ABSTRACT:
The aim of this study was to evaluate diuron sorption, desorption and degradation in two anthropogenic soils (Terra Preta de Índio - TPI) in contrast to a sandy soil (Quartzarenic Neosol - NQo). Sorption-desorption studies were performed by the batch equilibrium method and biodegradation in biometric bottles using radiolabeled diuron in 14C. Freundlich coefficient (Kf) values ranged from 13.50 to 50.41 µmol(1-1/n) L1/n kg-1 in TPI-2 and TPI-1, respectively, indicating very high diuron sorption in anthropogenic soils, following the order: TPI-1 ≥ TPI-2 > NQo (99.10, 98.95 and 60.8%, respectively). Diuron desorption was very low in anthropogenic soils, ranging from 1.36 (TPI-1) to 1.70% (TPI-2), and 24% to NQo. Accumulated diuron mineralization to 14C-CO2 was < 3% at 70 days after herbicide application, regardless of the assessed soil. Formation of 35 and 44% residue bound to TPI-2 and TPI-1 was observed, higher than to NQo (17%). In contrast, the residue extracted from NQo varied from 72 to 91%, ranging from 48 to 83% for TPI-1 and TPI-2 during the incubation period. The degradation half-life (DT50) of diuron in anthropogenic soils was of 66.65 and 68.63 days for TPI-1 and TPI-2, respectively, while a period of 88.86 days was observed for NQo. The formation of only one herbicide metabolite in all soils was evidenced. The application of diuron in arable areas in the presence of anthropogenic Amazonian soils may lead to inefficient chemical weed control, since these soils may reduce herbicide soil bioavailability due to high OC contents, where high sorption and low herbicide desorption are noted, as well as faster degradation compared to sandy soil.
Keywords:
bound residue; mineralization; sorption isotherms; amazonian soils
RESUMO:
Neste estudo, objetivou-se avaliar a sorção, dessorção e degradação do diuron em dois solos antropogênicos (Terra Preta de Índio - TPI) em contraste com um solo arenoso (Neossolo Quartzarênico órtico - NQo). O estudo de sorção-dessorção foi realizado por método de equilíbrio em lote, e a degradação, em frascos biométricos utilizando o diuron radiomarcado no 14C. Os valores do coeficiente de Freundlich (Kf) variaram de 13,50 a 50,41 µmol (1-1/n) L1/n kg-1 na TPI-2 e TPI-1, respectivamente, e indicaram uma sorção muito alta do diuron nos solos antropogênicos, seguindo a ordem: TPI-1 ≥ TPI-2 > NQo (99,10, 98,95 e 60,8%, respectivamente). A dessorção do diuron foi muito baixa nos solos antropogênicos, variando de 1,36 (TPI-1) a 1,70% (TPI-2), e de 24% para o NQo. A mineralização acumulada do diuron para 14C-CO2 foi < 3% aos 70 dias após a aplicação do herbicida, independentemente do solo estudado. Houve a formação de 35 e 44% de resíduo ligado para TPI-2 e TPI-1, maiores que para o NQo (17%). Em contraste, o resíduo extraído para o NQo variou de 72 a 91% e, para as TPI-1 e TPI-2, de 48 a 83% durante o tempo de incubação. O tempo de meia-vida da degradação (DT50) do diuron nos solos antropogênicos foi de 66,65 e 68,63 dias para TPI-1 e TPI-2, respectivamente, e para o NQo, de 88,86 dias. Evidenciou-se a formação de apenas um metabólito do herbicida em todos os solos. A aplicação de diuron em áreas agricultáveis na presença de solos antropogênicos da Amazônia pode ter um controle químico das plantas daninhas ineficiente, pois estes solos podem diminuir a biodisponibilidade do herbicida na solução do solo devido aos altos teores de CO, em que apresentou alta sorção e baixa dessorção do herbicida, além da degradação mais rápida, comparado com o solo arenoso.
Palavras-chave:
resíduo ligado; mineralização; isotermas de sorção; solos amazônicos
INTRODUCTION
“Terra Preta Antropogênica” or “Terra Preta de Índio” (TPI) is formed by archaeological sites distributed in virtually all Amazonian ecoregions and represents a large portion of high fertility soils in the Amazon territory. Its formation is attributed to the ancient agricultural customs of the peoples who inhabited the area during the pre-Columbian period (Woods and McCann, 2001Woods WI, McCann JM. El origen y persistencia de las tierras negras de la Amazonía. In: Hiraoka M, Mora S. Desarrollo sostenible en la Amazonía. Mito o realidad? Quito: Ediciones Abya-Yala. 2001. p.23-30.). Studies carried out by Glaser and Birk (2012Glaser B, Birk JJ. State of the scientific knowledge on properties and genesis of Anthropogenic Dark Earths in Central Amazonia (Terra Preta de Índio). Geochim Cosmochim Ac. 2012;82:39-51.) report that TPI contains high nutrient levels (mainly P, Ca and Mn) and organic matter (OM), greater microbiological activity than nearby soils and also contributes to carbon sequestration (Balliett, 2007Balliett A. Terra Preta. Magic Soil of the Lost Amazon. Acres. 2007;37:1-4.). TPI’s coal origin is known to be pyrogenetic, occurred at low oxygen concentration, with water evaporation, resulting in a low density coal (0.3-0.4 kg dm-3), capable of ensuring long carbon soil retention (carbon sequestration) (Balliett, 2007Balliett A. Terra Preta. Magic Soil of the Lost Amazon. Acres. 2007;37:1-4.; Petter and Madari, 2012Petter FA, Madari BE. Biochar: Agronomic and environmental potencial in Brazilian savannah soils. Rev Bras Eng Agríc Amb. 2012;16:761-68.).
Due to its characteristics, studies have sought to produce a similar material to increase agricultural crop productivity, giving rise to biochar, which is produced by burning any raw material in the absence of oxygen (practice coal origin of TPI) and at different temperatures (Rezende et al., 2011Rezende EIP, Angelo LC, Santos SS, Mangrich AS. Biocarvão (biochar) e sequestro de carbono. Rev Virtual Quím. 2011;5:426-33.). These carbonaceous materials display a very strong interaction with pesticides in the environment.
Due to the use of pesticides applied to TPI in arable areas to control pests, diseases and weeds, studies seeking to understand pesticide behavior in these anthropogenic soils are required, especially herbicides that are applied pre-emergently directly to the soil, such as diuron.
Diuron [3-(3,4-dichlorophenyl)-1,1-dimethylurea] is a herbicide belonging to the phenylamide family and phenylurea subclass, applied pre-emergency, as already reported. It is recommended for pineapple, alfalfa, cotton, banana, cocoa, coffee, citrus, rubber and grape crops, and, mainly in Brazil, for sugarcane cultivation (Prata, 2000Prata F, Lavorenti A, Regitano JB, Torniselo VL. Degradação e adsorção de diuron em solos tratados com vinhaça. Rev Bras Cienc Solo. 2000;24:217-23.). It acts in the control of certain weeds, such as Ipomoea grandifolia, Euphorbia heterophylla and Raphanus raphanistrum, among others (Campos et al., 2012Campos CF, Vitorino HS, Martins D. Controle de plantas daninhas com diuron em diferentes condições de luz. Rev Bras Herbic. 2012;11:258-68.). Its mechanism of action consists in the inhibition of the photosystem II (PSII), which in turn inhibits photosynthesis by binding the herbicide to the QB on protein D1, blocking electron transport from QA to QB and disrupting CO2 fixation and ATP and NADPH2 production, which are essential for plant growth (Giaccomazi and Cochet, 2004Giaccomazi N, Cochet N. Environmental impact of diuron transformation: a review. Chemosphere. 2004;56:1021-32.). According to the Pesticides Properties DataBase - PPDB (2019Pesticide Properties Database - PPDB. Footprint: creating tools for pesticide risk assessment and management in Europe. Developed by the Agriculture & Environment Research Unit (AERU), University of Hertfordshire, funded by UK national sources and the EU-funded FOOTPRINT project (FP6-SSP-022704), 2019.), diuron displays low water solubility (Sw = 35.6 mg L-1 at 20 oC), is slightly volatile, moderately persistent (half-life - DT50 = 89 days) and has poor soil mobility, as its octanol-water coefficient (Kow) is 589 and its Freundlich sorption coefficient (Kf) is 17.95 µmol (1-1 / n) L1/n kg-1.
Due to these characteristics, this herbicide displays an affinity for OM present in the soil, being less susceptible to water molecule loss by leaching and more susceptible to soil retention by sorption (Oliveira and Brighenti, 2011Oliveira MF, Brighenti AM, Comportamento de herbicidas no ambiente. In: Oliveira Jr. RS, Constantin J, Inoue MH (Eds.). Biologia e manejo de plantas daninhas. Curitiba: Omnipax. 2011 p.273-95.). Herbicides applied to TPI may have present increased degradation due to the higher nutrient supply to soil degrading microorganisms. On the other hand, the high organic carbon (OC) content of these soils may decrease product degradation, as more molecules are available to link to soil colloids, as reported previously diuron (Guimarães et al., 2018Guimarães ACD, Mendes KF, Reis FC, Campion TF, Chirstoffoleti PJ, Tornisielo VL. Role of soil physicochemical properties in quantifying the of diuron, hexazinone, and metribuzin. Environ Sci Pollut Res Int. 2018;25:12419-33.).
As it is an exclusively Amazon region soil, but displaying productive areas where weed chemical control are used, studies regarding herbicide behavior when applied to this soil are still scarce. Therefore, the aim of this study was to evaluate diuron sorption-desorption and degradation when applied to two anthropogenic soils, contrasting with a sandy soil, where this herbicide is more biologically available concerning weed absorption.
MATERIAL AND METHODS
Soils
Anthropogenic soil samples (TPI-1 and TPI-2) were collected from two different areas in Santarém-PA, at depths ranging from 0 to 10 cm: TPI-1, at 2o50’36" S and 54o58’32" W, 180 m elevation ; and TPI-2, at 2o38’25" S and 54o46’53" W, 155 m elevation, on the 67 and 30 km of the BR-163 highway, respectively. The sandy soil used for the comparative analysis, classified as Ortic quartzarenic (NQo), was collected in Pedro Velho-RN (6o26’21” S and 35o13’17” W, altitude 22 m), at depths between 0 and 10 cm.
The soils were dried and sieved through 2.0 mm sieves. Soil moisture content (M) and field capacity (FC) were determined for all soils. The FC of all soils was adjusted to 75%, using deionized water for the degradation study. Physicochemical soil properties are presented in Table 1.
Diuron
Radiolabelled 14C-diuron (phenyl-14C (U)) with a radiochemical purity of 98.7% and a specific activity of 2.43 MBq mg-1 was supplied by DuPont (Wilmington, DE, USA). Unmarked diuron (analytical standard) was kindly supplied by ChemService, Inc. (West Chester, PA, USA), at a chemical purity of 99.5%.
Diuron sorption and desorption were evaluated at five different concentrations: 0.83, 1.67, 3.33, 6.67 and 13.33 µg mL-1, equivalent to 1/4, 1/2, 1, 2 and 4 times the maximum recommended dose (MRD) for sugarcane cultivation, respectively, at a MRD of 4 kg a.i. ha-1. Radiolabeled solutions were diluted in a 0.01 mol L-1 CaCl2 solution. For the degradation study, the solution with the radiolabelled product and its respective analytical standard were diluted with 200 µL of acetone, and approximately 13,333.33 Bq were applied to each biometric vial using a microsyringe according to the MRD, as described above.
Sorption study
The sorption methodology was established in accordance to OECD (2000Organisation for Economic Co-operation and Development - OECD. OECD guidelines for the testing of chemicals. Test number 106, Adsorption - Desorption Using a Batch Equilibrium Method. Paris: OECD, 2000. 44p.) Guideline “106, Adsorption - Desorption Using a Batch Equilibrium Method”.
The volume of the 0.01 mol L-1 CaCl2 solution plus diuron concentrations was of 10 mL, resulting in a 1:1 (w v-1) soil: solution ratio. The vials containing the solutions were placed on a horizontal shaker table (model TE 140, Tecnal, Piracicaba, SP, Brazil), in a semi-dark room with controlled temperature at 20 ± 2 oC, and stirred for 24 h at 200 rpm. They were subsequently centrifuged at 3,000 rpm for 15 min in a refrigerated centrifuge at 4 oC (Hitachi CF16RXII, Hitachi Koki Co., Ltd., Indaiatuba, SP, Brazil); Two 1 mL aliquots of each were extracted from the supernatant and pipetted into a liquid scintillation vial containing 10 mL of a scintillating solution. The radioactivity of this aliquot was assessed by liquid scintillation spectrometry (LSS) for five minutes using a Tri-Carb 2910 TR counter (LSA Perkin Elmer, Waltham, MA, USA). The percentage of soil sorbed herbicide was evaluated by the difference between the radioactivity found in the equilibrium soil solution and the initially applied solution.
Desorption study
The desorption study was performed under the same conditions as the sorption study. One mL aliquots of the supernatant were collected from the teflon flasks containing soil and the previously sorbed diuron. The remaining solutions in the vials were then adequately discarded in order to add 10 mL of a new 0.01 mol L-1 CaCl2 solution. All flasks were homogenized again on an orbital shaker table for 24 h at 200 rpm in a semi-dark room with controlled temperature at 20 ± 2 oC. Following the shaking period, the vials were centrifuged at 3,000 rpm for 15 min in a refrigerated centrifuge, and 1 mL aliquots of the supernatant were pipetted in duplicate into a scintillation vial containing 10 mL of a scintillation solution and analyzed by LSS for five min each. The desorbed amount was calculated by the difference between the radioactivity sorbed in the soil and the remnant in the collected supernatant.
Sorption-desorption models
The data obtained in the sorption and desorption studies were adjusted to the Linear, Langmuir and Freundlich mathematical models, allowing for soil herbicide behavior assessments, varying according to concentration. The mathematical Langmuir (1), Freundlich (2) and Linear (3) isothermsmodels are be represented by the following equations:
where Cs = amount of diuron sorbed (mg g-1); Ce = equilibrium concentration of diuron in soil solution (mg mL-1); Cm = maximum amount of sorbed herbicide (mg g-1); 1/n = degree of linearity of the isotherm; K1 = sorption coefficient for the Langmuir model; Kf = Freundlich sorption constant; and Kd = Linear sorption coefficient.
To normalize the Linear sorption coefficient (Kd) to the soil OC (Koc) content, Equation (4) was used
The Langmuir (Kl) and Freundlich (Kf) coefficients were also normalized to the OC content, obtaining the Kloc and Kfoc coefficients, respectively, as presentes in Equations 5 and 6:
The hysteresis coefficient (H) was calculated by Equation 7 using the sorption and desorption parameters derived from Freundlich isotherms (Barriuso et al., 1994Barriuso E, Laird DA, Koskinen WC, Dowdy RH. Atrazine desorption from smectites. SSSA, 1994;58:1632-38.).
14C-CO2 mineralization study
Mineralization studies were performed according to the methods established by “Test number 307: Aerobic and Anaerobic Transformation in Soil” (OECD, 2002Organisation for Economic Co-Operation and Development - OECD. OECD Guideline for the Testing of Chemicals, 307, Aerobic and anaerobic transformation in soil. Paris: OECD, 2002. 17p.), in a completely randomized design with two replications for each soil at each sampled time. Each experimental unit was represented by a 300 mL biometric flask (Fisher C-4443-250).
A total of 30 g of soils (dry basis) were placed in appropriate biometric flasks preincubated in a dark room and heated at 20 ± 2 oC for seven days. Accumulated 14C-diuron mineralization was evaluated based on the amount of 14C-CO2 released and trapped in the 0.2 M NaOH solution contained in each biometric vial. The 0.2 M NaOH solutions (10 mL) of each bottle were analyzed for five minutes by LSS, using two 1 mL aliquots, referring to each incubation period, as follows: 7, 14, 21, 28, 35, 42, 49, 56, 63 and 70 days after application (DAA). For each evaluation time, a new NaOH solution (10 mL) was placed in the side tube of each biometric vial. Atmospheric CO2 entry into the vials was prevented by using soda lime filters.
Bound and extractable residue analysis
The extracted residues were quantified at 0, 35 and 70 DAA. Soil samples containing 14C-diuron underwent a process consisting of three extraction stages. First, the soils were transferred from biometric flasks to 250 mL Teflon tubes containing 100 mL of methanol (solvent) and shaken for one hour at 200 rpm on an orbital shaker table. Thenm the tubes were centrifuged at 3,000 rpm for 15 minutes and the supernatants were collected and transferred to Schott vials (500 mL). The methanol volumes for the second and third extraction were 80 and 70 mL, respectively, repeating the same procedure as the first extraction. The procedure used for diuron solvent extraction was carried out according to the methodology established by Guimarães et al. (2018Guimarães ACD, Mendes KF, Reis FC, Campion TF, Chirstoffoleti PJ, Tornisielo VL. Role of soil physicochemical properties in quantifying the of diuron, hexazinone, and metribuzin. Environ Sci Pollut Res Int. 2018;25:12419-33.).
After extraction, two 1 mL aliquots of each sample were pipetted into liquid scintillation vials for LSS measurements for five minutes. Remaining extract solutions in the Schott vials were evaporated under vacuum at 40 oC using a a rotavapor (vacuum controller V-850, rotavapor R-215, heating bath B-491, Buchi Brasil Ltda, Valinhos, SP, Brazil), and two 1 mL aliquots of the concentrated extracted residue of each vial were again analyzed by LSS for five minutes for radioactivity quantification. The bound residue was analyzed after drying the soil samples at 50 oC for 48 h in an air circulating oven and sample grinding in a mechanical mill (Marconi MA330, Piracicaba, SP, Brazil). Samples from each soil were weighed in triplicate at 0.2 g and oxidized using a biological oxidizer (OX500, RJ Harvey Instrument Corporation, Tappan, NY, USA), which burns the radioactive material, leaving all 14C-diuron present in the samples “trapped” in scintillation vials containing 10 mL of a scintillation solution, which were then analyzed by LSS for five minutes.
Metabolite and parental product quantification
The separation method of the parental compound (diuron) from its metabolites was performed by thin layer chromatography (TLC), according to an established method (Environmental Protection Agency - EPA, 1998Environmental Protection Agency - EPA. Soil thin layer chromatography. Washington: 1998. 6p. (EPA - Fate. transport and transformation test guidelines - OPPTS 835.1210).; Fried and Sharma, 1999Fried B, Sharma J. Thin-Layer chromatography. 4th ed. New York: Marcel Dekker, 1999. 499p.). Aliquots of all concentrated extracted residues (0.1 mL) and the 14C-diuron analytical standard were applied to TLC plates (60F254, EMD Milipore) with the aid of an electronic microsyringe (Hamilton PB6000 Dispenser, Hamilton, CO, USA) for chromatographic peak comparison. TLC plates were placed in a chamber saturated with 100 mL of 60:40 hexane: acetone as reported by Prata et al. (2000Prata F, Lavorenti A, Regitano JB, Torniselo VL. Degradação e adsorção de diuron em solos tratados com vinhaça. Rev Bras Cienc Solo. 2000;24:217-23.).
TLC plates were placed on phosphorescent films for 24 h, which were then subjected to a radioscanner (Packard Cyclone - Perkin-Elmer, Shelton, CT, USA) for chromatogram peak identification through herbicide radioactive intensity. The amount of each component (parental and metabolites) of the concentrated extracted residue was evaluated using retention factors (RF). The RF of a specific product is the ratio of the distance this product has moved above its point of origin to the distance traveled by the solvent above its point of origin.
Degradation and Mineralization Models for DT50 and MT50 determinations
Data on the amount of degraded and mineralized 14C-diuron were adequate for the first-order decay (C = C0 e-kt) and growth (C = C0 ekt) kinetic model, respectively, where Cis the herbicide concentration in time (%); C0is the herbicide concentration at time zero (%); kis a degradation or mineralization rate constant (days-1); and tis the incubation time (days). Degradation (DT50) or mineralization (MT50) half-lifes were defined as the time required for 50% of the applied herbicide to be degraded or mineralized, and were calculated using the following equation: DT50 or MT50 = ln2/k (Picton and Farenhorst, 2014Picton P, Farenhorst A. Factors influencing 2,4-D sorption and mineralization in soil. J Environ Sci Health Part B. 2004;39:367-79.).
Statistical analyses
All data are presented as the means and the standard deviation of the means (n= 2). Diuron degradation regressions and sorption-desorption isotherms for the applied three models were plotted using the Sigma Plot® software package (Windows version 10.0, Systat Software Inc., Point Richmond, CA, USA).
RESULTS AND DISCUSSION
Sorption isotherms
The parameters derived from the Langmuir, Freundlich and Linear models and the diuron sorption isotherms adapted for the three models are presented in Table 2 and Figure 1, respectively. Good data adequacy was observed for the three assessed models, as noted by the coefficient of determination (R²) values of 0.99 in all mathematical equations.
Sorption isotherm parameters of the Linear, Freundlich and Langmuir models for diuron applied in two anthropogenic Amazonian soils (TPI-1 and TPI-2) and a sandy soil (NQo)
Sorption (●) and desorption (○) isotherms of diuron applied to two anthropogenic Amazonian soils (TPI-1 and 2) and a sandy soil (NQo). Models: Linear (black), Freundlich (red) and Langmuir (blue).
It is known that diuron sorption is strongly influenced by CEC and soil OC content (Giori et al., 2014Giori FG, Tornisielo VL, Cerri CEP, Regitano JB. Sugarcane straw management and soil attributes on alachlor and diuron sorption in highly weathered tropical soils. J Environ Sci Health Part B. 2014;49:352-60.), which may justify the diferences observed between the anthropogenic and sandy soil coefficients (Tables 1and2). In the Linear model, the diuron linear sorption coefficient (Kd) was similar for the two anthropogenic soils, ranging from 21.93 (TPI-2) to 28.34 L kg-1 (TPI-1). Compared to TPI, the diuron Kd value observed for NQo was low, of Kd = 0.50 L kg-1. Kd values obtained by Carbo et al. (2007Carbo LL, Martins EL, Dores EFGC, Spadotto CA, Weber OLS, Freire EMDL. Acetamiprid, carbendazim, diuron and thiamethoxam sorption in two Brazilian tropical soils. Environ Sci Health, Part B. 2007;42:49-507.) when evaluating diuron sorption applied to Oxisols and Entisols ranged from 1.44 to 14.31 L kg-1 in Oxisol and from 4.34 to 10.99 L kg-1 in Entisol, lower than the anthropogenic soil coefficients observed in the present study, and with higher Kd than NQo. The Koc values of this herbicide in TPI-1 and TPI-2 were also higher (761.83 and 565.21 L kg-1, respectively) when compared to NQo (151.52 L kg-1). Studies carried out by Kile et al. (1999Kile DE, Wershaw RK, Chiou CT. Correlation of soil and sediment organic matter polarity to aqueous sorption of nonionic compounds. Environ Sci Technol. 1999;33:2053-6.), Salloum et al. (2001Salloum MJ, Dudas MJ, McGill WB. Variation of 1-naphthol sorption with organic matter fractionation: the role of physical conformation. Org Geochem. 2001;32:709-19.) and Smernik and Kookana (2015Smernik RJ, Kookana RS. The effects of organic matter-mineral interaction and organic matter chemistry on diuron sorption across a diverse range of soils. Chemosphere. 2015;119:99-104.) pointed out a relationship between Koc and soil OC content, which may be the most influential diuron sorption factor, evidenced in this study by five times the herbicide Koc value in TPI-1 compared to NQo, and about 3.7 times higher in TPI-2 (Table 2).
Freundlich coefficients (Kf) in anthropogenic soils presented values ranging from 6.38 to 8.74 µmol (1-1 / n) L1/n kg-1 in TPI-1 and TPI-2, respectively, and 0.41 µmol (1-1 / n) L1/n kg-1 in NQo (Table 2). Studies performed by Rocha et al. (2013Rocha PRR, Faria AT, Borges LGFC, Silva LOC, Silva AA, Ferreira EA. Sorção e dessorção do diuron em quatro Latossolos brasileiros. Planta Daninha. 2013;31:231-38.) evaluating diuron sorption in four types of Latosol report that, for Red-Yellow Latosol (LVA) and Humic Red-Yellow Latosol (LVAh), both displaying a pH of 5.8, Kf values were of 15.27 µmol (1-1 / n) L1/n kg-1 in LVA and 14.13 µmol (1-1 / n) L1/n kg-1 in LVAh, but increasing soil pH increased diuron sorption . It is noteworthy that the soils assessed herein and those investigated by Rocha et al. (2013) display similar OC values, differing only in pH. The Kf values found for NQo corroborate the studies carried out by Rubbio-Bellido (2016Rubbio-Bellido M, Morillo E, Villaverde J. Effect of addition of HPBCD on diuron adsorption-desorption, transport and mineralization in soils with different properties. Geoderma. 2016;265:196-203.), which found Kf ranging from 0.60 to 14.3 µmol (1-1 / n) L1/n kg-1 for diuron applied to sandy soil with the addition of hydroxypropyl-β-cyclodextrin (HPBCD), indicating a strong influence of soil texture on diuron sorption.
The Freundlich coefficient normalized for organic carbon (Kfoc) ranged from 164.43 (TPI-2) to 234.95 µmol (1-1/n) L1/n kg-1 (TPI-1) in anthropogenic soils and was of 124.24 µmol (1-1/n) L1/n kg-1 in the NQo (Table 2). The degrees of linearity (1/n) obtained by the Freundlich sorption isotherms were of 0.70 and 0.66 in TPI-1 and TPI-2, respectively, and 0.71 in sandy soil, with a nonlinear behavior (1/n≠1). This behavior was also reported by Boeira and Souza (2004Boeira RC, Souza MD. Sorção de diuron em solos com diferentes texturas. Jaguariúna: Embrapa Meio Ambiente, 2004. 5p. (Circular Técnica, 9)) when assessing diuron sorption applied to three soils with different textures, with 1/n values ranging from 0.60 to 0.94. Nonlinear behavior indicates that the sorption in question is a process that depends on the herbicide concentration.
In the Langmuir model, the Kl values found in both anthropogenic soils were of 0.014 L kg-1, and 0.364 L kg-1 in the NQo. Kl normalization in relation to organic carbon (Kloc) varied little among anthropogenic soils, ranging from 0.376 to 0.361 L kg-1 in TPI-1 and TPI-2, respectively, and from 110.3 L kg-1 in sandy soil. Langmuir isotherms suggest that sorption occurs on a flat surface, with a number of fixed functional groups that interact with the herbicide molecules to be sorbed and that maximum sorption occurs once all functional groups are filled (Linhares et al., 2008Linhares LA, Egreja Filho FB, Iahnez R, Santos EA. Aplicação dos modelos de Langmuir e Freundlich na adsorção de cádmio e chumbo em diferentes classes de solos brasileiros. Rev Tecnol. 2008;17:49-60.).
This kind of Kd, Kfoc and Kloc normalization is especially important in cases where herbicide sorption is directly influenced by soil OM (Inoue et al., 2006Inoue MH, Oliveira Jr RS, Regitano JB, Tormena CA, Constantin J, Tornisielo VL. Sorption-desorption of atrazine and diuron in soils from Southern Brazil. J Environ Sci Health Part B, 2006;41:605-21.), as observed for diuron applied to both anthropogenic soils. The sorbed amount of diuron in anthropogenic TPI-1 and TPI-2 soils was very high, noted at 99.10 and 98.95%, respectively. In contrast, the sorbed amount of this herbicide in NQo was of 60.88%, about 39% lower than anthropogenic soils.
Desorption Isotherms
As evidenced by the sorption isotherm parameters, the data were also well suited to the three models assessed concerning the diuron desorption isotherms in two anthropogenic and one sandy soil (R² > 0.99), as presented in Table 3.
Desorption isotherms parameters of the Linear, Freundlich and Langmuir models and hysteresis coefficient (H) for diuron applied in two anthropogenic Amazonian soils (TPI-1 and TPI-2) and a sandy soil (NQo)
The data obtained through diuron desorption indicate that both anthropogenic soils present low desorption rates, as the Kd(des) (Linear model) values in both Amazonian soils were of 132.00 and 74.17 L kg-1 in TPI-1 and TPI-2, respectively. These values were higher than those reported by Damin (2005Damin V. Biodegradação, sorção e dessorção do herbicida 14C-diuron em dois Latossolos tratados com lodo de esgoto [dissertation]. Piracicaba: Luiz de Queiroz College of Agriculture, 2005.), at Kd(des) of 37.22 L kg-1, when applying diuron to a typical dystrophic Red Latosol with sewage sludge concentration equivalent to 20 t ha-1. In NQo, diuron Kd(des) increased seven-fold in relation to its sorption Kd, but remained low in relation to both Amazonian soils. The Koc(des) of the anthropogenic soils ranged from 1911.60 (TPI-2) to 3548.39 L kg-1 (TPI-1), ans was observed at 1,075.76 L kg-1 in NQo (Table 3).
Similar to the sorption study, the Freundlich Kf(des) coefficient was higher in soils containing higher OC contents (Tables 1and3). The Kf(des) ranged from 13.50 (TPI-2) to 50.41 µmol (1-1/n) L1/n kg-1 (TPI-1) in anthropogenic soils and was equal to 3.05 µmol (1-1/n) L1/n kg-1 in sandy soil. The normalized Kf values for OC (Kfoc(des)) obtained in the diuron desorption experiments were 347.94 and 1355.11 µmol (1-1/n) L1/n kg-1 in TPI-1 and TPI-2, respectively. In NQo, diuron presented Kfoc(des) equal to 924.24 µmol (1-1/n) L1/n kg-1. As the Kf(des) of the three soils was higher than their respective sorption Kf, the diuron desorption process is noted as applying higher binding energy mechanisms than sorption processes (Arsego, 2009Arsego IB. Sorção dos herbicidas diuron e hexazinone em solos de texturas contrastantes [dissertation]. Piracicaba: Luiz de Queiroz College of Agriculture, 2009.).
Hysteresis (H) was also noted, which establishes a relationship between the degree of sorption and desorption linearity (1/n). The higher the index, the higher the occurrence of H, leading to irreversibility of the sorption process, evidenced by 1/n(des) < 1/n(sor) (Seybold and Mersie, 1996Seybold CA, Mersie W. Adsorption and desorption atrazine, deethlyazine, deisopropylatrazine, hydroxyatrazine and metolachlor in two soils from Virginia. J Environ Qual. 1996;25:1179-85., Moreau and Mouvet, 1997Moreau C, Mouvet C. Sorption and Desorption of atrazine, deethlyazine, and hydroxyatrazine by soil and aquifers solids. J Environ Qual. 1997;26:416-24.). These values can be observed in Tables 2 and3. The desorption values obtained in the Langmuir model for Kl(des) ranged from 0.004 to 0.008 L kg-1 in TPI-2 and TPI-1, respectively, and was of 0.382 L kg-1 in sandy soil. The Langmuir desorption coefficient normalized for soil OC (Kloc (des)) ranged from 0.103 (TPI-2) to 0.215 L kg-1 (TPI-1), reaching its highest value (115.76 L kg-1) in NQo.
The desorbed amount of diuron in the TPIs ranged from 1.36 (TPI-1) to 1.70% (TPI-2). The largest amount (24.02%) of this desorbed herbicide was observed in NQo soil, probably due to low OC content. The desorption isotherms and all calculated data and coefficients are displayed in Figure 2 and Table 3, respectively.
14C-CO2 accumulated and evolved from 14C-diuron mineralization during incubation at 70 days after application (DAA) in two anthropogenic Amazonian soils (TPI-1 and TPI-2) and a sandy soil (NQo).
Degradation mass balance
The mass balances of the three studied soils were calculated according to the sum of the amount of 14C-diuron in the mineralized, extracted and soil bound residues. The balance sheets ranged from 87.94 to 94.18%, 87.88 to 93.24% and 84.66 to 94.82% for TPI-1, TPI-2 and NQo, respectively. The calculated values are close to those established by the OECD (2002Organisation for Economic Co-Operation and Development - OECD. OECD Guideline for the Testing of Chemicals, 307, Aerobic and anaerobic transformation in soil. Paris: OECD, 2002. 17p.), which determines that the appropriate mass balance may vary ± 20% recovery of the initially applied radiolabelled herbicide.
Mineralization to 14C-CO2
The 14C-diuron mineralized to 14C-CO2 displayed similar accumulation between TPI-1 and TPI-2 until 42 DAA, and both were below the NQo throughout the incubation period. From 42 DAA in TPI-2, increased mineralization was observed, with values close to those noted in NQo at the end of the evaluation (at 70 DAA), of 3.29 and 3.33% in TPI-2 and NQo, respectively, while TPI-1 mineralized 3.10% of the applied diuron (Figure 2). The values found for diuron mineralization were much lower than those reported by Guimarães et al. (2018Guimarães ACD, Mendes KF, Reis FC, Campion TF, Chirstoffoleti PJ, Tornisielo VL. Role of soil physicochemical properties in quantifying the of diuron, hexazinone, and metribuzin. Environ Sci Pollut Res Int. 2018;25:12419-33.), who obtained up to 41.3% of mineralized diuron in a clay soil. El Sebai et al. (2010El Sebaï T, Devers-Lamrani M, Lagacherie B, Rouard N, Soulas G, Laurent-Martin F. Isoproturon mineralization in an agricultural soil. Biol Fert Soils. 2010;47:427-35.) also reported approximately 40% diuron mineralization in a soil presenting 1.7% OC. The low mineralized diuron values, lower for TPI compared to NQo, may also be related to the high amount of extracted residues and bound residue formation during the diuron incubation period in the assessed soils. Due to the fact that sorption is directly influenced by CEC and soil OC content, reduction diuron bioavailability in the soil solution may ocurr, where this compund becomes less available for mineralization by microorganisms. This relationship may justify the low mineralization values observed for anthropogenic soils.
Diuron MT50 presented its lowest value in NQo (1051.8 days), where the highest mineralization occurred; TPI-1 presented the lowest mineralization, followed by TPI-2, so that MT50 values in anthropogenic soils were of 1138.17 and 1064.7 days, respectively (Figure 2). Diuron MT50 is related to the amount mineralized during the soil incubation time, increasing with decreased mineralization. The higher the diuron MT50 value, the longer the persistence time of the product in the soil. This can lead to extended weed control if the product is available in the soil solution. However, this was not the case in the present study, as diuron is likely to be unavailable due to its high sorption and low desorption in anthropogenic soils, as reported.
Bound and extractable residues
A gradual increase in the formation of bound residues and a decrease in the extracted residues was observed over 70 DAA (Figure 3). The highest formation of extracted residue corresponded to the lowest bound residue formation. Soils subjected to solvent extraction also displayed similarity in the amount sextracted by each extraction step, always higher in the first step. At 0 DAA, 65, 66 and 78% diuron were extracted in the first step in TPI-1, TPI-2 and NQo, respectively; at 35 DAA, diuron extracted in the first stage in TPI-1, TPI-2 and NQo was of 64, 63 and 77%, respectively. This behavior was also evidenced at 70 DAA.
14C-diuron distribution in soils between solvent-extracted residues and bound residues at 70 days after application (DAA) in two anthropogenic Amazonian soils (TPI-1 and TPI-2) and a sandy soil (NQo).
Comparing extraction times and soils, NQo presented higher amounts of extracted residues during the entire incubation period, initially higher (at 0 DAA), reaching a total of 91.25% of herbicide solvent extraction, following a decreasing order concerning the amount of residue extracted at 35 and 70 DAA (77.1 and 71.9%, respectively) (Figure 3).
The superiority of NQo over anthropogenic soils regarding the amount of extracted residue can be explained by differences in OC and CEC levels, as displayed in Table 1. The low OC (0.33%) and CEC (2.2 mmolc dm-3) values in NQo were essential to increase the bioavailability of the herbicide in the soil solution, where low bound residue formation occurred as few diuron molecules were bound to soil particles, as noted in the sorption-desorption study. Thus, these same soil characteristics are responsible for the higher formation of bound residues over time in Amazonian soils, presenting higher values at 70 DAA: 44 and 35% for TPI-1 and TPI-2, respectively. The highest amount of diuron bound residue in NQo (17.6%) was observed at 35 DAA.
The tendency to reduce the amount of residue extracted during the incubation period corroborated the study developed by Guimarães et al. (2018Guimarães ACD, Mendes KF, Reis FC, Campion TF, Chirstoffoleti PJ, Tornisielo VL. Role of soil physicochemical properties in quantifying the of diuron, hexazinone, and metribuzin. Environ Sci Pollut Res Int. 2018;25:12419-33.), who evaluated diuron degradation and residue formation in five soils, two clayey, two sandy-sandy and one sandy soil. The authors observed similar behavior in soils containing higher clay contents, presenting higher soil-bound residue values throughout the evaluation period.
Metabolite and parental product formation
During the incubation period, only one metabolite resulting from diuron degradation was detected. The three soils were similar regarding parental product recovery and metabolite formation, and the amount of parent products was reduced in all assesssed soils throughout the incubation period (Figure 4). High parent product values were observed in NQo in relation to the anthropogenic soils throughout the incubation period, reaching 88, 68 and 50.45% diuron at 0, 35 and 70 DAA. Values were similar for all extraction times in the anthropogenic soils (79.5 and 77.3%; 53.6 and 54%; and 38.95 and 38.30% in TPI-1 and TPI-2 at 0, 35 and 70 DAA, respectively). Concomitantly, metabolite formation in Amazonian soils was higher than in NQo (5.6%) at 70 DAA, at 10 and 6.75% in TPI-1 and TPI-2, respectively.
Degradation of 14C-diuron extracted from parental compound and metabolites at 70 days after application (DAA) in two anthropogenic Amazonian soils (TPI-1 and TPI-2) and one sandy soil (NQo).
At 35 DAA, diuron in TPI-2 and NQo soils displayed the highest metabolite formation (8.83 and 9.00%, respectively), decerasing at the end of the incubation period (70 DAA), at 6.7% in TPI-2 and 5.6% in NQo. The highest amount of metabolites was found in TPI-1 at 70 DAA, at appoximately 10% of the total extracted residue (Figure 4).
The lowest diuron DT50 was observed in TPI-1, at 66.65 days, similar to TPI-2, at 68.63 days, which presentes the highest amount of formed metabolites, similar to TPI-2 (Figure 4). The highest DT50 (88.86 days) was found in the NQo, corresponding to the lowest percentage of formed metabolites. The DT50 values found in this study were possibly higher due to the OC levels in soils compared to the studies carried out by Dores et al. (2009Dores EFCG, Spadotto CA, Weber OLS, Carbo L, Vecchiato AB, Pinto AA. Environmental behaviour of metalachlor and diuron in a tropical soil in the central region of Brazil. Water Air Soil Pollut. 2009;197:175-83.), who found a 15 days DT50 for diuron applied to medium-sandy Latosol.
Given the above, diuron application in arable areas in the presence of anthropogenic Amazonian soils may lead to inefficient chemical weed control, as these soils may decrease herbicide soil bioavailability due to high OC levels, which presente high herbicide sorption and low desorption, as well as faster degradation rates wehn compared to the sandy soil.
ACKNOWLEDGEMENTS
The authors would like to thank the National Council for Scientific and Technological Development (CNPq) and the Luiz de Queiroz Foundation for Agricultural Studies (FEALQ), for financial support, and the Cellular and Molecular Biology Laboratory from the Center for Nuclear Energy in Agriculture (CENA/USP), for kindly donating the anthropogenic soils.
REFERENCES
- Arsego IB. Sorção dos herbicidas diuron e hexazinone em solos de texturas contrastantes [dissertation]. Piracicaba: Luiz de Queiroz College of Agriculture, 2009.
- Balliett A. Terra Preta. Magic Soil of the Lost Amazon. Acres. 2007;37:1-4.
- Barriuso E, Laird DA, Koskinen WC, Dowdy RH. Atrazine desorption from smectites. SSSA, 1994;58:1632-38.
- Boeira RC, Souza MD. Sorção de diuron em solos com diferentes texturas. Jaguariúna: Embrapa Meio Ambiente, 2004. 5p. (Circular Técnica, 9)
- Campos CF, Vitorino HS, Martins D. Controle de plantas daninhas com diuron em diferentes condições de luz. Rev Bras Herbic. 2012;11:258-68.
- Carbo LL, Martins EL, Dores EFGC, Spadotto CA, Weber OLS, Freire EMDL. Acetamiprid, carbendazim, diuron and thiamethoxam sorption in two Brazilian tropical soils. Environ Sci Health, Part B. 2007;42:49-507.
- Damin V. Biodegradação, sorção e dessorção do herbicida 14C-diuron em dois Latossolos tratados com lodo de esgoto [dissertation]. Piracicaba: Luiz de Queiroz College of Agriculture, 2005.
- Dores EFCG, Spadotto CA, Weber OLS, Carbo L, Vecchiato AB, Pinto AA. Environmental behaviour of metalachlor and diuron in a tropical soil in the central region of Brazil. Water Air Soil Pollut. 2009;197:175-83.
- El Sebaï T, Devers-Lamrani M, Lagacherie B, Rouard N, Soulas G, Laurent-Martin F. Isoproturon mineralization in an agricultural soil. Biol Fert Soils. 2010;47:427-35.
- Environmental Protection Agency - EPA. Soil thin layer chromatography. Washington: 1998. 6p. (EPA - Fate. transport and transformation test guidelines - OPPTS 835.1210).
- Fried B, Sharma J. Thin-Layer chromatography. 4th ed. New York: Marcel Dekker, 1999. 499p.
- Giaccomazi N, Cochet N. Environmental impact of diuron transformation: a review. Chemosphere. 2004;56:1021-32.
- Giori FG, Tornisielo VL, Cerri CEP, Regitano JB. Sugarcane straw management and soil attributes on alachlor and diuron sorption in highly weathered tropical soils. J Environ Sci Health Part B. 2014;49:352-60.
- Glaser B, Birk JJ. State of the scientific knowledge on properties and genesis of Anthropogenic Dark Earths in Central Amazonia (Terra Preta de Índio). Geochim Cosmochim Ac. 2012;82:39-51.
- Guimarães ACD, Mendes KF, Reis FC, Campion TF, Chirstoffoleti PJ, Tornisielo VL. Role of soil physicochemical properties in quantifying the of diuron, hexazinone, and metribuzin. Environ Sci Pollut Res Int. 2018;25:12419-33.
- Inoue MH, Oliveira Jr RS, Regitano JB, Tormena CA, Constantin J, Tornisielo VL. Sorption-desorption of atrazine and diuron in soils from Southern Brazil. J Environ Sci Health Part B, 2006;41:605-21.
- Kile DE, Wershaw RK, Chiou CT. Correlation of soil and sediment organic matter polarity to aqueous sorption of nonionic compounds. Environ Sci Technol. 1999;33:2053-6.
- Linhares LA, Egreja Filho FB, Iahnez R, Santos EA. Aplicação dos modelos de Langmuir e Freundlich na adsorção de cádmio e chumbo em diferentes classes de solos brasileiros. Rev Tecnol. 2008;17:49-60.
- Moreau C, Mouvet C. Sorption and Desorption of atrazine, deethlyazine, and hydroxyatrazine by soil and aquifers solids. J Environ Qual. 1997;26:416-24.
- Organisation for Economic Co-operation and Development - OECD. OECD guidelines for the testing of chemicals. Test number 106, Adsorption - Desorption Using a Batch Equilibrium Method. Paris: OECD, 2000. 44p.
- Organisation for Economic Co-Operation and Development - OECD. OECD Guideline for the Testing of Chemicals, 307, Aerobic and anaerobic transformation in soil. Paris: OECD, 2002. 17p.
- Oliveira MF, Brighenti AM, Comportamento de herbicidas no ambiente. In: Oliveira Jr. RS, Constantin J, Inoue MH (Eds.). Biologia e manejo de plantas daninhas. Curitiba: Omnipax. 2011 p.273-95.
- Petter FA, Madari BE. Biochar: Agronomic and environmental potencial in Brazilian savannah soils. Rev Bras Eng Agríc Amb. 2012;16:761-68.
- Picton P, Farenhorst A. Factors influencing 2,4-D sorption and mineralization in soil. J Environ Sci Health Part B. 2004;39:367-79.
- Pesticide Properties Database - PPDB. Footprint: creating tools for pesticide risk assessment and management in Europe. Developed by the Agriculture & Environment Research Unit (AERU), University of Hertfordshire, funded by UK national sources and the EU-funded FOOTPRINT project (FP6-SSP-022704), 2019.
- Prata F, Lavorenti A, Regitano JB, Torniselo VL. Degradação e adsorção de diuron em solos tratados com vinhaça. Rev Bras Cienc Solo. 2000;24:217-23.
- Rezende EIP, Angelo LC, Santos SS, Mangrich AS. Biocarvão (biochar) e sequestro de carbono. Rev Virtual Quím. 2011;5:426-33.
- Rocha PRR, Faria AT, Borges LGFC, Silva LOC, Silva AA, Ferreira EA. Sorção e dessorção do diuron em quatro Latossolos brasileiros. Planta Daninha. 2013;31:231-38.
- Rubbio-Bellido M, Morillo E, Villaverde J. Effect of addition of HPBCD on diuron adsorption-desorption, transport and mineralization in soils with different properties. Geoderma. 2016;265:196-203.
- Salloum MJ, Dudas MJ, McGill WB. Variation of 1-naphthol sorption with organic matter fractionation: the role of physical conformation. Org Geochem. 2001;32:709-19.
- Seybold CA, Mersie W. Adsorption and desorption atrazine, deethlyazine, deisopropylatrazine, hydroxyatrazine and metolachlor in two soils from Virginia. J Environ Qual. 1996;25:1179-85.
- Smernik RJ, Kookana RS. The effects of organic matter-mineral interaction and organic matter chemistry on diuron sorption across a diverse range of soils. Chemosphere. 2015;119:99-104.
- Woods WI, McCann JM. El origen y persistencia de las tierras negras de la Amazonía. In: Hiraoka M, Mora S. Desarrollo sostenible en la Amazonía. Mito o realidad? Quito: Ediciones Abya-Yala. 2001. p.23-30.
Publication Dates
-
Publication in this collection
17 Apr 2020 -
Date of issue
2020
History
-
Received
30 Nov 2018 -
Accepted
26 June 2019






 The vertical and horizontal bars represent the standard deviation of the means (n= 2) of Ce (equilibrium concentration) and Cs (sorbed soil concentration), respectively. Symbols may cover the bars.
The vertical and horizontal bars represent the standard deviation of the means (n= 2) of Ce (equilibrium concentration) and Cs (sorbed soil concentration), respectively. Symbols may cover the bars.
 The vertical bars represent the standard deviation (± SD) of the means (n= 2). Standard deviations were omitted when they were smaller than symbols. ** p<0.01 by the F test.
The vertical bars represent the standard deviation (± SD) of the means (n= 2). Standard deviations were omitted when they were smaller than symbols. ** p<0.01 by the F test.
 The vertical bars represent the standard deviation (± SD) of the means (n= 2).
The vertical bars represent the standard deviation (± SD) of the means (n= 2).
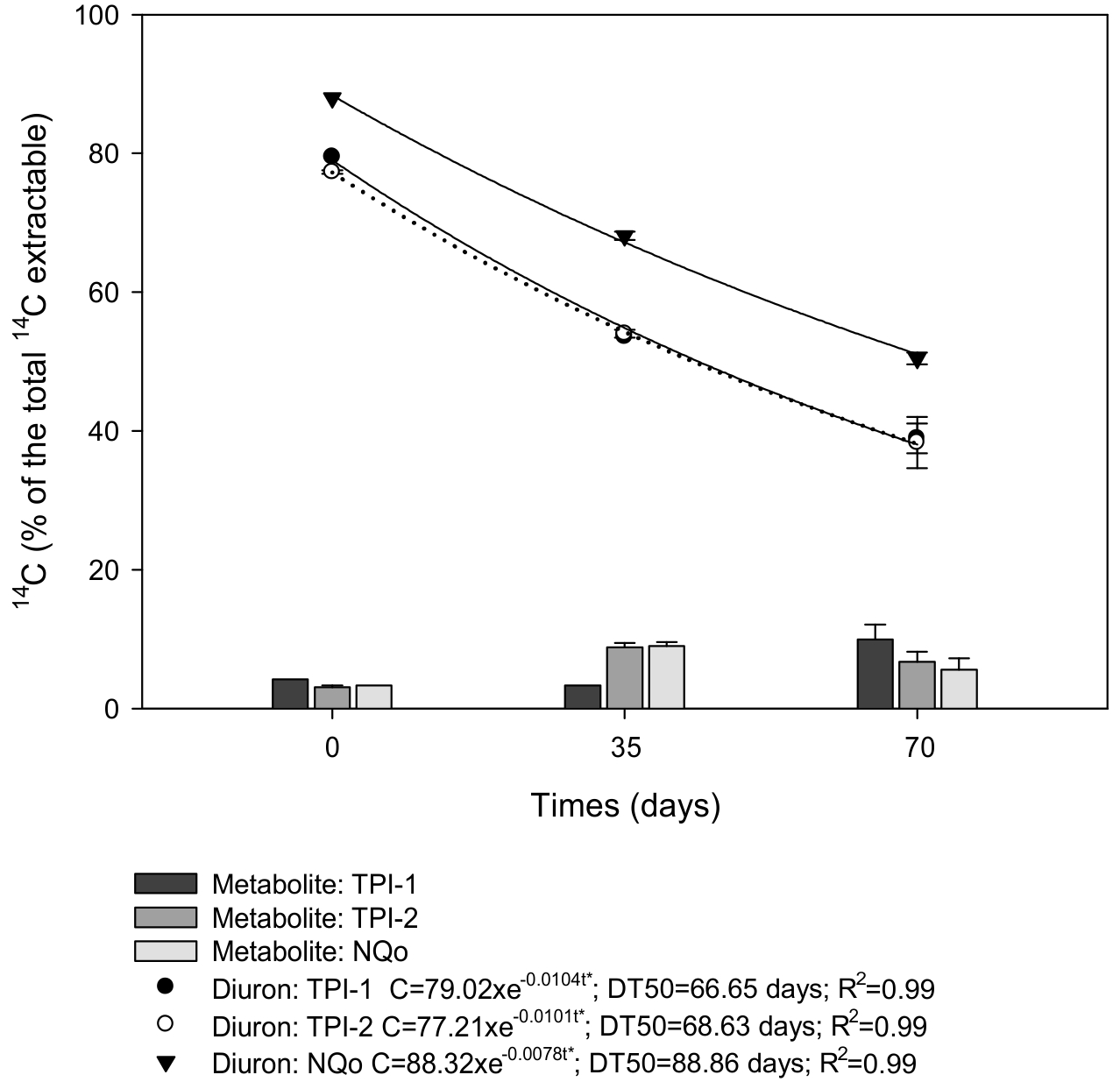 The vertical bars represent the standard deviation (± SD) of the means (n= 2). Standard deviations were omitted when smaller than symbols. **p<0.01 and *p<0.05 by the F test.
The vertical bars represent the standard deviation (± SD) of the means (n= 2). Standard deviations were omitted when smaller than symbols. **p<0.01 and *p<0.05 by the F test.