ABSTRACT:
Velvetleaf, an annual broadleaf weed, is a common and troublesome weed of cropping systems worldwide. Laboratory and field experiments were conducted to determine the effects of environmental factors on germination and emergence of velvetleaf. Seeds germinated over a range of constant temperatures from 10 to 40 oC regardless of light conditions, but no germination occurred at temperature below 5 oC and beyond 50 oC. Seeds germinated at alternating temperature regimes of 15/5 to 40/30 oC, with maximum germination (>90%) at alternating temperatures of 40/30 oC. Germination was sensitive to water stress, and only 0.4% of the seeds germinated at the osmotic potential of -0.4 MPa. There was no germination at ? 0.6 MPa. Moreover, germination was reduced by saline and alkaline stresses and no germination occurred at ³ 150 mM NaCl or ³ 200 mM NaHCO3 concentrations. However, pH values from 5 to 9 had no effect on seed germination. Seedling emergence was significantly affected by burial depth and maximum emergence (78.1-85.6%) occurred at the 1-4 cm depth. The results of this study have contributed to our understanding of the germination and emergence of velvetleaf and should enhance our ability to improve control strategies in cropping systems in central China.
Keywords:
burial depth; light; osmotic potential; saline and alkaline stress; temperature
RESUMO:
O malvão, planta daninha anual de folha larga, é uma espécie invasora comum e problemática para os sistemas de cultivo no mundo todo. Foram conduzidos experimentos em laboratório e em campo para determinar os efeitos de fatores ambientais sobre a germinação e a emergência do malvão. Foi observada a germinação de sementes em temperaturas constantes, que variaram entre 10 e 40 oC, independentemente das condições de luminosidade, mas não houve germinação em temperaturas menores que 5 oC e maiores que 50 oC. A germinação das sementes ocorreu em regimes alternados de temperatura de 15/5 oC a 40/30 oC, com máxima germinação (>90%) em temperaturas alternadas de 40/30 oC. A germinação foi sensível ao estresse hídrico, e apenas 0,4% das sementes germinaram no potencial osmótico de -0,4 MPa. Nenhuma germinação foi observada no potencial osmótico ? -0,6 MPa. Além disso, a germinação foi reduzida por estresse salino e alcalino e não ocorreu nas concentrações ³ 150 mM de NaCl ou ³ 200 mM de NaHCO3. No entanto, os valores de pH entre 5 e 9 não tiveram nenhum efeito sobre a germinação das sementes. A emergência das plântulas foi afetada de forma significativa pela profundidade de enterramento e houve máxima emergência (78,1-85,6%) a 1-4 cm de profundidade. Os resultados deste estudo contribuíram para o entendimento da germinação e da emergência do malvão e deverão aumentar a capacidade de melhoria das estratégias de controle nos sistemas de cultivo na região central da China.
Palavras-chave:
profundidade de enterramento; luminosidade; potencial osmótico; estresse salino e alcalino; temperatura
INTRODUCTION
Velvetleaf, also known as butterprint, Indian mallow, China jute, is an annual broadleaf weed of the Malvaceae family and thought to be native to China (Li, 1970Li HL. The origin of cultivated plants in Southeast Asia. Econ Bot. 1970;24:3-19.). However, this species has undergone rapid range extension and been widespread around the globe as a result of the attempted use of this plant species as a source of commercial fiber production (Dempsey, 1975Dempsey JM. Fiber crops. China Jute. Gainesville: The University of Florida press; 1975. p.397-413.; Spencer, 1984Spencer NR. Velvetleaf, Abutilon theophrasti (Malvaceae), history and economic impact in the United States. Econ Bot. 1984;38(4):407-16.; Holt and Boose, 2000Holt JS, Boose AB. Potential for spread of Abutilon theophrasti in California. Weed Sci. 2000;48:43-52.). Velvetleaf is now a common and troublesome weed in several crops such as maize (Zea mays L.) (Lindquist et al., 1996Lindquist JL, Mortensen DA, Clay SA, Schmenk R, Kells JJ, Howatt K et al. Stability of corn (Zea mays) - velvetleaf (Abutilon theophrasti) interference relationships. Weed Sci . 1996;44(2):309-13.; McDonald et al., 2004McDonald AJ, Riha SJ, Mohler CL. Mining the record: historical evidence for climatic influences on maize - Abutilon theophrasti competition. Weed Res. 2004;44:439-45.), soybean [Glycine max (L.) Merr.] (Akey et al., 1991Akey AC, Jurik TW, Dekker J. A replacement series evaluation of competition between velvetleaf (Abutilon theophrasti) and soybean (Glycine max). Weed Res. 1991;31:63-72.; Begonia et al., 1991Begonia GB, Aldrich RJ, Salisbury CD. Soybean yield and yield components as influenced by canopy heights and duration of competition of velvetleaf (Abutilon theophrasti Medik.). Weed Res. 1991;31(3):117-24.; Ziska, 2012Ziska L. Observed changes in soyabean growth and seed yield from Abutilon theophrasti competition as a function of carbon dioxide concentration. Weed Res. 2012;53:140-5.), cotton (Gossypium hirsutum L.) (Cortés et al., 2010Cortés JA, Mendiola MA, Castejón M. Competition of velvetleaf (Abutilon theophrasti M.) weed with cotton (Gossypium hirsutum L.). economic damage threshold. Span J Agric Res. 2010;8(2):391-9.), and grain sorghum [Sorghum bicolor (L.) Moench] (Traoré et al., 2003Traoré S, Mason SC, Martin AR, Mortensen DA, Spotanski JJ. Velvetleaf interference effects on yield and growth of grain sorghum. Agron J. 2003;95(6):1602-7.). In recent decades, velvetleaf has become an increasingly problematic weed in cotton field in central China (Ma et al., 2016).
As a summer annual weed, reproduction of velvetleaf is entirely by seeds, which replenishes the soil seed bank. The increasing problems of velvetleaf have been attributed to its high seed production (Clay et al., 2005Clay SA, Kleinjan J, Clay DE, Forcella F, Batchelor W. Growth and fecundity of several weed species in corn and soybean. Agron J. 2005;97(1):294-302.), high seed viability (Buhler and Hartzler, 2001Buhler DD, Hartzler RG. Emergence and persistence of seed of velvetleaf, common waterhemp, woolly cupgrass, and giant foxtail. Weed Sci. 2001;49(2):230-5.) and high latency (Nurse and DiTommaso, 2005Nurse RE, DiTommaso A. Corn competition alters the germinability of velvetleaf (Abutilon theophrasti) seeds. Weed Sci. 2005;53(4):479-88.). Ma et al. (2016Ma XY, Yang JY, Wu HW, Jiang WL, Ma YJ, Ma Y. Growth analysis of cotton in competition with velvetleaf (Abutilon theophrasti). Weed Technol. 2016;30:123-36.) demonstrated that seed production of velvetleaf is prodigious and a single plant can produce as many as 28,000 seeds. In addition, velvetleaf seeds can remain viable in soil for over 50 years as a result of physical dormancy caused by an impermeable seed coat (Dorado et al., 2009Dorado J, Fernández-Quintanilla C, Grundy AC. Germination patterns in naturally chilled and non-chilled seeds of fierce thornapple (Datura ferox) and velvetleaf (Abutilon theophrasti). Weed Sci. 2009;57(2):155-62.). Therefore, velvetleaf is difficult to eradicate once it has formed a well-established soil seed bank (Buhler and Hartzler, 2001). Moreover, atrazine-resistant velvetleaf biotypes have been identified (Pavlovic´ et al., 2007Pavlovic´ D, Vrbnièanin S, Bozic´ D, Simonèiè A. The study of methods for determination of metabolism based resistance ofAbutilon theophrastimedic. to atrazine. J Cent Eur Agric. 2007;8(4):435-42.; James and Cooper, 2012James TK, Cooper JM. Control of the recently-introduced weed butterprint (Abutilon theophrasti) in maize. New Zeal Plant Prot. 2012;65:64-8.; Heap, 2017Heap I. International Survey of Herbicide Resistant Weeds. [accessed: Feb. 20, 2017] 20, 2017] http://www.weedscience.org/summary/home.aspx
.
http://www.weedscience.org/summary/home....
), hence it is more challenging for growers to control the weed.
Successful weed management in an agro-ecosystem requires knowledge of seed germination and emergence behavior, which is regulated by several environmental factors, such as temperature, light, soil salinity, pH, moisture, and burial depth (Chauhan et al., 2006Chauhan BS, Gill G, Preston C. Factors affecting seed germination of annual sowthistle (Sonchus oleraceus) in southern Australia. Weed Sci. 2006;54(5):854-60.; Asgarpour et al., 2015Asgarpour R, Ghorbani R, Khajeh-Hosseini M, Mohammadvand E, Chauhan BS. Germination of spotted spurge (Chamaesyce maculata) seeds in response to different environmental factors. Weed Sci. 2015;63(2):502-10.; Li et al., 2015Li Q, Tan J, Li W, Yuan G, Du L, Ma S et al. Effects of environmental factors on seed germination and emergence of Japanese brome (Bromus japonicus). Weed Sci. 2015;63(3):641-6.). Weed seeds sensitive to light for germination, for example, would emerge when they are close to the soil surface (Milberg et al., 2000Milberg P, Anderson L, Thompson K. Large-seeded species are less dependent on light for germination than small-seeded ones. Seed Sci Res. 2000;10:99-104.). Soil salinization is a persistent ecological issue that adversely influences plant growth and development (Zhao et al., 2014Zhao Y, Lu Z, He L. Effects of saline-alkaline stress on seed germination and seedling growth of Sorghum bicolor (L.) Moench. Appl Biochem Biotechnol. 2014;173(7):1680-91.). In China, more than 7% of the 135 million ha of cropping soils are saline-alkaline, and salinization of soil is becoming even more serious as a result of secondary salinization caused by improper farming and irrigation (Dong et al., 2010Dong HZ, Kong XQ, Luo Z, Li WJ, Xin CS. Unequal salt distribution in the root zone increases growth and yield of cotton. Eur J Agron. 2010;33(4):285-92.). In northwest China, limited rainfall, high temperature and evapotranspiration, as well as reduction in freshwater availability, have led to an increase in soil salinity and alkalinity (Dong et al., 2008Dong HZ, Li W, Tang W, Zhang D. Furrow seedling with plastic mulching increases stand establishment and lint yield of cotton in a saline field. Agron J. 2008;100(6):1640-6.). Consequently, salt, alkali, high temperature and drought stresses are increasingly becoming important factors affecting crop growth and constraining agricultural production, and these factors can also affect seed germination and emergence of annual velvetleaf.
Despite the increasing problem of annual velvetleaf in farming systems around the world, knowledge of its seed germination ecology is limited (Sadeghloo et al., 2013Sadeghloo A, Asghari J, Ghaderi-Far F. Seed germination and seedling emergence of velvetleaf (Abutilon theophrasti) and barnyardgrass (Echinochloa crus-galli). Planta Daninha. 2013;31(2):259-66.). Therefore, the objectives of the present study were to evaluate the effects of temperature, light, osmotic and salinity stresses, pH, and sowing depth on seed germination and seedling emergence of velvetleaf. Studying these issues will help provide basic data and technical support for the integrative management of velvetleaf.
MATERIAL AND METHODS
Seed collection and pretreatment
Matured seed capsules of velvetleaf were manually collected in September 2015 from 50 randomly chosen plants located along the edges of the cotton fields belonging to Institute of Cotton Research, Chinese Agricultural Academy of Sciences (36.13o N, 114.85o E). The selected plants were less than 2 m apart from each other. The soil was a sandy loam soil (Typic Haplustepts) according to Soil Taxonomy, with 40% sand, 25% silt, 35% clay, 1.5% organic matter, pH 8.0, and electrical conductivity of 500 μs cm-1. The capsules of velvetleaf were hand-threshed, and then seeds were cleaned with an air cleaner to remove debris. Cleaned seeds were air-dried for several days before being stored in paper bags at room temperature (approximately 20 oC) in darkness until the start of the experiments in July 2016. Several days before the experiments were initiated, seed viability and dormancy were determined using 100 seeds in four replicates. Seed germination under light at 35 oC (Sadeghloo et al., 2013Sadeghloo A, Asghari J, Ghaderi-Far F. Seed germination and seedling emergence of velvetleaf (Abutilon theophrasti) and barnyardgrass (Echinochloa crus-galli). Planta Daninha. 2013;31(2):259-66.) was tested in petri dishes; germination percentage was more than 80%. Therefore, no treatments were carried out to break seed dormancy before experiments. Velvetleaf seeds were surface-sterilized with a 0.1% v/v sodium hypochlorite solution for 2 min and then rinsed with deionized water for 5 min. Sterilization had no negative effect on germination (data not shown).
General experimental arrangement
Four replicates of 25 seeds of velvetleaf were evenly placed in 90-mm-diam petri dishes on two layers of Whatman No. 1 filter paper moistened with 4 mL of deionized water or treatment solutions. Dishes were sealed with Parafilm to minimize water loss from evaporation. After that, the dishes were placed in an incubator at fluctuating day/night temperatures of 40/30 oC, unless specified otherwise. The photoperiod was set at 14 h to coincide with day length in July and August. White fluorescent bulbs provided approximately 150 μmol m-2 s-1 of photosynthetic photon flux density during the experiment. The number of germinated seeds was counted daily and terminated at 10 days after sowing (DAS) as no further seed germination occurred. Seeds with root length ³2 mm length were considered to be germinated seeds. In all treatments, the viability of non-germinated seeds was examined by squeezing the seeds with a pair of tweezers to see if they contained a firm embryo. Seed germination percentage was calculated by multiplying the ratio of germinated seeds to total number of viable seeds in a single petri dish by 100.
Constant temperature and light
Germination tests were conducted in incubators set at ten constant temperatures of 5, 10, 15, 20, 25, 30, 35, 40, 45, and 50 oC. Photoperiod was set at 14 h. Seeds were prepared for germination in complete darkness under a green safe light and then the dishes were wrapped in two layers of aluminum foil. Germination for the darkness treatments was evaluated once at 10 DAS.
Alternating temperature and light
Seed germination was evaluated in incubators at six fluctuating temperature regimes of 15/5, 20/10, 25/15, 30/20, 35/25, and 40/30 oC. Difference between minimum and maximum temperatures from April to October during the last five years in the region was about 10 oC (Table 1). Photoperiod was set at 14 h, and it coincided with the higher temperature. A set of petri dishes was wrapped in two layers of aluminum foil to study seed germination in the dark, and the number of germinated seeds was counted once at 10 DAS.
Table 1 - Average maximum (max), minimum (min) and mean temperatures (oC) in the experimental region of Anyang, Henan Province, China, during 2011-2015 (Anyang Meteorological Bureau)
Osmotic stress
Germination, as affected by osmotic stress, was determined at water potentials of 0, -0.2, -0.4, -0.6, -0.8, and -1.0 MPa, prepared by dissolving appropriate amounts of polyethylene glycol (PEG) 8000 in deionized water (Michel, 1983Michel BE. Evaluation of the water potentials of solutions of polyethylene glycol 8000 both in the absence and presence of other solutes. Plant Physiol. 1983;72:66-70.). Petri dishes were incubated at 40/30 oC day/night temperature with 14 h light. These conditions provided maximum germination in the temperature experiments. The number of germinated seeds in each dish was recorded daily.
Saline and alkaline stresses
The effect of saline and alkaline stresses on velvetleaf germination was determined by incubating seeds in dishes containing sodium chloride (NaCl) or sodium bicarbonate (NaHCO3) solutions of 0, 12.5, 25, 50, 100, 150, 200, and 250 mM. Petri dishes were managed and germinated seeds were assessed as described in the osmotic stress study.
pH
To examine the effects of pH on seed germination of velvetleaf, buffered solutions of pH 5 to 10 were prepared according to the method described by Chauhan and Johnson (2008Chauhan BS, Johnson DE. Germination ecology of goosegrass (Eleusine indica): an important grass weed of rainfed rice. Weed Sci. 2008;56(5):699-706.). Unbuffered deionized water (pH 6.8) was used as a control. Petri dishes were managed and germinated seeds assessed as previously described.
Seed burial depth
The effect of seed burial depth on seedling emergence was investigated in the experimental field at the Institute of Cotton Research, Chinese Agricultural Academy of Sciences between July and August 2016. The seed burial site was chosen because of the absence of velvetleaf. The top field soil used for this experiment was autoclaved and passed through a 2 mm sieve. Twenty seeds were placed on the soil surface or covered to depths of 1, 2, 3, 4, 6, and 8 cm with soil in the cylindrical pots (height/diameter 15 cm). Pots were manually subirrigated as needed to maintain adequate soil moisture. Seedlings were considered to be emerged when the two cotyledons were visible at the soil surface. Germinated seedlings were counted 20 d after sowing.
Statistical analyses
All laboratory treatments were conducted in a completely randomized design with four replicates. All experiments were repeated twice. Data from repeated experiments were subjected to ANOVA and there was no significant time-by-treatment interaction; therefore, data from two experiments were pooled before further analysis. Data on germination percentage were transformed before statistical analysis to ensure homogeneity of variance. Neither arcsine square nor log transformation of the data did improve homogeneity; thus, ANOVA and regression analysis were performed on the nontransformed germination percentage. Significant differences among treatment means were identified by Fisher’s protected LSD test.
Additionally, regression analysis was used to evaluate the effect of osmotic or salinity and alkalinity stresses on germination. Data were fitted to a functional three-parameter logistic model (Equation 1; Asgarpour et al., 2015Asgarpour R, Ghorbani R, Khajeh-Hosseini M, Mohammadvand E, Chauhan BS. Germination of spotted spurge (Chamaesyce maculata) seeds in response to different environmental factors. Weed Sci. 2015;63(2):502-10.). The applied model was
where G represents the total percentage of germination at salt or alkali concentration or osmotic potential x, Gmax is the maximum germination, x50 is the salt or alkali concentration or osmotic potential required for 50% inhibition of the maximum germination, and Grate indicates the slope. This model was also used to describe the effects of time (days after sowing) on germination (%). The model (Equation 2) fitted was
where E is the total germination (%) at time x, Emax is the maximum germination (%), x50 is the time to reach 50% of maximum germination, namely the germination rate, and Erate indicates the slope.
Coefficients of determination (r2) were calculated for nonlinear regressions and used to determine the goodness of fit to nonlinear models. All probabilities were two-tailed, and the significance level was set at p = 0.05. Analysis was performed with the statistical software SPSS 13.0.
RESULTS AND DISCUSSION
Effects of constant temperatures and light
Temperature significantly affected velvetleaf germination (p<0.001). No germination occurred at the constant temperature of 5 oC. Germination of 5.5% was recorded at 10 oC under 14 h light conditions. Germination percentage increased to 22.0-31.0% (14 h light) and 16.5-24.0% (dark) under 15-25 oC. As temperature increased to 35 oC, 83.0% and 78.5% of velvetleaf seeds germinated under 14 h light and complete dark conditions, respectively. There was maximum germination percentage at 40 oC but it decreased sharply to 0-3% at 45 oC. There was no seed germination when temperature reached 50 oC (Figure 1). Light had no significant effect on the percentage of velvetleaf germination at temperatures ranging from 10 to 45 oC (Figure 1). Sadeghloo et al. (2013Sadeghloo A, Asghari J, Ghaderi-Far F. Seed germination and seedling emergence of velvetleaf (Abutilon theophrasti) and barnyardgrass (Echinochloa crus-galli). Planta Daninha. 2013;31(2):259-66.) tested seed germination of velvetleaf at the same range of constant temperatures from 5 to 50 oC and found that no germination occurred at temperatures of 5 and 50 oC. Maximum germination (73-85%) occurred between 10 to 40 oC and there was no significant difference among these different temperatures. In our study, seeds also did not germinate at temperatures of 5 and 50 oC and maximum germination (94.0-94.5%) occurred at 40 oC, which was similar to the findings reported by Sadeghloo et al. (2013). However, seed germination at the temperature regimes between 10 and 30 oC was only 5.5-35.0% in the present study, which was much lower than the rate (about 80%) of Sadeghloo et al. (2013) under the same temperatures. This difference raised some questions for consideration concerning the influence of type of seeds - including storage conditions (Dorado et al., 2009Dorado J, Fernández-Quintanilla C, Grundy AC. Germination patterns in naturally chilled and non-chilled seeds of fierce thornapple (Datura ferox) and velvetleaf (Abutilon theophrasti). Weed Sci. 2009;57(2):155-62.), biotypes (Kremer and Lotz, 1998aKremer E, Lotz LAP. Germination and emergence characteristics of triazine-susceptible and triazine-resistant biotypes of Solanum nigrum. J Appl Ecol. 1998a;35(2):302-10.), and growth environment differences of maternal plants from which seeds were collected (Eslami, 2011Eslami SV. Comparative germination and emergence ecology of two populations of common lambsquarters (Chenopodium album) from Iran and Denmark. Weed Sci. 2011; 59:90-7.) - on germination capacity. In this study, velvetleaf seeds were stored at room temperature in the dark and under dry conditions, and no pretreatment was performed before germination tests. However, seeds were kept in a refrigerator at 5 ± 2 oC and soaked in boiling water for 5 s to break seed dormancy before germination tests in the study of Sadeghloo et al. (2013). Therefore, the significant differences (up to 45%) found for seed germination at temperatures 10-30 oC could be attributed to the differences of storage conditions and pretreatments of the seeds before the germination tests used in these two experiments.
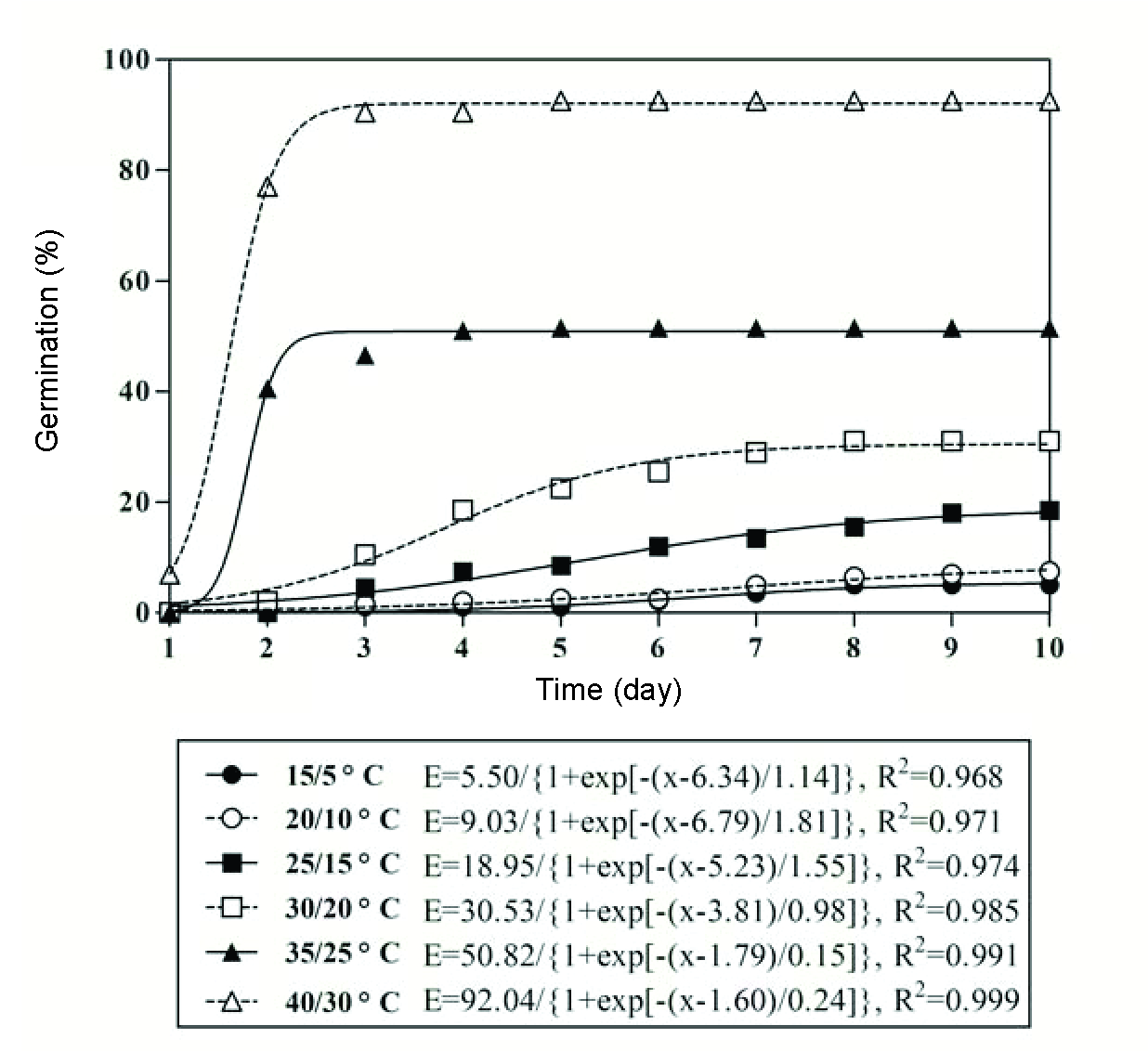
High temperature promoted the onset of germination, and the germination rate described by the time to reach 50% of maximum germination decreased from 8.61 to 1.81 d when temperature increased from 10 to 35 oC (Figure 2).
Effect of constant temperatures and light (light/dark and dark) on velvetleaf seed germination.
Effect of alternating temperature and light
Germination of 5.0-7.5% was recorded at day/night temperatures of 15/5 and 20/10 oC. Germination percentage increased as day/night temperature increased from 25/15 to 35/25 oC, and it reached its maximum rate (92.5%) at 40/30 oC (Figure 3). The germination rate decreased with the increase in the day/night temperatures from 15/5 to 40/30 oC (Figure 4).
Effect of alternating temperatures and light (light/dark and dark) on velvetleaf seed germination.
In comparison to the constant temperatures at 10 and 20 oC, the same mean temperature of alternating temperatures 15/5 and 25/15 oC did not improve germination values (Mann-Whitney U-Test, p>0.23). Under the light/dark condition, the germination percentage (7.5%) at alternating temperature 20/10 oC was significantly lower than that (18.0%) at the constant temperature of 15 oC (p=0.01). These results were consistent with those of Leon and Knapp (2004Leon RG, Knapp AD. Effect of temperature on the germination of common waterhemp (Amaranthus tuberculatus), giant foxtail (Setaria faberi), and velvetleaf (Abutilon theophrasti). Weed Sci. 2004;52:67-73.), who reported that temperature alternation did not typically increase germination of velvetleaf under mean temperatures of 14-26 oC. However, in this study, higher alternating temperatures of 35/25 and 40/30 oC (with a mean temperatures of 30 and 35 oC) resulted in better germination of velvetleaf than that at the constant temperatures of 30 and 35 oC regardless of light condition (p?0.04). This suggests that alternating temperatures may be more suitable for seed germination in velvetleaf at high temperatures including the optimum temperature of 35 oC.
There was no significant difference in germination between light and darkness at all tested temperature regimes (Figures 1and3). This indicated that velvetleaf was not sensitive to light conditions, allowing germination to occur below the soil surface. Varied germination responses to light have been reported among different weed species, ranging from stimulation, no effects to suppression. Light was an indispensable stimulus for seed germination of giant Parramatta (Sporobolus indicus) (Andrews, 1997Andrews TS. Factors affecting the germination of giant Parramatta grass. Aust J Exp Agric. 1997;37(4):439-46. ). Seed germination of some weed species could be stimulated by light, e.g. black nightshade (Solanum nigrum) (Taab and Andersson, 2009Taab A, Andersson L. Seed dormancy dynamics and germination characteristics of Solanum nigrum. Weed Res. 2009;49(5):490-8.), common lambsquarters (Chenopodium album) (Eslami, 2011Eslami SV. Comparative germination and emergence ecology of two populations of common lambsquarters (Chenopodium album) from Iran and Denmark. Weed Sci. 2011; 59:90-7.), eastern black nightshade (S. ptycanthum) (Zhou and Deckard, 2005Zhou JK, Deckard EL. Factors affecting eastern black nightshade (Solanum ptycanthum) seed germination. Weed Sci. 2005;53(5):651-6.), goosegrass (Eleusine indica) (Chauhan and Johnson, 2008Chauhan BS, Johnson DE. Germination ecology of goosegrass (Eleusine indica): an important grass weed of rainfed rice. Weed Sci. 2008;56(5):699-706.), and horseweed (Conyza canadensis) (Nandula et al., 2006Nandula VK, Eubank TW, Koger CH, Reddy KN. Factors affecting germination of horseweed (Conyza canadensis). Weed Sci. 2006;54(5):898-902.). However, light was not required for the germination of hairy nightshade (Zhou et al., 2005Zhou JK, Deckard EL, Ahrens WH. Factors affecting germination of hairy nightshade (Solanum sarrachoides) seeds. Weed Sci. 2005;53:41-5.), Japanese brome (Bromus japonicus) (Li et al., 2015), and muskweed (Myagrum perfoliatum) (Honarmand et al., 2016Honarmand SJ, Nosratti I, Nazari K, Heidari H. Factors affecting the seed germination and seedling emergence of muskweed (Myagrum perfoliatum). Weed Biol Manag. 2016;16(4):186-93.).
Effect of osmotic stress
Velvetleaf germination decreased sharply from 93.0% to 29.5% as water potentials decreased from 0 to -0.2 MPa, and it was completely inhibited at -0.4 MPa or below. A three-parameter logistic model was fitted to obtain the germination percentage at different osmotic potentials for velvetleaf. Osmotic potential for 50% inhibition of the maximum germination estimated from the fitted model was -0.19 MPa (Figure 5). The water potential for 50% inhibition of the maximum germination was -0.32 MPa for sowthistle (Sonchus oleraceus) (Chauhan et al., 2006Chauhan BS, Gill G, Preston C. Factors affecting seed germination of annual sowthistle (Sonchus oleraceus) in southern Australia. Weed Sci. 2006;54(5):854-60.), -0.42 MPa for wild safflower (Carthamus oxyacantha M. Bieb.) (Tanveer et al., 2012Tanveer A, Farid MZ, Tahir M, Javaid MM, Khaliq A. Environmental factors affecting the germination and seedling emergence of Carthamus oxyacantha M. Bieb. (Wild safflower). Pak J Weed Sci Res. 2012;18(2):221-35.), -0.52 MPa for spotted spurge (Asgarpour et al., 2015Asgarpour R, Ghorbani R, Khajeh-Hosseini M, Mohammadvand E, Chauhan BS. Germination of spotted spurge (Chamaesyce maculata) seeds in response to different environmental factors. Weed Sci. 2015;63(2):502-10.), and -0.77 for Japanese brome (Li et al., 2015Li Q, Tan J, Li W, Yuan G, Du L, Ma S et al. Effects of environmental factors on seed germination and emergence of Japanese brome (Bromus japonicus). Weed Sci. 2015;63(3):641-6.). These results indicated that seed germination of velvetleaf was more sensitive to soil moisture and drought was unfavorable for seed germination.
Relationship between water potential and seed germination percentage (mean ± SE) of velvetleaf. Seeds were placed in an incubator at 40/30 oC day/night temperature with a 14 h photoperiod for 10 d.
Effect of salinity and alkalinity stress
A three-parameter logistic model was fitted to germination values obtained at different concentrations of NaCl and NaHCO3 (Figure 6). Velvetleaf germination was greater than 94.5% up to a concentration of 50 mM NaCl, but it declined sharply to 0.5% at 100 mM NaCl, while no germination occurred at ³150 mM NaCl. The concentration required for 50% inhibition of maximum germination was 70.6 mM NaCl (Figure 6A). The maximum concentration at which no seed could germinate for barnyardgrass (Echinochloa crus-galli) (Sadeghloo et al., 2013Sadeghloo A, Asghari J, Ghaderi-Far F. Seed germination and seedling emergence of velvetleaf (Abutilon theophrasti) and barnyardgrass (Echinochloa crus-galli). Planta Daninha. 2013;31(2):259-66.), citronmelon (Ramirez et al., 2014Ramirez AHM, Jhala AJ, Singh M. Factors affecting germination of citronmelon (Citrullus lanatus var. citroides). Weed Sci. 2014;62:45-50.), Japanese brome (Li et al., 2015Li Q, Tan J, Li W, Yuan G, Du L, Ma S et al. Effects of environmental factors on seed germination and emergence of Japanese brome (Bromus japonicus). Weed Sci. 2015;63(3):641-6.), muskweed (Honarmand et al., 2016Honarmand SJ, Nosratti I, Nazari K, Heidari H. Factors affecting the seed germination and seedling emergence of muskweed (Myagrum perfoliatum). Weed Biol Manag. 2016;16(4):186-93.), and spotted spurge (Asgarpour et al., 2015Asgarpour R, Ghorbani R, Khajeh-Hosseini M, Mohammadvand E, Chauhan BS. Germination of spotted spurge (Chamaesyce maculata) seeds in response to different environmental factors. Weed Sci. 2015;63(2):502-10.) was 400, 300, 360, 250, and 160 mM NaCl, respectively. These results showed that velvetleaf is not tolerant to salinity as compared with the above weed species, which could germinate at high NaCl concentration.
Relationship between sodium chloride (NaCl, A) or sodium bicarbonate (NaHCO3, B) concentration and seed germination percentage (mean ± SE) of velvetleaf. Seeds were placed in an incubated at 40/30 oC day/night temperature with a 14 h photoperiod for 10 d.
Although the literature on weed germination is expanding, the majority of research has only studied the effects of NaCl on seed germination. Since different cations and anions and their combination in the soil play an important role in governing the changes in soil salt-alkalization, e.g. Na+, chloride (Cl-), bicarbonate (HCO3-), and sulfate (SO42-) (Wu et al., 2014Wu Y, Wang YX, Xie XJ. Spatial occurrence and geochemistry of soil salinity in Datong basin, northern China. J Soil Sediment. 2014;14(8):1445-55.; Zhao et al., 2014Zhao Y, Lu Z, He L. Effects of saline-alkaline stress on seed germination and seedling growth of Sorghum bicolor (L.) Moench. Appl Biochem Biotechnol. 2014;173(7):1680-91.), understanding seed germination characteristics under different salinity and alkalinity stresses is important for devising appropriate weed management strategies. Figure 6B showed that there was no change in seed germination up to the concentration of 50 mM NaHCO3 (98-99%); 64.0% germination occurred at 100 mM NaHCO3; germination was completely inhibited at the NaHCO3 concentration of 200 mM. The concentration for 50% inhibition of maximum germination was 106.4 mM NaHCO3 (Figure 6B). These data suggest that velvetleaf is more sensitive to NaCl than to NaHCO3.
The germination rate was reduced with increasing salinity and alkalinity; for example, the time for 50% germination increased from 1.51 d at 0 mM NaCl (control) to 4.09 d at 50 mM NaCl (Figure 7), and the time for 50% germination increased from 1.46 d at 0 mM NaHCO3 to 6.46 d at 100 mM NaHCO3 (Figure 8). Salinity stress (NaCl) also delayed the onset of germination of other species, such as spotted spurge (Asgarpour et al., 2015Asgarpour R, Ghorbani R, Khajeh-Hosseini M, Mohammadvand E, Chauhan BS. Germination of spotted spurge (Chamaesyce maculata) seeds in response to different environmental factors. Weed Sci. 2015;63(2):502-10.) and wild safflower (Tanveer et al., 2012Tanveer A, Farid MZ, Tahir M, Javaid MM, Khaliq A. Environmental factors affecting the germination and seedling emergence of Carthamus oxyacantha M. Bieb. (Wild safflower). Pak J Weed Sci Res. 2012;18(2):221-35.).
Effect of sodium chloride (NaCl) concentrations on cumulative germination of velvetleaf seeds.
Effect of sodium bicarbonate (NaHCO3) concentrations on cumulative germination of velvetleaf seeds.
Effect of pH
Velvetleaf germination varied slightly between 95.5-98.0% over a pH range of 5 to 9. Although germination significantly decreased at pH 10, 91.5% seeds still germinated (Figure 9). Our results were consistent with those of Sadeghloo et al. (2013Sadeghloo A, Asghari J, Ghaderi-Far F. Seed germination and seedling emergence of velvetleaf (Abutilon theophrasti) and barnyardgrass (Echinochloa crus-galli). Planta Daninha. 2013;31(2):259-66.) and showed that pH is not a limiting factor for velvetleaf germination and such a trait would help this weed species to colonize various habitats. Similar to velvetleaf, several weed species, such as spotted spurge (Asgarpour et al., 2015Asgarpour R, Ghorbani R, Khajeh-Hosseini M, Mohammadvand E, Chauhan BS. Germination of spotted spurge (Chamaesyce maculata) seeds in response to different environmental factors. Weed Sci. 2015;63(2):502-10.), Japanese brome (Li et al., 2015Li Q, Tan J, Li W, Yuan G, Du L, Ma S et al. Effects of environmental factors on seed germination and emergence of Japanese brome (Bromus japonicus). Weed Sci. 2015;63(3):641-6.), and silverleaf nightshade (S. elaeagnifolium Cav.) (Stanton et al., 2012Stanton R, Wu H, Lemerle D. Factors affecting silverleaf nightshade (Solanum elaeagnifolium) germination. Weed Sci. 2012;60:42-7.) were also able to germinate under a wide range of pH. The soil in central China has a pH value between 7 and 8, indicating the soil pH is not a limiting factor for the germination of velvetleaf.
Effect of seed burial depth
Seedling emergence of velvetleaf was significantly influenced by sowing depth (p<0.001). Only 5.0% germination occurred when the seeds were sown at the surface (0 cm). Velvetleaf seedling emergence remained constant at about 80% at the burial depths from 1 to 4 cm, it declined thereafter, with emergence reducing to 53.1% at the 6 cm burial depth. Only 1.9% emergence occurred when seeds were burial at the burial depth of 8 cm (Figure 10). However, Davis and Renner (2007Davis AS, Renner KA. Influence of seed depth and pathogens on fatal germination of velvetleaf (Abutilon theophrasti) and giant foxtail (Setaria faberi). Weed Sci. 2007;55:30-5.) reported that velvetleaf seeds could germinate to a depth of 10 cm with 25-49% seedling emergence. Similarly, Sadeghloo et al. (2013Sadeghloo A, Asghari J, Ghaderi-Far F. Seed germination and seedling emergence of velvetleaf (Abutilon theophrasti) and barnyardgrass (Echinochloa crus-galli). Planta Daninha. 2013;31(2):259-66.) found that 24% of velvetleaf seedlings emerged at a depth of 10 cm and no seedlings emerged from seeds buried at a depth of 12 cm. All published protocols showed that the optimal burial depth of velvetleaf is 1-4 cm and the depth of 10 cm is the maximum depth to emerge. The maximum depth of seedling emergence is affected by seed size (Benvenuti and Macchia, 2001Benvenuti S, Macchia M. Quantitative analysis of emergence of seedlings from buried weed seeds with increasing soil depth. Weed Sci. 2001;49(4):528-35.), temperature (Benvenuti and Macchia, 1993Benvenuti S, Macchia M. Calculation of threshold temperature for the development of various weeds. Agr Med. 1993;123:252-6.), light (Ballaré et al., 1992Ballaré CL, Scopel AL, Sànchez RA, Radosevich SR. Photomorphogenic processes in the agricultural environment. Photochem Photobiol. 1992;56(5):777-88.), soil properties and compaction (Pareja and Staniforth, 1985Pareja MR, Staniforth DW. Seed-soil characteristics in relation to weed seed germination. Weed Sci. 1985;33:190-5.), and soil water content (Roberts and Potter, 1980Roberts HA, Potter ME. Emergence patterns of weed seedlings in relation to cultivation and rainfall. Weed Res. 1980;20(6):377-86.). Moreover, Davis and Renner (2007) found that velvetleaf reductions in seedling emergence with increasing seed burial depth were partly due to increased fatal germinants, which were those germinated seeds that obviously had no chance of reaching the surface. This research proved once again that light is not a requirement for velvetleaf seed germination because 53% of the seeds germinated at a depth of 6 cm.
In this study, velvetleaf seeds placed on the surface of the soil had only 5% emergence, much lower than seeds planted at 1 cm depth. Mester and Buhler (1991Mester TC, Buhler DD. Effects of soil temperature, seed depth, and cyanazine on giant foxtail (Setaria faberi) and velvetleaf (Abutilon theophrasti) seedling development. Weed Sci. 1991;39:204-9.) also found that velvetleaf seedling survival was only 28% after germination on the soil surface and most individuals often failed to become established. Limited contact between soil and seed, heterogeneity of soil penetration resistance, or reduced water availability are some environmental conditions that may limit seed germination on the surface of the soil (Kremer and Lotz, 1998bKremer E, Lotz LAP. Emergence depth of triazine susceptible and resistant Solanum nigrum seeds. Ann Appl Biol. 1998b;132(2):277-88.; Sadeghloo et al., 2013Sadeghloo A, Asghari J, Ghaderi-Far F. Seed germination and seedling emergence of velvetleaf (Abutilon theophrasti) and barnyardgrass (Echinochloa crus-galli). Planta Daninha. 2013;31(2):259-66.).
In summary, the results of this study indicate that velvetleaf germination is affected by various environmental factors. This study showed that temperature is a limiting factor for germination of velvetleaf seeds. Velvetleaf germinated over a wide temperature range between 10 and 45 oC, which could allow for germination throughout the spring and summer months in many parts of China. As shown in Table 1, maximum temperatures from April to October at the experimental site in north China do not exceed 45 oC; therefore, minimum temperature is the key limiting factor for seed germination. The minimum germination temperature of 10 oC identified in this study has determined that field emergence of velvetleaf seedlings starts in early April and continues through mid October (unpublished data), as this coincided well with the minimum temperature distribution between April and October, with average minimum temperature ranging from 9.1 oC in April to the highest 22.6 oC in July and then back to 10.0 oC in October.
Velvetleaf could germinate over a wide range of burial depths but light did not promote seed germination. Moreover, physical dormancy made velvetleaf seeds remain viable in soil for many years (Dorado et al., 2009Dorado J, Fernández-Quintanilla C, Grundy AC. Germination patterns in naturally chilled and non-chilled seeds of fierce thornapple (Datura ferox) and velvetleaf (Abutilon theophrasti). Weed Sci. 2009;57(2):155-62.). These results suggest that strategic inversion tillage might not be useful to control velvetleaf. After moldboard plowing, weed seeds buried under 10 cm in the soil will cause fatal germination, and seeds in the upper 10 cm of the soil can germinate under optimal environmental conditions. Therefore, control of weeds must be carried out either just before they appear (pre-emergence) or after they have appeared (post-emergence). However, there is no registered herbicide for post-emergence control of broad-leaved weeds including velvetleaf in cotton and soybean fields in China. Moreover, transgenic glyphosate-resistant crops have not been adopted. Therefore, pre-emergence herbicides, like pendimethalin or oxyfluorfen, should be used in these crops to control velvetleaf. If necessary, post-emergence herbicides, such as glyphosate and glufosinate, can be applied through shielded spraying between crop rows.
Seed germination of velvetleaf was sensitive to water potential, and germination decreased as osmotic stress increased. However, crop-producing areas in central China receive an average of 600 to 1,600 mm of precipitation during spring and summer months, ensuring adequate soil moisture to support the emergence and growth of velvetleaf. Furthermore, seed germination of velvetleaf occurred over a broad pH range (5-10) and the soil of central China is slightly alkaline, with pH of 7-8. All these features could be attributed to its ability to colonize various habitats in this area. As an annual weed, velvetleaf reproduces by seed, and therefore prevention of seed production is the key to elimination of future dispersal. Crop growers need to adopt weed management programs to control velvetleaf early in the growing season to prevent seeds from developing and reducing soil seed bank.
ACKNOWLEDGEMENTS
The authors wish to acknowledge Yanrong Lu and Meirong Zhang for their assistance in collecting weed seeds and Bopeng Wang for his assistance in the laboratory. Financial support was provided by the Fundamental Research Funds for Central Public Welfare Research Institutes, China.
REFERENCES
- Akey AC, Jurik TW, Dekker J. A replacement series evaluation of competition between velvetleaf (Abutilon theophrasti) and soybean (Glycine max). Weed Res. 1991;31:63-72.
- Andrews TS. Factors affecting the germination of giant Parramatta grass. Aust J Exp Agric. 1997;37(4):439-46.
- Asgarpour R, Ghorbani R, Khajeh-Hosseini M, Mohammadvand E, Chauhan BS. Germination of spotted spurge (Chamaesyce maculata) seeds in response to different environmental factors. Weed Sci. 2015;63(2):502-10.
- Ballaré CL, Scopel AL, Sànchez RA, Radosevich SR. Photomorphogenic processes in the agricultural environment. Photochem Photobiol. 1992;56(5):777-88.
- Begonia GB, Aldrich RJ, Salisbury CD. Soybean yield and yield components as influenced by canopy heights and duration of competition of velvetleaf (Abutilon theophrasti Medik.). Weed Res. 1991;31(3):117-24.
- Benvenuti S, Macchia M. Calculation of threshold temperature for the development of various weeds. Agr Med. 1993;123:252-6.
- Benvenuti S, Macchia M. Quantitative analysis of emergence of seedlings from buried weed seeds with increasing soil depth. Weed Sci. 2001;49(4):528-35.
- Buhler DD, Hartzler RG. Emergence and persistence of seed of velvetleaf, common waterhemp, woolly cupgrass, and giant foxtail. Weed Sci. 2001;49(2):230-5.
- Chauhan BS, Gill G, Preston C. Factors affecting seed germination of annual sowthistle (Sonchus oleraceus) in southern Australia. Weed Sci. 2006;54(5):854-60.
- Chauhan BS, Johnson DE. Germination ecology of goosegrass (Eleusine indica): an important grass weed of rainfed rice. Weed Sci. 2008;56(5):699-706.
- Clay SA, Kleinjan J, Clay DE, Forcella F, Batchelor W. Growth and fecundity of several weed species in corn and soybean. Agron J. 2005;97(1):294-302.
- Cortés JA, Mendiola MA, Castejón M. Competition of velvetleaf (Abutilon theophrasti M.) weed with cotton (Gossypium hirsutum L.). economic damage threshold. Span J Agric Res. 2010;8(2):391-9.
- Davis AS, Renner KA. Influence of seed depth and pathogens on fatal germination of velvetleaf (Abutilon theophrasti) and giant foxtail (Setaria faberi). Weed Sci. 2007;55:30-5.
- Dempsey JM. Fiber crops. China Jute. Gainesville: The University of Florida press; 1975. p.397-413.
- Dong HZ, Kong XQ, Luo Z, Li WJ, Xin CS. Unequal salt distribution in the root zone increases growth and yield of cotton. Eur J Agron. 2010;33(4):285-92.
- Dong HZ, Li W, Tang W, Zhang D. Furrow seedling with plastic mulching increases stand establishment and lint yield of cotton in a saline field. Agron J. 2008;100(6):1640-6.
- Dorado J, Fernández-Quintanilla C, Grundy AC. Germination patterns in naturally chilled and non-chilled seeds of fierce thornapple (Datura ferox) and velvetleaf (Abutilon theophrasti). Weed Sci. 2009;57(2):155-62.
- Eslami SV. Comparative germination and emergence ecology of two populations of common lambsquarters (Chenopodium album) from Iran and Denmark. Weed Sci. 2011; 59:90-7.
- Heap I. International Survey of Herbicide Resistant Weeds. [accessed: Feb. 20, 2017] 20, 2017] http://www.weedscience.org/summary/home.aspx
» http://www.weedscience.org/summary/home.aspx - Holt JS, Boose AB. Potential for spread of Abutilon theophrasti in California. Weed Sci. 2000;48:43-52.
- Honarmand SJ, Nosratti I, Nazari K, Heidari H. Factors affecting the seed germination and seedling emergence of muskweed (Myagrum perfoliatum). Weed Biol Manag. 2016;16(4):186-93.
- James TK, Cooper JM. Control of the recently-introduced weed butterprint (Abutilon theophrasti) in maize. New Zeal Plant Prot. 2012;65:64-8.
- Kremer E, Lotz LAP. Germination and emergence characteristics of triazine-susceptible and triazine-resistant biotypes of Solanum nigrum J Appl Ecol. 1998a;35(2):302-10.
- Kremer E, Lotz LAP. Emergence depth of triazine susceptible and resistant Solanum nigrum seeds. Ann Appl Biol. 1998b;132(2):277-88.
- Leon RG, Knapp AD. Effect of temperature on the germination of common waterhemp (Amaranthus tuberculatus), giant foxtail (Setaria faberi), and velvetleaf (Abutilon theophrasti). Weed Sci. 2004;52:67-73.
- Li HL. The origin of cultivated plants in Southeast Asia. Econ Bot. 1970;24:3-19.
- Li Q, Tan J, Li W, Yuan G, Du L, Ma S et al. Effects of environmental factors on seed germination and emergence of Japanese brome (Bromus japonicus). Weed Sci. 2015;63(3):641-6.
- Lindquist JL, Mortensen DA, Clay SA, Schmenk R, Kells JJ, Howatt K et al. Stability of corn (Zea mays) - velvetleaf (Abutilon theophrasti) interference relationships. Weed Sci . 1996;44(2):309-13.
- Ma XY, Yang JY, Wu HW, Jiang WL, Ma YJ, Ma Y. Growth analysis of cotton in competition with velvetleaf (Abutilon theophrasti). Weed Technol. 2016;30:123-36.
- McDonald AJ, Riha SJ, Mohler CL. Mining the record: historical evidence for climatic influences on maize - Abutilon theophrasti competition. Weed Res. 2004;44:439-45.
- Mester TC, Buhler DD. Effects of soil temperature, seed depth, and cyanazine on giant foxtail (Setaria faberi) and velvetleaf (Abutilon theophrasti) seedling development. Weed Sci. 1991;39:204-9.
- Michel BE. Evaluation of the water potentials of solutions of polyethylene glycol 8000 both in the absence and presence of other solutes. Plant Physiol. 1983;72:66-70.
- Milberg P, Anderson L, Thompson K. Large-seeded species are less dependent on light for germination than small-seeded ones. Seed Sci Res. 2000;10:99-104.
- Nandula VK, Eubank TW, Koger CH, Reddy KN. Factors affecting germination of horseweed (Conyza canadensis). Weed Sci. 2006;54(5):898-902.
- Nurse RE, DiTommaso A. Corn competition alters the germinability of velvetleaf (Abutilon theophrasti) seeds. Weed Sci. 2005;53(4):479-88.
- Pareja MR, Staniforth DW. Seed-soil characteristics in relation to weed seed germination. Weed Sci. 1985;33:190-5.
- Pavlovic´ D, Vrbnièanin S, Bozic´ D, Simonèiè A. The study of methods for determination of metabolism based resistance ofAbutilon theophrastimedic. to atrazine. J Cent Eur Agric. 2007;8(4):435-42.
- Ramirez AHM, Jhala AJ, Singh M. Factors affecting germination of citronmelon (Citrullus lanatus var. citroides). Weed Sci. 2014;62:45-50.
- Roberts HA, Potter ME. Emergence patterns of weed seedlings in relation to cultivation and rainfall. Weed Res. 1980;20(6):377-86.
- Sadeghloo A, Asghari J, Ghaderi-Far F. Seed germination and seedling emergence of velvetleaf (Abutilon theophrasti) and barnyardgrass (Echinochloa crus-galli). Planta Daninha. 2013;31(2):259-66.
- Spencer NR. Velvetleaf, Abutilon theophrasti (Malvaceae), history and economic impact in the United States. Econ Bot. 1984;38(4):407-16.
- Stanton R, Wu H, Lemerle D. Factors affecting silverleaf nightshade (Solanum elaeagnifolium) germination. Weed Sci. 2012;60:42-7.
- Taab A, Andersson L. Seed dormancy dynamics and germination characteristics of Solanum nigrum Weed Res. 2009;49(5):490-8.
- Tanveer A, Farid MZ, Tahir M, Javaid MM, Khaliq A. Environmental factors affecting the germination and seedling emergence of Carthamus oxyacantha M. Bieb. (Wild safflower). Pak J Weed Sci Res. 2012;18(2):221-35.
- Traoré S, Mason SC, Martin AR, Mortensen DA, Spotanski JJ. Velvetleaf interference effects on yield and growth of grain sorghum. Agron J. 2003;95(6):1602-7.
- Wu Y, Wang YX, Xie XJ. Spatial occurrence and geochemistry of soil salinity in Datong basin, northern China. J Soil Sediment. 2014;14(8):1445-55.
- Zhao Y, Lu Z, He L. Effects of saline-alkaline stress on seed germination and seedling growth of Sorghum bicolor (L.) Moench. Appl Biochem Biotechnol. 2014;173(7):1680-91.
- Zhou JK, Deckard EL. Factors affecting eastern black nightshade (Solanum ptycanthum) seed germination. Weed Sci. 2005;53(5):651-6.
- Zhou JK, Deckard EL, Ahrens WH. Factors affecting germination of hairy nightshade (Solanum sarrachoides) seeds. Weed Sci. 2005;53:41-5.
- Ziska L. Observed changes in soyabean growth and seed yield from Abutilon theophrasti competition as a function of carbon dioxide concentration. Weed Res. 2012;53:140-5.
Publication Dates
-
Publication in this collection
2018
History
-
Received
06 July 2017 -
Accepted
29 Sept 2017












 Vertical bars represent standard errors of the means. Bars with the same letters are not significantly different (p=0.05).
Vertical bars represent standard errors of the means. Bars with the same letters are not significantly different (p=0.05).

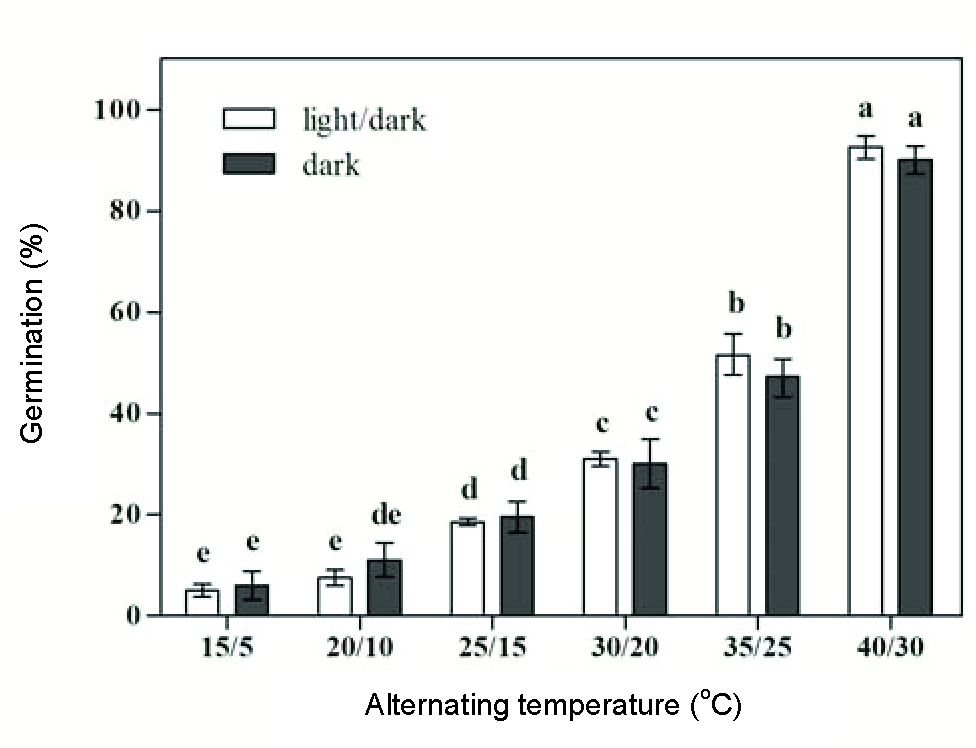 Vertical bars represent standard errors of the means. Bars with the same letters are not significantly different (p=0.05).
Vertical bars represent standard errors of the means. Bars with the same letters are not significantly different (p=0.05).




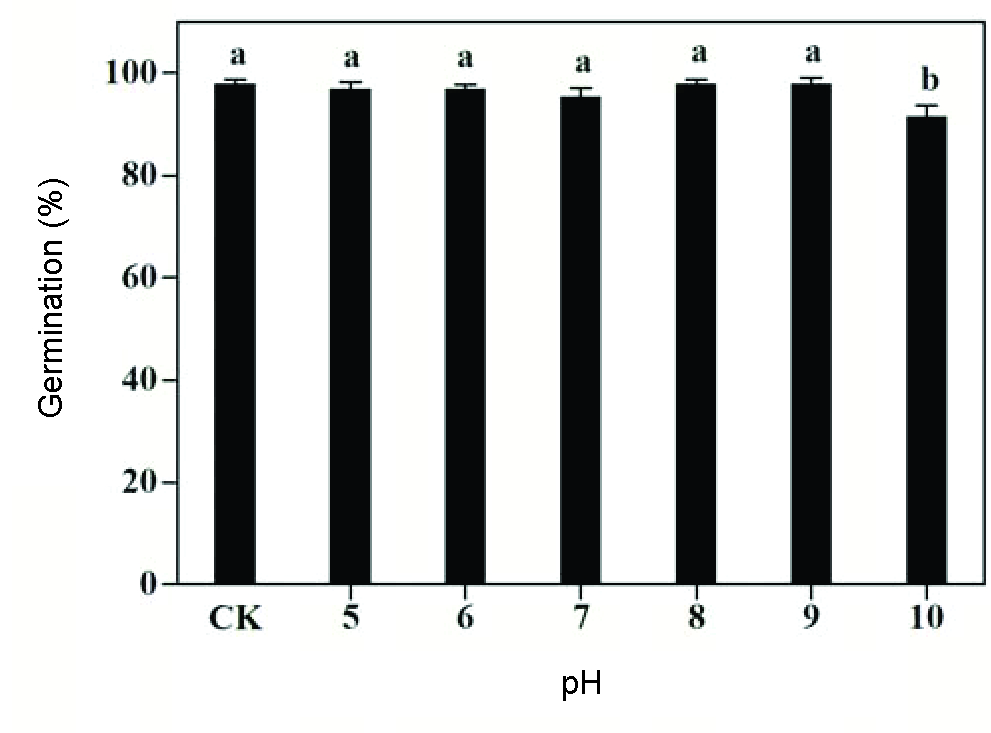 Bars with the same letters are not significantly different (p=0.05). Vertical bars represent standard errors of the means.
Bars with the same letters are not significantly different (p=0.05). Vertical bars represent standard errors of the means.
