiodic effect on flowering and endogenous hormonal status in Dendrobium (Orchidaceae) J Plant Physiol. 2004;161:1385-7.
- Chiurugwi T. et al. Floral meristem interdeterminacy depends on flower position and is facilitated by acarpellate gynoecium development in Impatiens balsamina New Phytol. 2007;173:79-90.
- Jana S., Sekhawat G.S. Plant growth regulators, adenine sulfate and carbohydrates regulate organogenesis and in vitro flowering of Anethum graveolens Acta Physiol Plant. 2011;33:305-11.
- Haque S.M., Ghosh B. In vitro completion of sexual life cycle: production of R1 plants of Ipomoea quamoclit L. Propag Ornam Plants. 2013;13:19-24.
- Kozlowska M. et al. Changes in carbohydrate contents of Zantedeschia leaves under gibberellins-stimulated flowering. Acta Physiol Plant . 2007;29:27-32.
- Kyungkul H., Stephens L.C. Growth regulators affect in vitro propagation two interspecific Impatiens hybrids. Sci Hortic. 1987;32:307-13.
- Kyungkul H. In vitro shoot regeneration from cotyledons of immature ovules of Impatiens platypetala Lindl. In vitro Cell Dev Biol Plant. 1993;30:108-12.
- Miles C.D., Hagen Jr C.W. The differentiation of pigmentation in flower. Parts IV. flavonoid elaborating enzymes from petals of Impatiens balsamina Plant Physiol. 1968;43:1347-54.
- Muller B.M. et al. The MADS-box gene DEFH28 from Antirrhinum is involved in the regulation of floral meristem identity and fruit development. Plant J. 2001;28:169-79.
- Murashige T., Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962;15:473-97.
- Murthy K.S.R. et al. In vitro flowering- A review. J Agric Technol. 2012;8:1517-36.
- Pouteau S., Tooke F., Battey N. Quantitative control of inflorescence formation in Impatiens balsamina Plant Physiol . 1998;118:1191-201.
- Saha S.P., Ghosh B. Micropropagation and in vitro flowering of Luffa acutangula (L.) Roxb-an important vegetable crop. Inter J Bio-Res Stress Manag. 2014;5:12-21.
- Sattler R., Lacroix C. Development and evolution of basal cauline placentation- Basella rubra Am J Bot. 1988;75:918-27.
- Schoen D.J. et al. The ecology and genetics of fitness in forest plants. Quantitative genetics of fitness components in Impatiens pallida (Balsaminacea). Am J Bot . 1994;81:232-9.
- Silva T.J.A.D. et al. Genetic control of flower development, color and senescence of Dendrobium orchids. Sci Hortic . 2014;175:74-86.
- Su W.R., et al. Changes in gibberellins levels in the flowering shoot of Phalaenopsis hybrida under high temperature conditions when flower development is blocked. Plant Physiol Biochemistry. 2001;39:45-50.
- Sudarshana M.S. et al. In vitro flowering, somatic embryogenesis and regeneration in Boerhaavia diffusa Linn- A medicinal plant. Global J Biotechnol Biochem. 2008;3:83-6.
- Sumanasiri H. et al. Effect of gibberellic acid on growth and flowering of Henkelia humboldtianus Gardner (Ceylon Rock Primrose). Sci Hortic . 2013;159:29-32.
- Taha A. et al. In vitro regeneration of Garden Balsam, Impatiens balsamina using cotyledons derived from seedlings. Biotechnology. 2009;8:44-52.
- Taha R.M. In vitro Flowering of Murraya paniculata (Jack) Linn. Asia Pacific J Molec Biol Biotechnol. 1997;5:68-71.
- Tisserat B. et al. In vitro flowering from Citrus limon lateral buds. J Plant Physiol. 1990;136:56-60.
- Wahyuni S. et al. Plant growth regulators and flowering of Brunonia and Calandrinia sp. Sci Hortic . 2011;128:141-5.
- Weiss D., Ori N. Mechanisms of crosstalk between gibberellin and other hormones. Plant Physiol. 2007;144:1240-6.
- Yamaguchi S. Gibberellin metabolism and its regulation. Ann Rev Plant Biol. 2008;59:225-51.
- Zhang T. Studies on in vitro Flowering and Fruiting of Perilla frutescens Agric Sci China. 2007;6:33-7.
- Zhang Y.W. et al. Within-season adjustment of sex expression in females and hermaphrodites of the clonal gynodioecious herb Glechoma longituba (Lamiaceae). Ecol Res. 2008;23:873-81.
- Ziv M., Naor V. Flowering of geophytes in vitro Prop Ornam Plants. 2006;6:3-16.
Publication Dates
-
Publication in this collection
2018
History
-
Received
15 June 2016 -
Accepted
12 Sept 2016