ABSTRACT:
The species Euphorbia dracunculoides and Astragalus are problematic weeds of arid chickpea in the chickpea mono-cropping system in Pakistan. The influence of various ecological factors on germination and seedling emergence characteristics of these weeds was determined under laboratory conditions. The results suggested that seed germination of both species was 50% at 15 oC under light conditions, and germination decreased when the temperature was increased. The increase in drought stress from 2.5 to 15% significantly decreased germination of E. dracunculoides and Astragalus spp. Both species failed to germinate at the osmotic potential of -3.02 MPa. The increase in field capacity from 25 to 100% increased emergence percentage and emergence index of both weeds. A pH range of 6 to 9 did not influence seed germination of both species and they were able to germinate at a wide range of pH conditions. Both weeds were very sensitive to salinity; however, a few seeds (10%) of Astragalusspp. germinated even at a 150 mM sodium chloride concentration. To check the effect of burial depth, seeds were placed in pots under seeding depths of 0 to 6 cm at an interval of 1 cm, respectively. Maximum emergence was attained at the soil surface and emergence declined with increasing depths. Seedling emergence of E. dracunculoides was higher than that of Astragalusspp. at all burial depths. Studies on germination ecology of these two weeds will offer insights into their behavior under different environmental conditions. Their germination responses and growth patterns under different ecological factors will help us to design an efficient management strategy to control these two troublesome weeds.
Keywords:
temperature; burial depth; light; management; emergence; salinity
RESUMO:
As espécies Euphorbia dracunculoides e Astragalus são plantas daninhas problemáticas do grão-de-bico no sistema monocultivo desta cultura no Paquistão. Em condições de laboratório, determinou-se a influência de vários fatores ecológicos nas características de germinação e emergência de plântulas dessas plantas daninhas. Os resultados sugeriram que a germinação de sementes de ambas as espécies foi de 50% a 15 oC sob condições de luz, e a germinação diminuiu com o aumento da temperatura. O aumento do estresse hídrico de 2,5 para 15% causou a diminuição significativa da germinação de E. dracunculoides e Astragalus spp. Ambas as espécies não germinaram no potencial osmótico de -3,02 MPa. O aumento na capacidade de campo de 25 para 100% aumentou a porcentagem de emergência e o índice de emergência de ambas as plantas daninhas. A variação do pH de 6 a 9 não influenciou a germinação de sementes de ambas as espécies, que foram capazes de germinar em uma ampla gama de condições de pH. Ambas as plantas daninhas foram muito sensíveis à salinidade; no entanto, algumas sementes (10%) de Astragalusspp. germinaram mesmo em uma concentração de cloreto de sódio de 150 mM. Para verificar o efeito da profundidade de semeadura, as sementes foram colocadas em vasos com profundidades de semeadura de 0 a 6 cm, em um intervalo de 1 cm, respectivamente. Foi obtida máxima emergência na superfície do solo e houve diminuição da emergência com o aumento da profundidade. A emergência de plântulas de E. dracunculoides foi maior que a de Astragalusspp. em todas as profundidades de semeadura. Estudos sobre a ecologia da germinação dessas duas plantas daninhas poderão elucidar seu comportamento sob diferentes condições ambientais. As respostas de germinação e os padrões de crescimento dessas espécies sob diferentes fatores ecológicos serão úteis para a elaboração de uma estratégia de gerenciamento eficiente para controlar essas duas plantas daninhas problemáticas.
Palavras-chave:
temperatura; profundidade de semeadura; luz; manejo; emergência; salinidade
INTRODUCTION
Euphorbia dracunculoides Lamk. is an annual, medium sized herb with a smooth stem. It belongs to the family Euphorbiaceae. It is usually 15-40 cm tall and its stem is often much branched from the base (Wang et al., 2014Wang L, Zang Z, Wang YF, Huang SX, Cao P, Zhao Y. Two new myrinsol diterpenoids from Euphorbia dracunculoides Lam. Chin Chem Lett. 2014;26:121-3.). This species has been reported in cultivated and sandy fields of Pakistan, Egypt, Iraq, Kuwait, Saudi Arabia, Afghanistan, India, and tropical Africa (Shanee et al., 2011Shanee S, Tanveer A, Javaid MM, Chaudhry KM, Aziz A, Khaliq A. et al. Phytotoxic effects of Euphorbia dracunculoides: a weed of rainfed chickpea-chickpea cropping system. Span J Agric Res. 2011;9(2):580-8.). Euphorbia dracunculoides density of 40 plants m-2 can cause chickpea grain yield losses by up to 63% (Tanveer et al., 2015Tanveer A, Javaid MM, Irfan M, Khaliq A, Yaseen M. Yield losses in chickpea with varying densities of dragon spurge (Euphorbia dracunculoides). Weed Sci. 2015;63(2):522-8.). Astragalusspp. is an annual, xerophytic shrub, which belongs to the family Fabaceae, and it normally achieves up to 40 cm height (Drobná, 2010Drobná J. Monitoring of endangered Astragalus species in the protected landscape area Dunajské luhy at the Danube Floodplains. Czech J Genet Plant Breed. 2010;46:S14-S18.). Genus Astragalus is found in North and South America, Asia, Europe, northern Africa, and Australia (Naghiloo et al., 2012Naghiloo S, Movafeghi A, Delazar A, Nazemiyeh H, Asnaashari S, Dadpour MR. Ontogenetic variation of volatiles and antioxidant activity in leaves of Astragalus compactus Lam.(Fabaceae). Excli J. 2012;11:436-43.). Both species are the most troublesome weeds of rainfed chickpea (Ikram et al., 2014Ikram RM, Tanveer A, Ata Z, Saqib M. Dormancy studies on Euphorbia dracunculoides and Astragalus spp.: major weeds of arid areas. Planta Daninha. 2014;32(4):747-53.). The salient features of this area include moisture shortage as a result of inadequate and erratic rains, low-temperature stress (frost) during December-January, marginal land, no use of fertilizers, and presence of weeds (Sijapati and Bhatt, 2012Sijapati S, Bhatt D. Perception and realities of the impact of global changes on water resources and agricultural practices - Preliminary findings of AGloCAP Project in Indrawati Basin, Nepal. Hydro Nepal: J Water Energy Environ. 2012;11:35-41.).
Understanding the biology and ecology of a weed is needed for effective management (Koger et al., 2004Koger CH, Reddy KN, Poston DH. Factors affecting seed germination, seedling emergence and survival of texasweed (Caperonia palustris). Weed Sci. 2004;52(6):989-95.; Fenner and Thompson, 2005Fenner M, Thompson K. The ecology of seeds. Cambridge: Cambridge University Press; 2005.; Chauhan, 2013Chauhan BS. Seed germination ecology of feather lovegrass [Eragrostis tenella (L.) Beauv. Ex Roemer and JA Schultes]. PloSONE. 2013;8(11):379-98. ). Knowledge based on research of critical life stage and seed germination offers a comprehensive explanation of plant development, seed dormancy patterns, ecological adaptation traits, distribution, and management strategies (Fandrich and Mallory-Smith, 2006Fandrich L, Mallory-Smith CA. Factor’s affecting germination of jointed goatgrass (Aegilops cylindrica) seed. Weed Sci. 2006;54(4):677-84.). Various studies on germination ecology stated that many factors, such as salinity, temperature, seed age, sowing depth, pH, light, and moisture, influence germination and emergence of a weed species (Chauhan and Johnson, 2008bChauhan BS, Johnson DE. Seed germination and seedling emergence of giant sensitive plant (Mimosa invisa). Weed Sci. 2008b;56(2):244-8., 2009Chauhan BS, Johnson DE. Germination ecology of spiny (Amaranthus spinosus) and slender amaranth (A. viridis): troublesome weeds of direct-seeded rice. Weed Sci. 2009;57(4):379-85.; Nakamura and Hossain, 2009Nakamura C, Hossain MA. Factors affecting the seed germination and seedling emergence of red flower ragleaf (Crassocephalum crepidioides). Weed Biol Manag. 2009;9:315-22. ).
Optimum temperature determines the ecological limitation and adaptation for the geographical distribution of a species (Nerson, 2007Nerson H. Seed production and germinability of cucurbit crops. Seed Sci Biotechnol. 2007;1:1-10. ; Turkoglu et al., 2009Turkoglu N, Alp E, Cig A. Effect of temperature on germýnatýon biology ýn Centaurea specýes. African J Agric Res. 2009;4:259-61.). Light is one of the environmental factors that can influence dormancy. Seeds of some species require light for germination and light exposure of less than a minute and, for some species, less than a second is enough to induce germination (Milberg et al., 1996Milberg P, Andersson L, Naronha A. Seed germination after short duration light exposure. Implication for the photo-control of weeds. J Appl Ecol. 1996;33(3):1469-78.). pH is also an important environmental factor that can severely restrict germination as a result of its effects on enzyme activity (Ali et al., 2013Ali HH, Tanveer A, Nadeem MA, Asghar HN, Javaid MM. Germination ecology of Rhynchosia capitata: an emerging summer weed in Asia. Planta Daninha. 2013;31(2);249-57. ). In this context, salts create water deficit along nutritional imbalance and are another reason for an unfavorable environment for seed germination (Achuo et al., 2006Achuo EA, Prinseh E, Hofte M. Influence of drought, salt stress and abscisic acid on the resistance of tomato to Botrytis cinerea and Oidium neolycopersici. Plant Pathol. 2006;55:178-86.). Water stress arising from drought is probably the most noteworthy abiotic feature limiting germination (van der Berg and Zeng, 2006van den Berg L, Zeng YJ. Response of south African indigenous grass species to drought stress induced by polyethylene glycol (PEG) 6000. S Afr J Bot. 2006;72(2):284-6.). In case of severe drought, germination stops. As a result, the embryo of seeds cannot not develop into radicle and plumule. Hence, moisture availability has a very dynamic role in seed germination (Hillel, 1972Hillel D. Soil moisture and seed germination. In: Kozlowski TT. editor. Water deficits and plant growth. New York: Academic Press; 1972. v.3. p.65-89.). Almansouri et al. (2001Almansouri M, Kinet JM, Lutts S. Effect of salt and osmotic stresses on germination in durum wheat (Triticum durum Desf.). Plant Soil. 2001;231(3):243-54.) reported that moisture and temperature are critical factors for seed germination. Seeding depth is one of the most important environmental factors affecting seed germination and seedling emergence because seeds are present in soil at different depths (Liu et al., 2011Liu HL, Shi X, Wang JC, Yin LK, Huang ZY, Zhang DY. Effects of sand burial, soil water content and distribution pattern of seeds in sand on seed germination and seedling survival of Eremosparton songoricum (Fabaceae), a rare species inhabiting the moving sand dunes of the Gurbantunggut Desert of China. Plant soil. 2011;345(1-2):69-87.). The behavior of seed germination changes with an increase or decrease in soil depth environments. The ideal soil depth varies with species (Gulshan and Dasti, 2006Gulshan AB, Dasti AA. Role of soil texture and depths on the emergence of buried weed seeds. J Agric Biol Sci. 2006;7(4):223-8.; Ali et al., 2013).
Euphorbia dracunculoides has been previously studied for its phytotoxicity (Shanee et al., 2011Shanee S, Tanveer A, Javaid MM, Chaudhry KM, Aziz A, Khaliq A. et al. Phytotoxic effects of Euphorbia dracunculoides: a weed of rainfed chickpea-chickpea cropping system. Span J Agric Res. 2011;9(2):580-8.). However, those studies did not address the germination ecology of E. dracunculoides. On the other hand, there is no work reported on Astragalus spp. Therefore, a significant understanding through ecological aspects is necessary. These weeds were particularly chosen because these are ubiquitous in the typical rainfed zone of Pakistan and known to occur in the mono cropping system for chickpea. Given the aforesaid importance of E. dracunculoides and Astragalusspp., the objective of present project was to understand the germination response of E. dracunculoides and Astragalusspp. to various environmental factors.
MATERIALS AND METHODS
Collection of weed seeds
Seeds of both weeds (E. dracunculoides and Astragalusspp.) were collected from plants at maturity from District Khushab of Punjab province, Pakistan, in October 2010. The collected seeds were isolated from fruits of E. dracunculoides and pods of Astragalusspp. and undesirable materials and immature/damaged seeds in the laboratory. These healthy, mature and uniform seeds were stored in paper bags under normal laboratory conditions after drying.
General germination test protocol
For all laboratory experiments, seeds were rinsed thoroughly with sterilized water four times and placed on double layered Whatman No. 10 filter papers in sterilized Petri dishes of 9 cm diameter. Initially, 5 mL of distilled water/respective solution of each treatment was applied per Petri dish and after this, the solution was added whenever needed. Each experiment was laid out in a completely randomized design with four replications, and 25 seeds were allocated per Petri dish.
Temperature
To evaluate the influence of temperature, 25 seeds for each species were evenly placed on double layer of filter papers in Petri plates, which were then placed in a germinator at the temperatures of 10, 15, 20, and 25 oC.
Light
The seeds of both species were evenly placed on Whatman no 10 filter papers in Petri dish and kept uncovered for complete light exposure. Similarly, to evaluate the effect of darkness, Petri dishes with seeds on double layered filter papers were wrapped with a single layer of aluminum foil and then placed in a germinator at 15 oC.
pH
The effect of pH on germination of E. dracunculoides and Astragalusspp. was studied by using buffer solutions of pH 6.0, 7.0, 8.0 and 9.0, prepared as described by Reddy and Singh (1992Reddy NK, Singh M. Germination and emergence of hairy beggarticks (Bidens pilosa). Weed Sci. 1992;40:195-9.). These pH ranges were selected to reflect the prevailing soil pH conditions in southern Punjab, Pakistan. A 2 mM solution of MES [2-(Nmorpholino) ethanesulfonic acid], HEPES [N-(2-hydroxymethyl) piperazine-N-(2-ethanesulfonic acid)], and tricine [N-tris(hydroxymethyl) methylglycine] were adjusted to pH 6, 7-8, and 9 with 1 N NaOH, respectively. Deionized water was used as a control treatment. Petri dishes were placed in a germinator at 15 oC.
Salt stress
To assess the germination ability of E. dracunculoides and Astragulusspp. under different levels of salt stress, 25 seeds of each species were evenly placed on Whatman No. 10 filter papers in 9 cm diameter Petri dish. Sodium chloride solutions of 0 (control), 25, 50, 75, 100, 125, and 150 mM concentrations were added to each Petri dish separately. These ranges of NaCl were selected to reflect the level of salinity occurring in the soils of southern Punjab. The Petri dishes were sealed with a strip of parafilm to prevent moisture loss and placed in a germinator at 15 oC.
Drought stress
The germination response of E. dracunculoides and Astragalusspp. under eight levels of drought stress was also assessed under laboratory environments. PEG-8000 was used as a drought stimulator with seven water stress levels. These were the osmotic stress levels applied: 0% (control), 2.5% (-0.17 MPa), 5.0% (-0.47 MPa), 7.5% (-0.91 MPa), 10.0% (-1.48 MPa), 12.5% (-2.18 MPa), and 15.0% (-3.02 MPa). These concentrations were developed according to the equation proposed by Michel (1983). After the application of the required treatments, Petri dishes were kept in a germinator at a temperature of 15 oC.
Field capacity
In this experiment, four field capacity levels of 25%, 50%, 75%, and 100% were maintained while using soil as the sowing medium. Soil was collected from the area where there were no weeds previously. The soil was then dried properly and placed into 14 cm diameter plastic pots. The analysis of soil showed that it was sandy loam soil with 0.7% carbon and pH of 7.1. Twenty-five seeds of each weed were evenly placed in plastic pots and different levels of field capacity were maintained (Tanveer et al., 2013Tanveer A, Tasneem M, Khaliq A, Javaid MM, Chaudhry MN. Influence of seed size and ecological factors on the germination and emergence of field bindweed (Convolvulus arvensis). Planta Daninha. 2013;31:39-51.). The pots were placed in a greenhouse. The average minimum and maximum temperatures in the greenhouse during the experiment were 15 oC and 20 oC. Field capacity was determined as per the procedure followed by Tanveer et al. (2013) by using the following formula:
Field capacity = Saturation percentage /2
The plastic pots were filled with soil and then water was applied. After being watered, the pots were weighed. Weight was measured of the pots containing moisture contents equal to 100%, 75%, 50%, and 25% field capacities (Tanveer et al., 2013Tanveer A, Tasneem M, Khaliq A, Javaid MM, Chaudhry MN. Influence of seed size and ecological factors on the germination and emergence of field bindweed (Convolvulus arvensis). Planta Daninha. 2013;31:39-51.).
Seeding depth
Twenty-five seeds each of E. dracunculoides and Astragalusspp. were separately placed in 14 cm diameter plastic pots on the soil surface (0 cm) or covered to depths of 1, 2, 3, 4, 5, and 6 cm. A mixture of sand, silt, and clay was used as media for germination in the pots. Initially, 100 mL distilled water was given to each pot with an irrigation shower so as to avoid soil disturbance. The average minimum and maximum temperatures recorded in the greenhouse during the experiment were 15 oC and 20 oC. Daily germination counts were performed for 3 weeks. Non-germinated seeds were subjected to the tetrazolium salt test to check viability. In the seed burial depth experiment, seedling emergence was considered as such when a cotyledon became visible on the surface. We recorded seedling emergence data up to 30 days.
The germination/emergence index (GI/EI) was assesed as formula given by the Association of Official Seed Analysts (AOSA, 1990Association of Official Seed Analysts - AOSA. Rules for testing seeds. J Seed Sci Technol. 1990;12(3):1-112.):
Germination energy (GE) was determined as suggested by the Association of Official Seed Analysts (AOSA, 1990Association of Official Seed Analysts - AOSA. Rules for testing seeds. J Seed Sci Technol. 1990;12(3):1-112.):
All experiments were repeated within 20 days after the end of the first run. The data from the repeated experiments were analyzed by using the ANOVA function of the MSTAT statistical computer package and least significance difference (LSD) at 5% probability was used to compare the treatment means (Steel et al., 1997Steel RGD, Torrie JH, Dickey DA. Principles and procedures of statistics. A biometrical approach. 3rd ed. Singapore: McGraw Hill Book; 1997. p.172-7.).
RESULTS AND DISCUSSION
Effect of temperature
Germination of E. dracunculoides and Astragalus species was significantly (P<0.05) affected by the temperatures tested (Figure 1) and it was significantly higher at 15 oC (50% for E. dracunculoides and 40% for Astragalus species) than at 10, 20, and 25 oC. GE and GI of both weeds were maximum at 15 oC and then decreased with a further increase or decrease in temperatures (Table 1). Similarly to the species E. dracunculoides and Astragalus, other weed species, including Caragana microphylla (Zhu et al. 2004Zhu XW, Huang ZY, Chu Y, Zhang SM, Liu HD, Dong M. Effects of burial in sand and seed size on seed germination and seedling emergence in two leguminous shrubs in the Otindag Sandland, China. Israel J Plant Sci. 2004;52(2):133-42.) and Rhynchosia capitata (Ali et al. 2013Ali HH, Tanveer A, Nadeem MA, Asghar HN, Javaid MM. Germination ecology of Rhynchosia capitata: an emerging summer weed in Asia. Planta Daninha. 2013;31(2);249-57. ), germinated over a wide range of temperature conditions.
Effect of temperature on seed germination of Euphorbia dracunculoides (A) and Astragalusspp. (B).
The ability of both weeds to germinate over the tested temperature range suggests that the weeds could emerge throughout the growing season of rainfed chickpea. Such a broad adaptation of these weeds to temperature also provides opportunities for seed production and weed proliferation.
Effect of light and darkness
Seed germination of E. dracunculoides and Astragalus species was significantly affected by light conditions. Germination (Figure 2) values for the weed species were 66% for E. dracunculoides and 44% for Astragalus species; GE and GI (Table 2) were significantly greater in light/dark (10/14 hr) than in the dark. Reports on germination response to light are inconsistent. For example, the higher germination of these weed seeds under light indicates that emergence will be favored by the seeds that are present at or near the soil surface, i.e., the condition that occurs under no-till chickpea cropping systems. Similarly to the results in the present study, seed germination of Sonchus oleraceus (Chauhan et al. 2006bChauhan BS, Gill G, Preston C. Factors affecting seed germination of annual sowthistle (Sonchus oleraceus) in southern Australia. Weed Sci. 2006b;54(5):854-60.) and Faloua villosa (Gina and Joseph, 2003Gina MP, Joseph CN. Light, temperature, seed burial, and mulch effects on mulberry weed (Fatoua villosa) seed germination. Weed Technol. 2003;17:213-8.) was stimulated by light instead of darkness. Our results are further supported by the findings of Gracia et al. (2005Gracia FP, Pita JM, Benito MEG, Iriondo JM. Effect of light, temperature and seed priming on germination of celery seeds (Apium graveolens L.). Seed Sci Technol. 2005;23(2):377-83.), who reported that absence of light inhibited germination of Triglochin maritimeChauhan et al. (2006cChauhan BS, Gill G, Preston C. Factor affecting turnipweed (Rapistrum rugosum) seed germination in South Australia. Weed Sci. 2006c;54(6):1032-6.) reported that in Rapistrum rugosum, germination was 87% under light/dark and 76% in the dark. They concluded that seed germination was stimulated by light. Lu et al. (2006Lu P, Sang W, Ma K. Effects of environmental factors on germination and emergence of Crofton weed (Eupatorim adenophorum). Weed Sci. 2006;54:452-7.) found that Crofton weed (Eupatorium adenophorum) was moderately photoblastic, with 17% germination occurring in the dark. It suggests that seed germination to light could be variable within weed species.
In the present study, seed germination of E. dracunculoides and Astragalus species was intensely stimulated by light, which means that seeds of these species are positively photoblastic. When seeds of both weeds were exposed to 10 h photoperiod, they showed significantly higher GE and GI compared to those seeds that remained under complete darkness. Greater seed germination of the species E. dracunculoides and Astragalus under light conditions suggests that germination and emergence of both weeds in the field will be ideal at or near the soil surface. No-till systems, in which a large proportion of weed seeds will be near the soil surface, would favor seed germination/emergence and their subsequent management.
Effect of pH
Seed germination of E. dracunculoides and Astragalus species was significantly affected by the tested range of pH solutions, and it varied from 52 to 25% in E. dracunculoides and 38 to 16% in Astragalus over the pH range of 6 to 9 (Figure 3). Germination of both weeds at pH 6 to 9 indicates that pH may not be a limiting factor for germination of these weeds. This kind of character leads a plant to become an invasive weed (Watanabe et al., 2002Watanabe O, Kurokawa S, Sasaki H, Nishida T, Onoue T, Yoshimura Y. Geographic scale distribution and occurrence pattern of invasive weeds. Grass Sci. 2002;48(5):440-50.). Germination of Galium tricornutum (Dandy) was found over a pH range from 4 to 10 (Chauhan et al., 2006aChauhan BS, Gill G, Preston C. Seed germination and seedling emergence of threehorn bedstraw (Galium tricornutum). Weed Sci. 2006a;54(5):867-72.) and Solanum nigrum at neutral pH showed a high percentage of germination (Suthar et al., 2009Suthar AC, Naik VR, Mulani RM. Seed and seed germination in Solanum nigrum Linn. Am Eurasian J Agric Environ Sci. 2009;5:179-83.). A higher germination percentage of E. dracunculoides than that of Astragalus over a pH range of 6 to 9 explains its wide range of adaptability. The results show that both species are slightly sensitive to pH 9 while both germinated well at other pH levels (Table 3). This kind of behavior of weeds suggests that they can adapt to a wide range of soil conditions.
Effect of salt stress
Germination of the species E. dracunculoides and Astragalus decreased after an increase in NaCl concentrations from 0 to 150 mM although germination of the former weed was completely inhibited at 125 and 150 mM NaCl (Figure 4). Seed germination of Astragalusspp. was more tolerant to high concentrations of NaCl than that of E. dracunculoides, which suggests that, under saline conditions, more proportions of Astragalus seeds may germinate, which could be a key feature of this species that enables it to colonize in saline areas more rapidly than E. dracunculoides. There was a linear decrease (Table 4) in GE and GI after an increase in salinity from 0 to 150 mM in E. dracunculoides and from 25 to 150 mM in Astragalus. These results are line with those of Chauhan and Johnson (2008aChauhan BS, Johnson DE. Germination ecology of two troublesome Asteraceae species of rainfed rice: Siam weed (Chromolaena odorata) and coat buttons (Tridax procumbens). Weed Sci. 2008a;56(4):567-73.), who reported that Corchorus olitorius and Melochia concatenata were moderately tolerant to salt stress.
Effect of salt stress on seed germination of Euphorbia dracunculoides (A) and Astragalusspp. (B).
Effect of drought stress
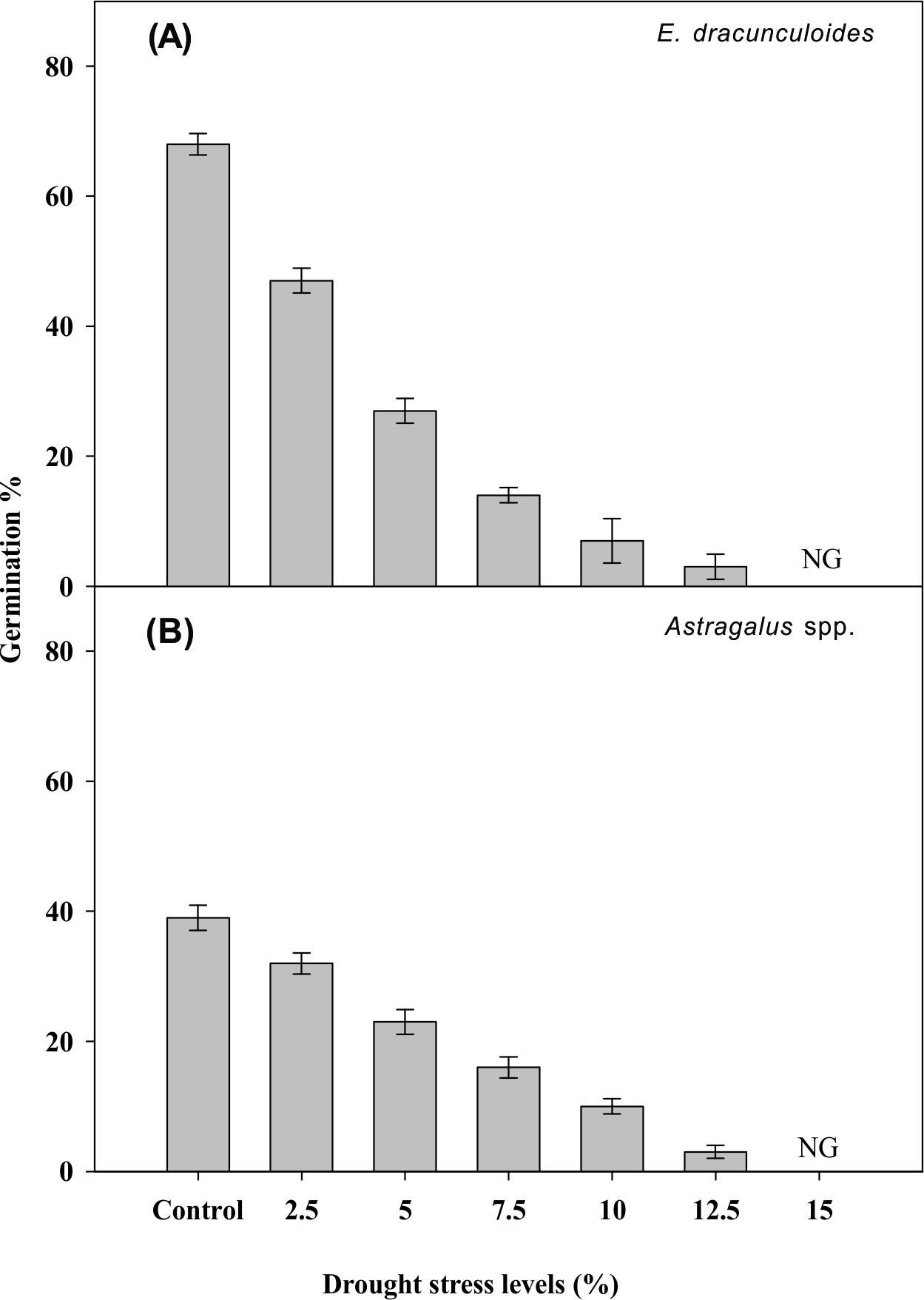
In both weeds, seed germination declined with decreasing osmotic potential, and seeds of neither species germinated at the osmotic potential of -3.02 MPa (Figure 5). Data also suggest that the response of E. dracunculoides seeds to the tested range of osmotic potential was slightly higher than that of the species Astragalus. GE and GI of the species E. dracunculoides and Astragalus were zero at osmotic stress higher than -0.47 and -0.91, respectively (Table 5). The results of the present study are supported by those of Zhou et al. (2005Zhou J, Deckard EL, Ahrens WH. Factors affecting germination of hairy nightshade (Solanum sarrachoides) seeds. Weed Sci. 2005;53:41-5.) and Tanveer et al. (2014Tanveer A, Sibtain M, Javaid MM, Ali HH. Germination ecology of wild onion: a rainfed crop weed. Planta Daninha. 2014;32:69-80.), who reported that in S. sarrachoides and A. tenuifolius, optimum germination occurred at an osmotic potential between 0 and -0.20 MPa. The response to low osmotic potential suggests that both weeds favor a moist environment for germination. Seeds of these weeds may germinate at the start of the sowing season (October) in moisture conserved from monsoon (July-August) rains and may germinate again if the rainfall occurs later in January-February (winter season).
Effect of drought stress on seed germination of Euphorbia dracunculoides (A) andAstragalusspp. (B).
Effect of field capacity
The progressive decrease in field capacity declined the emergence percentage of the species E. dracunculoides and Astragalus (Figure 6). At the 100% field capacity level, emergence was 60% and 47.50% for E. dracunculoides and Astragalus species, respectively. It decreased to 20% for E. dracunculoides and 5% for Astragalus at the 25% field capacity level. Emergence index of both weeds increased with the increase in field capacity level but E. dracunculoides showed higher emergence energy than the species Astragalus at the same field capacity level (Table 6). Some weeds such as E. adenophoum and taxsasweed Caperonia palustris are sensitive to drought stress (Koger et al., 2004Koger CH, Reddy KN, Poston DH. Factors affecting seed germination, seedling emergence and survival of texasweed (Caperonia palustris). Weed Sci. 2004;52(6):989-95.; Lu et al., 2006Lu P, Sang W, Ma K. Effects of environmental factors on germination and emergence of Crofton weed (Eupatorim adenophorum). Weed Sci. 2006;54:452-7.); by contrast, other weed species such as Hibiscus tridactylites and Solanum sarrachoides were tolerant to drought stress (Zhou et al., 2005Zhou J, Deckard EL, Ahrens WH. Factors affecting germination of hairy nightshade (Solanum sarrachoides) seeds. Weed Sci. 2005;53:41-5.; Chauhan, 2016Chauhan BS. Germination biology of Hibiscus tridactylites in Australia and the implications for weed management. Sci Rep. 2016;6. doi:10.1038/Srep26006
https://doi.org/10.1038/Srep26006...
).
Effect of field capacity on seed emergence of Euphorbia dracunculoides (A) and Astragalusspp. (B).
Effect of burial depth
Seed burial depth had a significant influence on the seedling emergence of E. dracunculoides and Astragalus (Figure 7). As the burial depth increased, seedling emergence decreased, and no Astragalus seedlings emerged when the seeds were buried at a depth of 5 and 6 cm (Figure 8). Emergence was higher (78% for E. dracunculoides and 38% for Astragalus) for seeds placed on the soil surface followed by seeds buried at 1 cm. There was a higher germination/emergence rate (78%) of E. dracunculoide in the pot trial as compared to that found in Petri dishes in the laboratory. It may have been due to the release of leachates in the presence of soil (Houseman and Mahoney, 2015Houseman GR, Mahoney AK. Intraspecific seed interactions alter seedling emergence of Lespedeza cuneata under field conditions. Popul Ecol. 2015;57(3):539-44. ), which may have increased the germination of E. dracunculoides. Maximum GE and GI values were recorded for both weed species when seeds were placed on the soil surface (Figure 7). There are several pieces of evidence of maximum seed germination at depths of 0 to 2 cm (Chauhan and Johnson, 2008bChauhan BS, Johnson DE. Seed germination and seedling emergence of giant sensitive plant (Mimosa invisa). Weed Sci. 2008b;56(2):244-8.; Xiao et al., 2010Xiao C, Wanga X, Xia J, Liu G. The effect of temperature, water level and burial depth on seed germination of Myriophyllum spicatum and Potamogeton malaianus. Aquatic Bot. 2010;92:28-32.) with the lowest germination at 6 cm (Liu and Han, 2008Liu GX, Han JG. Seedling establishment of wild and cultivated Leymus chinensis (Trin.) Tzvel. under different seeding depths. J Arid Environ. 2008;72(3):279-84.). Decreased seedling emergence resulting from increased burial depth has been reported in Asphodelus tenuifolius, an important weed of arid areas (Tanveer et al., 2014Tanveer A, Sibtain M, Javaid MM, Ali HH. Germination ecology of wild onion: a rainfed crop weed. Planta Daninha. 2014;32:69-80.). The higher rate of seedling emergence of E. dracunculoides as compared to Astragalus at all burial depths could have been due to large seed size, which is associated with higher tolerance to adverse conditions (Tanveer et al., 2013). On the other hand, species such as Astragalus (Figure 8) may not have sufficient energy reserves to support hypocotyls elongation. Greater seedling emergence from seeds placed on the soil surface suggests that no-till farming systems which are being used in the rainfed chickpea mono-cropping system of Pakistan would favor the emergence of E. dracunculoides and Astragalus.
Based on the results of the present study, it can be concluded that E. dracunculoides and Astragulus ssp. have the ability to germinate over a broad range of environmental conditions. Their tolerance against environmental stresses make these weed species troublesome and difficult to control. As these species are sensitive to dark conditions, and germination decreases by increasing depth, deep tillage will lead towards deep placement of these weed seeds, hence making them dormant or minimizing their germination. Thus, by observing their growth patterns, an environmentally friendly management strategy should be designed to focus on cultural controls.
REFERENCES
- Achuo EA, Prinseh E, Hofte M. Influence of drought, salt stress and abscisic acid on the resistance of tomato to Botrytis cinerea and Oidium neolycopersici Plant Pathol. 2006;55:178-86.
- Ali HH, Tanveer A, Nadeem MA, Asghar HN, Javaid MM. Germination ecology of Rhynchosia capitata: an emerging summer weed in Asia. Planta Daninha. 2013;31(2);249-57.
- Almansouri M, Kinet JM, Lutts S. Effect of salt and osmotic stresses on germination in durum wheat (Triticum durum Desf.). Plant Soil. 2001;231(3):243-54.
- Association of Official Seed Analysts - AOSA. Rules for testing seeds. J Seed Sci Technol. 1990;12(3):1-112.
- van den Berg L, Zeng YJ. Response of south African indigenous grass species to drought stress induced by polyethylene glycol (PEG) 6000. S Afr J Bot. 2006;72(2):284-6.
- Chauhan BS. Germination biology of Hibiscus tridactylites in Australia and the implications for weed management. Sci Rep. 2016;6. doi:10.1038/Srep26006
- Chauhan BS. Seed germination ecology of feather lovegrass [Eragrostis tenella (L.) Beauv. Ex Roemer and JA Schultes]. PloSONE. 2013;8(11):379-98.
- Chauhan BS, Gill G, Preston C. Factor affecting turnipweed (Rapistrum rugosum) seed germination in South Australia. Weed Sci. 2006c;54(6):1032-6.
- Chauhan BS, Gill G, Preston C. Factors affecting seed germination of annual sowthistle (Sonchus oleraceus) in southern Australia. Weed Sci. 2006b;54(5):854-60.
- Chauhan BS, Gill G, Preston C. Seed germination and seedling emergence of threehorn bedstraw (Galium tricornutum). Weed Sci. 2006a;54(5):867-72.
- Chauhan BS, Johnson DE. Germination ecology of spiny (Amaranthus spinosus) and slender amaranth (A. viridis): troublesome weeds of direct-seeded rice. Weed Sci. 2009;57(4):379-85.
- Chauhan BS, Johnson DE. Germination ecology of two troublesome Asteraceae species of rainfed rice: Siam weed (Chromolaena odorata) and coat buttons (Tridax procumbens). Weed Sci. 2008a;56(4):567-73.
- Chauhan BS, Johnson DE. Seed germination and seedling emergence of giant sensitive plant (Mimosa invisa). Weed Sci. 2008b;56(2):244-8.
- Drobná J. Monitoring of endangered Astragalus species in the protected landscape area Dunajské luhy at the Danube Floodplains. Czech J Genet Plant Breed. 2010;46:S14-S18.
- Fandrich L, Mallory-Smith CA. Factor’s affecting germination of jointed goatgrass (Aegilops cylindrica) seed. Weed Sci. 2006;54(4):677-84.
- Fenner M, Thompson K. The ecology of seeds. Cambridge: Cambridge University Press; 2005.
- Gina MP, Joseph CN. Light, temperature, seed burial, and mulch effects on mulberry weed (Fatoua villosa) seed germination. Weed Technol. 2003;17:213-8.
- Gracia FP, Pita JM, Benito MEG, Iriondo JM. Effect of light, temperature and seed priming on germination of celery seeds (Apium graveolens L.). Seed Sci Technol. 2005;23(2):377-83.
- Gulshan AB, Dasti AA. Role of soil texture and depths on the emergence of buried weed seeds. J Agric Biol Sci. 2006;7(4):223-8.
- Hillel D. Soil moisture and seed germination. In: Kozlowski TT. editor. Water deficits and plant growth. New York: Academic Press; 1972. v.3. p.65-89.
- Houseman GR, Mahoney AK. Intraspecific seed interactions alter seedling emergence of Lespedeza cuneata under field conditions. Popul Ecol. 2015;57(3):539-44.
- Ikram RM, Tanveer A, Ata Z, Saqib M. Dormancy studies on Euphorbia dracunculoides and Astragalus spp.: major weeds of arid areas. Planta Daninha. 2014;32(4):747-53.
- Koger CH, Reddy KN, Poston DH. Factors affecting seed germination, seedling emergence and survival of texasweed (Caperonia palustris). Weed Sci. 2004;52(6):989-95.
- Liu GX, Han JG. Seedling establishment of wild and cultivated Leymus chinensis (Trin.) Tzvel. under different seeding depths. J Arid Environ. 2008;72(3):279-84.
- Liu HL, Shi X, Wang JC, Yin LK, Huang ZY, Zhang DY. Effects of sand burial, soil water content and distribution pattern of seeds in sand on seed germination and seedling survival of Eremosparton songoricum (Fabaceae), a rare species inhabiting the moving sand dunes of the Gurbantunggut Desert of China. Plant soil. 2011;345(1-2):69-87.
- Lu P, Sang W, Ma K. Effects of environmental factors on germination and emergence of Crofton weed (Eupatorim adenophorum). Weed Sci. 2006;54:452-7.
- Milberg P, Andersson L, Naronha A. Seed germination after short duration light exposure. Implication for the photo-control of weeds. J Appl Ecol. 1996;33(3):1469-78.
- Naghiloo S, Movafeghi A, Delazar A, Nazemiyeh H, Asnaashari S, Dadpour MR. Ontogenetic variation of volatiles and antioxidant activity in leaves of Astragalus compactus Lam.(Fabaceae). Excli J. 2012;11:436-43.
- Nakamura C, Hossain MA. Factors affecting the seed germination and seedling emergence of red flower ragleaf (Crassocephalum crepidioides). Weed Biol Manag. 2009;9:315-22.
- Nerson H. Seed production and germinability of cucurbit crops. Seed Sci Biotechnol. 2007;1:1-10.
- Reddy NK, Singh M. Germination and emergence of hairy beggarticks (Bidens pilosa). Weed Sci. 1992;40:195-9.
- Shanee S, Tanveer A, Javaid MM, Chaudhry KM, Aziz A, Khaliq A. et al. Phytotoxic effects of Euphorbia dracunculoides: a weed of rainfed chickpea-chickpea cropping system. Span J Agric Res. 2011;9(2):580-8.
- Sijapati S, Bhatt D. Perception and realities of the impact of global changes on water resources and agricultural practices - Preliminary findings of AGloCAP Project in Indrawati Basin, Nepal. Hydro Nepal: J Water Energy Environ. 2012;11:35-41.
- Steel RGD, Torrie JH, Dickey DA. Principles and procedures of statistics. A biometrical approach. 3rd ed. Singapore: McGraw Hill Book; 1997. p.172-7.
- Suthar AC, Naik VR, Mulani RM. Seed and seed germination in Solanum nigrum Linn. Am Eurasian J Agric Environ Sci. 2009;5:179-83.
- Tanveer A, Javaid MM, Irfan M, Khaliq A, Yaseen M. Yield losses in chickpea with varying densities of dragon spurge (Euphorbia dracunculoides). Weed Sci. 2015;63(2):522-8.
- Tanveer A, Sibtain M, Javaid MM, Ali HH. Germination ecology of wild onion: a rainfed crop weed. Planta Daninha. 2014;32:69-80.
- Tanveer A, Tasneem M, Khaliq A, Javaid MM, Chaudhry MN. Influence of seed size and ecological factors on the germination and emergence of field bindweed (Convolvulus arvensis). Planta Daninha. 2013;31:39-51.
- Turkoglu N, Alp E, Cig A. Effect of temperature on germýnatýon biology ýn Centaurea specýes. African J Agric Res. 2009;4:259-61.
- Wang L, Zang Z, Wang YF, Huang SX, Cao P, Zhao Y. Two new myrinsol diterpenoids from Euphorbia dracunculoides Lam. Chin Chem Lett. 2014;26:121-3.
- Watanabe O, Kurokawa S, Sasaki H, Nishida T, Onoue T, Yoshimura Y. Geographic scale distribution and occurrence pattern of invasive weeds. Grass Sci. 2002;48(5):440-50.
- Xiao C, Wanga X, Xia J, Liu G. The effect of temperature, water level and burial depth on seed germination of Myriophyllum spicatum and Potamogeton malaianus Aquatic Bot. 2010;92:28-32.
- Zhou J, Deckard EL, Ahrens WH. Factors affecting germination of hairy nightshade (Solanum sarrachoides) seeds. Weed Sci. 2005;53:41-5.
- Zhu XW, Huang ZY, Chu Y, Zhang SM, Liu HD, Dong M. Effects of burial in sand and seed size on seed germination and seedling emergence in two leguminous shrubs in the Otindag Sandland, China. Israel J Plant Sci. 2004;52(2):133-42.
Publication Dates
-
Publication in this collection
13 June 2019 -
Date of issue
2019
History
-
Received
17 Jan 2017 -
Accepted
18 Oct 2017













 NG: Not germinated.
NG: Not germinated.
 NG = Not germinated.
NG = Not germinated.


