ABSTRACT:
Tebuthiuron is one of the most widely used herbicides in the sugarcane culture and its characteristic is the long persistence in soil. When used without knowing its interactions with soil attributes, it can reduce the sustainability of cropping systems and contaminate surface and groundwaters. In this research, by using a high-performance liquid chromatography, the effects of adding organic matter in a Red-Yellow Latosol were evaluated, as for the sorption and desorption of tebuthiuron. It was concluded that there is a direct relation between the sorption of tebuthiuron and the organic matter content in Red-Yellow Latosols and there is an inverse relation for desorption. The hysteresis index was lower in samples with high organic matter content.
Keywords:
chromatography; hysteresis index; persistence
RESUMO:
O tebuthiuron é um dos herbicidas mais utilizados na cultura da cana-de-açúcar e tem como característica a longa persistência no solo. Quando utilizado sem o conhecimento de suas interações com os atributos dos solos, pode reduzir a sustentabilidade dos sistemas de cultivos e contaminar águas superficiais e subterrâneas. Nesta pesquisa, utilizando a Cromatografia Líquida de Alta Eficiência, foram avaliados os efeitos da adição de matéria orgânica em um Latossolo Vermelho-Amarelo sobre a sorção e dessorção do tebuthiuron. Conclui-se que existe relação direta entre a sorção do tebuthiuron e os teores de matéria orgânica no Latossolo Vermelho-Amarelo e relação inversa para a dessorção. O índice de histerese foi menor nas amostras com alto conteúdo de matéria orgânica.
Palavras-chave:
cromatografia; índice de histerese; persistência
INTRODUCTION
Among the problems found by technicians and farmers in the management of weeds in the sugarcane cultivation, greater spacing between planting lines is the main limitation to the use of control programs with the exclusive use of herbicides. In order to solve this problem, there are two options: the first one consists in the pre- or post-emergence application of herbicides that have a short residual effect on the soil. In this case, for crops with a long critical period to prevent interferences, producers will have to repeat the operation several times, due to new infestations that may compromise the development and growth of the culture. Another option is using herbicides that have a long persistence in the soil (Chirukuri and Atmakuru, 2015Chirukuri R., Atmakuru R. Sorption characteristics and persistence of herbicide bispyribac sodium in different global soils. Chemosphere. 2015;138:932-9.). In this case, with a single application it is possible guarantee weed control until the end of the crop, preventing further infestations of the area. This technology, being the cheapest, is more used by most producers (Silva et al., 2013Silva V.P. et al. Efficiency and soil residual effect of herbicides in bean culture. Planta Daninha. 2013;31(4):961-70. ). Among the most commonly used herbicides in the sugarcane culture there is tebuthiuron, which has a long residual effect on the soil and is efficient in controlling several monocot and dicot species when applied before weed emergence.
However, regardless of whether the herbicide is applied during weed pre-or post-emergence, its final destination is the soil, in which this herbicide will undergo a redistribution process; it may be retained by colloids or it may be available in the soil solution. This part, available in the soil solution, may be absorbed by plants and microorganisms and degraded, and the rest may be leached to deeper layers of the soil profile (Lapworth and Gooddy, 2006Lapworth D.J., Gooddy D.C. Source and persistence of pesticides in a semi-confined chalk aquifer of southeast England. Environ Poll. 2006;144(3):1031-44.). Therefore, the main key factor of the herbicide behavior in the soil refers to its sorption and desorption capacity of mineral and organic colloids from the soil matrix (Cadková, 2013Cadková E. et al. Tebuconazole sorption in contrasting soil types. Soil Sed Cont Inter J. 2013;22(4):404-14.).
The energy for herbicide sorption to the soil is variable and depends on the nature of the herbicide-soil connection. These interactions may be Van der Waals, electrostatic and/or hydrogen bonding type (Low, 2001). When there is enough free energy to promote the return of the herbicides to the soil solution, the desorption process occurs (Rigi et al., 2015Rigi M.R. et al. Adsorption and Desorption Behavior of Herbicide Metribuzin in Different Soils of Iran. J Agric Sci Technol. 2015;17(3):777-87. ). This process is very important, since it will influence the availability of the herbicide over time, guaranteeing or not its efficiency in controlling weeds, that is, the residual effect of the herbicide (Sondhia, 2014Sondhia S. Herbicides residues in soil, water, plants and non-targeted organisms and human health implications: an Indian perspective. Ind J Weed Sci. 2014;46, n.(1):66-85. ).
For herbicides with basic or non-ionic characteristics, the soil organic matter content and quality are the most important attributes in the retention (sorption) of this compound by the soil matrix (Pereira Júnior, 2015Pereira Júnior E.V. et al. Effects of soil attributes and straw accumulation on the sorption of hexazinone and tebuthiuron in tropical soils cultivated with sugarcane. J Environ Sci Health Part B. 2015;50(4):238-46.). However, for acidic herbicides, other soil attributes, such as pH and the physical and chemical characteristics of mineral colloids, may be of greater importance. The sorption intensity is quantified by the partition coefficient (Kd), which refers to the ratio of the product sorbed by the colloids and that free part in the soil solution (Piwowarczyk and Holden, 2013Piwowarczyk A.A., Holden N.M. Phenoxyalkanoic acid herbicide sorption and the effect of co-application in a Haplic Cambisol with contrasting management. Chemosphere. 2013;90(2):535-41.). However, when intending to use mathematical models to infer the herbicide leaching capacity in the soil profile, such as the ERI (Environmental Risk Index), Koc or Kfoc are used; they represent Kd or Kf values normalized by the soil organic carbon (Oliver et al., 2016Oliver D.P. et al. Comparative environmental impact assessment of herbicides used on genetically modified and non-genetically modified herbicide-tolerant canola crops using two risk indicators. Sci Total Environ. 2016;557:754-63. ).
In Brazil, there are large areas of Red-Yellow Latosol used to cultivate sugarcane, and in these areas one of the mostly used herbicides is tebuthiuron. In addition, studies about tebuthiuron behavior in tropical soils are scarce. It is believed that the obtained results may be very important to make safe recommendations about tebuthiuron, both from the agronomical and environmental point of view.
The goal of this research was to quantify the sorption and desorption of tebuthiuron in samples of a Red-Yellow Latosol enriched with different organic carbon contents, using the High-Performance Liquid Chromatography.
MATERIAL AND METHODS
In samples of a Red-Yellow Latosol, collected at a 0 20 cm depth, air-dried and sewed in 4 mm mesh, different volumes of bovine manure were incorporated (Substrate 1: 0.0 kg OM/kg; Substrate 2: 0.250 kg OM/kg soil; Substrate 3: 0.500 kg OM/kg soil; Substrate 4: 0.750 kg OM/kg soil; Substrate 5: 1.00 kg OM/kg), increasing the organic matter content in the samples to 1.55, 2.84, 3.97, 6.35 and 7.28 dag kg-1, respectively (Table 1). The mixture of soil and bovine manure was made in a homogenous way and incubated for 30 days in 100 L plastic boxes. After that period, samples were collected and characterized chemically and physically (Tables 1 and 2). In order to determine tebuthiuron quantitatively, the high-performance liquid chromatography (HPLC) technique was used, according to the adjusted methodology described by Calvayrac et al. (2013Calvayrac C. et al. Photolysis of tembotrione and its main by-products under extreme artificial conditions: comparison with another â-triketone herbicide. Sci Total Environ. 2013;452:227-32.).
The quantification of tebuthiuron in soils was determined by a high-performance liquid chromatography with a Shimadzu LC 20AT model equipment, equipped with UV-Vis detector (Shimadzu SPD 20A) and C18 stainless steel column (Shimadzu VP-ODS Shim-pack 250 Mm x 4.6 mm d.i.). All used solvents were HPLC grade, and the other reagents were of analytical grade. Calcium chloride was supplied by Vetec, Brazil. Tebuthiron (4-amino-3,5,6-trichloropyridine-2-carboxylic acid, 99.6%) (Table 3) was obtained from Sigma-Aldrich, Germany. The stock solution of tebuthiuron was prepared using a 99.0% purity standard at the concentration of 1,000 mg L-1.
The chromatographic conditions for the analyses consisted in a mobile phase composed of a mixture of water and methanol in a 20:80 (v/v) ratio, with a 1.0 mL min-1 flow and a 20 μL injection volume. The column temperature was 40 oC, and the wavelength used for the reading was 254 nm. The areas obtained in the chromatograms were quantified and compared by through the external calibration method, and this methodology was satisfactory for all method validation parameters.
The equilibrium time required for tebuthiuron sorption was determined by the batch equilibrium method. The method consists in weighing inside polypropylene Falcon tubes 2.00 g of soil sample and, afterwards, adding 10 mL of herbicide solution with a concentration equal to 1.00 mg L-1 in 0.01 mol L-1 CaCl2. The tubes were agitated vertically for different time intervals (0.5, 1, 2, 4, 12, 16, 20, 24 and 30 h) at a temperature of 27 ± 2 oC and centrifuged for 4 minutes at 3,000 rpm. A portion of the supernatant was filtered in a 0.45 μm MCE membrane for further chromatographic analysis.
Tebuthiuron sorption in the soil samples was evaluated using working solutions prepared from the stock solution, at the concentrations of 0.50; 1.0; 1.5; 2.0; 2.5 and 3.0 mg L-1, in 0.01 mol L-1 CaCl2. From these solutions, 10 mL were transferred to polypropylene Falcon tubes containing 2.00 g of soils. These tubes were then agitated vertically for the equilibrium time determined in the previous tests and centrifuged for four minutes at 3,000 rpm; the supernatant was filtered on a 0.45 μm MCE membrane for further chromatographic analysis. All analyses were performed in triplicate, and the obtained data were submitted to regression analysis in order to interpret the results.
After analyzing the data, the amount of herbicide sorbed to the soil (Cs) in mg kg-1 was determined by the difference between the amount of standard solution initially added to the soil (Cp) in mg L-1 and the quantity found in the equilibrium solution (Ce), in mg L-1. With the encountered Ce and Cs values, the sorption constants were determined, where Kf and 1 n are empirical constants representing the sorption capacity and intensity, respectively.
The study about desorption was conducted by removing the supernatant from all tubes containing the soils after the sorption test, and adding to them 10 mL of the herbicide-free solution of CaCl2 0.01 mol L-1. In order to homogenize soil and solution, the tubes were shaken manually for 10 seconds. Subsequently, they were subjected to vertical agitation for the same time as the sorption tests and then centrifuged. The supernatant was filtered on a 0.45 μm MCE membrane for further chromatographic analysis.
In the desorption tests, calculations were also made to determine Kf and 1/n and, with this, to estimate the desorption capacity of the herbicide through soils with different organic matter contents. The hysteresis index was also calculated by dividing 1/n desorption values by the sorption ones (Silva et al., 2007Silva A.A., Silva J.F. Tópicos em manejo de plantas daninhas. Viçosa, MG: Universidade Federal de Viçosa, 2007. p.189-248.).
The analyses were performed in triplicate, and data were submitted to regression analysis to interpret the results; the coefficients of the equations tested by t test were at 5% significance.
RESULTS AND DISCUSSION
The equilibrium time of tebuthiuron (the concentration of the herbicide in the soil solution becomes constant) was 12 hours for all the used substrates (Figure 1); this differs from the 7 hour equilibrium time observed by Faria et al. (2013Faria A.T. et al. Atividade fisiológica da cana-de-açúcar após a aplicação de herbicidas em pré-emergência. Rev Bras Herb. 2013;12(2):171-8.) in Brazilian Latosols. The sorption rate depends on the kinetics of the molecule in the soil solution and on the availability of sites that are susceptible to adsorbing the herbicide; soil organic matter is one of the most important herbicide sorption sites (Mirzaei et al., 2013Mirzaei A. et al. Kinetic and equilibrium modeling of single and binary adsorption of methyl tert-butyl ether (MTBE) and tert-butyl alcohol (TBA) onto nano-perfluorooctyl alumina. Chem Eng J. 2013;231:550-60. ).
Concentrations of tebuthiuron in soil solution, according to time, in Red-Yellow Latosol with different organic matter (OM) contents.
Generally speaking, the sorption process occurs in two steps. Initially, the higher availability of adsorption sites on the surface of the aggregates and the high herbicide concentration in the soil solution promote the quick sorption of the molecule in the soil (Marco-Brown et al., 2014Marco-Brown J.L. et al. Adsorption of picloram herbicide on montmorillonite: kinetic and equilibrium studies. Coll Surf A: Physicochem Eng Aspects. 2014;449:121-8. ). In the second step, the sorption rate is lower, due to the lower diffusion and saturation of superficial sites. At this stage, the herbicide is adsorbed by the sites located in the micropores of the soil, but the herbicide molecules that are in the soil solution are repelled by the molecules adsorbed on the surface of the aggregate, reducing the contact between herbicide and micropore spaces. Moreover, the lower herbicide concentration in the soil solution reduces the diffusion of the molecule (Liu et al., 2010Liu Y. et al. Adsorption and desorption behavior of herbicide diuron on various Chinese cultivated soils. J Haz Mat. 2010;178(1):462-8. ; Faria et al., 2013Faria A.T. et al. Atividade fisiológica da cana-de-açúcar após a aplicação de herbicidas em pré-emergência. Rev Bras Herb. 2013;12(2):171-8.).
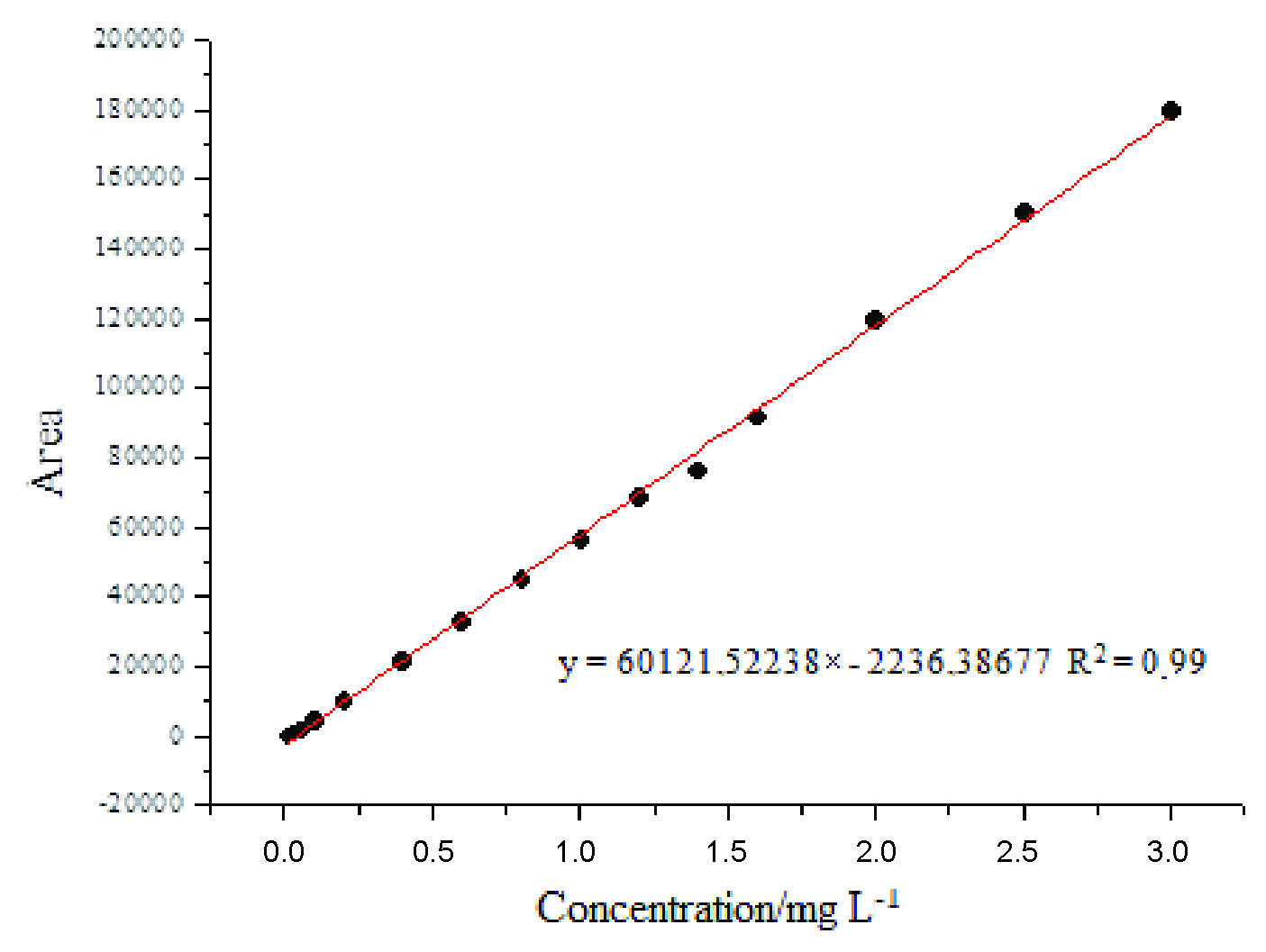
Tebuthiuron sorption was determined by an analytical curve constructed with growing herbicide concentrations. Linearity in the working range was determined at the concentration from 0.05 to 3.0 mg L-1, with a correlation coefficient of 0.99 (Figure 2), indicating good linearity of the method response to tebuthiuron at the used concentration. Detection and quantification limits were 0.64 and 2.97 mg L-1, respectively. In the chromatograms of the five substrates, no interferences were found as for the retention time of tebuthiuron; this indicates that the method is selective.
Tebuthiuron calibration curve in CaCl2 0.01 mol L-1, obtained by high performance liquid chromatography.
Tebuthiuron sorption was lower in the soil without the addition of organic matter (Figure 3). The sorption of herbicides in soil is associated with the presence of available sites that are capable of binding to the molecule (Kearns et al., 2014Kearns J.P. et al. 2,4-D adsorption to biochars: Effect of preparation conditions on equilibrium adsorption capacity and comparison with commercial activated carbon literature data. Water Res. 2014:62:20-8. ). Tebuthiuron is a non-ionic herbicide and it can bind with hydrophobic and hydrophilic groups that are present in both organic matter and clay colloids, through covalent hydrogen and Van der Waals bondings (Li et al., 2003; Kovaios et al., 2006Kovaios I.D. et al. Adsorption of atrazine on soils: Model study. J Coll Int Sci. 2006;299(1):88-94. ). Moreover, sandy soils have a low capacity to adsorb herbicides, due to their lower electrostatic surface, when compared to clayey soils (Firmino et al., 2008Firmino L.E. et al. Imazapyr sorption in soils with different textures. Planta Daninha. 2008;26(2):395-402.). Thus, the low sorption of tebuthiuron in substrate 1 may be associated to low organic matter content and soil texture.
Estimates of Freudlich sorption isotherms for tebuthiuron in Red-Yellow Latosol with different organic matter contents.
Tebuthiuron sorption was increased with a growth in the soil organic matter content (Figure 3). The sorption process of an herbicide in the soil may change as the physical-chemical characteristics of the herbicide are altered (Ololade et al., 2015Ololade I.A. et al. Kinetics and isotherm analysis of 2, 4-dichlorophenoxyl acetic acid adsorption onto soil components under oxic and anoxic conditions. J Environ Sci Health Part B. 2015;50(7):492-503.). The direct or conventional planting system and soil management practices, such as liming, adding gypsum and fertilization, can modify soil characteristics and affect the sorption of herbicides (Ouyang et al., 2016Ouyang W. et al. Typical agricultural diffuse herbicide sorption with agricultural waste-derived biochars amended soil of high organic matter content. Water Res. 2016;92:156-63.). Herbicides from the group of photosystem II inhibitors have their sorption reduced when the soil pH becomes more alkaline. This increases the motility of the molecule and increases the contamination potential of groundwater and springs by the herbicide (Refatti et al., 2014Refatti J.P. et al. Effect of liming on imazethapyr and imazapyr leaching in rice paddy soil. Ci Rural. 2014;44(6):1008-14.). In this case, when incubating with increasing amounts of bovine manure, the organic matter contents of the Red-Yellow Latosol increased, and so did the hydrophobic, carboxylic and hydroxylic sites capable of adsorbing the herbicide. Consequently, the sorption of tebuthiuron was increased.
As in the study about sorption, for the desorption of tebuthiuron, the parameters KF, 1/n and R2 for the isotherm were calculated and the hysteresis index for the five substrates was obtained. The substrates with higher contents of organic matter had lower hysteresis values (Table 4). The energy needed for the sorption and desorption of herbicides may be different and depends on the interaction force between soil and molecule. Low hysteresis indicates a greater difficulty for the previously sorbed herbicide to be desorbed (Koskinen et al., 2006Koskinen W.C. et al. Sorption-desorption of flucarbazone and propoxycarbazone and their benzenesulfonamide and triazolinone metabolites in two soils. Pest Manage Sci. 2006;62:598-602.), that is, the energy to promote the desorption of the herbicide is higher than the one required for the sorption process (Koskinen et al., 2006). The organic matter of the soil usually presents great group diversity (Velten et al., 2011Velten S. et al. Characterization of natural organic matter adsorption in granular activated carbon adsorbers. Water Res. 2011;45(13):3951-9.; Armanious et al., 2014Armanious A. et al. Dissolved organic matter adsorption to model surfaces: adlayer formation, properties, and dynamics at the nanoscale. Environ Sci Technol. 2014;48(16):9420-9.). Thus, herbicide molecules and organic matter in the soil establish distinct connections, and the covalents associated to secondary connections, such as hydrogen and Van der Waals bondings (Armanious et al., 2014Armanious A. et al. Dissolved organic matter adsorption to model surfaces: adlayer formation, properties, and dynamics at the nanoscale. Environ Sci Technol. 2014;48(16):9420-9.). The strength of the covalent bonding between components is high and, when other interactions occur simultaneously, the energy for the disruption of these bondings is high; this can reduce herbicide desorption (Benoit et al., 2008Benoit P. et al. Sorption and desorption of non ionic herbicides onto particulate organic matter from surface soils under different land uses. Eur J Soil Sci. 2008;59(2):178-89.; Trubetskaya et al., 2014Trubetskaya O. et al. Hydrophobicity of electrophoretic fractions of different soil humic acids. J Soils Sed. 2014;14(2):292-7. Okada et al., 2016Okada E. et al. Adsorption and mobility of glyphosate in different soils under no-till and conventional tillage. Geoderma. 2016;263:78-85.).
It is possible to conclude that the tebuthiuron sorption is directly proportional to the organic matter content, and the desorption is inversely proportional to the sorption. Based on this, it is possible to state that knowing the organic matter content in the soil, before recommending tebuthiuron, is an essential condition to recommend the application of doses, seeking agronomic efficiency and reducing the environmental risks deriving from the use of this herbicide.
REFERENCES
- Armanious A. et al. Dissolved organic matter adsorption to model surfaces: adlayer formation, properties, and dynamics at the nanoscale. Environ Sci Technol. 2014;48(16):9420-9.
- Benoit P. et al. Sorption and desorption of non ionic herbicides onto particulate organic matter from surface soils under different land uses. Eur J Soil Sci. 2008;59(2):178-89.
- Cadková E. et al. Tebuconazole sorption in contrasting soil types. Soil Sed Cont Inter J. 2013;22(4):404-14.
- Calvayrac C. et al. Photolysis of tembotrione and its main by-products under extreme artificial conditions: comparison with another â-triketone herbicide. Sci Total Environ. 2013;452:227-32.
- Chirukuri R., Atmakuru R. Sorption characteristics and persistence of herbicide bispyribac sodium in different global soils. Chemosphere. 2015;138:932-9.
- Faria A.T. et al. Atividade fisiológica da cana-de-açúcar após a aplicação de herbicidas em pré-emergência. Rev Bras Herb. 2013;12(2):171-8.
- Firmino L.E. et al. Imazapyr sorption in soils with different textures. Planta Daninha. 2008;26(2):395-402.
- Kearns J.P. et al. 2,4-D adsorption to biochars: Effect of preparation conditions on equilibrium adsorption capacity and comparison with commercial activated carbon literature data. Water Res. 2014:62:20-8.
- Koskinen W.C. et al. Sorption-desorption of flucarbazone and propoxycarbazone and their benzenesulfonamide and triazolinone metabolites in two soils. Pest Manage Sci. 2006;62:598-602.
- Kovaios I.D. et al. Adsorption of atrazine on soils: Model study. J Coll Int Sci. 2006;299(1):88-94.
- Lapworth D.J., Gooddy D.C. Source and persistence of pesticides in a semi-confined chalk aquifer of southeast England. Environ Poll. 2006;144(3):1031-44.
- Liu Y. et al. Adsorption and desorption behavior of herbicide diuron on various Chinese cultivated soils. J Haz Mat. 2010;178(1):462-8.
- Marco-Brown J.L. et al. Adsorption of picloram herbicide on montmorillonite: kinetic and equilibrium studies. Coll Surf A: Physicochem Eng Aspects. 2014;449:121-8.
- Mirzaei A. et al. Kinetic and equilibrium modeling of single and binary adsorption of methyl tert-butyl ether (MTBE) and tert-butyl alcohol (TBA) onto nano-perfluorooctyl alumina. Chem Eng J. 2013;231:550-60.
- Okada E. et al. Adsorption and mobility of glyphosate in different soils under no-till and conventional tillage. Geoderma. 2016;263:78-85.
- Oliver D.P. et al. Comparative environmental impact assessment of herbicides used on genetically modified and non-genetically modified herbicide-tolerant canola crops using two risk indicators. Sci Total Environ. 2016;557:754-63.
- Ololade I.A. et al. Kinetics and isotherm analysis of 2, 4-dichlorophenoxyl acetic acid adsorption onto soil components under oxic and anoxic conditions. J Environ Sci Health Part B. 2015;50(7):492-503.
- Ouyang W. et al. Typical agricultural diffuse herbicide sorption with agricultural waste-derived biochars amended soil of high organic matter content. Water Res. 2016;92:156-63.
- Pereira Júnior E.V. et al. Effects of soil attributes and straw accumulation on the sorption of hexazinone and tebuthiuron in tropical soils cultivated with sugarcane. J Environ Sci Health Part B. 2015;50(4):238-46.
- Piwowarczyk A.A., Holden N.M. Phenoxyalkanoic acid herbicide sorption and the effect of co-application in a Haplic Cambisol with contrasting management. Chemosphere. 2013;90(2):535-41.
- Refatti J.P. et al. Effect of liming on imazethapyr and imazapyr leaching in rice paddy soil. Ci Rural. 2014;44(6):1008-14.
- Rigi M.R. et al. Adsorption and Desorption Behavior of Herbicide Metribuzin in Different Soils of Iran. J Agric Sci Technol. 2015;17(3):777-87.
- Silva V.P. et al. Efficiency and soil residual effect of herbicides in bean culture. Planta Daninha. 2013;31(4):961-70.
- Silva A.A., Silva J.F. Tópicos em manejo de plantas daninhas. Viçosa, MG: Universidade Federal de Viçosa, 2007. p.189-248.
- Sondhia S. Herbicides residues in soil, water, plants and non-targeted organisms and human health implications: an Indian perspective. Ind J Weed Sci. 2014;46, n.(1):66-85.
- Trubetskaya O. et al. Hydrophobicity of electrophoretic fractions of different soil humic acids. J Soils Sed. 2014;14(2):292-7.
- Velten S. et al. Characterization of natural organic matter adsorption in granular activated carbon adsorbers. Water Res. 2011;45(13):3951-9.
Publication Dates
-
Publication in this collection
2018
History
-
Received
16 Sept 2016 -
Accepted
01 Feb 2017