ABSTRACT:
Non-target site (NTS) herbicide resistance by degradation enhancement is an increasing problem in several weeds around the world. In this study, the occurrence of degradation enhancement by cytochrome P450 monooxygenases (cytP450) was evaluated as the mechanism of resistance to imazethapyr in barnyardgrass. The cytP450 inhibitors malathion and piperonyl butoxide (PBO) and the inducer naphthalic anhydride (NA), applied in mixture or sequentially with imazethapyr, were evaluated on imidazolinone-susceptible and -resistant barnyardgrass byotipes. In addition, the degradation of imazethapyr was analyzed in plants treated with imazethapyr applied alone or two hours after malathion or NA. The spraying of malathion and PBO reduced the resistance factor (RF) from 15.92 to 3.44 and 4.99, respectively, in the resistant population PALMS01. Conversely, the cytP450 inducer NA increased the RF from 4.45 to 8.32. Malathion increased imazethapyr concentrations in resistant barnyardgrass in comparison with plants sprayed with the herbicide alone, indicating the inhibition of imazethapyr degradation. The simultaneous spraying of malathion and imazethapyr was less efficient than the previous application of this cytP450 inhibitor. These results indicate that degradation enhancement caused by cytP450 enzymes is involved in the resistance mechanism of barnyardgrass to imazethapyr, and appropriate measures should be taken to manage these populations.
Keywords:
Echinochloa crus-galli; herbicide detoxification; weed resistance; imidazolinones; cytP450
RESUMO:
A resistência não relacionada ao local de ação (NRLA) pelo incremento da degradação de herbicidas é um problema crescente em várias espécies ao redor do mundo. Neste estudo foi avaliada a ocorrência de incremento da degradação por enzimas citocromo P450 monoxigenases (cytP450) como mecanismo de resistência ao imazethapyr em capim-arroz. Os inibidores de cytP450 malathion e butóxido de piperonila (PBO) e o indutor anidrido naftálico (AN), aplicados em mistura ou sequencialmente ao imazethapyr, foram avaliados sobre biótipos de capim-arroz suscetíveis e resistentes às imidazolinonasàs imidazolinonas . Além disso, a degradação de imazethapyr foi analisada em plantas tratadas com esse herbicida, aplicado isoladamente ou duas horas após malathion ou AN. A pulverização de malathion e PBO reduziu o fator de resistência (FR) de 15,92 para 3,44 e 4,99, respectivamente, na população resistente PALMS01. Inversamente, o indutor de cytP450 AN aumentou o FR de 4,45 para 8,32. A pulverização prévia de malathion aumentou a concentração de imazethapyr em plantas de capim-arroz resistentes, em comparação com plantas que receberam apenas o herbicida, indicando a inibição da degradação de imazethapyr. A pulverização simultânea de malathion e imazethapyr foi menos eficiente que a aplicação prévia deste inibidor de cytP450. Esses resultados indicam que o incremento de metabolização causado por enzimas cytP450 está envolvido no mecanismo de resistência de capim-arroz ao imazethapyr, e medidas apropriadas devem ser tomadas para manejar essas populações.
Palavras-chave:
Echinochloa crus-galli; detoxificação de herbicidas; resistência de plantas daninhas; imidazolinonas; cytP450
INTRODUCTION
Barnyardgrass (Echinochloa crus-galli L. Beauv.) is a major weed of irrigated rice fields around the world (Chauhan and Johnson, 2011Chauhan BS, Johnson DE. Ecological studies on Echinochloa crus-galli and the implications for weed management in direct-seeded rice. Crop Prot. 2011;30:1385-91.). This weed can cause 21-79% yield rice losses, depending on its density, on the cultivar and the irrigation management (Bajwa et al., 2015Bajwa AA, Jabran K, Shahid M, Ali HH, Chauhan BS, Ehsanullah. Eco-biology and management of Echinochloa crus-galli. Crop Prot. 2015;75:151-62.), demanding the use of control methods. The chemical method is the most used to control this weed, and acetolactate synthase (ALS) enzyme inhibiting herbicides are widely used, including imidazolinones.
ALS inhibiting herbicides prevent the condensation reactions responsible for the synthesis of aliphatic-branched amino acids valine, leucine and isoleucine (Powles and Yu, 2010Powles SB, Yu Q. Evolution in action: plants resistant to herbicides. Ann Rev Plant Biol. 2010;61:317-47.). The favorable characteristics of these herbicides are: the use of small doses, broad spectrum control, large application window, high residual soil activity, large selectivity to several crops and low mammalian toxicity (Tranel and Wrigth, 2002Tranel PJ, Wright TR. Resistance of weeds to ALS-inhibiting herbicides: what have we learned? Weed Sci. 2002;50:700-12.). However, these compounds are prone to selecting herbicide resistant populations due to high natural mutation in the ALS gene (10-6 to 10-4) and the ability to be detoxified by plants (Preston and Powles, 2002Preston C, Powles SB. Evolution of herbicide resistance in weeds: initial frequency of target site-based resistance to acetolactate synthase-inhibiting herbicides in Lolium rigidum. Heredity. 2002;88:8-13. ; Carvalho et al., 2009Carvalho SJP, Nicolai1 M, Ferreira RR, Figueira AVO, Christoffoleti PJ. Herbicide selectivity by differential metabolism: considerations for reducing crop damages. Sci Agric. 2009;66:136-42.). The large use of ALS-inhibiting herbicides on practically all crops and weeds had selected weed populations of several species with resistance to these compounds in all production areas worldwide (Heap, 2018Heap I. The international survey of herbicide resistant weeds. 2018. [accessed: Jun. 27, 2018]. Available: Available: http:// www.weedscience.org/
http:// www.weedscience.org/...
). Herbicide resistance in E. crus-galli is currently one of the main problems of rice and other crops and the regulation of this problem in not fully understood.
The resistance to ALS inhibitors may be due to target-site resistance (TS), involving mutations in the ALS gene, making the ALS enzyme insensitive to herbicides. However, non-target site (NTS) mechanisms have been investigated as the cause of resistance in a great number of weeds species (Yu and Powles, 2014Yu Q, Powles S. Metabolism-based herbicides resistance and cross-resistance in crop weeds: a threat to herbicide sustainability and global crop protection. Plant Physiol. 2014;166:1106-18.). The increase in herbicide detoxification mediated by cytochrome P450 monooxygenases enzymes (cytP450) is one of the main mechanisms associated with NTS herbicide resistance (Powles and Yu, 2010). The cytP450 are key enzymes in phase I of the xenobiotics metabolism and have a central role in the herbicide detoxification in plants (Yun et al., 2005Yun MS, Yogo Y, Miura R, Yamasue Y, Fischer AJ. Cytochrome P-450 monooxygenase activity in herbicide-resistant and -susceptible late watergrass (Echinochloa phyllopogon). Pestic Biochem Physiol. 2005;83:107-14.). These enzymes are responsible for adding an oxygen atom to the herbicide molecule, reducing toxicity before it reaches the herbicide site of action (Werck-Reichhart et al., 2000Werck-Reichhart D. Cytochromes P450 for engineering herbicide tolerance. Trends Plant Sci. 2000;5:116-23.). The herbicide detoxification provided by cytP450 is also responsible for the selectivity on several crops to ALS-inhibiting herbicides and several other compounds (Liu et al., 2012Liu C, Liu S, Wang F, Wang Y, Liu K. Expression of a rice CYP81A6 gene confers tolerance to bentazon and sulfonylurea herbicides in both Arabidopsis and tobacco. Plant Cell Tissue Organ Cult. 2012;109:419-28.). Currently, the detoxification of herbicides is one of the most important processes of resistance due to the occurrence of cross and multiple resistances. In some species, this mechanism may be the resistance cause of various herbicides, including those that have not been marketed yet (Délye, 2013Délye C. Unravelling the genetic bases of non-target-site-based resistance (NTSR) to herbicides: a major challenge for weed science in the forthcoming decade. Pest Manage Sci. 2013;69:176-87.).
Some barnyardgrass populations were characterized in southern Brazil as to the mechanism of resistance to the herbicides imidazolinonas (Matzenbacher et al., 2015Matzenbacher FO, Bortoly ED, Kalsing A, Merotto Jr A. Distribution and analysis of the mechanisms of resistance of barnyardgrass (Echinochloa crus-galli) to imidazolinone and quinclorac herbicides. J Agric Sci. 2015;153:1044-58.). Resistant populations have already been found in practically all producing regions, including resistant populations with a mutation in theALSgene. Such mutations include substitution of tryptophan for leucine at position 574 (Trp574Leu) and substitution of serine for asparagine at position 653 (Ser653Asn). However, in some barnyardgrass populations resistant to imidazolinones, mutations were not observed in the ALS gene, indicating the occurrence of other resistance mechanisms, possibly NTSR.
The identification of the occurrence of the degradation enhancement of herbicides by cytP450 enzymes can be obtained with enzyme inhibitors (Yasuor et al., 2009Yasuor H, Osuna MD, Ortiz A, Saldaín NE, Eckert JW, Fischer AJ. Mechanism of resistance to penoxsulam in late watergrass [Echinochloa phyllopogon (Stapf) Koss.]. J Agric Food Chem. 2009;57:3653-60.). Piperonyl butoxide (PBO) and the organophosphate insecticide malathion have been used in several studies, such as in the detection of the resistance mechanism of Lolium rigidum to chlorsulfuron (Yu et al., 2009Yu Q, Abdallah I, Han H, Owen M, Powles S. Distinct non-target site mechanisms endow resistance to glyphosate, ACCase and ALS-inhibiting herbicides in multiple herbicide-resistant Lolium rigidum. Planta. 2009;230:713-23.) and Echinochloa phyllopogon to penoxsulam (Yasuor et al., 2009), as well as other examples (Siminszky, 2006Siminszky B. Plant cytochrome P450-mediated herbicide metabolism. Phytochem Rev. 2006;5:445-58.). Alternatively, some compounds act as inducers of cytP450 enzymes, increasing plant tolerance to herbicides. These compounds are known as safeners and among them there is the naphthalic anhydride (NA), a compound that induces the expression of cytP450 genes in Poaceae (Hatzios and Burgos, 2004Hatzios KK, Burgos N. Metabolism-based herbicide resistance: regulation by safeners. Weed Sci. 2004;52:454-67.). The objective of this study was to evaluate the occurrence of degradation enhancement as the resistance mechanism in barnyardgrass (E. crus-galli) resistant to imazethaphyr.
MATERIALS AND METHODS
Seed dormancy overcoming was carried out by soaking seeds in a KNO3 solution (0.2%) at 25 oC, until the beginning of germination (radicle protrusion), which occurred approximately four days after the treatment. After that, seeds were rinsed in distilled water and incubated in Petri dishes at 25 oC, until the beginning of the first leaf emission. The substrate used in both experiments was constituted by a mixture of ultisol and organic compound at a ratio of 10:1 and the mineral fertilizer (05-20-20 NPK complex) at 2.5 g kg-1. Plants were maintained in a greenhouse with temperatures ranging from 25 to 27 oC, relative humidity of about 70% and photoperiod of 14/10 hours (day/night).
Experiment 1 - Effect of the previous application of cytP450 inhibitors on barnyardgrass control with imazethapyr
Plants were grown singly in 200 mL pots, drilled and kept submerged in water until the soil level, with four replications. Treatments consisted of four populations of barnyardgrass (factor A), being two susceptible ones (SUSSP01 and MOSTS01) and two resistant ones (ARRGR01 and PALMS01) to imazethapyr. Barnyardgrass resistant populations were collected from paddy fields in South Brazil, and were selected based on the previous use of Clearfield®-rice cultivars and where escapes of control with imidazolinone herbicides had occurred. The susceptible population SUSSP01 was originally from an area where no herbicides had been applied and the MOSTS01 susceptible population was originally from a rice cultivated area with imidazolinone application history. In both susceptible populations, an efficient control had been obtained during previous studies using imazethapyr. The PALMS01 population is resistant to imidazolinones due to a target site alteration associated with the mutation Ser653Asn and also due to enhanced metabolism; in the ARRGR01 resistant population, there is no ALS gene mutation (Matzenbacher et al., 2015Matzenbacher FO, Bortoly ED, Kalsing A, Merotto Jr A. Distribution and analysis of the mechanisms of resistance of barnyardgrass (Echinochloa crus-galli) to imidazolinone and quinclorac herbicides. J Agric Sci. 2015;153:1044-58.). Factor B was represented by the inhibitors of cytP450 enzymes piperonyl butoxide (PBO) (Sigma-Aldrich) and malathion (Malathion 500 CE, 500 g a.i. L-1, Dipil), both sprayed at 1,000 g ha-1, two hours prior to herbicide application. Factor C was the herbicide imazethapyr, (Imazetapir Plus Nortox, 106 g a.i. L-1, Nortox S.A.) applied on susceptible populations at doses zero; 6.625; 13.25; 26.5; 53; 106 and 212 g ha-1, and zero; 26.5; 53; 106; 212; 424 and 848 g ha-1 on resistant populations. A preliminary study was conducted in order to establish the dose range for the susceptible and resistant populations. PBO and malathion doses were determined based on Matzenbacher et al. (2015). In all treatments, the adjuvant Dash HC (5% of oleic acid, 22.5% of polyoxyalkylene fatty alcohol phosphate esters, 37.5% of fatty acid methyl esters, Basf S.A.) was added at a rate of 0.5% v/v. Treatments were sprayed on plants at the three to four leaf stage, using an automated sprayer with TJ8002E nozzles, spray pressure of 42 lbs inch-2 and speed of 1.16 m s-1, resulting in a spray volume of 200 L ha-1.
The evaluation consisted of the plant injury at 7, 14 and 21 days after the treatment (DAT), using a visual percentage scale, where zero means no symptoms and 100% means plant death. The shoot dry matter (SDM) was evaluated by collecting plants at 28 DAT. Data were submitted to analysis of variance (ANOVA) and adjusted to the three parameter logistic model .
The determination of the C50 (dose value responsible for causing 50% of injury) was based on replacing the y value by 50 (50% of injury) (Ritz et al., 2015Ritz C, Kniss AR, Streibig JC. Research methods in weed science: Statistics. Weed Sci. 2015;63(sp1):166-187.). The effect of barnyardgrass populations and cytP450 enzyme inhibitors were compared by Tukey’s test (p<0.05). Data about the percentage of injury were transformed into √(x+0.5).
Experiment 2 - Effect of the previous application of naphthalic anhydride on barnyardgrass control with imazethapyr
The treatments related to barnyardgrass populations and imazetaphyr doses, plant growth conditions, spraying treatments, evaluations, and statistical analysis were performed as described above. Factor B consisted in the presence or absence of the inducer of cytP450 enzymes naphthalic anhydride (NA) (Sigma-Aldrich), applied at a dose of 1,000 g ha-1, two hours prior to herbicide application. The used dose was established based on Birk and Malefyt (1991Birk JH, Malefyt T, inventors; American Cyanamid Company, assignee. Method for safening gramineous crops against pyridine imidazolinone herbicides. US patent 4,922,092. 1991 Feb.).
Experiment 3 - Quantification of imazethapyr in barnyardgrass in response to the previous spraying of malathion and naphthalic anhydride
Seedlings were obtained as described above, and transplanted into plastic boxes containing a layer of substrate. In each box, 40 seedlings of the same population were transplanted, composing an experimental unit. Each treatment had two replicates. Two populations of barnyardgrass were used, one susceptible (SUSSP01) and another resistant one (PALMS01) to imazethaphyr. The PALMS01 population was selected based on the results of the first experiment, where the resistance to imazethapyr was partially reversed by the previous spraying of the cytP450 inhibitors, malathion and PBO.
Treatments are described in Table 1 and were applied when plants reached the three-leaf stage. The procedures of spraying treatments were similar to those described above. The shoot was collected at 7 DAT and immediately frozen at zero oC until sample preparation and quantification. Imazethapyr quantification was carried out through the High Performance Liquid Chromatography (HPLC) method in the NSF Bioensaios laboratory (Viamão, Rio Grande do Sul/Brazil). The averages were compared by Scott-Knott’s test (p<0.05).
Experiment 4 - Simultaneous mixture of imazethapyr with different doses of malathion on the control of barnyardgrass resistant to imidazolinones
The experiment was conducted in pots with a volume of 200 mL, with four replications. The population used was PALMS01, resistant to imazethapyr. Treatments consisted of the combination of the recommended dose of imazethapyr (106 g ha-1) with doses of malathion (0.67; 1.00; 1.50; 2.25; 3.37; 5.06 and 7.59 kg ha-1) to form dose-response curves. All treatments with imazethapyr had the addition of an adjuvant (Dash 0.5% v/v). The spraying of cytP450 inhibiting malathion was performed together with the herbicide, on plants at the three-leaf stage. The procedures of the treatments were similar to those described in experiment 1.
The injury was evaluated at 7 and 14 DAT, using a visual scale control, where zero means no symptoms and 100% means plant death. Shoot dry matter (SDM) was measured at 21 DAT. Data were submitted to analysis of variance (ANOVA) (p<0.05) and adjusted by the quadratic model .
RESULTS AND DISCUSSION
Experiment 1- Effect of the previous application of cytP450 inhibitors on barnyardgrass control with imazethapyr
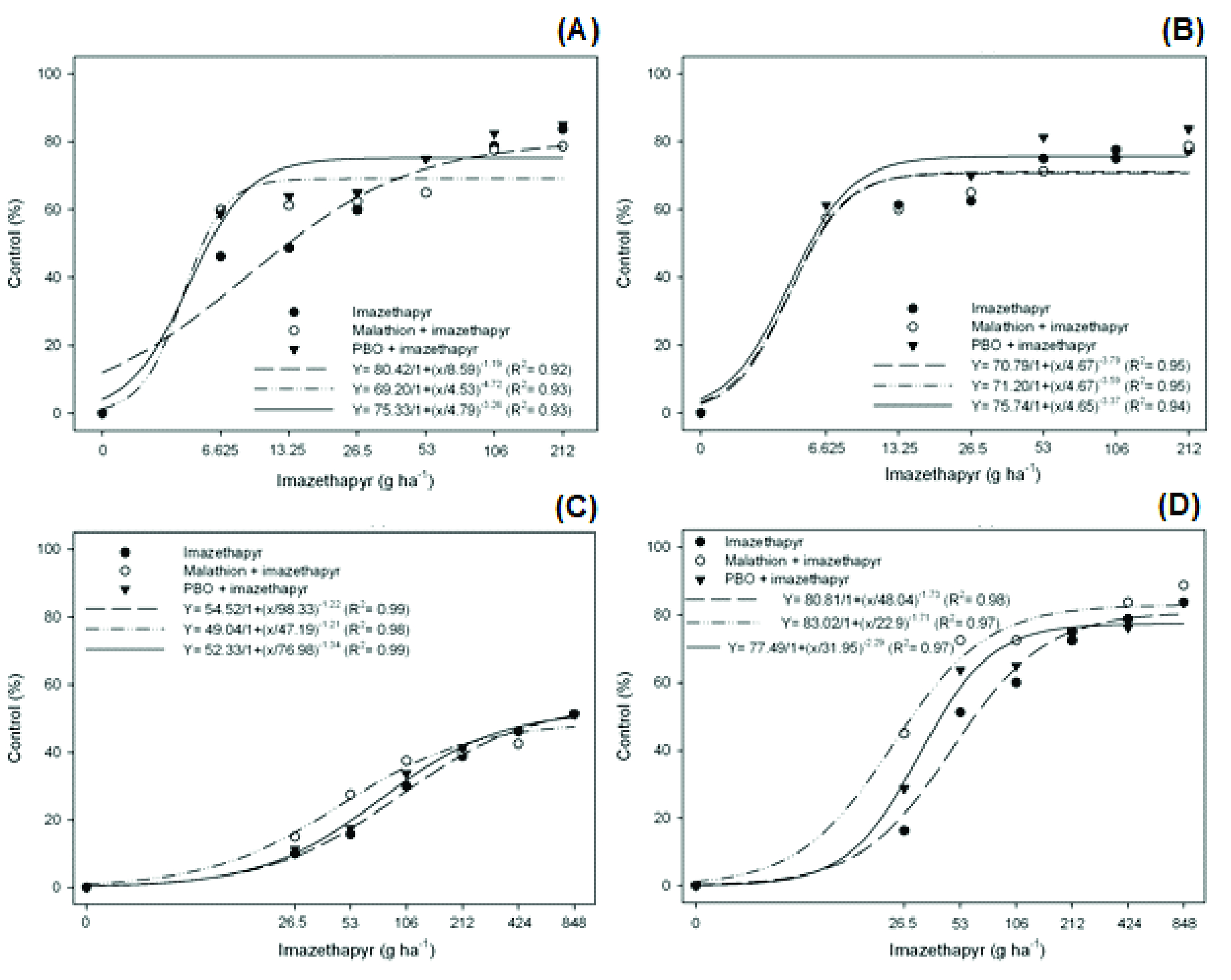
The ANOVA of plant injury at 7 DAT indicated a triple interaction between the factors, indicating that the effect of the previous application of cytP450 enzyme inhibitors varies according to the population of barnyardgrass and the dose of imazethapyr (Figure 1). The inhibition of the cytP450 enzymes was more significant in the PALMS01 resistant population (Figure 1D), mainly for malathion inhibitor and at lower doses of imazethapyr. In this population, the plant injury, at a dose of 26.5 g ha-1 of imazethapyr, increased by approximately 28% and 12.5% in response to a previous spraying of malathion and PBO, respectively, compared to plants treated with the herbicide alone, which had only 16% control. The previous spraying of cytP450 inhibitors had no effect on susceptible populations (Figure 1A, B). The resistance factor (RF) in the PALMS01 population decreased from 4.87 for treatments without the cytP450 inhibitors to 2.24 and 3.18 with the previous spraying of malathion and PBO, respectively (Table 2).
Control (%) of barnyardgrass treated with imazethapyr in response to the previous spraying of cytP450 enzyme inhibitors (malathion and PBO), 7 days after the application of treatments, in susceptible populations [SUSSP01 (A) and MOSTS01 (B)] and resistant populations [ARRGR01 (C) and PALMS01 (D)] to imazethapyr.
The injury at 14 DAT for the PALMS01 population was approximately 41% in plants treated with malathion at a dose of 26.5 g ha-1 of the herbicide imazethapyr (Figure 2). The effect of imazethapyr and cytP450 inhibitors can be observed in Figure 3. With the presence of inhibitors, plant growth reduced considerably, being more expressive for malathion (Figure 3B). The RF was 12.99 for plants treated only with imazethapyr, and 4.81 and 10.88 when plants were treated with malathion and PBO, respectively. In the ARRGR01 resistant population, similar effects occurred at small proportions. The effect of cytP450 inhibitors at 21 DAT were similar to those described above (data not shown).
The results of the shoot dry matter (SDM) (Figure 4) were similar to those observed for plant injury. The PALMS01 population showed greater reductions in SDM accumulation in response to the previous spraying of enzyme inhibitors, mainly malathion, and for the smallest doses of imazethapyr (26.5, 53 and 106 g ha-1) (Figure 4D). The RFs for the SDM were significantly reduced by the previous spraying of inhibitors (Table 2). The RF for the PALMS01 population was 15.92, whereas for plants previously treated with malathion and PBO, it was 3.44 and 4.94, respectively. For other populations, there was no significant reduction in SDM accumulation and FR with the previous spraying of cytP450 inhibitors.
Control (%) of barnyardgrass treated with imazethapyr in response to the previous spraying of cytP450 enzyme inhibitors (malathion and PBO), 14 days after the application of treatments, in susceptible populations [SUSSP01 (A) and MOSTS01 (B)] and resistant populations [ARRGR01 (C) and PALMS01 (D)] to imazethapyr.
Effect of imazethapyr (A) and inhibitors of cytP450 enzymes, malathion (B) and PBO (C), on barnyardgrass resistant (PALSM01) to imazethapyr, 14 days after the treatment.
Shoot dry matter (SDM) of barnyardgrass treated with imazethapyr in response to the previous spraying of cytP450 enzyme inhibitors (malathion and PBO), 28 days after the treatments, on susceptible [SUSSP01 (A) and MOSTS01 (B)] and resistant populations [ARRGR01 (C) and PALMS01 (D)] to imazethapyr.
Experiment 2 - Effect of the previous application of naphthalic anhydride on barnyardgrass control with imazethapyr
The ANOVA of visual control at 7 DAT indicated the occurrence of significant interaction between population, dose of imazethapyr and naphthalic anhydride (NA). The greatest differences were observed in the susceptible population SUSSP01 (Figure 5A) and in the resistant population PALMS01 (Figure 5D). In this population, the effect of the previous spraying of NA on the plant injury occurred at all herbicide doses, reaching 23% compared to plants treated with imazetaphyr sprayed at 424 g ha-1. NA increased the RF from 17.5 to a value above 150.35 in the PALMS01 population and from 102 to a value above 150.35 in the ARRGR01 population (Table 3).
Control (%) of barnyardgrass treated with imazethapyr in response to the previous spraying of cytP450 enzyme inducer naphthalic anhydride, 7 days after the treatment, on susceptible [SUSSP01 (A) and MOSTS01 (B)] and resistant populations [ARRGR01 (C) and PALMS01 (D)] to imazethapyr.
The effect of NA on plant injuries evaluated at 14 and 21 DAT among populations and dose of imazethapyr was similar to that at 7 DAT, as described above (data not shown). The FR at 14 and 21 DAT for the resistant population PALMS01 increased from 8.32 to 28.28 and 10.95 to 18.89, respectively, as a consequence of the NA application (Table 3). For other populations, although there were variations in visual injuries, there were no effects on the FR values.
The interaction between the factors population, dose of imazethapyr and NA was significant for the variable SDM. For the population PALMS01, the previous spraying of NA and the doses of imazethapyr 26.5, 53 and 106 g ha-1, increased SDM by approximately 33%, 50% and 63%, respectively (Figure 6D). For this population, in plants that received only imazethapyr, the FR was 4.45, while NA-treated plants had an RF of 8.32 (Table 3).
Shoot dry matter (SDM) of barnyardgrass treated with imazethapyr in response to the previous spraying of the cytP450 enzyme inducer naphthalic anhydride, 28 days after the treatments, on susceptible [SUSSP01 (A) and MOSTS01 (B)] and resistant populations [ARRGR01 (C) and PALMS01 (D)] to imazethapyr.
Experiment 3 - Quantification of imazethapyr in barnyardgrass in response to the previous spraying of malathion and naphthalic anhydride
The results obtained in this experiment corroborate the effect of malathion in the detoxification of imazethapyr on resistant plants (PALMS01), observed in experiment 1. Concentrations of imazethapyr were higher in susceptible plants (SUSSP01), since they have less ability to detoxify the herbicide. Resistant plants treated only with imazethapyr had a lower concentration of the herbicide compared to susceptible plants treated with imazethapyr and to resistant ones that received malathion followed by imazethapyr. Resistant plants previously treated with malathion had practically the same concentration of imazethapyr that was found in susceptible plants, indicating that malathion inhibited the activity of cytP450 enzymes (Figure 7). These results demonstrate that resistant plants, when pre-treated with malathion, behave as susceptible ones, since the concentration of imazethapyr at 7 DAT was equal. The previous application of NA had no effect as an inducer of cytP450 enzymes.
Effect of previous applications of malathion and naphthalic anhydride on the concentration of imazethapyr (mg kg-1 of shoot) on barnyardgrass.
Experiment 4 - Simultaneous mixture of imazethapyr with different doses of malathion on the control of barnyardgrass resistant to imidazolinones
The previous results (experiments 1 and 3) indicate that cytP450s inhibitors decrease the resistance of barnyardgrass to imazethapyr. However, the requirement of the previous application in relation to the herbicide spraying results in a high control cost and operation time. The simultaneous mixture of malathion, even at high a dose, was partially efficient as an inhibitor of cytP450s in order to increase the effect of imazethapyr on herbicide-resistant barnyardgrass. Even at very high doses of malathion (7.59 kg ha-1), the control increase was only of 8.5% and 10% at 7 and 14 DAT, respectively (Figure 8). This high dose reduced the SDM at 14 DAT by 30%. Although there was an increase in the injury with the addition of malathion, it was not close to the 80 to 90% of control required for most of the herbicide use.
Control (%) at 7 and 14 days after the treatments, and relative shoot dry matter (SDM) of barnyardgrass treated with imazethapyr (106 g ha-1) in combination with different doses of malathion.
The effect of cytP450 inhibitors, malathion and PBO, on the reduction of the RFs for plant injury and SDM indicate that the resistance to imazethapyr in the PALMS01 population is associated with degradation enhancement, combined with the ALS gene mutation Ser653Asn, as previously highlighted (Matzenbacher et al., 2015Matzenbacher FO, Bortoly ED, Kalsing A, Merotto Jr A. Distribution and analysis of the mechanisms of resistance of barnyardgrass (Echinochloa crus-galli) to imidazolinone and quinclorac herbicides. J Agric Sci. 2015;153:1044-58.). The most pronounced effect was observed when the herbicide was applied at smaller or equal to the recommended dose (106 g ha-1) combined with the previous spraying of malathion and plants belonging to PALMS01 population. In the ARRGR01 resistant population, although there was no mutation in the ALS gene so far, the effect of the previous spraying of inhibitors was not significant. This can be explained by the lack of affinity of the inhibitors used with cytP450 enzymes that are active in this population, or the occurrence of other resistance mechanisms.
The effect of malathion and PBO as in indicator of the occurrence of herbicide resistance associated with detoxication enhancement is reported for a number of species and herbicides. In L. rigidum, the use of malathion reversed the resistance to the herbicide chlorsulfuron (Yu et al., 2009Yu Q, Abdallah I, Han H, Owen M, Powles S. Distinct non-target site mechanisms endow resistance to glyphosate, ACCase and ALS-inhibiting herbicides in multiple herbicide-resistant Lolium rigidum. Planta. 2009;230:713-23.). In E. phyllopogon, both malathion and PBO increased the phytotoxicity of bispyribac in resistant plants (Fischer et al., 2000Fischer AJ, Bayer DE, Carriere MD, Yim Kyu-Ock. Mechanisms of resistance to bispyribac-sodium in an Echinochloa phyllopogon accession. Pestic Biochem Physiol. 2000;68:156-65.). Likewise, in E. phyllopogon, the use of malathion increased the phytotoxicity of penoxsulam in resistant plants, due to a lower detoxification of the herbicide (Yasuor et al., 2009Yasuor H, Osuna MD, Ortiz A, Saldaín NE, Eckert JW, Fischer AJ. Mechanism of resistance to penoxsulam in late watergrass [Echinochloa phyllopogon (Stapf) Koss.]. J Agric Food Chem. 2009;57:3653-60.). The same cytP450 inhibitor was used to detect the resistance mechanism of Alopercurus myosuroides. With the combination of malathion, there was a reduction in the resistance factor to the herbicide flupyrsulfuron (Letouze and Gasquez, 2003Letouze A, Gasquez J. Enhanced activity of several herbicide-degrading enzymes: a suggested mechanism responsible for multiple resistance in blackgrass (Alopecurus myosuroides Huds.). Agronomie. 2003;23:601-8. ).
CytP450s enzymes act by catalyzing oxygen- and NADPH- dependent monooxygenation reactions (Yuan et al., 2007Yuan JS, Tranel PJ, Stewart CN Jr. Non-target-site herbicide resistance: a family business. Trends Plant Sci. 2007;12:6-13.), making herbicide molecules less toxic for weeds. Inhibition by malathion occurs when the sulfur atom released by oxygenated organophosphate inhibits the activity of the cytP450 enzyme, increasing the sensitivity of plants to herbicides (Werk-Reichhart et al., 2000). The inhibition by PBO results from the formation of a complex between the heme group of the cytP450 enzyme and a carbene molecule (divalent carbon) derived from the hydroxylation of PBO molecules, inhibiting the activity of the herbicide (Hodgson and Levi, 1998Hodgson E, Levi PE. Interactions of Piperonyl Butoxide with cytochrome P450. In Jones DG, editor. Piperonyl Butoxide: the insecticide synergist. San Diego: Academic Press; 1998. p.41-54.).
The injury reduction caused by imazethapyr in barnyardgrass plants previously treated with NA demonstrates the effect of this compound on the herbicide detoxification enhancement for cytP450 enzymes. Similar results have been obtained in others species. The treatment of maize seeds with NA induced the expression of CYP92A1 and CYP72A5 genes, increasing herbicide tolerance (Persans et al., 2001Persans MW, Wang J, Schuler MA. Characterization of maize cytochrome P450 monooxygenases induced in response to safeners and bacterial pathogens. Plant Physiol. 2001;125:1126-38.). In another study on maize plants treated with NA, the phytotoxicity caused by imazethapyr was lower, and the cytP450 enzyme content in plants that received NA was greater than in untreated plants (Barrett and Maxon, 1991Barrett M, Maxon JM. Naphthalic anhydride induces imazethapyr metabolism and cytochrome P-450 activity in maize. Z Naturforschung. 1991;46:897-900.). This compound is capable of inducing the expression of cytP450 genes and results in greater enzyme content and higher capacity of detoxification (Persans et al., 2001).
The previous application of malathion provided higher control rates compared to the application in a mixture with imazethapyr. In the PALMS01 resistant population, at a dose of 106 g ha-1 of herbicide and 1.00 kg ha-1 of malathion, the previous application caused a reduction of 55% in the SDM (see experiment 1). With a simultaneous application, the reduction was only 15% (see experiment 4). Some studies corroborate these results, indicating that the previous spraying of cytP450 enzymes inhibitors is more efficient (Yasuor et al., 2009Yasuor H, Osuna MD, Ortiz A, Saldaín NE, Eckert JW, Fischer AJ. Mechanism of resistance to penoxsulam in late watergrass [Echinochloa phyllopogon (Stapf) Koss.]. J Agric Food Chem. 2009;57:3653-60.; Yu et al., 2009Yu Q, Abdallah I, Han H, Owen M, Powles S. Distinct non-target site mechanisms endow resistance to glyphosate, ACCase and ALS-inhibiting herbicides in multiple herbicide-resistant Lolium rigidum. Planta. 2009;230:713-23.). The simultaneous spraying of malathion (1.00 kg ha-1) with imazethapyr in E. crus-galli caused a reduction of only 5% to 10% of SDM in comparison with plants treated with just the herbicide (Riar et al., 2012Riar DS, Norsworthy JK, Bond JA, Bararpour MT, Wilson MJ, Scott RC. Resistance of Echinochloa crus-galli populations to acetolactate synthase-inhibiting herbicides. Int J Agron. 2012;2012:1-8.). The lower effect of malathion sprayed in mixture with imazethapyr is possibly due to the physicochemical characteristics of these products, and with the epicuticular composition of barnyardgrass sheets. The epicuticular coating of barnyardgrass leaves is predominantly composed of polar components (72%) (Tulloch and Bergter, 1980Tulloch AP, Bergter L. Epicuticular wax composition of Echinochloa crusgalli. Phytochemistry. 1980;19:145-6.). Due to the lower polarity of the malathion (log Kow: 2.75), the absorption is slower compared to imazethapyr (log Kow: 1.04) (Senseman, 2007Senseman SA. Herbicide handbook. 9th. ed. Lawrence: Weed Science Society of America; 2007.). Therefore, malathion should be sprayed before the herbicide absorption, in order to have the inhibition of cytP450 associated with herbicide resistance. A simultaneous mixture of propanil with the organophosphate piperophos (400 g L-1 + 40 g L 1) is efficient for the control of barnyardgrass resistant to propanil (Valverde et al., 2007Valverde BE. Status and management of grass-weed herbicide resistance in Latin America. Weed Technol. 2007;21:310-23.). The lipophilicity coefficient of propanil (log Kow: 2.28) is higher than that of imazethapyr; this results in a slower absorption, enabling the inhibition of the enzymes associated with the herbicide resistance even with simultaneous spray.
Summarizing, the previous spraying of malathion and PBO resulted in an increased control of barnyardgrass plants treated with imazethapyr, as indicated by lower FRs values in resistant populations. The most significant responses were observed for malathion, in particular on the PALMS01 population. Conversely, the previous spraying of the cytP450-inducer naphthalic anhydride decreases the control of resistant populations. The simultaneous spraying of imazethapyr and malathion, even at high doses of this cytP450 inhibitor, does not promote the control of barnyardgrass plants, as obtained with the previous spraying inhibitor. The concentration of imazethapyr on resistant plants was smaller in comparison with susceptible plants, and this effect was reversed in plants treated with the cytP450 inhibitor malathion.
The obtained results indicated that the enhanced metabolism of imazethapyr by cytP450 enzymes is involved as a mechanism of resistance in barnyardgrass. This mechanism of resistance is of particular concern, since the control of resistant plants is difficult due to the frequent occurrence of multiple resistance. The elucidation of the resistance mechanism is important to define adequate measures of weed management. The general measures of herbicide resistance management could not be adequate for herbicide-resistant weeds caused by different resistance mechanisms, mainly to enhanced degradation. Measures such as label rate use and weed control at early stages may contribute for the decrease of the evolution of herbicide-resistant weeds. Finally, the use of synergistic compounds, including products that inhibit detoxifying enzymes, like malathion and PBO, improves the efficiency of herbicides and can be crucial in the prevention of herbicide resistance caused by degradation enhancement.
ACKNOWLEDGMENTS
To the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), for the scholarship and fellowship.
REFERENCES
- Bajwa AA, Jabran K, Shahid M, Ali HH, Chauhan BS, Ehsanullah. Eco-biology and management of Echinochloa crus-galli Crop Prot. 2015;75:151-62.
- Barrett M, Maxon JM. Naphthalic anhydride induces imazethapyr metabolism and cytochrome P-450 activity in maize. Z Naturforschung. 1991;46:897-900.
- Birk JH, Malefyt T, inventors; American Cyanamid Company, assignee. Method for safening gramineous crops against pyridine imidazolinone herbicides. US patent 4,922,092. 1991 Feb.
- Carvalho SJP, Nicolai1 M, Ferreira RR, Figueira AVO, Christoffoleti PJ. Herbicide selectivity by differential metabolism: considerations for reducing crop damages. Sci Agric. 2009;66:136-42.
- Chauhan BS, Johnson DE. Ecological studies on Echinochloa crus-galli and the implications for weed management in direct-seeded rice. Crop Prot. 2011;30:1385-91.
- Délye C. Unravelling the genetic bases of non-target-site-based resistance (NTSR) to herbicides: a major challenge for weed science in the forthcoming decade. Pest Manage Sci. 2013;69:176-87.
- Fischer AJ, Bayer DE, Carriere MD, Yim Kyu-Ock. Mechanisms of resistance to bispyribac-sodium in an Echinochloa phyllopogon accession. Pestic Biochem Physiol. 2000;68:156-65.
- Hatzios KK, Burgos N. Metabolism-based herbicide resistance: regulation by safeners. Weed Sci. 2004;52:454-67.
- Heap I. The international survey of herbicide resistant weeds. 2018. [accessed: Jun. 27, 2018]. Available: Available: http:// www.weedscience.org/
» http:// www.weedscience.org/ - Hodgson E, Levi PE. Interactions of Piperonyl Butoxide with cytochrome P450. In Jones DG, editor. Piperonyl Butoxide: the insecticide synergist. San Diego: Academic Press; 1998. p.41-54.
- Letouze A, Gasquez J. Enhanced activity of several herbicide-degrading enzymes: a suggested mechanism responsible for multiple resistance in blackgrass (Alopecurus myosuroides Huds.). Agronomie. 2003;23:601-8.
- Liu C, Liu S, Wang F, Wang Y, Liu K. Expression of a rice CYP81A6 gene confers tolerance to bentazon and sulfonylurea herbicides in both Arabidopsis and tobacco. Plant Cell Tissue Organ Cult. 2012;109:419-28.
- Matzenbacher FO, Bortoly ED, Kalsing A, Merotto Jr A. Distribution and analysis of the mechanisms of resistance of barnyardgrass (Echinochloa crus-galli) to imidazolinone and quinclorac herbicides. J Agric Sci. 2015;153:1044-58.
- Persans MW, Wang J, Schuler MA. Characterization of maize cytochrome P450 monooxygenases induced in response to safeners and bacterial pathogens. Plant Physiol. 2001;125:1126-38.
- Powles SB, Yu Q. Evolution in action: plants resistant to herbicides. Ann Rev Plant Biol. 2010;61:317-47.
- Preston C, Powles SB. Evolution of herbicide resistance in weeds: initial frequency of target site-based resistance to acetolactate synthase-inhibiting herbicides in Lolium rigidum Heredity. 2002;88:8-13.
- Riar DS, Norsworthy JK, Bond JA, Bararpour MT, Wilson MJ, Scott RC. Resistance of Echinochloa crus-galli populations to acetolactate synthase-inhibiting herbicides. Int J Agron. 2012;2012:1-8.
- Ritz C, Kniss AR, Streibig JC. Research methods in weed science: Statistics. Weed Sci. 2015;63(sp1):166-187.
- Senseman SA. Herbicide handbook. 9th. ed. Lawrence: Weed Science Society of America; 2007.
- Siminszky B. Plant cytochrome P450-mediated herbicide metabolism. Phytochem Rev. 2006;5:445-58.
- Tranel PJ, Wright TR. Resistance of weeds to ALS-inhibiting herbicides: what have we learned? Weed Sci. 2002;50:700-12.
- Tulloch AP, Bergter L. Epicuticular wax composition of Echinochloa crusgalli Phytochemistry. 1980;19:145-6.
- Valverde BE. Status and management of grass-weed herbicide resistance in Latin America. Weed Technol. 2007;21:310-23.
- Werck-Reichhart D. Cytochromes P450 for engineering herbicide tolerance. Trends Plant Sci. 2000;5:116-23.
- Yasuor H, Osuna MD, Ortiz A, Saldaín NE, Eckert JW, Fischer AJ. Mechanism of resistance to penoxsulam in late watergrass [Echinochloa phyllopogon (Stapf) Koss.]. J Agric Food Chem. 2009;57:3653-60.
- Yu Q, Abdallah I, Han H, Owen M, Powles S. Distinct non-target site mechanisms endow resistance to glyphosate, ACCase and ALS-inhibiting herbicides in multiple herbicide-resistant Lolium rigidum Planta. 2009;230:713-23.
- Yu Q, Powles S. Metabolism-based herbicides resistance and cross-resistance in crop weeds: a threat to herbicide sustainability and global crop protection. Plant Physiol. 2014;166:1106-18.
- Yuan JS, Tranel PJ, Stewart CN Jr. Non-target-site herbicide resistance: a family business. Trends Plant Sci. 2007;12:6-13.
- Yun MS, Yogo Y, Miura R, Yamasue Y, Fischer AJ. Cytochrome P-450 monooxygenase activity in herbicide-resistant and -susceptible late watergrass (Echinochloa phyllopogon). Pestic Biochem Physiol. 2005;83:107-14.
Publication Dates
-
Publication in this collection
2018
History
-
Received
10 May 2017 -
Accepted
11 July 2017















 S: susceptible (SUSSP01); R: resistant (PALMS01); imi: imazethapyr (106 g ha-1); mal: malathion (1,000 g ha-1, two hours before the application of imazethapyr); NA: naphthalic anhydride (1,000 g ha-1, two hours before the application of imazethapyr); letters indicate the Scott-Knott test (p<0.05).
S: susceptible (SUSSP01); R: resistant (PALMS01); imi: imazethapyr (106 g ha-1); mal: malathion (1,000 g ha-1, two hours before the application of imazethapyr); NA: naphthalic anhydride (1,000 g ha-1, two hours before the application of imazethapyr); letters indicate the Scott-Knott test (p<0.05).
