ABSTRACT:
This study was conducted to explore the growth stimulating effect of foliage applied brassica water extract on growth and productivity of bread wheat (cv. Punjab 2011) at low and high fertilizer doses. The brassica water extract (5%) and the commercial growth regulator benzyl amino purine (BAP) (5 ppm) were applied alone and in combination at 30 and 45 days after sowing (DAS) under low fertilizer dose (125 kg ha-1 N and 90 kg ha-1 P) and high fertilizer doses (225 kg ha-1 N and 150 kg ha-1 P). Application of the brassica water extract (5%) significantly improved morphological traits such as crop growth rate, leaf elongation, leaf area index, plant height and number of productive tillers under both fertilizer regimes. Similarly, growth regulator benzyl amino purine (5 ppm) application enhanced the growth and yield components of wheat. However, maximum grain yield (6.20 t ha-1) was recorded with combined application of the brassica water extract (5%) and BAP (5 ppm) under the high fertilizer dose followed by individual application of the brassica water extract (5%) and BAP where 5.39 and 5.94 t ha-1 grain yields were recorded. Biological yield also showed an almost similar trend under the influence of the allelopathic water extract of brassica and BAP. Economic and marginal net benefits of 1521.6 and 237.0 USD ha-1 were respectively achieved with the application of the brassica water extract under the lower and higher fertilizer applications, respectively. The foliage applied 5% brassica water extract and BAP (5 ppm) was the most effective and had a stimulating impact on the growth and productivity of wheat.
Keywords:
fertilizer; allelopathic water extracts; plant growth regulators; Brassinolide
RESUMO:
Este estudo foi realizado para investigar o efeito estimulador do extrato aquoso de plantas do gênero Brassica, sob aplicação foliar, no crescimento e na produtividade do trigo panificável (cv. Punjab 2011) em doses baixa e alta de fertilizantes. O extrato aquoso de Brassica (5%) e o regulador de crescimento benzilaminopurina (BAP) (5 ppm) foram aplicados isoladamente e combinados aos 30 e 45 dias após a semeadura (DAS) sob dose baixa de fertilizante (125 kg ha-1 N e 90 kg ha-1 P) e dose elevada de fertilizante (225 kg ha-1 N e 150 kg ha-1 P). A aplicação do extrato aquoso de Brassica (5%) melhorou de forma significativa as características morfológicas, como taxa de crescimento da cultura, alongamento da folha, índice de área foliar, altura das plantas e número de perfilhos produtivos, nos dois regimes de fertilizantes. Da mesma forma, a aplicação do regulador do crescimento benzil amino purina (5 ppm) gerou aumento nos componentes de crescimento e produção de trigo. No entanto, o rendimento máximo de grãos (6,20 t ha-1) foi registrado com a aplicação combinada do extrato aquoso de Brassica (5%) e BAP (5 ppm) sob dose elevada de fertilizante, seguido por aplicação individual de extrato aquoso de Brassica (5%) e BAP, tendo sido registrado rendimento de grãos de 5,39 e 5,94 t ha-1. O rendimento biológico também mostrou tendência quase semelhante sob a influência do efeito alelopático do extrato aquoso de Brassica e BAP. Foram obtidos benefícios econômicos líquidos e marginais de 1.521,6 e 237,0 USD ha-1 com a aplicação do extrato aquoso de Brassica nas doses inferior e superior de fertilizante, respectivamente. A aplicação foliar a 5% do extrato aquoso de Brassica e BAP (5 ppm) foi a mais eficaz e exerceu impacto estimulante sobre o crescimento e a produtividade do trigo.
Palavras-chave:
fertilizantes; extratos aquosos com efeito alelopático; reguladores de crescimento de plantas; Brassinolide
INTRODUCTION
Allelopathy is responsive to the release of secondary metabolites known as allelochemicals. These allelochemicals released by plants, bacteria, viruses and fungi influence the growth of plants across their vicinity either positively or negatively (Farooq et al., 2011aFarooq M, Habib M, Rehman AU, Wahid A, Munir R. Employing aqueous allelopathic extracts of sunflower in improving salinity tolerance in rice. J Agric Soc Sci. 2011a;7:75-80.; Abbas et al., 2017aAbbas T, Nadeem MA, Tanveer A, Syed S, Zohaib A, Farooq N et al. Allelopathic influence of aquatic weeds on agro-ecosystems: a review [online]. Planta Daninha. 2017a;35:e017163146. ). These allelochemicals are byproducts of different physiological processes; they are released into the environment and play a vital role in determining nutrient dynamics, soil chemical characteristics, mycorrhizae, microbial activity, plant diversity, succession, invasion and climax of natural vegetation (Bhowmik and Inderjit, 2003Bhowmik PC, Inderjit. Challenges and opportunities in implementing allelopathy for natural weed management. Crop Prot. 2003;22:661-7.; Bhadoria, 2011Bhadoria PBS. Allelopathy: a natural way towards weed management. Am J Exp Agric. 2011;1:7-20.; Farooq et al., 2011a; Al-Sherif et al., 2013Al-Sherif E, Hegazy AK, Gomaa NH, Hassan MO. Allelopathic effect of black mustard tissues and root exudates on some crops and weeds. Planta Daninha. 2013;31(1):11-9.; Koocheki et al., 2013Koocheki A, Lalegani B, Hosseini SA. Ecological consequences of Allelopathy. In: Cheema ZA, Farooq M, Wahid A editors. Allelopathy: Current trends and future applications. Berlin: Springer; 2013. p.23-38.; Abbas et al., 2017aAbbas T, Nadeem MA, Tanveer A, Syed S, Zohaib A, Farooq N et al. Allelopathic influence of aquatic weeds on agro-ecosystems: a review [online]. Planta Daninha. 2017a;35:e017163146. ).
Plant materials (roots, shoots, leaves and flowers) have been used in the form of water extracts to investigate their effect on different field crops (Iqbal et al., 2017Iqbal J, Rauf HA, Shah AN, Shahzad B. Allelopathic effects of rose wood, guava, eucalyptus, sacred fig and jaman leaf litter on growth and yield of wheat (Triticum aestivum L.) in wheat based agroforestry system. [onlne]. Planta Daninha 2017;35:e017166992.). The inhibitory effect of allelochemicals is well explored and previously it was the only known dimension of allelopathy. Several authors extensively explored the inhibitory potential of different allelopathic crops and trees for weed management (Cheema et al., 2004Cheema ZA, Khaliq A, Saeed S. Weed control in maize (Zea mays L.) through sorghum allelopathy. J Sustain Agric. 2004;23:73-86.; Iqbal et al., 2007Iqbal J, Cheema ZA, An M. Intercropping of field crops in cotton for the management of purple nutsedge (Cyperus rotundus L.). Plant Soil. 2007;300:163-71.; Jamil et al., 2009Jamil M, Cheema ZA, Mushtaq MN, Farooq M, Cheema MA. Alternative control of wild oat and canary grass in wheat fields by allelopathic plant water extracts. Agron Sustain Dev. 2009;29:475-82.; Farooq et al., 2011bFarooq M, Jabran K, Cheema ZA, Wahid A, Siddique KH. The role of allelopathy in agricultural pest management. Pest Manage Sci. 2011b;67(5):493-506. ). However, these allelochemicals also have potential to promote crop growth (Abbas et al., 2017bAbbas T, Nadeem MA, Bhagirath AT, Chauhan S. Can hormesis of plant-released phytotoxins be use d to boost and sustain crop production? Crop Prot. 2017b;93:69-76.; Shah et al., 2017Shah AN, Iqbal J, Fahad S, Tanveer M, Yang G, Khan EA. Allelopathic Influence of Sesame and Green Gram Intercrops on Cotton in a Replacement Series. Clean Soil Air Water. 2017;45(1):1500469. ) and induce resistance against abiotic stresses when applied at lower concentrations (Farooq et al., 2011a, b). For instance, seed treatment with moringa water extract increased sorghum germination, maize radical length and wheat hypocotyl length by 29.0, 77.8 and 14.5% respectively (Phiri, 2010Phiri C. Influence of Moringa oleifera leaf extract on germination and early seedling development of major cereals. Agric Biol J Am. 2010;1:774-7.). These secondary metabolites at low concentrations help to induce germination by breaking seed dormancy (Nickell, 1982Nickell LG. Plant growth regulators agricultural uses. New York: Springer Verlag; 1982.), promote root growth by improving moisture availability and temperature regulation (Mackay and Barber, 1985Mackay AD, Barber SA. Soil moisture effects on root growth and phosphorus uptake by corn. Agron J. 1985;77:519-23.), enhance mineralization of nutrients and improve their uptake (Barber, 1984Barber SA. Soil nutrient bioavailability: A mechanistic approach. New York: John Wiley and Sons; 1984.). Furthermore, physiological processes such as seed germination, root growth, chlorophyll accumulation, photosynthesis, transpiration, leaf expansion, translocation and genetic encodings (Gamalero and Glick, 2011Gamalero E, Glick BR. Mechanisms used by plant growth promotion bacteria. In: Maheshwari DK editor. Bacteria in agrobiology: plant nutrient management. Berlin: Springer-Verlag; 2011.), process of mitosis as a result of tissue formation and ultra-structure are affected positively.
Brassica napus contains several allelochemicals such as glucosinolates (Agerbirk and Olsen, 2012Agerbirk N, Olsen CE. Glucosinolate structures in evolution. Phytochemistry. 2012;77:16-45.), thiocyanates, isothiocyanates (Romanowski and Klenk, 2000Romanowski F, Klenk H. Thiocyanates and isothiocyanates, organic; Ullmann’s Encyclopedia of Industrial Chemistry [on line]. Wiley-VCH Verlag GmbH; 2000.) and brassinolide (Grove et al., 1979Grove MD, Spencer GF, Rohwedder WK, Mandava NB, Worley JF, Warthen JD et al. Brassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen. Nature. 1979. 281(5728):216-7.). Brassinolide affects the various developmental processes e.g., seed germination, growth, flowering and senescence. Some studies suggest that brassinosteroids and their derivatives improve the growth and productivity of crops both under normal and stressed conditions (Arora et al., 2008Arora N, Bhardwaj R, Sharma P, Arora HK. Effects of 28-homobrassinolide on growth, lipid peroxidation and antioxidative enzyme activities in seedlings of Zea mays L. under salinity stress. Acta Physiol Plant. 2008;30:833-9. ). Up to 20 to 60% increase in crop yield has also been attained by genetic manipulation of brassinosteroids (Divi and Krishna, 2009Divi UK, Krishna P. Brassinosteroid: a biotechnological target for enhancing crop yield and stress tolerance. New Biotechnol. 2009;26(3-4):131-6.). Foliar application of 28-homobrassinolide improved the growth and yield of wheat, rice, brassica, potato and groundnut when applied at lower concentration at different growth stages (Ramraj et al., 1997Ramraj VM, Vyas BN, Godrej NB, Mistry KB, Swami BN, Singh N. Effects of 28-homobrassinolide on yields of wheat, rice, groundnut, mustard, potato and cotton. J Agric Sci. 1997;128:405-13.). Kwak et al. (2009Kwak MS, Kim IH, Kim SK. Effects of Brassinolide with Naphthalene acetic acid on the formation of adventitious roots, trichome-like roots and calli from cultured tobacco leaf segments, and the expression patterns of CNT103. J Plant Biol. 2009;52:511-7.) reported that brassinolide supplementation at a lower concentration on tobacco plant increased the length of adventitious roots; however, higher concentrations interfered with root initiation and caused cluster formation that further hindered root differentiation. Brassinolide in higher concentrations affected the shape of rice seedlings and decreased leaf sheath size as compared with plants which received lower concentration of brassinolide (Chon et al., 2008Chon NM, Nishikawa-Koseki N, Takeuchi Y, Abe H. 2008. Role of ethylene in abnormal shoot growth induced by high concentration of brassinolide in rice seedlings. J Pest Sci. 2008;33(1):67-72.). However, some other studies also have been reviewed suggesting beneficial role of brassinolide in mediating several abiotic stresses such as heavy metal and herbicide induced oxidative stress in plants (Rehman et al., 2018Rehman S, Shahzad B, Bajwa AA, Hussain S, Rehman A, Cheema SA, Abbas T, Ali A, Shah L, Adkins S, Li P. Utilizing the Allelopathic Potential of Brassica Species for Sustainable Crop Production: A Review. J Plant Growth Reg. 2018;1-14.; Shahzad et al., 2018Shahzad B, Tanveer M, Che Z, Rehman A, Cheema SA, Sharma A, Song H, Rehman S, Zhaorong D. Role of 24-epibrassinolide (EBL) in mediating heavy metal and pesticide induced oxidative stress in plants: A review. Ecotoxicol and Environ Saf. 2018:935-944.; Sharma et al., 2018Sharma A, Kumar V, Kumar R, Shahzad B, Thukral AK, Bhardwaj R. Brassinosteroid-mediated pesticide detoxification in plants: A mini-review. Cogent Food & Agri. 2018:1436212.).
Upon release of allelochemicals into the rhizosphere, they regulate solubilization, mobilization, release and chelation of mineral nutrients (Jabran et al., 2013Jabran K, Farooq M, Aziz T, Siddique KHM. Allelopathy and crop nutrition. In: Cheema ZA, Farooq M, Wahid A editors. Allelopathy: Current trends and future applications. Berlin: Springer Verlag; 2013. p.113-43.). Moreover, allelochemicals reduce nutrient losses and improve nutrient use efficiency. Recently, a lot of work has been under progress regarding the role of allelopathy in improving growth promotion through application of crop residues, mulching as well as cultivation of companion crops. However, growth behavior of wheat crop in response to the brassica water extract under different fertilizer regimes has been rarely studied. Because of their diverse mode of action on the physiology and morphology of plants and nutrient dynamics, the current study was aimed at examining the influence of allelopathic water extracts of brassica on wheat growth and yield, compared with Benzyl amino purine (BAP) (a plant growth regulator) under different fertilizer regimes.
MATERIAL AND METHODS
Experimental site and plant growth conditions
This study was conducted between 2012 and 2013 at Agronomic Research Area, University of Agriculture Faisalabad Pakistan. The experimental site lies between latitude 31.25o N and longitude 73.09o E. The experimental soil was sandy clay loam in nature with pH 8.2, electrical conductivity (EC) 0.37 dSm-1, organic matter (OM) 0.64%, total nitrogen (N) 0.04%, available phosphorus (P) 8.0 ppm and available potassium (K) 170 ppm. The climate of experimental site was semi-arid with an average rainfall of 346 mm and weather data during the course of experiment is given in Table 1. The experiment was designed in a randomized complete block design with a split-plot arrangement with four replications.
Treatments
Wheat was fertilized with two rates, (low fertilizer dose (F1), 125 kg ha-1 and 90 kg P ha-1) and (high fertilizer dose (F2), 225 kg N ha-1 and 150 kg P ha-1. Urea (46% N) and DAP (46% P2O5) were used as a source of N and P, respectively. All of the phosphorus and half of the nitrogen were applied as a basal dose, while the remaining half of the nitrogen was applied at the time of second irrigation (booting stage). For foliar application treatments, five treatments were employed such as no treatment as control (T1), water spray (WS) (T2), 5% brassica water extract (BWE) (T3), 5 ppm benzyl amino purine (BAP) (T4) and BWE (5%) + BAP (5 ppm) (T5). Foliar application treatments were applied at 30 and 45 days after sowing (DAS) of wheat.
Preparation and application of the brassica water extracts
Water extract of Brassica napus was prepared according to Cheema and Khaliq (2000Cheema ZA, Khaliq A. Use of sorghum allelopathic properties to control weeds in irrigated wheat in semi-arid region of Punjab. Agric Ecosyst Environ. 2000;79:105-112.). Air dried plant herbage of Brassica napus was chopped with a fodder cutter into 2-3 cm pieces and kept under cover to avoid possible leaching by rainwater. Chopped brassica material was soaked in water (1:10 w/v) for 24 h at room temperature. Extracts were sieved, packed and labeled as 100% brassica water extract. The volume of the spray (300 L ha-1) was determined by calibration as described by Rao et al. (1987Rao MR, Shetty SVR, Reddy SLN, Sharma MM.Weed managementinimproved rainfed cropping systemsinsemi-arid India. In: Soil, crop, and water management systems for rainfed agriculture in the sudano-sahelian zone. Proceedings of an International Workshop, 11-16 Jan. 1987. Patancheru: ICRISAT Sahelian Center; 1989. p.303-16.). Foliar application was done with a Knapsack hand sprayer fitted with a T-Jet nozzle maintaining a pressure of 207 KPa.
Measurement of growth traits
To determine the length and width of the 5th and 7th leaves, ten plants were randomly tagged and periodic data were taken with the help of a meter ruler. Leaf area index (LAI) was calculated with the ratio of leaf area to land area. From each plot, 1m2 area was harvested to determine dry matter accumulation. Samples were weighed for fresh weight, dried under sunlight and placed in an oven at 70±5 ?C up to constant weight. Crop growth rate (CGR) was measured by using the following formulae given by Hunt (1978Hunt R. Plant growth analysis. The InstBio Studies Bio/Edward Arnold: 1978.) and it was measured with an interval of 15 days after 60 DAS.
At physiological maturity, twenty plants from each replication were randomly selected for plant height, which was measured with the help of a meter rod from the soil surface to the top of the spike excluding the awns.
Determination of yield and yield contributing traits
Number of spikelets per spike and number of grains per spike were counted from 5 randomly selected plants in each experimental plot. Grain yield and biological yield per replication were measured separately and then an average was taken.
Statistical Analysis
The significance of variance (p≤0.05) was determined after analyzing the data collected using Fisher’s analysis of variance technique with computer software Statistix 9 under a split-plot design. Differences among treatment means were compared by using the least significant difference (LSD) test at 5% (Steel et al., 1997Steel RGD, Torrie JH, Dickey D. Principles and procedures of statistics: a biometrical approach. New York McGraw Hill Book; 1997.).
RESULTS AND DISCUSSION
Growth and related traits
Application of a plant growth regulator (BAP) significantly improved the crop growth rate of wheat. In the low fertilizer dose (F1), the allelopathic brassica water extract (5%) improved the growth of wheat. All treatments were alike for CGR until 65 DAS. Maximum crop growth was recorded with application of the brassica water extract (5%) alone and with combination of the brassica water extract (5%) + BAP (5 ppm) (Figure 1). In the high fertilizer dose (F2), the allelopathic brassica water extract (5%) substantially improved the growth of wheat; however, maximum crop growth was recorded with application of the allelopathic brassica water extract (5%) alone and with combination of the brassica water extract (5%) + BAP (5 ppm), respectively (Figure 1). The interaction was found to be non-significant.
Influence of foliage applied brassica water extract (5%) on CGR of wheat in the low fertilizer dose (F1) and high fertilizer dose (F2) ±S.E.
Length of fifth and seventh leaves
Application of the allelopathic brassica water extract (5%) significantly improved the length of 5th leaf of wheat. In the low fertilizer dose, all treatments improved the length of 5th leaf in comparison with control. Similarly, application of the brassica water extract (5%) and BAP (5 ppm) continuously improved the length of the 5th leaf. In the high fertilizer dose, the combined application of the brassica water extract (5%) and BAP (5 ppm) improved the length of the 5th leaf from 30 DAS and continued to improve the length of the 5th leaf. However, all other treatments did not improve the length of 5th leaf and were alike the control treatment (Figure 2). The length of the 7th leaf was substantially improved by application of the brassica water extract (5%). None of the other treatments improved the length of the 7th leaf and were alike the control treatment in the low fertilizer dose. However, in the high fertilizer dose, the combined application of the brassica water extract (5%) and the growth regulator BAP (5 ppm) substantially improved the length of 7th leaf of wheat while all other treatments did not improve the length of the 7th leaf and were alike the control treatment. (Figure 3). Moreover, the interaction between the allelopathic water extracts and the fertilizer was non-significant.
Influence of foliage applied brassica water extract (5%) on the 5th leaf length in the low fertilizer dose (F1) and high fertilizer dose (F2) ±S.E.
Influence of the foliage applied brassica water extract (5%) on the 7th leaf length in the low fertilizer dose (F1) and high fertilizer dose (F2) ±S.E.
Leaf area index
Leaf area index (LAI) was substantially improved by application of the brassica water extract (5%). In the low fertilizer dose, LAI was significantly improved by application of the brassica water extract (5%) alone and when the brassica water extract (5%) was combined with BAP (5 ppm). The brassica water extract (5%) alone and the combination of the brassica water extract (5%) + BAP (5 ppm) improved LAI until 90 DAS; later on, it started to decline towards maturity (Figure 4). In the high fertilizer dose, the allelopathic brassica water extract improved the LAI of wheat. Application of the brassica water extract (5%) and the combination of the brassica water extract (5%) + BAP (5 ppm) improved LAI until 90 DAS. (Figure 4).
Influence of the foliage applied brassica water extract (5%) on LAI of wheat in the low fertilizer dose (F1) and high fertilizer dose (F2) ±S.E.
Plant height
Analysis of variance (Table 2) showed that fertilizer dose significantly affected the plant height of wheat. Similarly, foliar application of the allelopathic brassica water extract (5%) substantially affected the plant height of wheat. However, the interaction between the allelopathic brassica water extract (5%) and fertilizer dose was non-significant for plant height (Table 2). Among the fertilizer doses, maximum plant height (99.56 cm) was recorded with the high fertilizer dose (Table 3). Maximum plant height (100.93 cm) was recorded with application of allelopathic brassica water extract (5%) followed by BAP (5 ppm), when plant height (100.75 cm) was recorded. Minimum plant height (96.62 cm) was recorded in control with no spray.
Yield parameters
Numbers of productive tillers
Foliar application of the allelopathic brassica water extract (5%) significantly affected the number of productive tillers of wheat. Interaction between the allelopathic brassica water extract (5%) and fertilizer dose was non-significant. Among the fertilizer doses, maximum number of productive tillers/m2 (317.35) was recorded with the higher fertilizer dose (Table 3). Among plant growth regulators, the allelopathic brassica water extract (5%) and BAP (5 ppm) improved the number of productive tillers of wheat. Maximum number of productive tillers (321.75) was achieved with the combined application of the allelopathic brassica water extract (5%) and BAP (5 ppm) followed by benzyl amino purine (5 ppm) alone, and about 311 productive tillers were recorded. Minimum number of productive tillers (253.8 m-2) was recorded in control with no spray.
Number of spikelets per spike
Application of the allelopathic brassica water extract (5%) significantly affected the number of spikelets per spike of wheat. However, the interaction between the allelopathic brassica water extract (5%) and the fertilizer dose was non-significant for number of spikelets per spike of wheat. Among the fertilizer doses, maximum number of spikelets per spike (14.90) was recorded with the high fertilizer dose (Table 3). Moreover, the allelopathic brassica water extract (BWE) 5% improved the number of spikelets per spike of wheat. Maximum number of spikelets per spike (15.38) was found with BAP (5 ppm) followed by application of the allelopathic brassica water extract (5%), and about 15 spikelets per spike were recorded. Minimum number of spikelets per spike (13.25) was recorded in control with no spray.
Spike length
Table 2 shows that fertilizer dose did not affect the spike length of wheat. Similarly, application of the plant growth regulator (BAP) and the allelopathic water extract (5%) was also non-significant for spike length of wheat. In the same lines, the interaction between the fertilizer dose and the plant growth regulators was non-significant for spike length. None of the fertilizer doses improved spike length of wheat. Moreover, application of the plant growth regulator BAP (5 ppm) and the allelopathic brassica water extract (5%) with both fertilizer doses did not improve spike length of wheat and were similar to control.
Grains per spike
The higher the number of grains per spike, the higher grain yield was. The data in Table 2 showed that number of grains per spike was not affected by fertilizer dose. However, the allelopathic brassica water extract (5%) substantially improved the number of grains per spike. Additionally, the interaction between the plant growth regulator and the fertilizer dose did not affect the number of grains per spike of wheat. Among the fertilizer doses, maximum number of grains per spike (48.53) was recorded with the high fertilizer dose. Application of the allelopathic brassica water extract (5%) and BAP (5 ppm) increased the number of grains per spike of wheat. Maximum number of grains per spike (54.41) was achieved in the combined application of the brassica water extract (5%) and BAP (5 ppm) followed by solo application of BAP (5 ppm) and about 50 grains per spike were recorded (Table 4). Minimum number of grains per spike (42.60) was recorded in control with no spray.
Grain weight
Analysis of variance (Table 2) showed that fertilizer dose substantially affected the 1000 grain weight of wheat. Similarly, foliar application of the allelopathic brassica water extract (5%) also significantly affected the 1000 grain weight of wheat. However, the interaction between the fertilizer dose and the plant growth regulators was non-significant for the 1000 grain weight of wheat. Among fertilizer doses, maximum 1000 grain weight (48.92 g) was recorded with the high fertilizer dose (Table 4). Among plant growth regulators, benzyl amino purine (5 ppm) improved the 1000 grain weight of wheat. Maximum 1000 grain weight (49.05 g) was achieved with application of BAP (5 ppm) followed by the allelopathic brassica water extract (5%), and 48.28 g was recorded. Minimum 1000 grain weight (45.91 g) was recorded in control with no spray.
Biological yield
Application of the allelopathic brassica water extract (5%) substantially affected the biological yield of wheat. However, the interaction between the fertilizer dose and the allelopathic water extract (5%) was non-significant for the biological yield of wheat. Among the fertilizer doses, maximum biological yield (15.26 t ha-1) was achieved with the high fertilizer dose (Table 4). Among plant growth regulators, the combination of the allelopathic brassica water extract (5%) and BAP (5 ppm) improved the biological yield of wheat. Maximum biological yield (16.11 t ha-1) was achieved with the combined application of the allelopathic brassica water extract (5%) and BAP (5 ppm) followed by solo application of BAP (5 ppm), and 15.17 t ha-1 biological yield was achieved. Minimum biological yield (12.0 t ha-1) was recorded in control with no spray.
Grain yield
The grain yield is the combined effect of all individual parameters of yield components under the particular set of ecological conditions. Analysis of variance (Table 2) showed that application of fertilizer dose significantly affected the grain yield of wheat. Similarly, foliar application of the brassica water extract (5%) significantly affected the grain yield of wheat. However, the interaction between fertilizer dose and the brassica water extract (5%) was non-significant for the grain yield of wheat. Among fertilizer doses, maximum grain yield (5.50 t ha-1) was recorded when the high fertilizer dose was applied (Table 4). Among plant growth regulators, the allelopathic brassica water extract (5%) and BAP (5 ppm) increased the grain yield of wheat. Maximum grain yield (6.20 t ha-1) was achieved in the combined application of the allelopathic brassica water extract (5%) and BAP (5 ppm) followed by BAP (5 ppm) alone, and 5.94 t ha-1 grain yield was gained. Minimum grain yield (4.33 t ha-1) was achieved in control with no spray.
In the present study, application of the allelopathic brassica water extracts (5%) improved the growth-related traits, including plant height, leaf area index, leaf elongation and dry matter accumulation under both fertilizer levels. The performance of the allelopathic water extracts in terms of growth improvement was better than water application alone (Figures 1, 2, 3 and 4). Application of allelochemicals is positively correlated with improvement in crop growth and development (Cohen and Meudt, 1983Cohen JD, Meudt WJ. Investigations on the mechanism of the brassinosteroid response: I. Indole-3-acetic acid metabolism and transport. Plant Physiol. 1983;72(3):691-4.) and lower concentrations of phenolic compounds present in Brassica spp. stimulate the growth of target plants (Al-Sherif et al., 2013Al-Sherif E, Hegazy AK, Gomaa NH, Hassan MO. Allelopathic effect of black mustard tissues and root exudates on some crops and weeds. Planta Daninha. 2013;31(1):11-9.). Application of the brassica water extracts (5%) further improved crop growth as it contains glucosinolates (Agerbirk and Olsen, 2012Agerbirk N, Olsen CE. Glucosinolate structures in evolution. Phytochemistry. 2012;77:16-45.), thiocyanates, isothiocyanates (Romanowski and Klenk, 2000Romanowski F, Klenk H. Thiocyanates and isothiocyanates, organic; Ullmann’s Encyclopedia of Industrial Chemistry [on line]. Wiley-VCH Verlag GmbH; 2000.) and brassinolide (Grove et al., 1979Grove MD, Spencer GF, Rohwedder WK, Mandava NB, Worley JF, Warthen JD et al. Brassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen. Nature. 1979. 281(5728):216-7.) which might have stimulated growth, chlorophyll accumulation, photosynthesis, transpiration, leaf expansion, translocation and genetic encodings (Rice, 1984Rice EL. Allelopathy. New York: Academic Press; 1984.; Gamalero and Glick, 2011Gamalero E, Glick BR. Mechanisms used by plant growth promotion bacteria. In: Maheshwari DK editor. Bacteria in agrobiology: plant nutrient management. Berlin: Springer-Verlag; 2011.). The growth-stimulating effect of the brassica water extracts on wheat seedling growth could have been due to several aspects which suggest that allelochemicals affect hormone synthesis and using them further triggers vital physiological aspects such as cell division, cell elongation, microscopic structure, membrane permeability and protein synthesis (Al-Sherif et al., 2013).
In the present study, improved performance of the fertilizer dose applied, in terms of crop growth rate, leaf area index, leaf elongation and plant height under the influence of the allelopathic brassica water extracts (5%) and growth regulator benzyl amino purine (5 ppm) (Figures 1, 2, 3 and 4), might be due to the interaction between the fertilizer and the allelopathic brassica water extracts, as Jabran et al. (2013Jabran K, Farooq M, Aziz T, Siddique KHM. Allelopathy and crop nutrition. In: Cheema ZA, Farooq M, Wahid A editors. Allelopathy: Current trends and future applications. Berlin: Springer Verlag; 2013. p.113-43.) showed a positive correlation between nutrients and application of allelochemicals. Furthermore, improved plant height under application of both the fertilizer and the allelopathic water extract (5%) is attributed to the allelochemicals as described by Sassa et al. (1992Sassa H, Hirano H, Ikehashi H. Self-incompatibility-related RNases in styles of Japanese pear (Pyrus serotina Rehd.). Plant Cell Physiol. 1992;33(6):811-4.) and Jeyakumar et al. (2008). Moreover, Field and Osbourn (2008Field B, Osbourn AE. Metabolic diversification-independent assembly of operon-like gene clusters in different plants. Science. 2008;320(5875):543-7.) stated that allelochemicals have the potential to modulate the physiological and metabolic functions of plants, which might have stimulated plant height (Table 3).
In the present study, an overall increase in all the yield related attributes of wheat was recorded under the influence of the allelopathic brassica water extracts (5%) and the growth regulator benzyl amino purine (5 ppm) except for spike length, which was non-significant under all the treatments (Table 3). Furthermore, the combined application of the allelopathic water extracts (5%) and benzyl amino purine (5 ppm) resulted in a higher number of productive tillers and number of spikelets per spike, which illustrates the synergistic effect of allelochemicals and benzyl amino purine ;as Mandava (1988Mandava NB. Plant growth-promoting brassinosteroids. Ann Rev Plant Physiol Plant Molec Biol. 1988;39:23-52.) suggested, auxins mediate the effect of brassinosteroids through increasing tissue sensitivity to endogenous auxins, which might have improved the number of productive tillers in this study. Similarly, improvement in number of grains per spike and 1000 grain weight under the influence of the combined application of the brassica water extract (5%) and BAP (5 ppm) might be due to the action of allelochemicals on the translocation of photosynthates at the grain filling stage (Fujii and Saka, 1992Fujii S, Saka H. Growth regulating action of brassinolide on plants: II. Effect of brassinolide on the translocation of assimilate in rice plants during the ripening stage. Jpn J Crop Sci. 1992;61:193-9. ; Sairam, 1994Sairam RK. Effects of homobrassinolide application on plant metabolism and grain yield under irrigated and moisture-stress conditions of two wheat varieties. Plant Growth Regulation. 1994;14(2):173-81. ; Shahbaz and Ashraf, 2007Shahbaz M, Ashraf M. Influence of exogenous application of brassinosteriod on growth and mineral nutrients of wheat under saline conditions. Pakistan J Bot. 2007;39:513-22.; Sharma and Bhardwaj, 2007Sharma P, Bhardwaj R. Effect of 24-epibrassinlide on seed germination, seedling growth and heavy metal uptake in Brassica juncea L. Genet Appl Plant Physiol. 2007;33:59-73.; Rehman et al., 2012Rehman A, Biswas MS, Sarder A, Khan MIK. Allelopathic effect of brassica biomass on yield of wheat. J Exp Biosci. 2012;3(2):13-8.).
Application of the allelopathic brassica water extract (5%) and BAP (5ppm) improved biological and grain yield under both fertilizer levels (Table 4). It is therefore substantiated that application of both BWE and BAP could lead to a mutual interaction which uplifted grain and biological yield either through their action on cell division, photosynthesis, respiration or source sink relationship (Rice, 1984Rice EL. Allelopathy. New York: Academic Press; 1984.; Gamalero and Glick, 2011Gamalero E, Glick BR. Mechanisms used by plant growth promotion bacteria. In: Maheshwari DK editor. Bacteria in agrobiology: plant nutrient management. Berlin: Springer-Verlag; 2011.).
An economic analysis was made to judge grain and straw yield in economic returns and returns from each treatment for final recommendation for adaptation of the methodology. The economic analysis (Table 5) revealed that there was an overall increase in net benefits in different treatments with the brassica water extract. These treatments were beneficial for maximum profit as compared to control. Among the treatments, the brassica water extract (5%) + benzyl amino purine (5 ppm) gave the maximum net benefits of (1708.7 USD ha-1) with the high fertilizer dose (Table 5). Economic and marginal net benefits of 1521.6 and 237.0 USD ha-1 were achieved by applying the brassica water extract under the high fertilizer application (Table 6). Use of the allelopathic water extracts of different crops along with commercial growth regulators can have promotive effects on target plants. It is quite clear from the above-mentioned results that the allelopathic brassica water extracts have a major impact on bread wheat growth and productivity, and they gave a net benefit of USD. 1708 ha-1. Use of allelopathic water extracts is also safe, environment friendly and easy to operate at field conditions.
In conclusion, the foliage applied allelopathic brassica water extract (5%) significantly improved the growth and yield components; however, the combined application of the brassica water extract (5%) and BAP (5 ppm) applied at 30 and 45 DAS was the most effective in improving growth and grain yield of wheat, which caused the maximum net benefits of (1708.7 USD ha-1).
REFERENCES
- Abbas T, Nadeem MA, Tanveer A, Syed S, Zohaib A, Farooq N et al. Allelopathic influence of aquatic weeds on agro-ecosystems: a review [online]. Planta Daninha. 2017a;35:e017163146.
- Abbas T, Nadeem MA, Bhagirath AT, Chauhan S. Can hormesis of plant-released phytotoxins be use d to boost and sustain crop production? Crop Prot. 2017b;93:69-76.
- Agerbirk N, Olsen CE. Glucosinolate structures in evolution. Phytochemistry. 2012;77:16-45.
- Al-Sherif E, Hegazy AK, Gomaa NH, Hassan MO. Allelopathic effect of black mustard tissues and root exudates on some crops and weeds. Planta Daninha. 2013;31(1):11-9.
- Arora N, Bhardwaj R, Sharma P, Arora HK. Effects of 28-homobrassinolide on growth, lipid peroxidation and antioxidative enzyme activities in seedlings of Zea mays L. under salinity stress. Acta Physiol Plant. 2008;30:833-9.
- Barber SA. Soil nutrient bioavailability: A mechanistic approach. New York: John Wiley and Sons; 1984.
- Bhadoria PBS. Allelopathy: a natural way towards weed management. Am J Exp Agric. 2011;1:7-20.
- Bhowmik PC, Inderjit. Challenges and opportunities in implementing allelopathy for natural weed management. Crop Prot. 2003;22:661-7.
- Cheema ZA, Khaliq A. Use of sorghum allelopathic properties to control weeds in irrigated wheat in semi-arid region of Punjab. Agric Ecosyst Environ. 2000;79:105-112.
- Cheema ZA, Khaliq A, Saeed S. Weed control in maize (Zea mays L.) through sorghum allelopathy. J Sustain Agric. 2004;23:73-86.
- Chon NM, Nishikawa-Koseki N, Takeuchi Y, Abe H. 2008. Role of ethylene in abnormal shoot growth induced by high concentration of brassinolide in rice seedlings. J Pest Sci. 2008;33(1):67-72.
- Cohen JD, Meudt WJ. Investigations on the mechanism of the brassinosteroid response: I. Indole-3-acetic acid metabolism and transport. Plant Physiol. 1983;72(3):691-4.
- Divi UK, Krishna P. Brassinosteroid: a biotechnological target for enhancing crop yield and stress tolerance. New Biotechnol. 2009;26(3-4):131-6.
- Farooq M, Habib M, Rehman AU, Wahid A, Munir R. Employing aqueous allelopathic extracts of sunflower in improving salinity tolerance in rice. J Agric Soc Sci. 2011a;7:75-80.
- Farooq M, Jabran K, Cheema ZA, Wahid A, Siddique KH. The role of allelopathy in agricultural pest management. Pest Manage Sci. 2011b;67(5):493-506.
- Fujii S, Saka H. Growth regulating action of brassinolide on plants: II. Effect of brassinolide on the translocation of assimilate in rice plants during the ripening stage. Jpn J Crop Sci. 1992;61:193-9.
- Field B, Osbourn AE. Metabolic diversification-independent assembly of operon-like gene clusters in different plants. Science. 2008;320(5875):543-7.
- Gamalero E, Glick BR. Mechanisms used by plant growth promotion bacteria. In: Maheshwari DK editor. Bacteria in agrobiology: plant nutrient management. Berlin: Springer-Verlag; 2011.
- Grove MD, Spencer GF, Rohwedder WK, Mandava NB, Worley JF, Warthen JD et al. Brassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen. Nature. 1979. 281(5728):216-7.
- Hunt R. Plant growth analysis. The InstBio Studies Bio/Edward Arnold: 1978.
- Iqbal J, Cheema ZA, An M. Intercropping of field crops in cotton for the management of purple nutsedge (Cyperus rotundus L.). Plant Soil. 2007;300:163-71.
- Iqbal J, Rauf HA, Shah AN, Shahzad B. Allelopathic effects of rose wood, guava, eucalyptus, sacred fig and jaman leaf litter on growth and yield of wheat (Triticum aestivum L.) in wheat based agroforestry system. [onlne]. Planta Daninha 2017;35:e017166992.
- Jabran K, Farooq M, Aziz T, Siddique KHM. Allelopathy and crop nutrition. In: Cheema ZA, Farooq M, Wahid A editors. Allelopathy: Current trends and future applications. Berlin: Springer Verlag; 2013. p.113-43.
- Jamil M, Cheema ZA, Mushtaq MN, Farooq M, Cheema MA. Alternative control of wild oat and canary grass in wheat fields by allelopathic plant water extracts. Agron Sustain Dev. 2009;29:475-82.
- Koocheki A, Lalegani B, Hosseini SA. Ecological consequences of Allelopathy. In: Cheema ZA, Farooq M, Wahid A editors. Allelopathy: Current trends and future applications. Berlin: Springer; 2013. p.23-38.
- Kwak MS, Kim IH, Kim SK. Effects of Brassinolide with Naphthalene acetic acid on the formation of adventitious roots, trichome-like roots and calli from cultured tobacco leaf segments, and the expression patterns of CNT103. J Plant Biol. 2009;52:511-7.
- Mackay AD, Barber SA. Soil moisture effects on root growth and phosphorus uptake by corn. Agron J. 1985;77:519-23.
- Mandava NB. Plant growth-promoting brassinosteroids. Ann Rev Plant Physiol Plant Molec Biol. 1988;39:23-52.
- Nickell LG. Plant growth regulators agricultural uses. New York: Springer Verlag; 1982.
- Phiri C. Influence of Moringa oleifera leaf extract on germination and early seedling development of major cereals. Agric Biol J Am. 2010;1:774-7.
- Ramraj VM, Vyas BN, Godrej NB, Mistry KB, Swami BN, Singh N. Effects of 28-homobrassinolide on yields of wheat, rice, groundnut, mustard, potato and cotton. J Agric Sci. 1997;128:405-13.
- Rao MR, Shetty SVR, Reddy SLN, Sharma MM.Weed managementinimproved rainfed cropping systemsinsemi-arid India In: Soil, crop, and water management systems for rainfed agriculture in the sudano-sahelian zone. Proceedings of an International Workshop, 11-16 Jan. 1987. Patancheru: ICRISAT Sahelian Center; 1989. p.303-16.
- Rehman A, Biswas MS, Sarder A, Khan MIK. Allelopathic effect of brassica biomass on yield of wheat. J Exp Biosci. 2012;3(2):13-8.
- Rehman S, Shahzad B, Bajwa AA, Hussain S, Rehman A, Cheema SA, Abbas T, Ali A, Shah L, Adkins S, Li P. Utilizing the Allelopathic Potential of Brassica Species for Sustainable Crop Production: A Review. J Plant Growth Reg. 2018;1-14.
- Rice EL. Allelopathy. New York: Academic Press; 1984.
- Romanowski F, Klenk H. Thiocyanates and isothiocyanates, organic; Ullmann’s Encyclopedia of Industrial Chemistry [on line]. Wiley-VCH Verlag GmbH; 2000.
- Sairam RK. Effects of homobrassinolide application on plant metabolism and grain yield under irrigated and moisture-stress conditions of two wheat varieties. Plant Growth Regulation. 1994;14(2):173-81.
- Sassa H, Hirano H, Ikehashi H. Self-incompatibility-related RNases in styles of Japanese pear (Pyrus serotina Rehd.). Plant Cell Physiol. 1992;33(6):811-4.
- Shah AN, Iqbal J, Fahad S, Tanveer M, Yang G, Khan EA. Allelopathic Influence of Sesame and Green Gram Intercrops on Cotton in a Replacement Series. Clean Soil Air Water. 2017;45(1):1500469.
- Shahbaz M, Ashraf M. Influence of exogenous application of brassinosteriod on growth and mineral nutrients of wheat under saline conditions. Pakistan J Bot. 2007;39:513-22.
- Shahzad B, Tanveer M, Che Z, Rehman A, Cheema SA, Sharma A, Song H, Rehman S, Zhaorong D. Role of 24-epibrassinolide (EBL) in mediating heavy metal and pesticide induced oxidative stress in plants: A review. Ecotoxicol and Environ Saf. 2018:935-944.
- Sharma A, Kumar V, Kumar R, Shahzad B, Thukral AK, Bhardwaj R. Brassinosteroid-mediated pesticide detoxification in plants: A mini-review. Cogent Food & Agri. 2018:1436212.
- Sharma P, Bhardwaj R. Effect of 24-epibrassinlide on seed germination, seedling growth and heavy metal uptake in Brassica juncea L. Genet Appl Plant Physiol. 2007;33:59-73.
- Steel RGD, Torrie JH, Dickey D. Principles and procedures of statistics: a biometrical approach. New York McGraw Hill Book; 1997.
Publication Dates
-
Publication in this collection
2018
History
-
Received
10 Apr 2017 -
Accepted
13 July 2017






 T1= Control, T2= water Spray, T3= Brassica Water extract (5%), T4= BAP (5 ppm), T5= Brassica water extract (5%) + BAP (5ppm).
T1= Control, T2= water Spray, T3= Brassica Water extract (5%), T4= BAP (5 ppm), T5= Brassica water extract (5%) + BAP (5ppm).
 T1= Control, T2= water Spray, T3= Brassica Water extract (5%), T4= BAP (5ppm), T5= Brassica water extract (5%) + BAP (5ppm).
T1= Control, T2= water Spray, T3= Brassica Water extract (5%), T4= BAP (5ppm), T5= Brassica water extract (5%) + BAP (5ppm).
 T1= Control, T2= water Spray, T3= Brassica Water extract (5%), T4= BAP (5 ppm), T5= Brassica water extract (5%) + BAP (5ppm).
T1= Control, T2= water Spray, T3= Brassica Water extract (5%), T4= BAP (5 ppm), T5= Brassica water extract (5%) + BAP (5ppm).
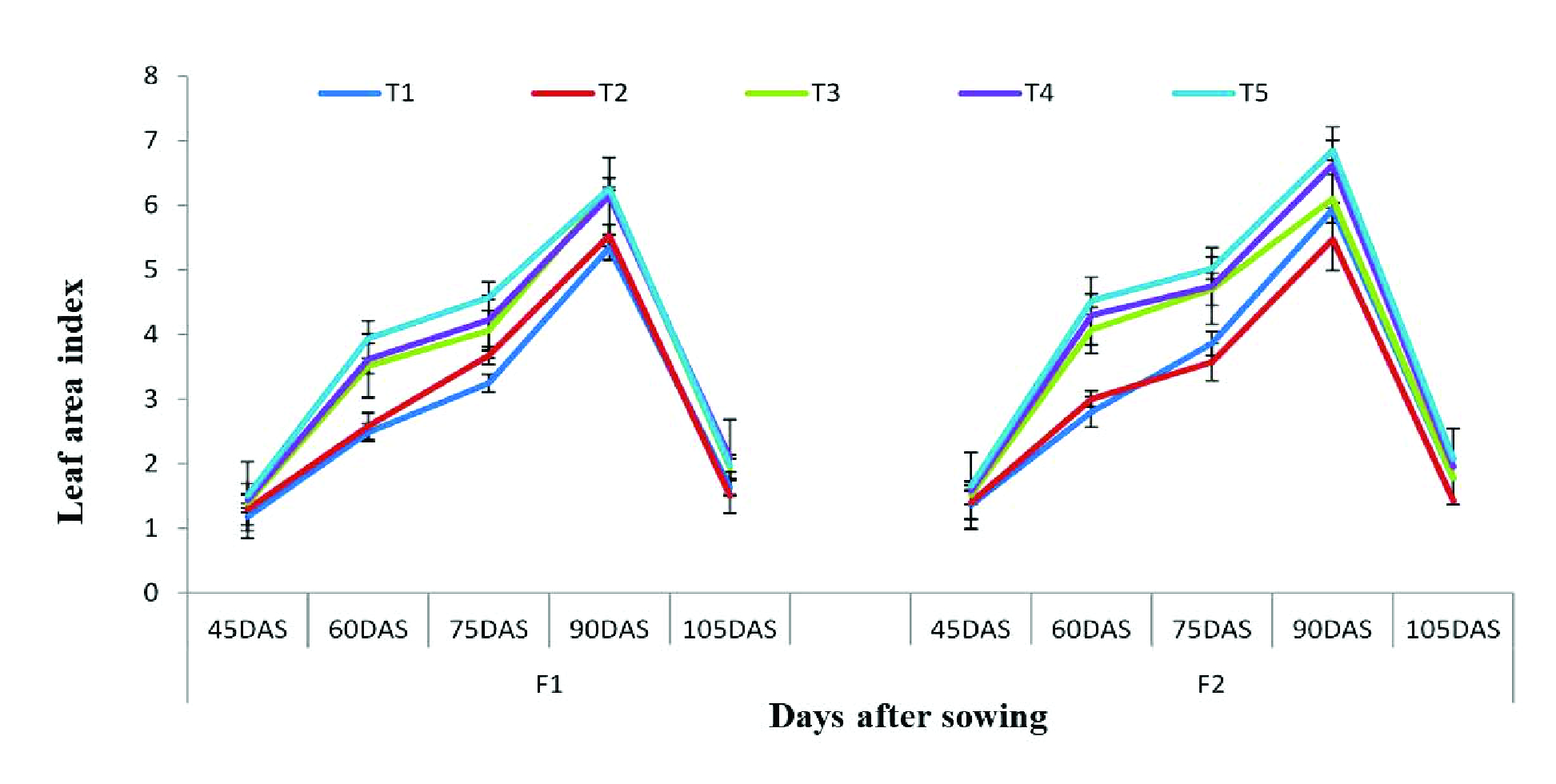 T1= Control, T2= water Spray, T3= Brassica Water extract (5%), T4= BAP (5 ppm), T5= Brassica water extract (5%) + BAP (5ppm).
T1= Control, T2= water Spray, T3= Brassica Water extract (5%), T4= BAP (5 ppm), T5= Brassica water extract (5%) + BAP (5ppm).