ABSTRACT:
Our knowledge about seed dormancy breaking and environmental factors affecting seed germination of greater bur-parsley (Turgenia latifolia) is restricted. This study has addressed some seed dormancy breaking techniques, including different concentrations of gibberellic acid (GA3) and potassium nitrate (KNO3), leaching duration, physical scarification as well as some environmental factors effective on seed germination such as salt and drought stresses, pH and seed planting depth. Seed germination was promoted with lower concentrations of KNO3 (0.01 to 0.02 g L-1), while higher concentrations reduced germination percentage. Seed dormancy was declined by low concentrations of GA3 up to 100 ppm. Seeds of greater bur-parsley germinated in a range of pH from 3 to 7. With enhancement of drought and salt stresses, seed germination decreased. Also, there was no seed germination in a high level of stresses. Seedling emergence reduced as planting depth increased. Use of GA3, KNO3, leaching and physical scarification had a positive effect on seed dormancy breaking of greater bur-parsley. The information from the study increases our knowledge about seed dormancy breaking techniques, response of germination to drought and salt stresses and also determination of distribution regions of greater bur-parsley in the future.
Keywords:
environmental factors; gibberellic acid; physical scarification; salt and osmotic stresses
RESUMO:
O conhecimento sobre a quebra da dormência de sementes e fatores ambientais que afetam a germinação de sementes de Grande Bur-Parsley (Turgenia latifolia) é restrito. Neste estudo, foram investigadas algumas técnicas de quebra da dormência de sementes, incluindo diferentes concentrações de ácido giberélico (GA3) e nitrato de potássio (KNO3), durações de lixiviação, escarificação física, bem como alguns fatores ambientais efetivos na germinação de sementes, como estresse salino e seca, pH e profundidade de plantio semente. A germinação de sementes foi promovida com as menores concentrações de KNO3 (0,01 a 0,02 g L-1), enquanto as maiores concentrações reduziram a porcentagem de germinação. A dormência da semente foi diminuída por baixas concentrações de GA3 até 100 ppm. As sementes de Grande Bur-Parsley germinaram em pH na faixa de 3 a 7. Com o aumento dos estresses de seca e sal, a germinação de sementes diminuiu. Além disso, não houve germinação de sementes em alto nível de estresse. A emergência de plântulas se reduziu à medida que se aumentou a profundidade de plantio. O uso de GA3, KNO3, a lixiviação e a escarificação física afetaram positivamente a quebra de dormência de sementes de Grande Bur-Parsley. Os resultados deste estudo aumentam nosso conhecimento sobre técnicas de quebra de dormência de sementes, resposta de germinação à seca e estresses de sal e também sobre a determinação de regiões de distribuição de Grande Bur-Parsley no futuro.
Palavras-chave:
fatores ambientais; ácido giberélico; escarificação física; estresse salino e osmótico
INTRODUCTION
Greater bur-parsley (Turgenia latifolia (L.) Hoffmann) is an annual plant that belongs to the Apiaceae family. The seeds of this plant can germinate in the autumn and the flowers appear in the spring. Greater bur-parsley is common in natural ecosystems, orchards, crops and vegetables in Northern Provinces of Iran (Karimi, 2001Karimi H. Weeds of Iran. 2nd.ed. Center for University Publications; 2001. p.347.).
Dormancy is considered as a temporary suspension of growth of any plant structure containing a meristem (Lang et al., 1987Lang GA, Early JD, Martin GC, Darnell RL. Endo-, para-, and ecodormancy: physiological terminology and classification for dormancy research. Hort Sci. 1987;22:371-7.). These phenomena can help plants to adapt to a variety of habitats and climates and survive in diferent ecosystems. Seed dormancy can serve to synchronize germination so that probability of seedling survival is optimized (Baskin and Baskin, 1998Baskin CC, Baskin JM. Seeds-ecology, biogeography, and evolution of dormancy and germination. San Diego: Academic Press; 1998.).
Seed dormancy is genetically determined through environmental factors and/or plant hormones such as abscisic acid (ABA) (Graeber et al., 2010Graeber K, Linkies A, Müller K, Wunchova A, Rott A, Leubner-Metzger G. Cross-species approaches to seed dormancy and germination: conservation and biodiversity of ABA-regulated mechanisms and the Brassicaceae DOG1 genes. Plant Mol Biol. 2010;73:67-87.). Many chemicals and growth regulator treatments can overcome seed dormancy without after-ripening (Bewley and Black, 1994Bewley JD, Black M. Seeds: Physiology of development and germination. New York: Plenum; 1994.). Gibberellic acid plays a key role in dormancy breaking of seeds and promotion of germination (Kucera et al., 2005Kucera B, Cohn MA, Leubner-Metzger G. Plant hormone interactions during seed dormancy release and germination. Seed Sci Res. 2005;15:281-307.). Also, some free-living soil bacteria known as plant growth-promoting rhizobacteria (PGPR), including Acetobacter, Arthrobacter, Azospirillium, Azotobacter and Bacillus (Sudhakar et al., 2000Sudhakar P, Chattopadhyay GN, Gangwar SK, Ghosh JK. Effect of foliar application of Azotobacter, Azospirillum and Beijerinckia on leaf yield and quality of mulberry (Morus alba). J Agric Sci. 2000;134:227-34.) either directly or indirectly facilitate seed germination and growth of plants by releasing phytohormones in the soil.
Seed germination is imposed by several environmental factors. Optimum temperature, light, pH, soil moisture and burial depth play a decisive role in seed germination (Egley and Duke, 1985Egley GH, Duke SO. Physiology of weed seed dormancy and germination. In: Duke, SO editor. Weed physiology. Reproduction and ecophysiology. Boca Raton: CRC; 1985. p.27-64 ). The impact of soil moisture on germination of plant species depends on rainfall, temperature, and soil type. Plant-available water in the soil lies between field capacity (20.03 MPa) and permanent wilting point (21.5 MPa) (Miller and Donahue, 2004Miller RW, Donahue L. Soil water properties: In Soils in our environment. New Jersey: Upper Saddle River, Prentice Hall; 2004. p.62-97.), and reduction of soil moisture potential can be considered as an obstacle to germination.
Little information is available on seed dormancy breaking and germination requirements of greater bur-parsley. Therefore, the objectives of this study were to investigate: 1) the effects of some chemichal, physical and biological factors on seed dormancy breaking and 2) some environmental factors which influence the germination of greater bur-parsley.
MATERIALS AND METHODS
Seed collection
Seeds of greater bur-parsley were harvested from 500 natural ripened plants in crop fields at Islamic Azad University, Qaemshahr Branch, Mazandaran province, Iran in June, 2012. Seed were cleaned and stored in paper bags and kept at 20?5 oC in the lab until the beginning of the experiments in November, 2012. Thousand-seed weight, seed length and width were determined. The seeds were tested for viability with the use of 1% tetrazolium chloride solution before each trial (Peters, 2000Peters J. Association of Official Seed Analysis Tetrazolium Testing Handbook. Lincoln: Association of Official Seed Analysis; 2000. (Contribution, 29)).
General germination test
Seeds were sterilized with soaking in 1% sodium hypochlorite solution for one minute and then rinsed several times with deionized water. Thirty seeds were placed on two layers of filter paper in a 9 cm plastic petri dish. The filter paper was moistened with 6 mL of deionized water. Petri dishes were sealed with Parafilm to inhibit moisture loss and placed in a germinator at 25/15 ?C (day/night). The photoperiod was set at 12/12 hours (day/night). Fluorescent lamps were used to supply light intensity of 300 μmmol m-2 s-1. For complete darkness, petri dishes were covered with two layers of aluminium foil. The number of germinated seeds was calculated 15 days after the start of the test. The seeds were determined as germinated as they produced visible radicle higher than 1mm length.
Dormancy breaking treatments
Impact of KNO3 on seed dormancy
The influence of KNO3 concentration on greater bur-parsley seed dormancy breaking was investigated by 0, 0.01, 0.02, 0.04, 0.08 and 0.16 g L-1 KNO3 solutions. Seeds were sterilized with 1% sodium hypochlorite solution for one minute and then rinsed with deionized water. Thirty seeds were placed on two layers of filter paper in petri dishes and treated with a 6 mL KNO3 treatment solution. Petri dishes were sealed with Parafilm and placed in a germinator at 25/15 ?C (day/night) at a photoperiod that was set at 12/12 hours (day/night) with light intensity of 300 µmol m-2 s-1. Germinated seeds were calculated after 15 days.
Impact of GA3 and light duration on seed dormancy
An evaluation was made of the influence of different concentrations of GA3 (including 0, 50, 100, 150, 200 and 250 ppm) both at light/dark (12/12h) and complete darkness regimes on seed dormancy breaking. The sterilized seeds were placed in petri dishes on two layers of filter papers and moistened with 6 mL GA3 solutions. The dishes were sealed with Parafilm and incubated at a temperature of 25/15 ?C (day/night) and a 12/12 hour (day/night) photoperiod with light intensity of 300 µmol m-2 s-1 for light/dark regime. For the complete darkness regime, the petri dishes were completely covered by two layers of aluminum foil to prevent light penetration. The germinated seeds were calculated after 15 days.
Impact of nitroxin on seed dormancy
Nitroxin is a biofertilizer that contains plant growth promoting rhizobacteria, including Azotobacter and Azospirillum. This study investigated the effect of nitroxin as a biological agent to induce seed germination of greater bur-parsley. The treatments included control, 20% and 40% concentrations of nitroxin. The seeds were sterilized and placed on two layers of filter paper and treated with 6 ml nitroxin solutions. Petri dishes were incubated at a temperature of 25/15 ?C (day/night) and a 12/12 hour (day/night) photoperiod with light intensity of 300 µmol m-2 s-1. The germinated seeds were evaluated after 15 days.
Impact of ethanol on seed dormancy
An evaluation was made of the influence of different concentrations of ethanol 95% (MERCK, Germany) (Bewley and Black, 1982Bewley JD, Black M. Physiology and biochemistry of seeds in relation to germination. Viability, dormancy and environmental control. New York: Springer-Verlag; 1982. v.2.), including 0, 5, 10, 15 and 20%, on seed dormancy. Thirty sterilized seeds were placed on two layers of filter paper containing 6 ml treatment solutions. Petri dishes were incubated at a temperature of 25/15 ?C (day/night) and a 12/12 hour (day/night) photoperiod with light intensity of 300 mmol m-2 s-1. Seed germination of greater bur-parsley was evaluated after 15 days.
Impact of mechanical scarification on seed dormancy
Seeds were scarified by rubbing between two layers of sandpaper for 1, 2 and 3 minutes. The seeds were sterilized and placed on two layers of filter paper and moistened with 6 mL deionized water. Dishes were sealed with Parafilm and incubated at a fluctuating day/night temperature of 25/15 ?C in a 12/12 hour photoperiod. Light intensity was set at 300 µmol m-2 s-1. Germination was evaluated after 15 days.
Impact of environmental factors on seed germination
Impact of drought stress on seed germination - An evaluation was made of the effect of drought stress with different osmotic potentials, including 0, -0.1, -0.2, -0.4, -0.6, -0.8 and -1 MPa, on seed germination of greater bur-parsley. Solutions were prepared by dissolving 0, 4.34, 7.73, 10.16, 12.81, 15.06 and 17.04 g of polyethylene glycol (PEG 6000) in 100 mL deionized water, respectively. The seeds were sterilized and then moistened with 6 mL polyethylene glycol and placed in a germinator at a temperature of 25/15 ?C and a 12/12 hour photoperiod with light intensity of 300 µmol m-2 s-1. The germinated seeds were counted after 15 days.
Impact of salt stress on seed germination - The impact of salt stress on seed germination was studied by using solutions containing 0, 25, 50, 100, 200 and 400 mM NaCl (Merck, Germany). The seeds were treated with 6 mL saline solutions. Petri dishes were sealed with Parafilm and incubated at a temperature of 25/15 ?C and a 12/12 hour photoperiod with light intensity of 300 µmol m-2 s-1. The germinated seeds were evaluated after 15 days.
Impact of pH on seed germination - To evaluate the effect of pH, buffer solutions of pH including 3, 5, 7, 9 and 11 were prepared according to the method of Chachalis and Reddy (2000Chachalis D, Reddy KN. Factors affecting Campsis radicans seed germination and seedling emergence. Weed Sci. 2000;48:212-6.). The seeds were treated with 6 mL pH solutions and isolated petri dishes were incubated at a 12/12 hour photoperiod and temperature of 25/15 ?C with light intensity of 300 µmol m-2 s-1. The germinated seeds were counted after 15 days.
Impact of leaching duration on germination - To study the effect of leaching duration on germination of greater bur-parsley, thirty seeds were placed in polyethylene bags and were leached for 12, 24, 48 and 96 hours. Water temperature was 10?3 ?C. The bags were exhumed and the seeds were sterilized and incubated at a 12/12 hour photoperiod with temperature of 25/15 ?C and light intensity of 300 µmol m-2 s-1. Seed germination was evaluated after 15 days.
Impact of burial depth and flooding duration on seed germination - Thirty seeds were placed in polyethylene bags and buried in the soil in plastic pots (20 cm by 30 cm) at depths of 0, 5 and 10 cm and flooded for 2 and 4 weeks. The pots were placed outdoors and kept flooded at the durations. Bags were exhumed from the pots and rinsed in tap water. In each bag, the germinated seeds were counted. The non-germinated seeds were placed in petri dishes and incubated in a 12/12 hour photoperiod at a temperature of 25/15 ?C and light intensity of 300 μmol m-2 s-1. The germinated seeds were counted after 15 days. Total germination percentage was the sum of germinated seeds in the pots and germinated seeds after incubation.
Impact of sowing depth on seedling emergence
Forty seeds of greater bur-parsley were planted in 20 cm diam plastic pots at depths of 0, 4, 8 and 12 cm. The control pots were considered to indicate that there was no background seed bank of greater bur-parsley in the soil. The soil used for this experiment was a silty soil with clay 26.30%; silt 55.70%; sand 18.00%; pH 7.6; and organic carbon 0.63%. The pots were irrigated as needed to maintain soil moisture at field capacity. Greenhouse temperature was set at 25/15 ?C (day/night) with a natural photoperiod. Seedling emergence was recorded as the appearance of the two cotyledons and was evaluated 45 days after planting.
Data analysis
All the experiments were set as a complete randomized design with 3 replicates. Each experiment was repeated twice. The experimental data were combined as there were no significant differences over time. The data were subjected to arcsin transformation to improve homogenity and transformed data were used for statistical analysis.
Regression analysis was used to determine the effect of salinity and drought stress on seed germination. ANOVA was performed on data about KNO3, GA3, nitroxin, ethanol, mechanichal scarification, pH, leaching duration, flooding duration, and sowing depth experiments with the the SAS software using PROC GLM. Means were separated by either standard error bars and using the LSD test at P=0.05.
RESULTS AND DISCUSSION
General seed traits
Thousand-seed weight, seed length and width of greater bur-parsley were 4.20 g, 4.93 mm and 1.31 mm, respectively. According to the tetrazolium chloride test, seed viability was 100%. Initial seed germination in light/dark and complete darkness was 54.51% and 51.56%, respectively.
Seed dormancy breaking treatments
Potassium nitrate
The effect of KNO3 on seed germination of greater bur-parsley was significant (Table 1). Enhancement of KNO3 concentration from 0.01 g L-1 to 0.04 g L-1 markedly promoted germination of greater bur-parsley seeds (32.71%). The concentrations of KNO3 above 0.04 g L-1 reduced germination and reached 5.79% at 0.16 g L-1 (Figure 1). Nitrogen-containing compounds such as nitrite, nitrate, nitrogen dioxide, ammonium, azide, and cyanide are used as seed dormancy braeking treaments (Bradford et al., 2007Bradford KJ, Nonogaki H. Seed development, dormancy and germination. Oxford: Blackwell; 2007.). Potassium nitrate is well documented as a compound, which increases the germination of photo-dormant seeds (Shanmugavalli et al., 2007Shanmugavalli M, Renganayaki PR, Menaka C. Seed dormancy and germination improvement treatments in fodder sorghum. Int Crops Res Inst Semi-Arid Tropics. 2007;3:1-3.). Bewley and Black (1994Bewley JD, Black M. Seeds: Physiology of development and germination. New York: Plenum; 1994.) indicated that KNO3 raises the ambient oxygen levels by making less oxygen available for citric acid cycle.
Effect of KNO3 concentrations on seed germination of greater bur-parsley after 15 days of incubation at 25/15 ºC day/night temperatures and a 12 hour photoperiod.
Gibberellic acid and light duration
In complete darkness, increasing GA3 concentration from 50 up to 100 ppm increased germination compared to control. However, in a higher concentration of GA3 than those of 100 ppm, seed germination was reduced (Figure 2). Gibberellic acid regulates seed dormancy and germination positively by means of a complex interaction with abscisic acid and environmental conditions (Kucera et al., 2005Kucera B, Cohn MA, Leubner-Metzger G. Plant hormone interactions during seed dormancy release and germination. Seed Sci Res. 2005;15:281-307.). Gonai et al. (2004Gonai T, Kawahara S, Tougou M, Satoh S, Hashiba T, Hirai N et al. Abscisic acid in the thermoinhibition of lettuce seed germination and enhancement of its catabolism by gibberellin. J Exp Bot. 2004;55:111-8.) reported that GA3 affects temperature responsiveness of the seed germination process through ABA metabolism. Also, germination of seeds in the darkness regime at different concentrations of GA3 was higher than in the light/dark regime (Figure 2). Gibberellic acid increased germination of dark-moistened non-photodormant tobacco seeds. Thus, GA3 releases dormancy and promotes germination rate and onset (Kamiya and Garcia-Martinez, 1999Kamiya Y, Garcia-Martinez JL. Regulation of gibberellin biosynthesis by light. Curr Opin Plant Biol. 1999;2:398-403.; Yamaguchi and Kamiya, 2002Yamaguchi S, Kamiya Y. Gibberellins and lightstimulated seed germination. J Plant Growth Regul. 2002;20:369-76.). Our result suggests that soaking of greater bur-parsley seeds with 100 ppm GA3 in the complete darknesss regime is more effective than that of the treatment of seeds with GA3 in the alternating photoperiod.
Effect of GA3 concentrations on seed germination of greater bur-parsley after 15 days of incubation at 25/15 ºC day/night temperatures and a 12 hour photoperiod.
Nitroxin
Application of nitroxin did not significantly influence germination of greater bur-parsley seeds (Table 1). Increased nitroxin concentration did not significantly enhance germination (Data not shown). Egamberdiyeva (2007Egamberdiyeva D. The effect of plant growth promoting bacteria on growth and nutrient uptake of maize in two different soils. Appl Soil Ecol. 2007;36:184-9.) showed that microorganisms can promote germination and growth of different plant species. Vrbnicanin et al. (2011Vrbnicanin S, Bozic D, Saric M, Pavlovic D, Raicevic V. Effect of plant growth promoting rhizobacteria on Ambrosia artemisiifolia L. seed germination. Pestic Phytomed (Belgrade). 2011;26:141-6.) showed that rhizobacteria had diverse effects (stimulative or inhibitory) on seed germination of Ambrosia artemisiifolia. Azotobacter and Azospirillum containing in nitroxin effect on seed germination through synthesis of plant growth promoters and plant growth regulators (Vrbnicanin et al., 2011).
Ethanol
Ethanol significantly influenced germination of greater bur-parsley seeds (Table 1). Seed germination was promoted by 0.5% ethanol. Nevertheless, in concentrations of ethanol higher than 0.5%, seed germination decreased (Figure 3). Our results are similar to those of Johnson (2012Johnson F. The effects of ethanol on the seed germination rates of vegetables at varying concentrations. California State Science Fair, 2012, Project summary, California, USA.), who found that an ethanol concentration lower than 0.5% was effective in seed dormancy breaking. However, any benefits from ethanol disappeared quickly at a higher concentration. Our findings are also in agreement with the conclusion of Rezvani and Fani Yazdi (2013Rezvani M, Fani Yazdi SA. Factors affecting seed germination of black nightshade (Solanum nigrum). Acta Bot Hung. 2013;55:397-408.), who showed that germination of black nightshade (Solanum nigrum) was increased at low concentrations of ethanol but inhibited at higher concentrations.
Effect of ethanol concentrations on seed germination of greater bur-parsley after 15 days of incubation at 25/15 ºC day/night temperatures and a 12 hour photoperiod.
Scarification duration
Scarification duration significantly influenced seed germination (Table 1). The dormancy of greater bur-parsley was markedly broken by physical scarification of seed coat. Compared with the control treatment, enhancement of scarification duration increased seed germination by about 27% (Figure 4). For many species, mechanical scarification of seeds has been proved to break dormancy and promote germination (Fang et al., 2006Fang S, Wang J, Wei Z, Zhu Z. Methods to break seed dormancy in Cyclocaria paliurus (Batal). Iljinskaja, Sci Hortici. 2006;110:305-9.). These results are in accordance with the studies of Silva et al. (2004Silva EA, Toorop PE, van Aelst AC, Hilhorst HW. Abscisic acid controls embryo growth potential and endosperm cap weakening during coffee (Coffea arabica cv. Rubi) seed germination. Planta. 2004;220:251-61.), Pinto et al. (2007Pinto LVA, Silva EAA, Davide AC, Jesus VAM, Toorop PE, Hilhorst EWM. Mechanism and control of Solanum lycocarpum seed germination. Ann Bot. 2007;100:1175-87.) and Sánchez et al. (1990Sánchez RA, Sunell L, Labavitch JM, Bonner BA. Changes in the endosperm cell walls of two Datura species before radicle protrusion. Plant Physiol. 1990;93:89-97.) about different plant species.
Effect of scarification duration on seed dormancy breaking of greater bur-parsley after 15 days of incubation at 25/15 ºC day/night temperatures and a 12 hour photoperiod.
Leaching duration
Leaching duration influenced germination (Table 1). In comparison with the control treatment, the increase in leaching duration partially increased seed germination. Germination of seeds that were imposed to 96-hour leaching duration increased about 13.41% compared with control (Figure 5). Leaching can be considered as a seed treatment procedure to release dormancy. Badgery et al. (1999Badgery W. et al. A simple method to break the dormancy of saffron thistle seed. In: Bishop AC, Boersma M, Barnes CD. Twelfth Australian Weeds Conference, Tasmania, Hobart, Australia: 1999. p.176-77.) showed that 48-hour leaching of saffron thistle (Carthamus lanatus) seeds increased germination. A previous study by Ansley and Abernethy (1985Ansley RJ, Abernethy RH. Environmental factors influencing gardner saltbush seed dormancy alleviation. J Range Manage. 1985;38:331-5.) showed that leachate seeds of gardner saltbush (Atriplex gardneri) germinated more than those of non-leachate ones.
Effect of leaching duration on seed germination of greater bur-parsley after 15 days of incubation at 25/15 ºC day/night temperatures and a 12 hour photoperiod.
Environmental treatments
Osmotic stress
Seed germination of greater bur-parsley decreased as poly ethylene glycol concentration increased (Figure 6). Germination was 57.8% when osmotic potential was -0.2 MPa, but enhancement of osmotic stress from -0.2 MPa up to -0.8 MPa markedly reduced germination, and then seed germination reached zero at a range of -1 to -1.2 MPa osmotic potential (Figure 6). A negative effect of osmotic stress on seed germination was reported in different plants including shepherd’s purse (Capsella bursapastoris) (Rezvani et al., 2014Rezvani M, Zaefarian F, Amini V. Effect of chemical treatments and environmental factors on seed dormancy and germination of Shepherd’s purse (Capsella bursapastoris (L.) Medic.). Acta Bot Bras. 2014;28:495-501. ), black nightshade (Rezvani and Fani Yazdi, 2013Yazdi SAF, Rezvani M, Mohassel MHR, Ghanizadeh H. Factors affecting seed germination and seedling emergence of sheep sorrel (Rumex acetosella). Romanian Agric Res. 2013;30:373-80.) and sheep sorrel (Rumex acetosella) (Fani Yazdi et al., 2013). In the north of Iran, greater bur-parsley seed germination occurs from mid November. With the climate change and changes of rainfall patterns in fall season, low rain following to the temporary drought stress that maybe affect on greater bur-parsley seed germination and establishement.
Effect of osmotic stress on seed germination of greater bur-parsley after 15 days of incubation at 25/15 ºC day/night temperatures and a 12 hour photoperiod.
Salt stress
A polynomial model was fitted for the effect of salt stress on seed germination. A partial reduction in seed germination was observed compared with control, when NaCl concentration increased from 0 to 50 mM. Germination markedly decreased with increasing of NaCl concentration from 50 mM. No seed germination was occured at a NaCl concentration of 400 mM (Figure 7). Germination of 26.81% of seeds at NaCl concentration of 200 mM indicates that even at high soil salinity, a portion of greater bur-parsley seeds is able to germinate. This trait could be important for a wide spread of the plant into the region of north Iran which is subjected to salinity.
Effect of salt stress on seed germination of greater bur-parsley after 15 days of incubation at 25/15 ºC day/night temperatures and a 12 hour photoperiod.
pH
The effect of pH on greater bur-parsley germination was significant (Table 1). Maximum seed germination occurred at pH 7 (Figure 8). A low percentage of seed germination was found at pH 9 and 11. Seed germination at pH 3 and 5 was 42.81% and 40.28%, respectively, which was significantly lower than germination at pH 7 (Figure 8). The results of Pérez-Fernández et al. (2006Pérez-Fernández MA, Calvo-Magro E, Montanero-Fernández J, Oyola-Velasco JA. Seed germination in response to chemicals: Effect of nitrogen and pH in the media. J Environ Biol. 2006;27:13-20.) showed that three apiaceae species including Foeniculum vulgare Miller, Daucus carota and Thapsia villosa had high germination at a pH range from 4.7 to 7.7. Pérez-Fernández and Rodríguez-Echeverría (2003Pérez-Fernández MA, Rodríguez-Echeverría S. Effect of smoke, charred wood and nitrogenous compounds on seed germination of ten species from woodland in Central-Western Spain. J Chem Ecol. 2003;29:237-51.) also showed that seed germination of annual and perennial plants at acidic pH was higher than those of alkaline ones. Mayer and Poljakoff-Mayber (1989Mayer AM, Poljakoff-Mayber A. The germination of seeds. Oxford: Pergamon Press; 1989.) showed that in high values of pH, germination declines by means of several mechanisms of action. High germination of greater bur-parsley seeds at acidic pH could be significant for adaptation and distribution of greater bur-parsley in acidic soils.
Effect of pH on seed germination of greater bur-parsley after 30 days of incubation at 25/15 ºC day/night temperatures and a 12 hour photoperiod.
Burial depth and flooding duration
Burial depth and flooding for 15 and 30 days significantly influenced germination (Table 1). After 15 days of flooding duration, germination of seeds at all burial depths increased in comparison with control. Maximum germination was found in seeds that had been buried at a depth of 10 cm (Figure 9). Germination of seeds buried for 30 days was reduced by enhancement of burial depth to 5 cm. An enhancement was occurred by increasing burial depth to 10 cm, but it was not significant in comparison with control (Figure 9).
Effect of burial depth and flooding duration on seed germination of greater bur-parsley after 30 days of incubation at 25/15 ºC day/night temperatures and a 12 hour photoperiod.
Sowing depth
Sowing depth significantly influenced seedling emergence (Table 1). By increasing planting depth, seedling emergence of greater bur-parsley was decreased (Figure 10). There was maximum seedling emergence in seeds that had been planted on the soil sourface while 13.92% of seeds were emerged at the depth of 12 cm (Figure 10). Higher seedling emergence of greater bur-parsley planted on the soil surface is due to the germination promotion effect of light, which is a vital factor for seeds with low carbohydrate storage. Rezvani et al. (2014Rezvani M, Zaefarian F, Amini V. Effect of chemical treatments and environmental factors on seed dormancy and germination of Shepherd’s purse (Capsella bursapastoris (L.) Medic.). Acta Bot Bras. 2014;28:495-501. ) showed that seedling emergence of shepherd’s purse was reduced as seed planting depth was increased. Thomas et al. (2006Thomas WE, Burke IC, Spears JE. Influence of environmental factors on slender amaranth (Amaranthus viridis) germination. Weed Sci. 2006;54:316-20. ); Rezvani and Fani Yazdi (2013Yazdi SAF, Rezvani M, Mohassel MHR, Ghanizadeh H. Factors affecting seed germination and seedling emergence of sheep sorrel (Rumex acetosella). Romanian Agric Res. 2013;30:373-80.) and Fani Yazdi et al. (2013) also suggested that light in small seeds with low levels of carbohydrate reserves is a limiting factor to germination.
Effect of sowing depth on seedling emergence of greater bur-parsley. Means followed by the same letter are not significantly different according to the LSD test.
Application of lower concentrations of KNO3 (0.04 g L-1), GA3 (100 ppm) and ethanol (5%) could be a successful strategy for seed dormancy breaking and induction of germination of greater bur-parsley. Also, leaching and scarification of seeds increased germination. Both drought and salt stresses negatively affected seed germination. There was maximum percentage of seed germination in a range of pH from 3 to 7, which shows that distribution of greater bur-parsley can occur in soil with pH from 3 to 7. Data from the effect of sowing depth suggest that seedling emergence of greater bur-parsley was reduced as planting depth increased. Light and water conditions of soil and seed carbohydrate reserves are effective factors in seed germination at different soil depths. This result indicates that a depth tillage of soil above 12 cm may be an effective approach to reduce seedling emergence in crops.
REFERENCES
- Ansley RJ, Abernethy RH. Environmental factors influencing gardner saltbush seed dormancy alleviation. J Range Manage. 1985;38:331-5.
- Badgery W. et al. A simple method to break the dormancy of saffron thistle seed. In: Bishop AC, Boersma M, Barnes CD. Twelfth Australian Weeds Conference, Tasmania, Hobart, Australia: 1999. p.176-77.
- Baskin CC, Baskin JM. Seeds-ecology, biogeography, and evolution of dormancy and germination. San Diego: Academic Press; 1998.
- Bewley JD, Black M. Seeds: Physiology of development and germination. New York: Plenum; 1994.
- Bewley JD, Black M. Physiology and biochemistry of seeds in relation to germination. Viability, dormancy and environmental control. New York: Springer-Verlag; 1982. v.2.
- Bradford KJ, Nonogaki H. Seed development, dormancy and germination. Oxford: Blackwell; 2007.
- Chachalis D, Reddy KN. Factors affecting Campsis radicans seed germination and seedling emergence. Weed Sci. 2000;48:212-6.
- Silva EA, Toorop PE, van Aelst AC, Hilhorst HW. Abscisic acid controls embryo growth potential and endosperm cap weakening during coffee (Coffea arabica cv. Rubi) seed germination. Planta. 2004;220:251-61.
- Egamberdiyeva D. The effect of plant growth promoting bacteria on growth and nutrient uptake of maize in two different soils. Appl Soil Ecol. 2007;36:184-9.
- Egley GH, Duke SO. Physiology of weed seed dormancy and germination. In: Duke, SO editor. Weed physiology. Reproduction and ecophysiology. Boca Raton: CRC; 1985. p.27-64
- Fang S, Wang J, Wei Z, Zhu Z. Methods to break seed dormancy in Cyclocaria paliurus (Batal). Iljinskaja, Sci Hortici. 2006;110:305-9.
- Yazdi SAF, Rezvani M, Mohassel MHR, Ghanizadeh H. Factors affecting seed germination and seedling emergence of sheep sorrel (Rumex acetosella). Romanian Agric Res. 2013;30:373-80.
- Gonai T, Kawahara S, Tougou M, Satoh S, Hashiba T, Hirai N et al. Abscisic acid in the thermoinhibition of lettuce seed germination and enhancement of its catabolism by gibberellin. J Exp Bot. 2004;55:111-8.
- Graeber K, Linkies A, Müller K, Wunchova A, Rott A, Leubner-Metzger G. Cross-species approaches to seed dormancy and germination: conservation and biodiversity of ABA-regulated mechanisms and the Brassicaceae DOG1 genes. Plant Mol Biol. 2010;73:67-87.
- Johnson F. The effects of ethanol on the seed germination rates of vegetables at varying concentrations. California State Science Fair, 2012, Project summary, California, USA.
- Karimi H. Weeds of Iran. 2nd.ed. Center for University Publications; 2001. p.347.
- Kamiya Y, Garcia-Martinez JL. Regulation of gibberellin biosynthesis by light. Curr Opin Plant Biol. 1999;2:398-403.
- Kucera B, Cohn MA, Leubner-Metzger G. Plant hormone interactions during seed dormancy release and germination. Seed Sci Res. 2005;15:281-307.
- Lang GA, Early JD, Martin GC, Darnell RL. Endo-, para-, and ecodormancy: physiological terminology and classification for dormancy research. Hort Sci. 1987;22:371-7.
- Mayer AM, Poljakoff-Mayber A. The germination of seeds. Oxford: Pergamon Press; 1989.
- Miller RW, Donahue L. Soil water properties: In Soils in our environment. New Jersey: Upper Saddle River, Prentice Hall; 2004. p.62-97.
- Pérez-Fernández MA, Calvo-Magro E, Montanero-Fernández J, Oyola-Velasco JA. Seed germination in response to chemicals: Effect of nitrogen and pH in the media. J Environ Biol. 2006;27:13-20.
- Pérez-Fernández MA, Rodríguez-Echeverría S. Effect of smoke, charred wood and nitrogenous compounds on seed germination of ten species from woodland in Central-Western Spain. J Chem Ecol. 2003;29:237-51.
- Peters J. Association of Official Seed Analysis Tetrazolium Testing Handbook. Lincoln: Association of Official Seed Analysis; 2000. (Contribution, 29)
- Pinto LVA, Silva EAA, Davide AC, Jesus VAM, Toorop PE, Hilhorst EWM. Mechanism and control of Solanum lycocarpum seed germination. Ann Bot. 2007;100:1175-87.
- Rezvani M, Fani Yazdi SA. Factors affecting seed germination of black nightshade (Solanum nigrum). Acta Bot Hung. 2013;55:397-408.
- Rezvani M, Zaefarian F, Amini V. Effect of chemical treatments and environmental factors on seed dormancy and germination of Shepherd’s purse (Capsella bursapastoris (L.) Medic.). Acta Bot Bras. 2014;28:495-501.
- Sánchez RA, Sunell L, Labavitch JM, Bonner BA. Changes in the endosperm cell walls of two Datura species before radicle protrusion. Plant Physiol. 1990;93:89-97.
- Shanmugavalli M, Renganayaki PR, Menaka C. Seed dormancy and germination improvement treatments in fodder sorghum. Int Crops Res Inst Semi-Arid Tropics. 2007;3:1-3.
- Sudhakar P, Chattopadhyay GN, Gangwar SK, Ghosh JK. Effect of foliar application of Azotobacter, Azospirillum and Beijerinckia on leaf yield and quality of mulberry (Morus alba). J Agric Sci. 2000;134:227-34.
- Thomas WE, Burke IC, Spears JE. Influence of environmental factors on slender amaranth (Amaranthus viridis) germination. Weed Sci. 2006;54:316-20.
- Vrbnicanin S, Bozic D, Saric M, Pavlovic D, Raicevic V. Effect of plant growth promoting rhizobacteria on Ambrosia artemisiifolia L. seed germination. Pestic Phytomed (Belgrade). 2011;26:141-6.
- Yamaguchi S, Kamiya Y. Gibberellins and lightstimulated seed germination. J Plant Growth Regul. 2002;20:369-76.
-
ERRATUM
In the article “Factors Affecting Seed Dormancy and Germination of Greater Bur-Parsley (Turgenia latifolia)”, DOI http://dx.doi.org/10.1590/S0100-83582018360100125, published in the Planta Daninha Journal, volume 36, p. 1 to 12,on the page 1 Where you read: “Received: August 18, 2016 Approved: November 30, 2016”It should be read: “Received: November 30, 2016 Approved: August 24, 2017”on the pages 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, and 12 Where you read: “AREZVANI, M., SADATIAN, S.A., and NIKKHAHKOUCHAKSARAEI, H.”It should be read: “REZVANI, M., SADATIAN, S.A., and NIKKHAHKOUCHAKSARAEI, H.”
Publication Dates
-
Publication in this collection
2018
History
-
Received
30 Nov 2016 -
Accepted
24 Aug 2017












 Means followed by the same letter are not significantly different according to the LSD test. Vertical bars represent standard errors of the means.
Means followed by the same letter are not significantly different according to the LSD test. Vertical bars represent standard errors of the means.
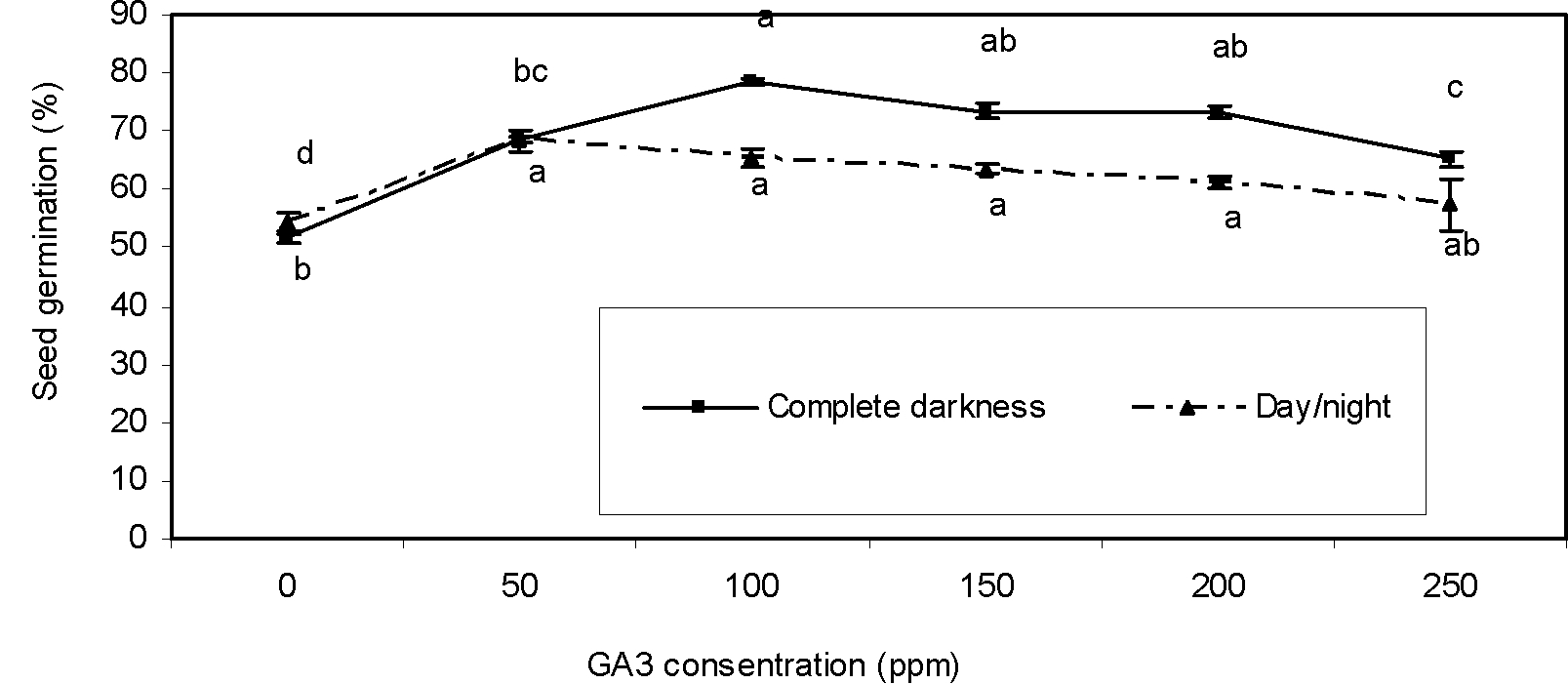 Means followed by the same letter are not significantly different according to the LSD test. Vertical bars represent standard errors of the means.
Means followed by the same letter are not significantly different according to the LSD test. Vertical bars represent standard errors of the means.
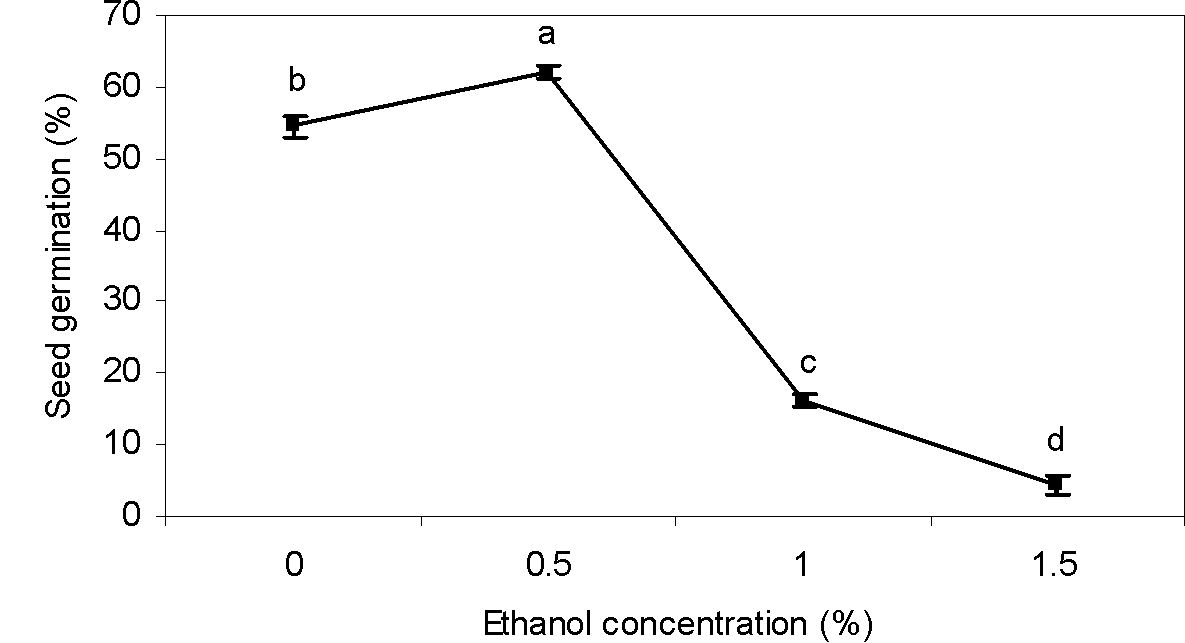 Means followed by the same letter are not significantly different according to the LSD test. Vertical bars represent standard errors of the means.
Means followed by the same letter are not significantly different according to the LSD test. Vertical bars represent standard errors of the means.
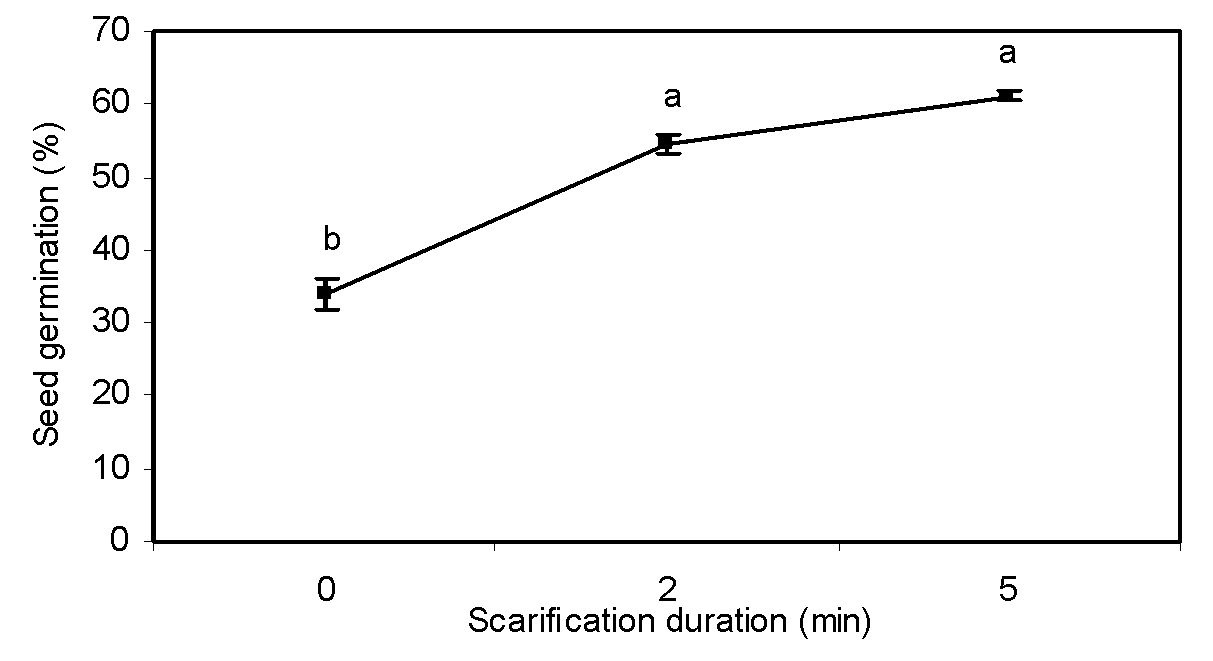 Means followed by the same letter are not significantly different according to the LSD test. Vertical bars represent standard errors of the means.
Means followed by the same letter are not significantly different according to the LSD test. Vertical bars represent standard errors of the means.
 Means followed by the same letter are not significantly different according to the LSD test. Vertical bars represent standard errors of the means.
Means followed by the same letter are not significantly different according to the LSD test. Vertical bars represent standard errors of the means.
 Vertical bars represent standard errors of the means.
Vertical bars represent standard errors of the means.
 Vertical bars represent standard errors of the means.
Vertical bars represent standard errors of the means.
 Means followed by the same letter are not significantly different according to the LSD test. Vertical bars represent standard errors of the means.
Means followed by the same letter are not significantly different according to the LSD test. Vertical bars represent standard errors of the means.
 In each flooding duration, means followed by the same letter are not significantly different according to the LSD test. Vertical bars represent standard errors of the means.
In each flooding duration, means followed by the same letter are not significantly different according to the LSD test. Vertical bars represent standard errors of the means.
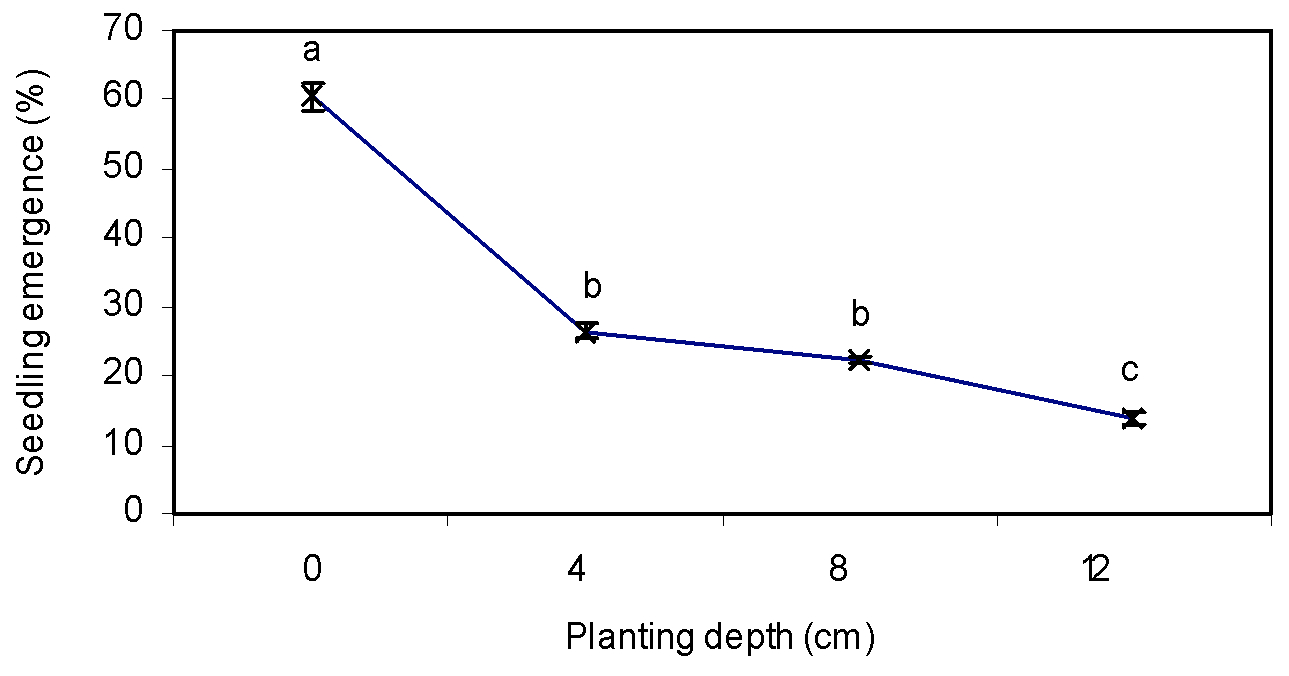 Vertical bars represent standard errors of the means.
Vertical bars represent standard errors of the means.