ABSTRACT:
The cultivation of transgenic soybean plants using the glyphosate resistant gene (RR soybeans), takes up about 47% of the world’s cropping area. Despite the large area planted with soybeans resistant to glyphosate, there are very few studies of the environmental impact of this technology, especially in tropical areas. Thus, this study aimed to evaluate the impact of the cultive of RR soybeans and the use of glyphosate on the community of soil surface arthropods. The experiment was conducted in Coimbra, Minas Gerais state for two agricultural years. The experimental design was conducted in randomized blocks with five replications. The treatments were: non-transgenic soybean with mechanical weeding; RR soybean with mechanical weeding; RR soybean with one application of glyphosate and RR soybean with three applications of glyphosate. The populations of the soil surface arthropods were sampled over two years of cultivation (2007/2008 and 2008/2009). The cultive of RR soybean did not affect the richness and abundance of arthropods. A lower number of predators and detritivorous arthropods were observed in the treatments with one or three applications of glyphosate. Lower densities of arthropods were observed on the cultive of transgenic soybeans with three applications of glyphosate compared to the other treatments, especially the predators Achaearaneasp. (Araneae: Theridiidae), Oxypodinisp. (Coleoptera: Staphylinidae), Solenopsisspp. (Hymenoptera: Formicidae), the detritivorous Entomobryidae (Collembola), Hypogastrurasp. (Collembola: Hypogastruridae) and Xyleborussp. (Coleoptera: Scolytidae). The results indicate that the insertion of the glyphosate resistant gene does not affect the richness and abundance of the arthropods, however the use of glyphosate reduce the densities of predators and detritivorous on the soil surface.
Keywords:
Glycine max; insect pests; natural enemies and detritivorous
RESUMO:
O cultivo de plantas transgênicas de soja com genes de resistência ao glyphosate (soja RR) ocupa cerca de 47% da área mundial com essa cultura. Apesar da grande área cultivada com soja resistente ao glyphosate, são escassos os estudos sobre o impacto ambiental dessa tecnologia, principalmente em áreas tropicais. Assim, o objetivo deste estudo foi avaliar o impacto da soja RR e do glyphosate sobre a comunidade de artrópodes da superfície do solo. O experimento foi realizado no município de Coimbra, Estado de Minas Gerais, durante dois anos agrícolas. O delineamento experimental foi em blocos casualizados com cinco repetições. Os tratamentos estudados foram: soja não transgênica com capina mecânica das plantas daninhas; soja RR com capina mecânica das plantas daninhas; soja RR com uma aplicação de glyphosate; e soja RR com três aplicações de glyphosate. As populações de artrópodes da superfície do solo foram amostradas em dois cultivos: o primeiro no biênio 2007/2008 e o segundo no biênio 2008/2009. A soja transgênica RR não afetou a riqueza e a abundância dos artrópodes. Menores riquezas de artrópodes predadores e detritívoros foram observadas nos tratamentos que receberam uma ou três aplicações de glyphosate. Foram observadas menores densidades de artrópodes na soja transgênica com três aplicações de glyphosate do que nos demais tratamentos, sobretudo dos predadores Achaearaneasp. (Araneae: Theridiidae), Oxypodinisp. (Coleoptera: Staphylinidae) e Solenopsissp. (Hymenoptera: Formicidae) e dos detritívoros Entomobryidae (Collembola), Hypogastrurasp. (Collembola: Hypogastruridae) e Xyleborussp. (Coleoptera: Scolytidae). Os resultados indicam que a inserção do gene de resistência ao glyphosate não afeta a riqueza e abundância dos artrópodes e que o uso de três aplicações do herbicida reduz as densidades de predadores e detritívoros da superfície do solo.
Palavras-chave:
Glycine max; insetos-praga; inimigos naturais e detritívoros
INTRODUCTION
One of the greatest advances in agriculture in the last century was the introduction of genetically modified plants (Kremer and Means, 2009Kremer R.J., Means N.E. Glyphosate and glyphosate-resistant crop interactions with rhizosphere microorganisms. Eur J Agron. 2009;31:153-61.). Among herbicide resistant transgenic crops, stands out the glyphosate resistant soybeans (RR soybeans). The use of glyphosate resistant soybeans increased the volume and frequency of application of this herbicide (Schneider et al., 2009Schneider M.I. et al. Impact of glyphosate on the development, fertility and demography of Chrysoperla externa (Neuroptera: Chrysopidae): ecological approach. Chemosphere. 2009;76:1451-5. ; Owen et al., 2015Owen M.D. et al. Integrated pest management and weed management in the United States and Canada. Pest Manage Sci. 2015;71:357-76.).
Despite the increase of RR soybeans cultivated areas, there are very few studies about the environmental impact of this technology, especially in tropical areas. Thus, it is important to develop research about the direct impact of this biotechnology and the indirect effects due to the use of glyphosate (Prosser et al., 2016Prosser R.S. et al. Indirect effects of herbicides on biota in terrestrial edge-of- field habitats: A critical review of the literature. Agric Ecosyst Environ. 2016;232:59-72.). Such studies can be performed by the assessment of the community of soil arthropods, which are considered bioindicators of environmental impact in agroecosystems (Pereira et al., 2010Pereira J.L. et al. Influence of crop management practices on bean foliage arthropods. Bull Entomol Res. 2010;100:679-88.; Zhang et al., 2014Zhang L. et al. Relationship between land use pattern and the structure and diversity of soil meso-micro arthropod community. Ecotoxicology. 2014;23:707-17.).
Bioindicators are characterized by rapid response to environmental changes and present broad geographic distribution (Noss, 1990Noss R.F. Indicators for monitoring biodiversity: a hierarchical approach. Conserv Biol. 1990;4:241-3. ). The important criteria in choosing a bioindicator are: its behavior specificity to a certain factor and its sensitivity to a stressing agent (van Straalen et al., 1997van Straalen N.M. Community structure of soil arthropods as a bioindicator of soil health. In: Pankhurst C.E.; Doube B.M.; Gupta V.V.S.R., editors. Biological indicators of soil health. Wallingford: Centre for Agriculture and Biosciences International, 1997.). The community of soil arthropods presents great diversity of species, being composed by insect pests, natural enemies and detritivorous feeding insects. Therefore, the assessments of changes in the community of these organisms can reflect on the effects caused by the use of glyphosate. These effects can be caused by the reduction of the soil vegetal cover such as weeds, reducing the source of food and shelter to some insects.
The understanding of the impact of glyphosate resistant crops on the community of arthropods is fundamental in the planning of agroecosystem management practices. Thus, the objective of this study was to evaluate the impact of RR soybean cultivation and the use of glyphosate on the community of soil surface arthropods in soybean crops.
MATERIAL AND METHODS
The experiment was conducted in Coimbra, Minas Gerais state (20o50’58'’ S, 42o47’28'’W), during two cultives: the first one during 2007/2008 and the second one during 2008/2009. The varieties used in the experiment were the transgenic soybean BRS Favorita RR (Roundup Ready®) and the non-transgenic soybean MG/BR-46 Conquista. These varieties are similar, taking an average cycle of 115 days, determinate growth habit and the same pattern of disease resistance, being recommended for cultivation in the center-south region of Brazil. The direct sowing of soybean was done in the first fortnight of December during the first cultive and in the second fortnight of November during the second cultive.
The experimental design was conducted in randomized blocks with five replications. Each experimental plot consisted of an area of 10 x 10 m, row spacings of 0.5 m and planting density of 18 seeds per metre. The treatments were: 1 - non-transgenic soybean with mechanical weeding; 2 - transgenic soybean with mechanical weeding; 3 - transgenic soybean with one application of glyphosate (1.080 g ha-1) 15 days after plant emergence; and 4 - transgenic soybean with three applications of glyphosate (1.080 g ha-1) 15, 30 and 45 days after plant emergence.
The populations of soil surface arthropods were evaluated in the first cultive at 10, 26, 39, 52, 65 and 92 days after the emergence of the plants. On the second cultive, these populations were evaluated at 10, 17, 31, 49, 72, 90 and 106 days after the emergence of the plants. The samples were collected using pitfall traps, as proposed by Luff (1975Luff M.L. Some features influencing the efficiency of pitfall traps. Oecologia. 1975;19:345-57. ). Samples from field collection were stored in glass jars containing 70% alcohol. Subsequently, these samples were transfered to Petri dishes (9 cm diameter x 2 cm height) to count the total number of arthropods, using a stereomicroscope (12x).
The arthropods were separated in morphospecies, and the total number of each morphospecies per sample was determinated. The collected specimens were then sent to taxonomists for identification.
Species richness was represented by the total number of species and the number of species present in each guild per treatment in both cultives (2007/2008; 2008/2009).
The data of relative abundance in both cultives were subjected to a selective process, in order to determine which of the most abundant species explained the observed variance (PROC STEPDISC with selection STEPWISE and SAS Institute, 2013SAS Institute. SAS User’s Manual, Version 9.4. Cary: 2013.). The data of relative abundance of the selected species were subjected to Canonical Analysis of Variance (CAV). The significant difference in the abundance of the community of arthropods in function of the treatments was verified by the test F at p<0,05, using the Mahalanobis distance among the classes of canonical means.
From the main species found, curves of population fluctuation were made (mean ± standard error) for each treatment in both cultives. The data of relative abundance of these species were subjected to analysis of variance of repeated measurements, to study which periods the treatments affected the number of species of arthropods.
RESULTS AND DISCUSSION
In the first cultive a total of 149 species of arthropods were observed on the soil surface including: 25 chewing phytophagous, 11 sucking phytophagous, 49 predators, 5 parasitoids and 59 detritivrous feeding insects. The total arthropods richness in the first cultive varied from 103 species in the non-transgenic soybean with no application of glyphosate (i.e., mechanical weeding) to 82 species on the transgenic soybean with one application of glyphosate. In the second cultive, 125 species of arthropods were observed including: 20 chewing phytophagous, 14 sucking phytophagous, 40 predators, 6 parasitoids and 45 detritivorous feeding insects. Species richness in the second cultive varied from 95 species in the non-transgenic soybean with no application of glyphosate to 70 species in the transgenic soybean with three application of glyphosate (Table 1).
Total of species of detritorous feeding arthropods, chewing phytophagous, sucking phytophagous, predators, parasitoids and total of species per guild on the soil surface of the transgenic soybeans (TS) and non-trangenic soybeans (NTS) with one and three applications of glyphosate (1Gly and 3Gly). Coimbra, MG. 2007-2009
From the 149 and 125 species of arthropods observed on the soybean soil surface during the first and second cultive, respecively, 24 species had frequency of occurence greater than 10%. The most frequent arthropods in each guild in both cultives were the predators Achaearaneasp., Galumnidae, Gnamptogenys striatula, Hypoaspissp., Neivamyrmexsp., Oxypodinisp., Pachycondyla striata, Scytodes itapevi, Solenopsissp. and Tapinomasp.; the detritivorous Ataeniussp., Drosophilasp., Entomobryidae, Hypogastrurasp., Isotomidae, Onychiuridae, larva of Scirtidae, Sericoderussp., Silvanidae, Tomocerussp. and Xyleborussp.; the chewing phytophagous Carpophilussp. and Gryllussp.; and the sucking phytophagous Cyrtonemus mirabilis (Tables 2and3).
The predators Achaearanea sp., S. itapevi, Solenopsissp. and Oxypodinisp. and the detritivorous Entomobryidae, Hypogastrurasp. and Xyleborussp. were the species that best explained the maximum difference among the treatments, being included in the canonical analysis of variance (Table 4).
Summary of the selection by STEPWISE with procedure STEPDISC of SAS STEPWISE, to select species of phytophagous, natural enemies and detritivorous insects to be included in the analysis of canonical variables, obtaining the maximum discrimination between the treatments. Coimbra, MG. 2007-2009
Based on the canonical coefficients, the species that most positively contributed to the divergence among the treatments on the canonical axes were S. itapevi (axes 1 and 2 on the second cultive), Oxypodinisp. (axis 1 on both cultives), Entomobryidae (axis 1 on the first cultive) and Achaearaneasp. (axis 2 on the second cultive). The species that most negatively contributed to the divergence among the treatments in the canonical axes was Achaearaneasp. (axis 2 on the first cultive and axis 1 on the second cultive) (Table 5). Therefore, the predators Achaearaneasp., S. itapevi, Solenopsissp., Oxypodinisp. and the detritivorous feeding insects Entomobryidae were the main species able to be used to predict the impacts of the treatments.
Canonical axes and ther coefficients (among canonical structure) of the effect of non-transgenic soybeans (NTS) and the transgenic soybenas (TS) with one or three applications of glyphosate (1Gly e 3Gly) on the species of predators and detritivorous on the soil surface, selected by STEPWISE with STEPDISC procedure of SAS STEPWISE. Coimbra, MG. 2007-2009
The canonical analysis of variance indicated significant diferences among the treatments in the first (Wilks’ lambda = 0.4522 and F = 3.49 and df (numerator/denominator) = 21/230 and p<0,0001) and second cultive (Wilks’ lambda = 0.5100 and F = 3.48 and df (numerator/denominator) = 21/276 and p<0.0001). Three canonical axes were calculated with two being significant in the first cultive (p<0.0001 e p = 0.0095) and two being significant in the second cultive (p<0.0001 and p = 0.060). The first and second canonical axes explained in the first cultive 63 and 26% and, in the second cultive, 57 and 38% of the accumulated variance, respectively (Table 5).
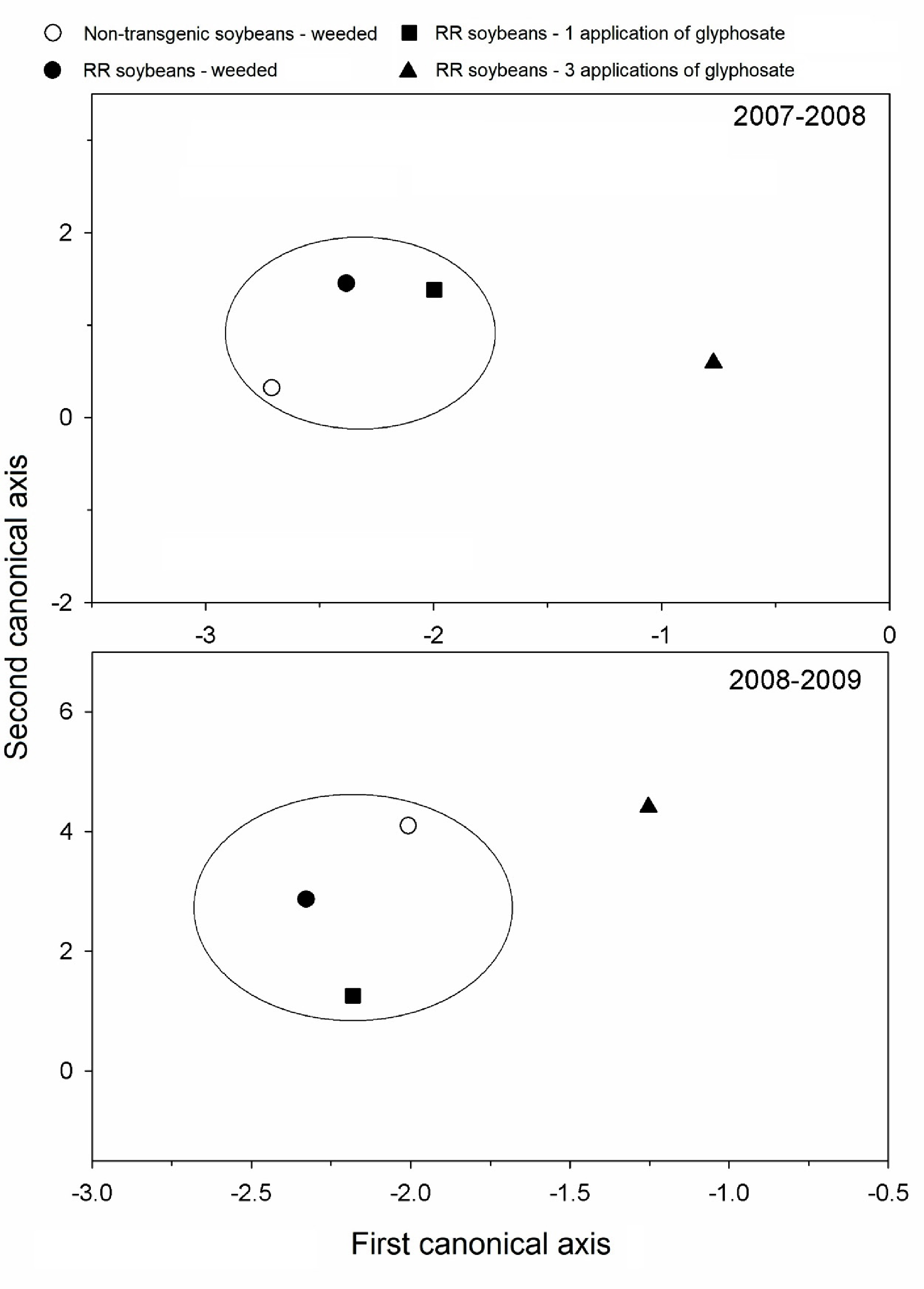
There was no significant difference among the transgenic and non-transgenic soybeans with mechanical weeding in both cultives. The treatment transgenic soybean with three applications of glyphosate reduced the total abundance of arthropods in both cultives (Figures 1and2).
Diagram of ordenation (CAV) of the community of arthropods on the soil surface on the cultive of soybeans. Treatments outside of the same circle differ by the Test F at p<0,05, based on the distance of Mahalanobis among the means of classes. Coimbra, MG. 2007-2009.
Abundance (mean ± standard error) of predators arthropods on the soil surface of non-transgenic soybeans (NTS) and transgenic soybeans (TS) with one or three applications of glyphosate (1Gly and 3Gly). Coimbra, MG. 2007-2009.
No significant differences were detected in the densities of the main species of predators and detritivorous arthropods on the soil surface, among the transgenic and non-transgenic soybeans in both cultives (Tables 6and7; Figures 2and3). Thus, it was demonstrated that the genetic cost related to the incorporation of the glyphosate resistant gene (CP4 EPSPS) from the bacteria Agrobacterium estirpe CP4 did not affect these groups of arthropods.
Multivariate analysis by repeated measurements on the abundance of predators on the soil surface of non-transgenic soybeans (NTS) and transgenic soybeans (TS) with one or three applications of glyphosate (1Gly and 3Gly). Coimbra, MG. 2007-2009
Multivariate analysis by repeated measurements on the abundance of arthropods detritivorous on the soil surface of non-transgenic soybeans (NTS) and transgenic soybeans (TS) with one or three applications of glyphosate (1Gly and 3Gly). Coimbra, MG. 2007-2009
3 - Abundance (mean ± standard error) of arthropods detritivorous on the soil surface of non-transgenic soybeans (NTS) and transgenic soybeans (TS) with one or three applications of glyphosate (1Gly and 3Gly). Coimbra, MG. 2007-2009.
However, using three applications of glyphosate on the transgenic soybeans reduced the abundance of the predators Achaearaneasp. and Oxypodinisp. during the first cultive and Solenopsissp. in both cultives (Table 7 and Figure 2). It was observed the lowest density of the detritivorous Entomobryidae, Hypogastrurasp. and Xyleborussp. in both cultives on the treatment transgenic soybeans with three applications of glyphosate (Table 7 and Figure 3). These results can be explained by the poor vegetal cover on the soils of the areas where glyphosate was applied. The vegetal cover promotes greater soil protection, avoiding losses of organic matter, which is important in the maintenance of water and nutrients and are essential resources in the preservation of species of arthropods, allowing them to cohabit within the same environment.
Some field investigations of the impact of glyphosate did not give attention to the populations of soil arthropods (Cerdeira et al., 2011Cerdeira A.L. et al. Agricultural impacts of glyphosate-resistant soybean cultivation in South America. J Agric Food Chem. 2011;59:5799-807. ; Prosser et al., 2016Prosser R.S. et al. Indirect effects of herbicides on biota in terrestrial edge-of- field habitats: A critical review of the literature. Agric Ecosyst Environ. 2016;232:59-72.). In the studies that have shown the impact of the use of glyphosate on soil insects, this pattern of response has been justified by the modification of the agroecosystem and not by the toxic effect of the molecule itself (Norris and Kogan, 2004Norris R.F., Kogan M. Ecology of interactions between weeds and arthropods. Ann Rev Entomol. 2004;50:479-503. ; Pereira et al., 2005Pereira J.L. et al. Effects of herbicide and insecticide interaction on soil entomofauna under maize crop. J Environ Scid Health, Part B: Pest Food Contam Agric Wastes. 2005;40:45-54. ). The glyphosate, when applied in the field, is rapidly translocated by the plants and adsorbed by the soil (Hoagland, 1980Hoagland R.E. Effects of glyphosate on metabolism phenolic compounds. Weed Sci. 1980;28:393-400.), not directly affecting the population of arthropods. However, the application of glyphosate is responsible for the alteration in the community of weeds.
Studies involving different species of arthropods have shown that these species have their population densities influenced by the diversity of host plants present in the environment (Norris and Kogan, 2004Norris R.F., Kogan M. Ecology of interactions between weeds and arthropods. Ann Rev Entomol. 2004;50:479-503. ). For example, the specie Scaphytopius acutus showed higher population density in peach trees associated with flowering weeds such as the red clover when compared with peach trees cultivated in areas with grass weeds (McClure et al., 1982McClure M.S.et al. Manipulating orchard cover to reduce invasion by leafhopper vectors of peach X-disease. J Econ Entomol. 1982;75:64-8. ). These data suggest that the lower diversity of resources present in the treatments with three applications of glyphosate (i.e lower density of weeds), may have favoured the reduction of the species of arthropods when compared to other treatments, as many species of weeds can serve as an alternative host to pests in agroecosystems (Castro et al., 2011Castro G.S.A. et al. Sistemas de produção de grãos e incidência de plantas daninhas. Planta Daninha. 2011;29:1001-10.).
Thus, the results of this research demonstrated that the insertion of the gene of resistance to glyphosate (CP4 EPSPS) from the bacteria Agrobacterium estirpe CP4 did not affect the richness and abundance of arthropods on the soybean soil surface. Whilst the application of glyphosate showed an impact on the abundance of species of predators and detritivorous feeding insects on the soil surface on the cultive of soybeans.
ACKNOWLEDGEMENTS
We would like to thank the Brazilian Agencies FAPEMIG, CNPq and CAPES.
REFERENCES
- Castro G.S.A. et al. Sistemas de produção de grãos e incidência de plantas daninhas. Planta Daninha. 2011;29:1001-10.
- Cerdeira A.L. et al. Agricultural impacts of glyphosate-resistant soybean cultivation in South America. J Agric Food Chem. 2011;59:5799-807.
- Hoagland R.E. Effects of glyphosate on metabolism phenolic compounds. Weed Sci. 1980;28:393-400.
- Kremer R.J., Means N.E. Glyphosate and glyphosate-resistant crop interactions with rhizosphere microorganisms. Eur J Agron. 2009;31:153-61.
- Luff M.L. Some features influencing the efficiency of pitfall traps. Oecologia. 1975;19:345-57.
- McClure M.S.et al. Manipulating orchard cover to reduce invasion by leafhopper vectors of peach X-disease. J Econ Entomol. 1982;75:64-8.
- Norris R.F., Kogan M. Ecology of interactions between weeds and arthropods. Ann Rev Entomol. 2004;50:479-503.
- Noss R.F. Indicators for monitoring biodiversity: a hierarchical approach. Conserv Biol. 1990;4:241-3.
- Owen M.D. et al. Integrated pest management and weed management in the United States and Canada. Pest Manage Sci. 2015;71:357-76.
- Pereira J.L. et al. Effects of herbicide and insecticide interaction on soil entomofauna under maize crop. J Environ Scid Health, Part B: Pest Food Contam Agric Wastes. 2005;40:45-54.
- Pereira J.L. et al. Influence of crop management practices on bean foliage arthropods. Bull Entomol Res. 2010;100:679-88.
- Prosser R.S. et al. Indirect effects of herbicides on biota in terrestrial edge-of- field habitats: A critical review of the literature. Agric Ecosyst Environ. 2016;232:59-72.
- SAS Institute. SAS User’s Manual, Version 9.4. Cary: 2013.
- Schneider M.I. et al. Impact of glyphosate on the development, fertility and demography of Chrysoperla externa (Neuroptera: Chrysopidae): ecological approach. Chemosphere. 2009;76:1451-5.
- van Straalen N.M. Community structure of soil arthropods as a bioindicator of soil health. In: Pankhurst C.E.; Doube B.M.; Gupta V.V.S.R., editors. Biological indicators of soil health. Wallingford: Centre for Agriculture and Biosciences International, 1997.
- Zhang L. et al. Relationship between land use pattern and the structure and diversity of soil meso-micro arthropod community. Ecotoxicology. 2014;23:707-17.
Publication Dates
-
Publication in this collection
2018
History
-
Received
05 Feb 2017 -
Accepted
14 June 2017