ABSTRACT:
Antifungal potential of aerial parts of an allelopathic grass Cenchrus pennisetiformis (Hochst. & Steud.) Wipff. was evaluated against Fusarium oxysporum f. sp. lycopersici Snyder & Hansen, a fungal pathogen causing wilt disease in tomato (Solanum lycopersicum L.). Different concentrations (1% to 6%) of methanolic leaf, stem and inflorescence extract of the grass significantly reduced fungal biomass by 40-88%, 13-89%, and 26-76%, respectively. Methanolic shoot (leaf + stem) extract was fractionated using four organic solvents viz. n-hexane, chloroform, ethyl acetate and n-butanol. All the sub-fractions of methanolic shoot extract showed remarkable antifungal potential to variable extents. Different concentrations (1.56-200 mg mL-1) of ethyl acetate sub-fraction exhibited the best antifungal activity resulting in 49-100% suppression in the fungal biomass. GC-MS analysis of ethyl acetate sub-fraction showed the presence of 10 compounds. Phenol, 2,4-bis{1,1-dimethlethyl}- was the major compound (30.99%) followed by hexadecanoic acid, ethyl-ester (21.72%), benzofuran 2,3-dihydro (10.65%), 1-propanol-2-2-hydroxypropxy (10.60%) and 1-eicosene (8.32%).
Keywords:
antifungal activity; GC-MS analysis; methanolic extracts
RESUMO:
Foi realizada uma avaliação do potencial antifúngico da parte aérea da gramínea alelopática Cenchrus pennisetiformis (Hochst. & Steud.) Wipff. contra Fusarium oxysporum f. sp. lycopersici Snyder & Hansen, um patógeno fúngico que causa a doença de murcha no tomate (Solanum lycopersicum L.). Diferentes concentrações (1% a 6%) do extrato metanólico da folha, do caule e da inflorescência da gramínea reduziram significativamente a biomassa fúngica em 40-88%, 13-89% e 26-76%, respectivamente. O extrato metanólico (folha + caule) foi fracionado, utilizando-se quatro solventes orgânicos: n-hexano, clorofórmio, acetato de etila e n-butanol. Todas as subfrações de extrato metanólico da parte aérea apresentaram significativo potencial antifúngico em graus variáveis. Concentrações diferentes (1,56-200 mg mL-1) da subfração do acetato de etila exibiram a melhor atividade antifúngica, com supressão de 49-100% na biomassa fúngica. A análise CG-EM da subfração do acetato de etila revelou a presença de 10 compostos. O principal composto (30,99%) foi o fenol, 2,4-bis{1,1-dimetiletil}, seguido de ácido hexadecanoico, éster etílico (21,72%), 2,3-di-hidrobenzofurano (10,65%), 1-propanol-2-2-hidroxipropil (10,60%) e 1-eicoseno (8,32%).
Palavras-chave:
atividade antifúngica; análise por CG-EM; extratos metanólicos
INTRODUCTION
Tomato (Solanum lycopersicum) is an important horticultural crop around the globe. The importance of tomato as a food item is due to presence of dietary fibers, vitamins A, B and C, sugar, amino acids and various nutrient elements including N, K, P, Ca, Mg, Fe (Kumar et al., 2008Kumar K.R. et al. Enhanced growth promotion of tomato and nutrient uptake by plant growth promoting rhizobacterial isolates in presence of tobacco mosaic virus pathogen. Karnataka J Agric Sci. 2008;21:309-11.). Pakistan ranks 35th in the world’s tomato production; this crop is cultivated in 58.2 thousands hectares with total production of 574.1 thousand tonnes. The average yield of tomato in Pakistan is 9.9 ton ha-1 (Pakistan, 2013Pakistan. Ministry of National Food Security & Research. Government of Pakistan. Agricultural Statistics of Pakistan 2012-2013. Islamabad: 2013), which is very low. Various biotic and abiotic factors are responsible for this low yield of tomato in Pakistan. Among the various biotic factors, fungal diseases are the most important in damaging this crop. One of the important fungal diseases is Fusarium wilt, caused by Fusarium oxysporum f. sp. lycopersici (Abdel-Fattah and Al-Amri, 2012Abdel-Fattah G.M., Al-Amri S.M. Induced systemic resistance in tomato plants against Fusarium oxysporum f. sp. lycopersici by different kinds of compost. Afr J Biotechnol. 2012;11:12454-63.). The pathogen has three races (Morid et al., 2012Morid B., Hajmansoor S., Kakvan N. Screening of resistance genes to Fusarium root rot and Fusarium wilt diseases in tomato (Lycopersicon esculentum) cultivars using RAPD and CAPs markers. Eur J Exp Biol. 2012;2:931-9.), among which races 1 and 2 have worldwide distribution while race 3 has a limited geographical range (Reis and Boiteux, 2007Reis A., Boiteux L. Outbreak of Fusarium oxysporum f. sp. lycopersici race 3 in commercial fresh-market tomato fields in Rio de Janeiro State. Hortic Bras. 2007;25:451-4.).
Chemical fungicides, namely prochloraz and carbendazim, have been proved highly effective against in vitro growth of F. oxysporum f. sp. lycopersici (Song et al., 2004Song W. et al. Tomato Fusarium wilt and its chemical control strategies in a hydroponic system. Crop Prot. 2004;23:243-7.). Likewise, Amini and Sidovich (2012Amini J., Sidovich D.F. The effects of fungicides on Fusarium oxysporum f. sp. lycopersici associated with Fusarium wilt of tomato. J Plant Protec Res. 2010;50:172-8.) reported that prochloraz and bromuconazole were highly effective against this pathogen both in vitro and in vivo, followed by carbendazim and benomyl. However, because of hazardous effects of synthetic pesticides on health and the environment, scientists are focusing on alternative environment-friendly disease management strategies, including the use of natural antifungal compounds from plants (Javaid et al., 2015Javaid A. et al. Management of Macrophomina phaseolina by extracts of an allelopathic grass Imperata cylindrica. Pak J Agric Sci. 2015;15:37-41.; Sana et al., 2016aSana N., Shoaib A., Javaid A. Antifungal potential of leaf extracts of leguminous trees against Sclerotium rolfsii. Afr J Trad Complement Altern Med. 2016a;13. (in press)). Many recent studies have shown that extracts of allelopathic grasses, namely Dicanthium annulatum (Forssk.) Stapf., Sorghum halepense (L.) Pers. and Imperata cylindrica (L.) P. Beauv, exhibit antifungal activity against Macrophomina phaseolina and F. oxysporum f. sp. cepae (Javaid et al., 2012Javaid A., Naqvi S.F., Shoaib A. Antifungal activity of methanolic extracts of Sorghum halepense against Macrophomina phaseolina. Afr J Microbiol Res. 2012;6:5814-8.; Naqvi et al., 2012Naqvi S.F., Javaid A., Shoaib A. Evaluation of antifungal activity of methanolic extracts of Dicanthium annulatum for the management of Macrophomina phaseolina. Afr J Microbiol Res. 2012;6:5882-6.; Javaid et al., 2015). C. pennisetiformis is a drought tolerant, allelopathic grass commonly growing along roadsides and open places in Pakistan. It is known to possess herbicidal activity against parthenium (Parthenium hysterophorus L.) and antifungal activity against M. phaseolina (Javaid and Anjum, 2006Javaid A., Anjum T. Control of Parthenium hysterophorus L. by aqueous extracts of allelopathic grasses. Pak J Bot. 2006;38:139-45.; Javaid and Naqvi, 2012Javaid A., Naqvi S.F. Evaluation of antifungal potential of Cenchrus pennisetiformis for the management of Macrophomina phaseolina. World Acad Sci Eng Technol. 2012;69:832-5.). Given the above, the present study was carried out to evaluate the antifungal activity of its shoot gainst F. oxysporum f. sp. lycopersici.
MATERIALS AND METHODS
Preparation of methanolic extracts
Stem, leaves and inflorescence of C. pennisetiformis were collected from different areas of Lahore, Pakistan. Each part was rinsed with tap water and dried in an electric oven at 45 oC. Two hundred grams of each of the ground plant parts were soaked in 2 L methanol in air tight glass jars separately for 15 days at room temperature. Afterwards, the soaked materials were passed through cheese cloth to separate debris and then filtered through Whatman No. 1 filter paper and methanol was evaporated on a rotary evaporator at 45 oC under reduced pressure. Finally, thick pastes of 13.1 g, 14.1 g and 12.9 g of leaf, stem, and inflorescence extracts, respectively, were obtained (Banaras et al., 2017Banaras S., Javaid A., Shoaib A., Ahmed E. Antifungal activity of Cirsium arvense extracts against a phytopathogenic fungus Macrophomina phaseolina. Planta Daninha. 2017;35:e017162738. ).
Evaluation of antifungal activity of methanolic extracts
Methanolic extracts (12.6 g) of each of the three plant parts were dissolved in 6 mL dimethyle sulphoxide (DMSO) and distilled autoclaved water was added to prepare 21 mL of the stock solutions. Similarly, the control solution was prepared by adding 6 mL DMSO in 15 mL distilled water. Seventy-four milliliters of malt extract was autoclaved in a 250 mL flask and cooled at room temperature. Six concentrations viz. 1%, 2%, 3%, 4%, 5% and 6% were prepared by adding 1, 2, 3, 4, 5 and 6 mL of stock solution along with 5, 4, 3, 2, 1 and 0 mL of control solution, respectively, to produce final volume of 80 mL for each concentration. The 80 mL volume of each treatment was divided into four equal portions in 100 mL flask to serve as replicates. The control treatment was prepared by adding 6 mL control solution to 74 mL of malt extract. The purpose of the control solution was to maintain the same quantity of DMSO in all the treatments. The flasks were inoculated with 500 μL of conidial suspension (1 x 109 conidia mL-1) of F. oxysporum f. sp. lycopersici. Flasks were incubated for 10 days in an incubator at 27 oC. Fungal mycelium was harvested by filtering the fungal mat through pre-weighed filter papers followed by oven drying at 60 oC to get dry biomass from each flask (Javaid and Akhtar, 2015Javaid A., Bashir A. Radish extracts as natural fungicides for management of Fusarium oxysporum f. sp. lycopersici, the cause of tomato wilt. Pak J Bot. 2015;47:321-4.).
Bioassays with sub-fractions of methanolic shoot extract
C. pennisetiformis shoot (leaf + stem) extract was prepared by soaking 2 kg crushed plant material in 7 L methanol for 15 days. After filtration and evaporation on a rotary evaporator, the extract was mixed in 300 mL sterilized distilled water and partitioned in a separating funnel using n-hexane, chloroform, ethyl acetate and n-butanol. After partitioning, the solvents were evaporated on a rotary evaporator to obtain n-hexane (15.74 g), chloroform (12.15 g), ethyl acetate (10.9 g), n-butanol (9.8 g) and aqueous (25.21 g) sub-fractions. These fractions were evaluated for their antifungal activity using the serial dilution method as described by Javaid et al. (2015Javaid A., Bashir A. Radish extracts as natural fungicides for management of Fusarium oxysporum f. sp. lycopersici, the cause of tomato wilt. Pak J Bot. 2015;47:321-4.). The inoculum was prepared by suspending fungal conidia in distilled water. For this purpose, 1.2 g of each of the five sub-fractions of methanolic extract was dissolved in 1 mL DMSO and added to 5 mL autoclaved malt extract broth. This stock solution (200 mg mL-1) was serially double diluted by adding malt extract broth to prepare lower concentrations viz., 100, 50, 25, 12.5, 6.25, 3.125 and 1.15 mg mL-1. For control, 1 mL of DMSO was dissolved in 5 mL malt extract broth and serially double diluted to prepare controls corresponding to various extract concentrations. Bioassays were conducted in 10 mL volume glass test tubes each containing 1 mL of growth medium. Test tubes were inoculated aseptically with 15 μL of conidial suspension of F. oxysporum f. sp. lycopersici. Each treatment was replicated three times. Test tubes were incubated at 27 oC for 7 days and then fungal biomass in each test tube was filtered, dried to constant weight and weighed. The inhibitory effect of different sub-fractions of methanolic shoot extracts against F. oxysporum f. sp. lycopersici was calculated in terms of fungal biomass produced in each treatment and compared with fungal biomass in the corresponding control treatment.
GC-MS analysis of ethyl acetate sub-fraction of the shoot
GC-MS analysis was performed in a Perkin Elmer Turbo Mass Spectrophotometer (Norwalk, CTO6859, and USA). Helium was used as a carrier gas with a flow rate of 0.5 mL min-1. Inlet temperature of the instrument was 250 oC. Oven was preset at 110 oC for 4 min, raised up to 280 oC and run time was finished in 90 minutes. Temperature of the MS transfer line was 200 oC and that of the source was 180 oC. One microliter of sample was used in injection. Electron impact ionization (70 eV) was used for identification of compounds. For measurement of peak areas and data processing, the Turbo-Mass-OCPTVS-Demo SPL software was used.
Statistical analysis
All the data of laboratory bioassays were subjected to ANOVA followed by separation of treatment means by Tukey’s HSD test at 5% level of significance using the computer software Statistix 8.1.
RESULTS AND DISCUSSION
Antifungal activity of mathanolic extracts
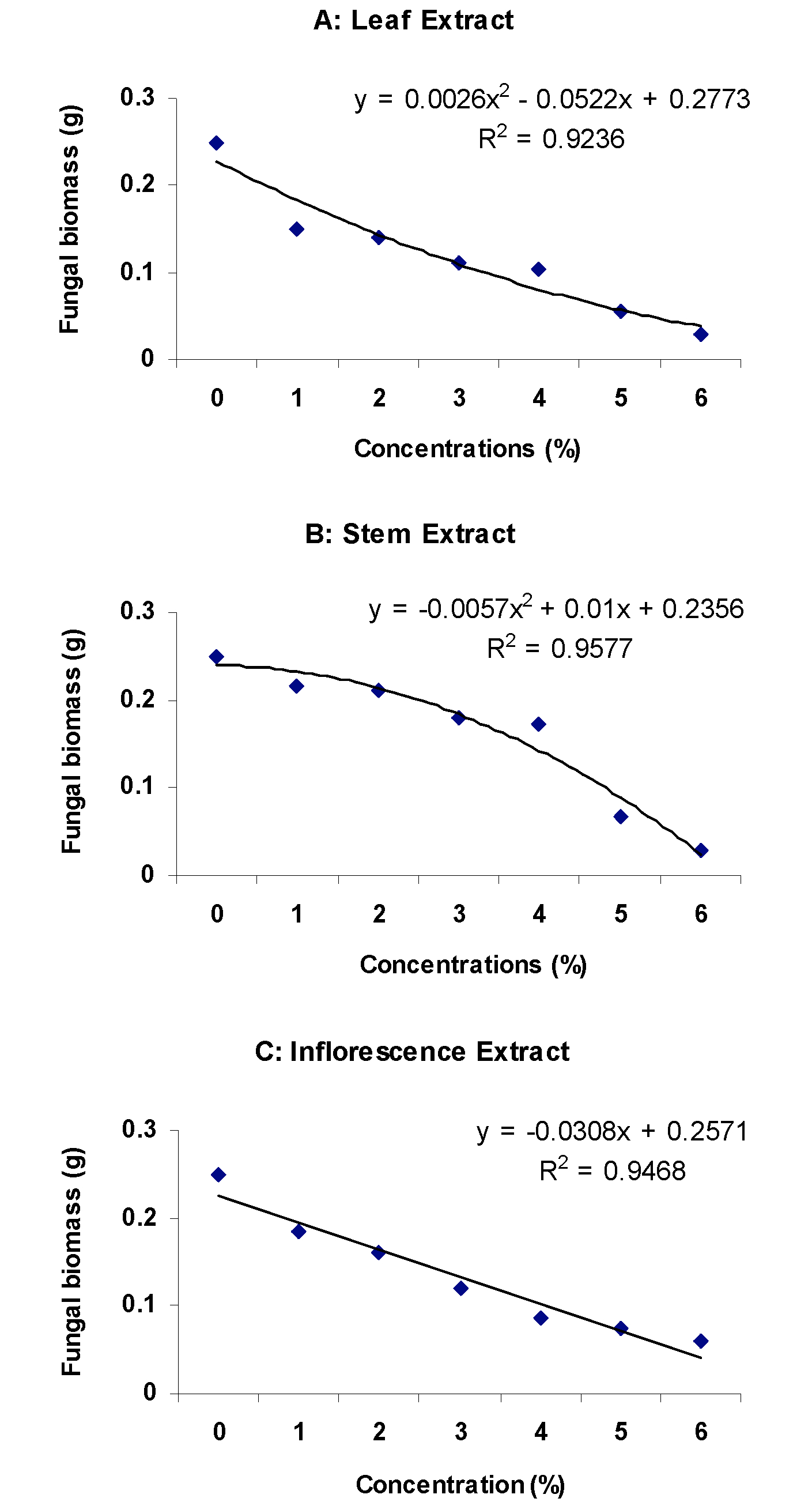
Methanolic extracts of all the three parts of C. pennisetiformis were found highly effective in suppressing in vitro growth of F. oxysporum f. sp. lycopersici. Different concentrations (1-6%) of leaf, stem, and inflorescence extracts significantly (P?0.05) reduced fungal biomass by 40-88%, 13-89%, and 26-76%, respectively (Figure 1). There was a polynomial relationship between fungal biomass and concentrations of methanolic extract of leaf and stem with R2 = 0.9236 and 0.9577, respectively. On the other hand, the relationship between fungal biomass and different concentrations of inflorescence extract was linear with R2 = 0.9468 (Figure 2). Previous studies showed that C. pennisetiformis has antifungal potential against M. phaseolina (Javaid and Naquvi, 2012Javaid A., Saddique A. Control of charcoal rot fungus Macrophomina phaseolina by extracts of Datura metel. Nat Prod Res. 2012;26:1715-20.). The use of methanolic extracts is a very useful technique for screening different parts of plants for their antifungal activity (Javaid and Bashir, 2015Javaid A., Bashir A. Radish extracts as natural fungicides for management of Fusarium oxysporum f. sp. lycopersici, the cause of tomato wilt. Pak J Bot. 2015;47:321-4.; Sana et al., 2016bSana N., Javaid A., Shoaib A. Antifungal activity of methanolic leaf extracts of allelopathic trees against Sclerotium rolfsii. Bangladesh J Bot. 2016b;45. (in press)). In the present study, screening trials showed that the leaf and stem extracts possessed greater antifungal activity than the inflorescence extract; therefore, the leaf + stem extracts were selected for further experiments to assess antifungal activity of sub-fractions of the methanolic extract.
Effect of different concentrations of methanolic extracts of aerial parts of Cenchrus pennisetiformis on growth of Fusarium oxysporum f. sp. lycopersici.
Relationship between concentrations of different methanolic extracts of Cenchrus pennisetiformis and biomass of Fusarium oxysporum f. sp. lycopersici.
Antifungal activity of sub-fractions of the methanolic shoot extract
Among the five sub-fractions of the methanolic shoot extract, the ethyl acetate sub-fraction exhibited the highest antifungal activity against F. oxysporum f. sp. lycopersici (Figure 3). Lower concentrations (1.56-6.25 mg mL-1) of this sub-fraction significantly (P?0.05) reduced fungal biomass by 50-60%, and further increase in concentrations (12.5-200 mg mL-1) resulted in 100% growth inhibition. The other highly effective antifungal sub-fractions were chloroform and n-hexane, where different concentrations generally showed a significant adverse effect, resulting in 40-100% decline in fungal biomass. The n-butanol sub-fraction also proved effective in inhibiting fungal biomass by 24-50% in the concentrations of 1.56-25 mg mL-1, and by 100% in the rest of the concentrations. The aqueous fraction was found to be effective at higher concentrations (100 and 200 mg L-1). A similar variable antifungal potential of different organic solvent fractions of methanolic extracts of Withania somnifera, Coronopus didymus, Chenopodium album and Datura metel has also been reported against various fungal pathogens including F. oxysporum (Iqbal and Javaid, 2012Iqbal D., Javaid A. Bioassays guided fractionation of Coronopus didymus for its antifungal activity against Sclerotium rolfsii. Nat Prod Res. 2012;26:1638-44.; Javaid and Munir, 2012Javaid A., Munir R. Bioassay guided fractionation of Withania somnifera for the management of Ascochyta rabiei. Int J Agric Biol. 2012;14:797-800.; Javaid and Saddique, 2012Javaid A., Saddique A. Control of charcoal rot fungus Macrophomina phaseolina by extracts of Datura metel. Nat Prod Res. 2012;26:1715-20.; Javaid et al., 2015Javaid A., Akhtar R. Antifungal activity of methanolic root extract of Withania sommnifera against Fusarium oxysporum f. sp. cepae. Afr J Trad Complement Altern Med. 2015;12:22-7.). Variability in antifungal activity of different sub-fractions possibly happened because of different polarity compounds in different organic solvents (Rauf and Javaid, 2013Rauf S., Javaid A. Antifungal activity of different extracts of Chenopodium album against Fusarium oxysporum f. sp. cepae the cause of onion basal rot. Int J Agric Biol. 2013;15:367-71.). It could be speculated that some compounds could be more active in the ethyl acetate sub-fraction as compared to other organic solvent fractions. Increase in inhibition of fungal growth with increased concentrations of the extract could result from the intensification of antioxidant potential of secondary metabolites in the extract (Pandey et al., 2010Pandey A.K. et al. Therapeutic potential of C. zeylanicum extracts: an antifungal and antioxidant perspective. Inter J Biol Med Res. 2010;1:228-33. ).
Effect of different concentrations of sub-fractions of the methanolic shoot extract of Cenchrus pennisetiformis on the growth of Fusarium oxysporum f. sp. lycopersici.
GC-MS analysis of the ethyl acetate sub-fraction
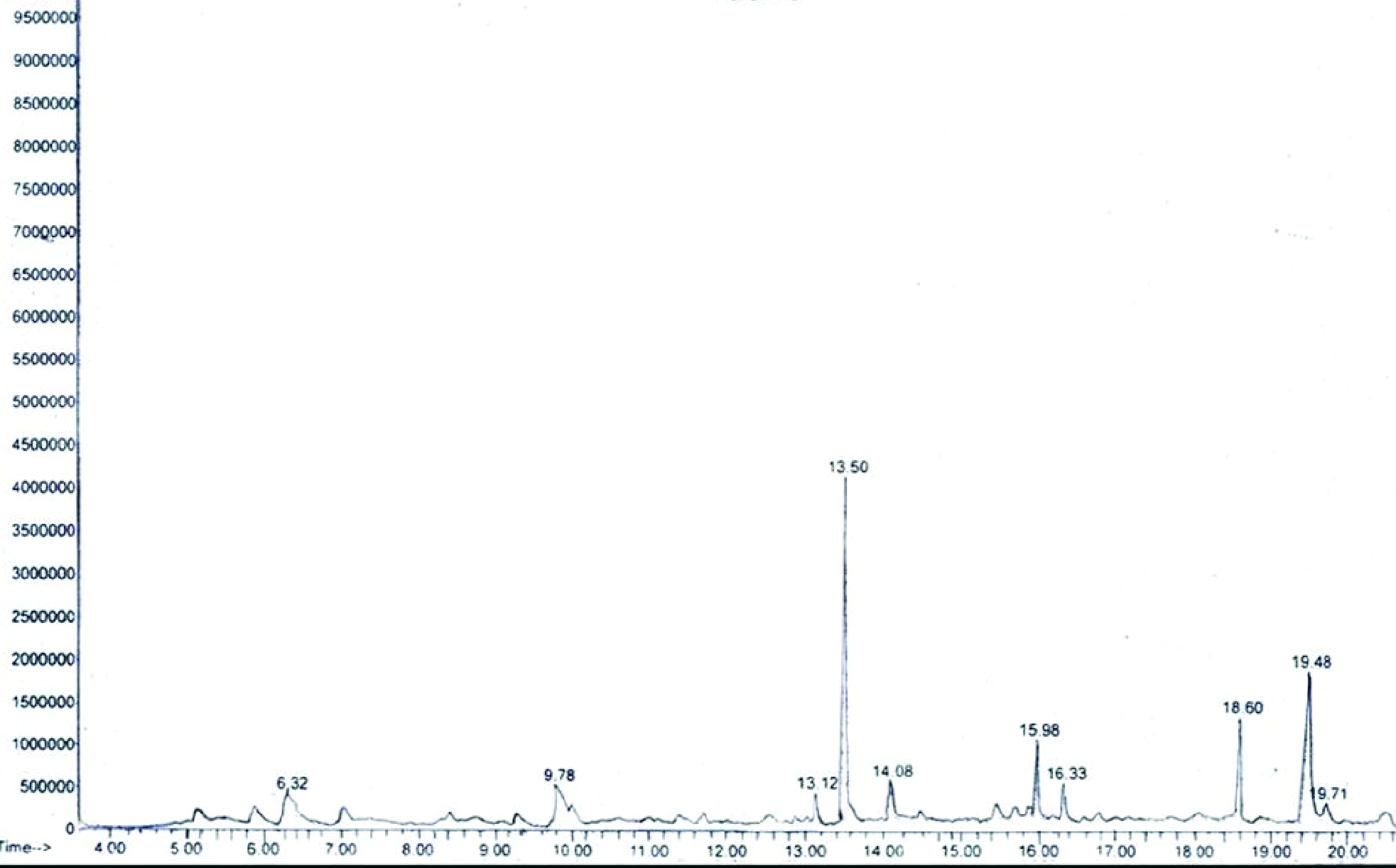
A total of 10 phytoconstituents were recorded in the ethyl-acetate sub-fraction of the methanolic shoot extract of C. pennisetiformis (Figure 4and5; Table 1). These compounds were phenol, 2,4-bis{1,1-dimethlethyl}-(30.99%); hexadecanoic acid, ethyl-ester (21.72%); 2,3-dihydro 1-benzofuran (10.65%); 1-propanol-2-2-hydroxypropxy (10.60%); 1-eicosene (8.32%); E-15-heptadecenal (5.80%); heptanal, 2 [phenylmethylene]- (3.59%); ethanone 1-[2,4,5-trimethoxypenyl]-(3.05%); benzene, 1 {1,1-dimethylethyl]-3,5-dimethyl-2,4,6-trinitro (2.90%) and hexadecanol, 2 methyl- (2.39%). Presence of phenol, 2,4-bis{1,1-dimethlethyl} in the highest amount could provide the basis of great fungicidal activity in C. pennisetiformis. Phenol, 2,6-bis(1,1-dimethylethyl)-4-methyl, commonly known as butylated hydroxytoluene, is an antioxidant and has been demonstrated as an antimicrobial agent (Sova, 2012Sova M. Antioxidant and antimicrobial activities of cinnamic acid derivatives. Mini Rev Med Chem. 2012;12:749-67.). 1-Eicosene has been isolated from many plants of medicinal values and hold significant antimicrobial potential (Nwodo et al., 2015Nwodo N.J. et al. Anti-trypanosomal activity of Nigerian plants and their constituents. Molecules. 2015;20:7750-71.). 1-[2,4,5 triethoxyphenyl] ethanone is widespread in plants and its fungicidal action has been reported against many devastating phytopathogens such as Colletotrichum capsici and Rhizoctonia cerealis (Yuqin et al., 2015Yuqin J. et al. Design, synthesis and antifungal activity of novel paeonol derivatives linked with 1,2,3-triazole moiety by the click reaction. J Chem Res. 2015;39:191-250.). Organic compounds, e.g., hexadecanoic acid, ethyl-ester and hexadecanol, 2 methyl are well-known for their antiviral, insecticidal and antibacterial activity (Sujatha et al., 2014Sujatha M. et al. GC-MS analysis of phytocomponents and total antioxidant activity of hexane extract of Sinapis alba. Int J Pharm Chem Biol Sci. 2014;4:112-7.; Mihigo et al., 2015Mihigo S.O. et al. Preliminary GC-MS Profiling and Anti-bacterial activity Investigation of Acanthospermum hispidum DC (Asteraceae). Int J Chem Aquat Sci. 2015;1:20-9.). Benzofuran, also known as methylcoumaran, is an important class of heterocyclic compounds; it has a significant position in medical chemistry and has shown biological activity against a wide spectrum of bacteria and fungi (Kossakowski et al., 2010Kossakowski J. et al. Synthesis and preliminary evaluation of the antimicrobial activity of selected 3-benzofurancarboxylic acid derivatives. Molecules. 2010;15:4737-49.). Many compounds such as 1-propanol-2-2-hydroxypropxy; benzene and 1-{1,1-dimethylethyl]-3,5-dimethyl-2,4,6-trinitro have been isolated from the methanolic extract of Aegle marmelos (golden apple or bael) and hold antimicrobial activity (Mujeeb et al., 2014Mujeeb F., Bajpai P., Pathak N. Phytochemical evaluation, antimicrobial activity, and determination of bioactive components from leaves of Aegle marmelos. [on linne] BioMed Res Int. 2014; 2014:11. Article ID 497606). Therefore, the presently reported antifungal potential of C. pennisetiformis against F. oxyporum f. sp. lycopersici could be attributed to the occurrence of many important compounds belonging to phenolics.
GC-MS chromatogram of the ethyl acetate sub-fraction of the methanolic shoot extract of C. pennisetiformis.
Structures of compounds identified from the ethyl acetate sub-fraction of the methanolic shoot extract of Cenchrus pennisetiformis through GC-MS analysis.
REFERENCES
- Abdel-Fattah G.M., Al-Amri S.M. Induced systemic resistance in tomato plants against Fusarium oxysporum f. sp. lycopersici by different kinds of compost. Afr J Biotechnol. 2012;11:12454-63.
- Amini J., Sidovich D.F. The effects of fungicides on Fusarium oxysporum f. sp. lycopersici associated with Fusarium wilt of tomato. J Plant Protec Res. 2010;50:172-8.
- Banaras S., Javaid A., Shoaib A., Ahmed E. Antifungal activity of Cirsium arvense extracts against a phytopathogenic fungus Macrophomina phaseolina Planta Daninha. 2017;35:e017162738.
- Iqbal D., Javaid A. Bioassays guided fractionation of Coronopus didymus for its antifungal activity against Sclerotium rolfsii Nat Prod Res. 2012;26:1638-44.
- Javaid A., Akhtar R. Antifungal activity of methanolic root extract of Withania sommnifera against Fusarium oxysporum f. sp. cepae Afr J Trad Complement Altern Med. 2015;12:22-7.
- Javaid A., Anjum T. Control of Parthenium hysterophorus L. by aqueous extracts of allelopathic grasses. Pak J Bot. 2006;38:139-45.
- Javaid A. et al. Management of Macrophomina phaseolina by extracts of an allelopathic grass Imperata cylindrica Pak J Agric Sci. 2015;15:37-41.
- Javaid A., Bashir A. Radish extracts as natural fungicides for management of Fusarium oxysporum f. sp. lycopersici, the cause of tomato wilt. Pak J Bot. 2015;47:321-4.
- Javaid A., Munir R. Bioassay guided fractionation of Withania somnifera for the management of Ascochyta rabiei Int J Agric Biol. 2012;14:797-800.
- Javaid A., Naqvi S.F. Evaluation of antifungal potential of Cenchrus pennisetiformis for the management of Macrophomina phaseolina World Acad Sci Eng Technol. 2012;69:832-5.
- Javaid A., Naqvi S.F., Shoaib A. Antifungal activity of methanolic extracts of Sorghum halepense against Macrophomina phaseolina Afr J Microbiol Res. 2012;6:5814-8.
- Javaid A., Saddique A. Control of charcoal rot fungus Macrophomina phaseolina by extracts of Datura metel Nat Prod Res. 2012;26:1715-20.
- Kossakowski J. et al. Synthesis and preliminary evaluation of the antimicrobial activity of selected 3-benzofurancarboxylic acid derivatives. Molecules. 2010;15:4737-49.
- Kumar K.R. et al. Enhanced growth promotion of tomato and nutrient uptake by plant growth promoting rhizobacterial isolates in presence of tobacco mosaic virus pathogen. Karnataka J Agric Sci. 2008;21:309-11.
- Mihigo S.O. et al. Preliminary GC-MS Profiling and Anti-bacterial activity Investigation of Acanthospermum hispidum DC (Asteraceae). Int J Chem Aquat Sci. 2015;1:20-9.
- Morid B., Hajmansoor S., Kakvan N. Screening of resistance genes to Fusarium root rot and Fusarium wilt diseases in tomato (Lycopersicon esculentum) cultivars using RAPD and CAPs markers. Eur J Exp Biol. 2012;2:931-9.
- Mujeeb F., Bajpai P., Pathak N. Phytochemical evaluation, antimicrobial activity, and determination of bioactive components from leaves of Aegle marmelos [on linne] BioMed Res Int. 2014; 2014:11. Article ID 497606
- Naqvi S.F., Javaid A., Shoaib A. Evaluation of antifungal activity of methanolic extracts of Dicanthium annulatum for the management of Macrophomina phaseolina Afr J Microbiol Res. 2012;6:5882-6.
- Nwodo N.J. et al. Anti-trypanosomal activity of Nigerian plants and their constituents. Molecules. 2015;20:7750-71.
- Pandey A.K. et al. Therapeutic potential of C. zeylanicum extracts: an antifungal and antioxidant perspective. Inter J Biol Med Res. 2010;1:228-33.
- Pakistan. Ministry of National Food Security & Research. Government of Pakistan. Agricultural Statistics of Pakistan 2012-2013. Islamabad: 2013
- Rauf S., Javaid A. Antifungal activity of different extracts of Chenopodium album against Fusarium oxysporum f. sp. cepae the cause of onion basal rot. Int J Agric Biol. 2013;15:367-71.
- Reis A., Boiteux L. Outbreak of Fusarium oxysporum f. sp. lycopersici race 3 in commercial fresh-market tomato fields in Rio de Janeiro State. Hortic Bras. 2007;25:451-4.
- Sana N., Shoaib A., Javaid A. Antifungal potential of leaf extracts of leguminous trees against Sclerotium rolfsii Afr J Trad Complement Altern Med. 2016a;13. (in press)
- Sana N., Javaid A., Shoaib A. Antifungal activity of methanolic leaf extracts of allelopathic trees against Sclerotium rolfsii Bangladesh J Bot. 2016b;45. (in press)
- Song W. et al. Tomato Fusarium wilt and its chemical control strategies in a hydroponic system. Crop Prot. 2004;23:243-7.
- Sova M. Antioxidant and antimicrobial activities of cinnamic acid derivatives. Mini Rev Med Chem. 2012;12:749-67.
- Sujatha M. et al. GC-MS analysis of phytocomponents and total antioxidant activity of hexane extract of Sinapis alba Int J Pharm Chem Biol Sci. 2014;4:112-7.
- Yuqin J. et al. Design, synthesis and antifungal activity of novel paeonol derivatives linked with 1,2,3-triazole moiety by the click reaction. J Chem Res. 2015;39:191-250.
Publication Dates
-
Publication in this collection
2018
History
-
Received
14 July 2016 -
Accepted
19 Aug 2016







 The vertical bars show standard errors of means of four replicates. Values with different letters at their top show significant difference (p≤0.05) as determined by Tukey’s HSD test.
The vertical bars show standard errors of means of four replicates. Values with different letters at their top show significant difference (p≤0.05) as determined by Tukey’s HSD test.
 The vertical bars show standard errors of means of four replicates. The values with different letters at their top show significant difference (p≤0.05) as determined by Tukey’s HSD Test.
The vertical bars show standard errors of means of four replicates. The values with different letters at their top show significant difference (p≤0.05) as determined by Tukey’s HSD Test.